Abstract
The antiretroviral restriction factor TRIM5 has recently emerged as an important mediator of innate immunity and species-specific inhibition of retroviral replication in mammals. Selection pressure from pathogenic infection has driven rapid evolution of TRIM5 genes, leading to the antiviral specificities we see today. Remarkably, the New World owl monkey (Aotus trivirgatus) encodes a TRIM5 protein in which the antiviral determinants in the B30.2 domain have been replaced by cyclophilin A (CypA) encoded by a retrotransposed cDNA. The owl monkey TRIMCyp protein restricts infection by a subset of lentiviruses that recruit CypA to their capsids, including HIV-1 and feline immunodeficiency virus. Here, we show that the Old World monkey, rhesus macaque (Macaca mulatta), also encodes a TRIMCyp protein that has arisen independently from that in owl monkeys. The rhesus TRIMCyp is encoded by a single, but common, allele (Mamu7) of the rhesus TRIM5 gene, among at least six further alleles that encode full-length TRIM5 proteins with no homology to CypA. The antiviral specificity of the rhesus TRIMCyp is distinct, restricting infection of HIV-2 and feline immunodeficiency virus but not HIV-1. Restriction by rhesus TRIMCyp is before reverse transcription and inhibited by blocking CypA binding, with cyclosporine A, or by mutation of the capsid CypA binding site. These observations suggest a mechanism of restriction that is conserved between TRIMCyp proteins. The lack of activity against HIV-1 suggests that Mamu7 homozygous animals will be null for TRIM5-mediated restriction of HIV-1 and could contribute to improved animal models for HIV/AIDS.
Keywords: cyclophilin, lentivirus, restriction, TRIM5, zoonosis
TRIM5 has recently been identified as a powerful restriction factor responsible for species-specific restriction of retroviral infectivity as part of the innate immune system (1–6). TRIM5 has a tripartite, or RBCC, motif consisting of RING, B Box 2, and coiled coil domains with the characteristic ordering and spacing that defines the TRIM family. Together with many other members of the TRIM family, TRIM5 has a C-terminal PRY/SPRY, or B30.2, domain. In the case of TRIM5, this domain defines antiviral specificity, probably by interacting directly with the incoming viral capsid (7–13). Antiretroviral TRIM5 variants have been described in primates, cattle, and rabbits (1–3, 14–16). These TRIM5 sequences form a monophyletic group, indicating that they are derived from a common ancestor that probably had antiviral properties (16). TRIM5 blocks retroviral infectivity by an incompletely characterized mechanism that involves the proteasome (6, 17, 18) and may involve capsid uncoating (13, 19). In the New World owl monkey, the TRIM5 locus has been modified by insertion of a cyclophilin A (CypA) cDNA by retrotransposition into the seventh intron (20, 21), leading to the expression of a TRIM5 variant (TRIMCyp), in which CypA replaces the exon eight-encoded B30.2 domain. This change effectively replaces the antiviral specificity determinant, with CypA, leading to restriction of retroviruses that recruit CypA to their incoming capsid, including HIV-1, feline immunodeficiency virus (FIV), and simian immunodeficiency virus from Tantalus monkey (SIVtan) (20–23).
Here, we demonstrate that rhesus macaques also encode an antiviral TRIMCyp molecule that has evolved independently from that in owl monkeys. Convergent evolution is indicated by the two CypA sequences being in different positions in the owl monkey and rhesus TRIM5 loci. Furthermore, unlike the situation in owl monkeys, the rhesus TRIMCyp fusion is encoded by a single TRIM5 allele, among at least six further alleles that encode an intact B30.2 domain.
Results
Identification of a TRIMCyp in Rhesus Macaques.
