Abstract
Antigen-specific transplantation tolerance in the absence of immunosuppressive drugs is a rarely achieved goal. Immune responses to Y chromosome-encoded transplantation antigens (HY) can have life-threatening consequences in the clinic. Here, we have adopted a procedure developed in T cell antigen receptor (TCR)-transgenic mice to convert naïve T cells into male-specific Foxp3+ regulatory T cells (Tregs) in WT female mice. For this purpose, female mice were infused by osmotic minipumps with a single class II MHC-presented HY peptide and Tregs visualized by tetramer staining. As a result, animals developed Treg-mediated long-term tolerance to all HY transplantation antigens, irrespective of whether they were recognized by CD4 or CD8 T cells, on skin or hematopoietic grafts from male donors.
Keywords: Foxp3, induction of tolerance, transplantation tolerance
Several mechanisms account for self–nonself discrimination by the immune system: Clonal deletion of autoreactive T cells within the thymus represents one important mechanism. Despite its high efficiency, it is insufficient to prevent the accumulation of self-reactive T cells in peripheral lymphoid tissues. These escapees can be kept in check by CD4+CD25+ Foxp3-expressing regulatory T cells (Tregs), which suppress their activation and effector function (1–3). Importantly, upon antigenic stimulation, Tregs can control responses of neighboring T cells with different antigen specificity in vivo (2, 4, 5).
A variety of experiments indicated that Tregs can delay or cure mice of diabetes, colitis, and graft-versus-host disease (GVHD) or interfere with graft rejection (6–14). Some of these experiments indicate that the efficacy of Treg-based immune therapy crucially depends on the antigen specificity of Tregs. On the other hand, one of the major limitations to devise strategies for the clinical use of Tregs is the difficulty in obtaining antigen-specific Tregs.
Antigen-specific Foxp3+ Tregs can be induced by either expressing antigens in certain tissue, such as thymic epithelial cells, or delivering antigen under subimmunogenic conditions (15–18). In T cell antigen receptor (TCR)-transgenic mice, foreign TCR agonist peptides, when supplied over a 2-week period through infusion by implanted osmotic minipumps or by antigen-DEC205 fusion antibodies targeting dendritic cells, could be used to instruct naïve T cells to become Foxp3+ Tregs, which mediated tolerance and could persist for long periods of time in the absence of their inducing antigen (17, 18).
The de novo induction of antigen-specific Foxp3+ Tregs has been documented unambiguously with T cells with transgenic TCRs specific for a limited number of antigens (17, 18), consistent with earlier work that reported the accumulation of Tregs under similar experimental conditions (19–21). It is important to establish that lessons learned from artificial transgenic systems can be translated to nontransgenic organisms. Such a task requires visualization of Tregs with defined antigen specificity in WT mice, where the very low frequency of T cells with a given antigen specificity makes it difficult especially because antigen-induced conversion takes place mostly in nondividing cells (17, 18).
Here, we attempted to generate HY-specific Tregs with the goal to interfere with HY antigen-specific graft rejection, which represents a serious complication in human transplantation (22) and can be conveniently studied in mice. In H-2b mice, the HY-specific response is largely restricted to single immunodominant class II and class I MHC-presented epitopes (22, 23). Because the supply of antigen by implanted osmotic minipumps is effective in inducing Tregs over a wide dose range of peptides, whereas delivery via DEC205 fusion antibodies works in a narrow dose range, C57BL/6 (B6) female mice were infused with the immunodominant class II MHC-presented HY peptide with osmotic minipumps. The impact of peptide infusion on both CD4 and CD8 T cell responses was directly analyzed by visualizing male-specific CD8 and CD4 T cells with the aid of MHC class I and class II HY peptide tetramers.
The results show that the supply of peptide under subimmunogenic conditions can induce complete transplantation tolerance in WT mice by converting naïve HY-specific CD4+ T cells into Foxp3+ Tregs, which in turn suppress the response of male-specific CD4 and CD8 T cells even when the latter recognize peptides from a different HY protein.
Results
Induction of Transplantation Tolerance to Male Tissues in WT Mice.
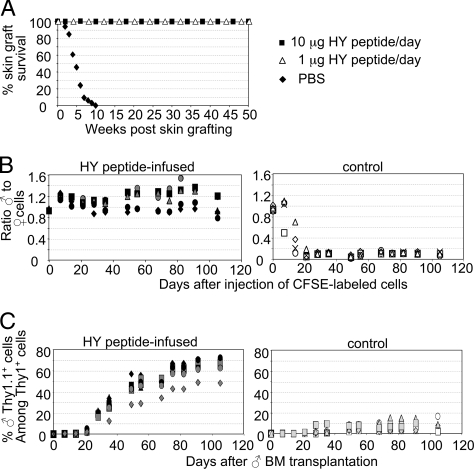
To test whether peptide infusion of WT animals would result in antigen-specific tolerance, B6 female mice were s.c. implanted with osmotic minipumps infusing daily for 14 days either 1 or 10 μg of the immunodominant I-Ab-restricted, DbY-encoded HY peptide, NAGFNSNRANSSRSS (HY peptide), or PBS. Peptide-infused and PBS-infused female mice were subsequently grafted with male skin. HY peptide-infused female mice retained their grafts indefinitely irrespective of the supplied peptide dose (Fig. 1A). In contrast, control mice rejected male skin within 5–10 weeks (Fig. 1A). All subsequent experiments were carried out by using a daily dose of 10 μg of HY peptide.
Fig. 1.
Induction of transplantation tolerance to male tissues in WT female mice. (A) Twenty female mice were implanted with osmotic minipumps infusing either the HY peptide (filled squares, 10 μg/day; open triangles, 1 μg/day) or PBS (filled