The rhesus macaque TRIM5 gene has been demonstrated to be polymorphic, particularly within exon eight, which encodes the B30.2 domain and determines antiviral specificity (24). This finding suggests balancing selection at the TRIM5 locus, under selective pressure from pathogenic retroviruses. We sought to further characterize the degree of polymorphism in rhesus macaques by sequencing exon eight from a cohort of rhesus macaques (Macaca mulatta), which revealed the existence of a TRIM5 allele that we named Macaca mulatta TRIM5 allele seven (Mamu7) (Fig. 1A). Mamu7 has a frame shift in exon eight leading to a B30.2 domain that is truncated by 59 aa and a G to T mutation in the splice acceptor site at the end of the sixth intron that is likely to cause exon skipping (Fig. 1B). Inspection of the rhesus genome sequence revealed CypA sequences downstream of TRIM5 (data not shown). Knowing that owl monkeys encode a TRIM5 protein with a CypA domain, encoded by a retrotransposed cDNA, in place of its B30.2 domain (20, 21), we sought an RNA transcript in rhesus cells in which the TRIM5 RBCC domain is fused to CypA by PCR. Remarkably, we were able to amplify a rhesus macaque TRIMCyp (rhTRIMCyp) cDNA from a rhesus macaque cell line heterozygous for Mamu7 and Mamu1 (LLC-MK2) by using primers specific to the RING and to CypA (Fig. 1C). The sequence of the Mamu7 coding sequence from a homozygous rhesus macaque and LLC-MK2 cells were identical (data not shown). RhTRIMCyp and owl monkey TRIMCyp (omTRIMCyp) protein sequences are shown aligned (Fig. 1C). The rhTRIMCyp protein is distinct from the owl monkey protein in that it is encoded by exons 2 to 6 of TRIM5 and a CypA sequence, which replaces exons seven and eight. The owl monkey protein is encoded by exons two to seven of TRIM5 and a CypA cDNA inserted into the owl monkey genome by retrotransposition (20, 21). We then used genomic primers to PCR-amplify the CypA cDNA, and we sequenced it, thus revealing that it is in a different position than that in owl monkeys, indicating independent evolution of the two TRIMCyps. In owl monkeys, the CypA cDNA lies between exons seven and eight, whereas in the rhesus genome, the CypA cDNA is ≈900 nt downstream of the end of exon eight (Fig. 1D). The Mamu7 CypA sequence has the features of a cDNA that has been reinserted into the genome by retrotransposition by an L1 line element, including target site duplication and a polyadenylation sequence (Fig. 1E).
Fig. 1.
Identification of a rhesus TRIMCyp. (A) Diagram of the TRIM5α protein showing polymorphisms between proteins encoded by Mamu1 and Mamu7. The position of nonsynonymous (above) and synonymous differences (below) are shown. (B) The sixth intron of Mamu7 bears a mutation at the splice acceptor site (star). Exon7 sequence is shown (boxed). Conserved nucleotides are indicated (asterisk). (C) Alignment of the protein sequences of rhesus TRIMCyp (Mamu7) and owl monkey TRIMCyp (TCyp). Asterisk, identical residue; colon, conserved substitution; period, semiconserved substitution; gap, no conservation. RING, Bbox2 (BB), coiled coil (CC), and CypA domains are shown. (D) Diagram indicating splicing of TRIM5α encoded by Mamu1, rhesus TRIMCyp (Mamu7), and owl monkey TRIMCyp. Noncoding (gray) and coding (black) exons and CypA (striped) sequences are shown. In owl monkey, TRIMCyp CypA is encoded by a CypA cDNA in the seventh intron (20). (E) Genomic sequence of the Mamu7 CypA sequence. Target site duplication (TSD), splice acceptor (asterisk), start codon (circle), and polyadenylation signal and poly(A) sequences (underlined), which are typical of L1-mediated retrotransposition, are shown. (F) PCR using primers on either side of the CypA insertion indicates that it is unique to the Mamu7 allele. Mamu genotypes, as determined by sequencing the exon eight PCR product, are shown. H2O denotes water control.
To examine the frequency of Mamu7 (TRIMCyp) in rhesus macaques, we PCR-amplified TRIM5 exon eight from DNA purified from 31 Indian and 38 Chinese Macaca mulatta. Fifteen of 31 Indian animals were heterozygous for Mamu7, and 1 was homozygous (Table 1). Strikingly, we did not detect Mamu7 in any of the 38 Chinese macaques analyzed. A PCR screen using oligos directed against the genomic sequence either side of the CypA cDNA sequence was consistent with the exon eight sequencing data and revealed that the CypA insertion is unique to the Mamu7 allele (Fig. 1F). It also confirms the fact that FRhK4 cells do not encode a TRIMCyp and that LLC-MK2 cells are heterozygous for Mamu7 and Mamu1.
Table 1.
Frequency of TRIM5 alleles in rhesus macaque cohort
| Macaca mulatta origin | Allelic frequency |
||||
|---|---|---|---|---|---|
| Mamu1 (%) | Mamu3 (%) | Mamu4 (%) | Mamu5 (%) | Mamu7 (%) | |
| Indian | 12/62 (19.35) | 19/62 (30.65) | 7/62 (11.29) | 8/62 (12.9) | 16/62 (25.81) |
| Chinese | 4/76 (5.26) | 37/76 (48.68) | 25/76 (32.89) | 10/76 (13.16) | 0/76 (0) |
Rhesus TRIMCyp Restricts HIV-2 and FIV but Not HIV-1, SIV from Macaques (SIVmac), Equine Infectious Anemia Virus (EIAV), or Murine Leukemia Virus (MLV).
To examine the restriction specificity of rhTRIMCyp, we expressed it in permissive feline CRFK cells, as described in ref. 23. As a negative control we expressed empty vector, and as positive controls we expressed rhesus TRIM5α encoded by Mamu1 or omTRIMCyp. We then determined infectious titers of vesicular stomatitis virus (VSV)-G pseudotyped GFP-encoding retroviral vectors derived from HIV-1, HIV-2, FIV, SIVmac, MLV, and EIAV on pools of transduced cells, as well as on unmodified CRFK cells, as described in ref. 23 (Fig. 2). Remarkably, rhTRIMCyp was able to strongly restrict retroviral infectivity but had a significantly different antiviral specificity from omTRIMCyp. RhTRIMCyp strongly restricted both HIV-2 and FIV but not HIV-1. OmTRIMCyp restricted HIV-1 and FIV, as has been described (20–22). SIVmac, EIAV, and MLV were not restricted by either of the TRIMCyp proteins. Rhesus TRIM5α restricted HIV-1, HIV-2, FIV, and EIAV as has been described (1, 3, 24–26). Expression levels of TRIM5α, owl monkey TRIMCyp, and rhTRIMCyp were shown to be similar by Western blotting for the N-terminal HA tag (Fig. 2H). β-Actin served as a loading control.
Fig. 2.
Rhesus TRIMCyp restricts HIV-2 and FIV but not HIV-1, SIVmac, EIAV, or MLV. (A–G) Infectious titers of the viruses indicated were determined on unmodified feline cells (CRFK), CRFK cells bearing empty vector (EXN), CRFK cells expressing TRIM5α (Mamu1), rhesus (Mamu7), or owl monkey TRIMCyp (OMTC). Errors are standard deviations of titers determined at three multiplicities of infection and are representative of experiments performed with independent viral stocks. (H) Expression levels of rhesus TRIM5α (Mamu1), rhTRIMCyp (Mamu7), or owl monkey TRIMCyp (OMTC) were compared in CRFK cells or CRFK cells bearing empty vector (EXN) by Western blot analysis for the HA tag. β-actin was detected on parallel blots as a loading control.
Overexpression of Rhesus TRIMCyp Inhibits Restriction by Rhesus TRIM5α.
The fact that rhesus TRIM5α and TRIMCyp have different antiviral specificities suggests that each factor might have dominant negative activity against the other. To test this possibility, we transiently overexpressed rhTRIMCyp in rhesus FRhK4 cells, which are homozygous for Mamu1 (Fig. 1 and data not shown). As a positive control, we expressed human TRIM34. We then tested the permissivity of the modified FRhK4 cells to HIV-1, HIV-2, or MLV-B vectors encoding GFP (Fig. 3). Expression of RhTRIMCyp was strongly dominant negative against the antiviral activity of rhesus TRIM5α against HIV-1 (Fig. 3A). HIV-2 infectivity was slightly reduced by TRIMCyp expression, presumably because it is sensitive to both factors (Fig. 3B). Expression of human TRIM34 also was strongly dominant negative against rhesus TRIM5α, rescuing infectivity of HIV-1 and HIV-2. Infectivity of unrestricted MLV-B remained unaffected by expression of rhTRIMCyp, TRIM5α, or TRIM34 (Fig. 3C). These data suggest that, when overexpressed, TRIMCyp forms heteromultimers with endogenous TRIM5α that cannot restrict HIV-1. Exogenous expression of TRIM5α (Mamu1) reduced infectivity of both HIV-1 and HIV-2, as expected. Viral stocks encoding rhTRIMCyp, TRIM5α, and TRIM34 were equalized by measuring provirus copy number in infected cells by quantitative PCR (Q-PCR) as described in ref. 27 (data not shown). Expression levels of expressed TRIM34, TRIM5α, and rhTRIMCyp proteins were similar, as shown by Western blot analysis (Fig. 3D). The phenomenon of dominant negative activity of TRIM5-related proteins, on overexpression, has been described for related TRIMs (23) and deleted TRIM5 (7) and natural shorter splice variants of TRIM5 itself, TRIM5γ or -δ (1, 28). We presume that the dominant negative activity is caused by overexpression and that at endogenous levels both factors are likely to restrict in a codominant way. This finding is true for the murine restriction factor Fv1, which is codominant in the mouse (29), but dominant negative when overexpressed in homozygous cell lines in experiments similar to those described here (30).
Fig. 3.
Overexpression of rhesus TRIMCyp inhibits restriction by rhesus TRIM5α. FRhK4 cells were transduced with equal doses of MLV vector encoding TRIM5α or rhesus TRIMCyp. A human TRIM34 expression vector was used as a positive control. GFP-encoding VSV-G pseudotypes of HIV-1 (A), HIV-2 (B), or MLV-B (C) were titrated on the modified cells 48 h later. Titers are expressed as infectious units/ml (IU/ml) determined as described (Fig. 2). Errors are standard deviations derived from triplicate experiments and are representative of at least two experiments using independent viral stocks. (D) Parallel infections were harvested at 48 h, and TRIM protein expression was determined by Western blotting analysis for the N-terminal HA tag.
Drugs or Mutations That Inhibit CypA Recruitment to Capsid Prevent rhTRIMCyp Antiviral Activity.
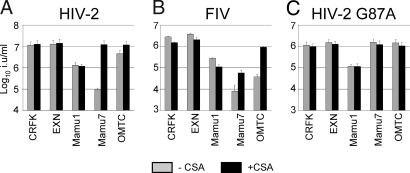
It is likely that, like omTRIMCyp, the rhesus TRIMCyp protein restricts infection via recruitment of the TRIM5 RBCC domain to incoming capsids via CypA–capsid interactions. To test this, we used the drug cyclosporine A (CSA), which binds and blocks the CypA active site and rescues omTRIMCyp-restricted infection (20, 21, 31). We infected CRFK cells expressing rhTRIMCyp with HIV-2- or FIV-derived vectors in the presence and absence of 5 μM CSA. Blocking the CypA binding site with CSA specifically rescued HIV-2 or FIV infectivity when restricted by rhTRIMCyp but not when restricted by rhesus TRIM5α (Fig. 4 A and B). HIV-2 infectivity is completely rescued by CSA and FIV infectivity is rescued specifically, although not completely. Titration of CSA up to 15 μM indicated that even high concentrations of CSA did not completely rescue FIV infectivity (data not shown). We interpret these observations as showing that, like owl monkey TRIMCyp, rhTRIMCyp restricts virus via recruitment of the CypA domain to the viral capsid.
Fig. 4.
Drugs or mutations that inhibit CypA recruitment to capsid prevent rhTRIMCyp antiviral activity. Titers of wild-type HIV-2 (A), FIV (B), or HIV-2 bearing capsid mutation G87A (C) were measured on CRFK cells (CRFK), CRFK cells bearing empty vector (EXN), CRFK cells expressing TRIM5α (Mamu1), CRFK cells expressing rhesus TRIMCyp (Mamu7), or CRFK cells expressing owl monkey TRIMCyp (OMTC) in the presence (black bars) or absence (gray bars) of 5 μM CSA. Errors are standard deviations of titers determined at three multiplicities of infection and are representative of experiments performed with independent viral stocks.
Previous work has shown that, unlike HIV-1, HIV-2 does not recruit CypA into viral particles (32). However, HIV-2 does have a conserved glycine-proline motif homologous to the CypA binding motif of HIV-1. To test whether this motif is responsible for sensitivity to rhTRIMCyp, we used an HIV-2 in which this motif has been mutated to a sequence that does not recruit CypA in the context of HIV-1 (HIV-2 capsid G87A). In fact, HIV-2 capsid G87A was insensitive to rhTRIMCyp (Fig. 4C), suggesting that this motif recruits rhTRIMCyp. Importantly, HIV-2 G87A remains restricted by rhesus TRIM5α (Fig. 4B). An FIV capsid mutant capsid P90A has been described as being insensitive to restriction by omTRIMCyp, suggesting that the CypA domain is recruited to this proline (33). However, in our hands, this mutant is three to four orders of magnitude less infectious than the wild-type virus, making it difficult to test whether it is sensitive to rhTRIMCyp (data not shown).
Rhesus TRIMCyp Blocks Restricted Viral Reverse Transcription in a Proteasome-Dependent Way.
Both TRIM5α and omTRIMCyp strongly block retroviral DNA synthesis in most cases of restricted infectivity. Moreover, restricted viral DNA synthesis, but not infectivity, is rescued by inhibition of the proteasome (17, 18), which suggests that restricted virions are rapidly degraded by the proteasome, before they can reverse-transcribe, but that the proteasome is not required for the block to infectivity. We examined whether restriction by rhTRIMCyp conserves these features. We infected CRFK cells expressing rhTRIMCyp with HIV-2 or FIV in the presence and absence of proteasome inhibitor and measured infection, or DNA synthesis, in parallel samples (Fig. 5). The results show that rhTRIMCyp causes a strong block to HIV-2 and FIV infectivity and DNA synthesis. We note that, as is often the case, the block to infectivity is greater than the block to DNA synthesis, suggesting that some restricted virions are able to reverse-transcribe. Importantly, DNA synthesis, but not infectivity, of rhTRIMCyp-restricted virus is rescued by inhibition of the proteasome. These observations are similar to those reported for restriction of HIV-1 by rhesus TRIM5α and omTRIMCyp (17, 18). It therefore is likely that the mechanism of restriction is conserved between TRIM5α and TRIMCyp. Indeed, the only significant difference between TRIM5α and the two TRIMCyps is likely to be the mechanism of recruitment to the viral capsid, by a B30.2 domain or a CypA domain, respectively. Boiling the virus before infection abrogates the production of viral DNA (Fig. 5 B and D), indicating that the Q-PCR signal is caused by viral reverse transcription and not plasmid contamination.
Fig. 5.
Rhesus TRIMCyp blocks restricted viral reverse transcription in a proteasome-dependent way. Cells expressing rhTRIMCyp (Mamu7), or unmodified cells (CRFK), were infected with HIV-2 (A and B) or FIV (C and D) in triplicate in the presence (black bars) or absence (gray bars) of proteasome inhibitor. One sample was subjected to FACS to determine infectious titer at 48 h (A and C), and total DNA was purified from the second and third sample 6 h after infection for measurement of viral DNA synthesis (B and D). Viral DNA synthesis is expressed as molecules of GFP template per 100 ng of total DNA. Errors are standard deviations of values derived from parallel infections, and data are representative of independent experiments performed by using independent stocks of virus.
Discussion
This study describes a TRIMCyp protein in Indian rhesus macaques. The protein is expressed from a single allele of the TRIM5 gene (Mamu7) by exon skipping from exon six to a CypA sequence derived from a reverse-transcribed, integrated CypA cDNA, probably generated by L1-mediated retrotransposition (Fig. 1). Remarkably, this is the second case of fusion of a simian TRIM5 RBCC domain to CypA. In the New World owl monkey, a similar L1-mediated retrotransposition event has inserted a CypA cDNA into the seventh intron of the TRIM5 gene, leading to the expression of the owl monkey TRIMCyp. This protein strongly restricts infection by a variety of unrelated retroviruses (20–23). Importantly, the different locations of the CypA cDNA in the owl monkey and rhesus TRIM5 loci indicate that they have evolved independently (Fig. 1E).
Despite their similarity, the two TRIMCyp proteins have distinct antiviral specificities, differentiating between viruses that have arisen from different SIV lineages, i.e., HIV-1 and HIV-2 (Fig. 2). The Mamu7 CypA sequence differs from the rhesus CypA sequence, from which it was derived, at two positions close to the active site (D369N and R372H), which suggests that the rhesus and owl monkey proteins have been under selection pressures from different pathogenic viruses, consistent with their Old and New World origins, respectively. Such evidence for change at the site of virus recruitment is reminiscent of positive selection in the TRIM5 B30.2 domain, which also leads to differences in antiviral specificity between species (24, 34). The observation that Mamu7 is common in Indian but not Chinese rhesus macaques is consistent with geographic variation in selection pressure on the TRIM5 locus. The fact that rhTRIMCyp does not restrict HIV-1 (Fig. 2) suggests that Mamu7 homozygous animals will be more permissive to HIV-1 replication and therefore useful in the development of a macaque animal model for HIV-1 infection.
RhTRIMCyp-restricted infectivity is rescued by the competitive inhibitor of CypA binding, CSA, indicating that the factor is recruited to virions via CypA–capsid interactions. The reason for the incomplete rescue of FIV infectivity by CSA is unclear, but high concentrations of the factor in the transduced cells, or high affinity binding between rhTRIMCyp and the FIV capsid, are possible explanations. Importantly, HIV-2 is rendered insensitive to restriction by mutation of the glycine-proline motif homologous to the site in HIV-1 that recruits CypA. It is surprising that rhTRIMCyp restricts HIV-2, a virus that has hitherto been thought not to recruit CypA to its capsid. However, we speculate that lentiviruses in general may recruit CypA after entering the target cell cytoplasm as evidenced by the conservation of proline-rich structures in primate lentiviral capsid sequences (6, 22). We propose that these motifs lead to the restriction of HIV-1, SIVtan, and FIV by omTRIMCyp and FIV and HIV-2 by rhTRIMCyp (Fig. 2) (20–23, 33). The role of CypA in lentiviral infection remains unclear. It has been suggested to be involved in uncoating, but mutation of the CypA recruiting motif does not significantly reduce infectivity of, for example, HIV-1 capsid G89V in feline cells (35) or HIV-2 capsid G87A in human cells (25). More recently, it has been suggested that CypA acts to influence sensitivity of retroviruses to restriction factors (6, 31, 35, 36).
It is remarkable that the same two genes have been fused together by a process of exon shuffling by L1-mediated retrotransposition on more than one occasion. It is unclear which property of TRIM5 makes it an attractive candidate for fusion to CypA, especially given that artificial fusion of CypA to divergent TRIMs (37), and unrelated molecules (38–40) can make effective restriction factors. It is also unclear which viruses might have been responsible for providing selection, but it is notable that a growing number of unrelated viruses have been shown to be influenced by cyclophilins, including vaccinia virus (41), hepatitis C (42), and murine cytomegalovirus (43). If cyclophilins are commonly recruited to virion proteins, they may be particularly effective as virus binding domains for restriction factors. The attraction of the TRIM5 RBCC domain remains unclear but is presumably mechanistic. The fact that restriction by TRIM5α and TRIMCyp leads to a strong block to reverse transcription that is sensitive to inhibition of the proteasome (Fig. 5) (17, 18) suggests a common mechanism. We presume that restricted complexes are rapidly recruited to the proteasome, but that virus infectivity is lost, even if the restricted virus is protected from degradation by inhibition of the proteasome (17, 18). We hypothesize that disruption of capsid rearrangement, uncoating, or trafficking causes this proteasome-independent block to infectivity.
Transposition of Alu elements and processed pseudogenes is thought to have played an important role in mammalian evolution. It is assumed that in most cases, retrotransposition and integration of cDNA sequences into the genome leads to the production of inactive pseudogenes. These sequences decay in the absence of purifying selection and usually are mutated when compared with the original cDNA sequence. Our data suggest that mammalian genomes may have sequences that appear to be pseudogenes but are in fact part of a functional gene modified by retrotransposition, as is the case with TRIMCyps. Characterization of open, and relatively intact, “pseudogenes” may well lead to the discovery of further examples of exon shuffling mediated by retrotransposition in mammalian genomes.
Methods
Cloning Rhesus TRIMCyp.
Rhesus TRIMCyp was cloned from cDNA prepared from the LLC-MK2 Macaca mulatta rhesus kidney cell line RNA by using SuperScript (Invitrogen) according to manufacturer's instructions. PCR was performed by using primers (forward) 5′-GATCGAATTCATGGCTTCTGAAATCCTGCTTAATG-3′ and (reverse) 5′-CAAGTTCGAATTATTTGAGTTGCCCACAGTCAGC-3′ and pfu turbo (Stratagene). Sequences of three independent clones were determined to ensure accuracy. CDNAs encoding TRIM5 Mamu1, omTRIMCyp, rhTRIMCyp, or human TRIM34 were cloned into the LNCX2-based MLV retroviral vector pEXN (a gift from Paul Bieniasz, The Rockefeller University, New York) between the EcoR1 and Csp54I/ClaI sites (underlined) in frame with an N-terminal HA tag (23). The MLV vectors were packaged into VSV-G pseudotyped Moloney MLV cores and expressed in permissive feline CRFK cells as described (23, 44). Transduced CRFK cells were selected in G418 (1 mg/ml; Invitrogen), and pools of cells were used for subsequent titration experiments. The rhesus TRIMCyp sequence has GenBank accession no. EU157763.
Sequencing TRIM5 Alleles from Rhesus Monkeys (Macaca mulatta).
Exon eight was amplified by PCR from rhesus macaque genomic DNA samples (Biomedical Primate Research Centre, Rijswijk, The Netherlands) by using primers (forward) 5′-CTTCTGAACAAGTTTCCTCCCAG-3′ and (reverse) 5′-ATGAGATGCACATGGACAAGAGG-3′. PCR products were sequenced directly. Exon eight heterozygosity was resolved by cloning PCR products and sequencing multiple clones. Sequences were analyzed by using DNADynamo (Bluetractor Software). PCR of the CypA insertion used primers (forward) 5′-TGACTCTGTGCTCACCAAGCTCTTG-3′ and (reverse) 5′-ACCCTACTATGCAATAAAACATTAG-3′ and GoTaq polymerase (Promega) according to manufacturer's instructions. The PCR product for the CypA insertion was sequenced directly.
Viral Vectors.
VSV-G protein pseudotyped retroviral vectors encoding GFP and derived from HIV-1, HIV-2, SIVmac, FIV, EIAV, or MLV were produced by triple transfection of 293T cells as described (44, 45). Viral stocks were titrated onto cells and infected cells enumerated by fluorescence-activated cell sorting (FACS), as described in ref. 46. Titers were determined at multiplicities of infection between 0.01 and 0.3. The HIV-2 capsid mutant G87A has been described (25). Cyclosporine (Sandoz) was diluted in DMSO and added to cells at the time of infection, where stated, at 5 μM.
Q-PCR.
Retroviral DNA synthesis by reverse transcription was measured by Taqman Q-PCR as described (27, 44). Samples were infected with DNase-treated virus (70 units/ml; Promega) in the presence or absence of MG132 (1 μg/ml; Sigma) for 6 h. Total DNA was purified (QiaAmp; Qiagen) and subjected to Taqman PCR (GFP amplicon) (27, 44). A parallel sample was subjected to FACS to determine an infectious titer.
ACKNOWLEDGMENTS.
We thank Welkin Johnson and Ruchi Newman for sharing unpublished observations, Salvatore Butera for helpful discussion and advice, and Ivonne Nieuwenhuis, Claire Pardieu, Imogen Lai, Paul Clapham, Paul Bieniasz, Eric Poeschla, Andrew Lever, Jonathan Stoye, Didier Trono, Francois Loic Cosset, Kyriacos Mitrophanous, and Adrian Thrasher for reagents. We thank the Wellcome Trust for funding this work through a senior fellowship (to G.J.T.).
Footnotes
References
- 1.Stremlau M, et al. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 2.Keckesova Z, Ylinen LM, Towers GJ. The human and African green monkey TRIM5α genes encode Ref1 and Lv1 retroviral restriction factor activities. Proc Natl Acad Sci USA. 2004;101:10780–10785. doi: 10.1073/pnas.0402474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatziioannou T, Perez-Caballero D, Yang A, Cowan S, Bieniasz PD. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5α. Proc Natl Acad Sci USA. 2004;101:10774–10779. doi: 10.1073/pnas.0402361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap MW, Nisole S, Lynch C, Stoye JP. Trim5α protein restricts both HIV-1 and murine leukemia virus. Proc Natl Acad Sci USA. 2004;101:10786–10791. doi: 10.1073/pnas.0402876101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perron MJ, et al. TRIM5α mediates the postentry block to N-tropic murine leukemia viruses in human cells. Proc Natl Acad Sci USA. 2004;101:11827–11832. doi: 10.1073/pnas.0403364101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towers GJ. The control of viral infection by tripartite motif proteins and cyclophilin A. Retrovirology. 2007;4:40. doi: 10.1186/1742-4690-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Caballero D, Hatziioannou T, Yang A, Cowan S, Bieniasz PD. Human tripartite motif 5{α} domains responsible for retrovirus restriction activity and specificity. J Virol. 2005;79:8969–8978. doi: 10.1128/JVI.79.14.8969-8978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yap MW, Nisole S, Stoye JP. A single amino acid change in the SPRY domain of human Trim5α leads to HIV-1. Restriction Curr Biol. 2005;15:73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 9.Stremlau M, Perron MJ, Welikala S, Sodroski J. Species-specific variation in the B30.2(SPRY) domain of TRIM5α determines the potency of human immunodeficiency virus restriction. J Virol. 2005;79:3139–3145. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perron MJ, Stremlau M, Sodroski J. Two surface-exposed elements of the B30.2/SPRY domain as potency determinants of N-tropic murine leukemia virus restriction by human TRIM5α. J Virol. 2006;80:5631–5636. doi: 10.1128/JVI.00219-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkura S, Yap MW, Sheldon T, Stoye JP. All three variable regions of the TRIM5α B30.2 domain can contribute to the specificity of retrovirus restriction. J Virol. 2006;80:8554–8565. doi: 10.1128/JVI.00688-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebastian S, Luban J. TRIM5α selectively binds a restriction-sensitive retroviral capsid. Retrovirology. 2005;2:40. doi: 10.1186/1742-4690-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stremlau M, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5α restriction factor. Proc Natl Acad Sci USA. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ylinen LM, et al. Isolation of an active Lv1 gene from cattle indicates that tripartite motif protein-mediated innate immunity to retroviral infection is widespread among mammals. J Virol. 2006;80:7332–7338. doi: 10.1128/JVI.00516-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Si Z, et al. Evolution of a cytoplasmic tripartite motif (TRIM) protein in cows that restricts retroviral infection. Proc Natl Acad Sci USA. 2006;103:7454–7459. doi: 10.1073/pnas.0600771103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaller T, Hue S, Towers GJ. An active TRIM5 in rabbits indicates an antiviral common ancestor for mammalian TRIM5 proteins. J Virol. 2007;81:11713–11721. doi: 10.1128/JVI.01468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. Proteasome inhibitors uncouple rhesus TRIM5α restriction of HIV-1 reverse transcription and infection. Proc Natl Acad Sci USA. 2006;103:7465–7470. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson JL, et al. Proteasome inhibition reveals that a functional preintegration complex intermediate can be generated during restriction by diverse TRIM5 proteins. J Virol. 2006;80:9754–9760. doi: 10.1128/JVI.01052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perron MJ, et al. The human TRIM5{α} restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid. J Virol. 2007;81:2138–2148. doi: 10.1128/JVI.02318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin A retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430:569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- 21.Nisole S, Lynch C, Stoye JP, Yap MW. A Trim5–cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci USA. 2004;101:13324–13328. doi: 10.1073/pnas.0404640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Griffero F, et al. Requirements for capsid-binding and an effector function in TRIMCyp-mediated restriction of HIV-1. Virology. 2006;351:404–417. doi: 10.1016/j.virol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Hatziioannou T, Perez-Caballero D, Derse D, Bieniasz PD. Antiretroviral potential of human tripartite motif-5 and related proteins. Virology. 2006;353:396–409. doi: 10.1016/j.virol.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Newman RM, et al. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5α. Proc Natl Acad Sci USA. 2006;103:19134–19139. doi: 10.1073/pnas.0605838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ylinen L, Keckesova Z, Wilson SJ, Ranasinghe S, Towers GJ. Differential restriction of HIV-2 and SIVmac by TRIM5α alleles. J Virol. 2005;79:11580–11587. doi: 10.1128/JVI.79.18.11580-11587.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saenz DT, Teo W, Olsen JC, Poeschla EM. Restriction of feline immunodeficiency virus by Ref1, Lv1, and primate TRIM5α proteins. J Virol. 2005;79:15175–15188. doi: 10.1128/JVI.79.24.15175-15188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Towers GJ, et al. One step screening of retroviral producer clones by real time quantitative PCR. J Gene Med. 1999;1:352–359. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<352::AID-JGM57>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Passerini LD, Keckesova Z, Towers GJ. Retroviral restriction factors Fv1 and TRIM5{α} act independently and can compete for incoming virus before reverse transcription. J Virol. 2006;80:2100–2105. doi: 10.1128/JVI.80.5.2100-2105.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pincus T, Rowe WP, Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses II: Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971;133:1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bock M, Bishop K, Towers G, Stoye JP. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J Virol. 2000;74:7422–7430. doi: 10.1128/jvi.74.16.7422-7430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towers GJ, et al. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat Med. 2003;9:1138–1143. doi: 10.1038/nm910. [DOI] [PubMed] [Google Scholar]
- 32.Braaten D, Franke EK, Luban J. Cyclophilin A is required for the replication of group M human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus SIV (CPZ)GAB but not group O HIV-1 or other primate immunodeficiency viruses. J Virol. 1996;70:4220–4227. doi: 10.1128/jvi.70.7.4220-4227.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin TY, Emerman M. Cyclophilin A interacts with diverse lentiviral capsids. Retrovirology. 2006;3:70. doi: 10.1186/1742-4690-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawyer SL, Wu LI, Emerman M, Malik HS. Positive selection of primate TRIM5α identifies a critical species-specific retroviral restriction domain. Proc Natl Acad Sci USA. 2005;102:2832–2837. doi: 10.1073/pnas.0409853102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keckesova Z, Ylinen L, Towers GJ. Cyclophilin A renders HIV-1 sensitive to old world monkey but not human TRIM5a antiviral activity. J Virol. 2006;80:4683–4690. doi: 10.1128/JVI.80.10.4683-4690.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berthoux L, Sebastian S, Sokolskaja E, Luban J. Cyclophilin A is required for TRIM5{α}-mediated resistance to HIV-1 in Old World monkey cells. Proc Natl Acad Sci USA. 2005;102:14849–14853. doi: 10.1073/pnas.0505659102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yap MW, Dodding MP, Stoye JP. Trim-cyclophilin A fusion proteins can restrict human immunodeficiency virus type 1 infection at two distinct phases in the viral life cycle. J Virol. 2006;80:4061–4067. doi: 10.1128/JVI.80.8.4061-4067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaller T, Ylinen LM, Webb BL, Singh S, Towers GJ. Fusion of cyclophilin A to fv1 enables cyclosporine-sensitive restriction of human and feline immunodeficiency viruses. J Virol. 2007;81:10055–10063. doi: 10.1128/JVI.00616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yap MW, Mortuza GB, Taylor IA, Stoye JP. The design of artificial retroviral restriction factors. Virology. 2007;365:302–314. doi: 10.1016/j.virol.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Javanbakht H, et al. The ability of multimerized cyclophilin A to restrict retrovirus infection. Virology. 2007;367:19–29. doi: 10.1016/j.virol.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro AP, Carvalho TM, Moussatche N, Damaso CR. Redistribution of cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles. J Virol. 2003;77:9052–9068. doi: 10.1128/JVI.77.16.9052-9068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watashi K, et al. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol Cell. 2005;19:111–122. doi: 10.1016/j.molcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Kawasaki H, Mocarski ES, Kosugi I, Tsutsui Y. Cyclosporine inhibits mouse cytomegalovirus infection via a cyclophilin-dependent pathway specifically in neural stem/progenitor cells. J Virol. 2007;81:9013–9023. doi: 10.1128/JVI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Besnier C, Takeuchi Y, Towers G. Restriction of lentivirus in monkeys. Proc Natl Acad Sci USA. 2002;99:11920–11925. doi: 10.1073/pnas.172384599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatziioannou T, Cowan S, Goff SP, Bieniasz PD, Towers GJ. Restriction of multiple divergent retroviruses by Lv1 and Ref1. EMBO J. 2003;22:385–394. doi: 10.1093/emboj/cdg042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towers G, et al. A conserved mechanism of retrovirus restriction in mammals. Proc Natl Acad Sci USA. 2000;97:12295–12299. doi: 10.1073/pnas.200286297. [DOI] [PMC free article] [PubMed] [Google Scholar]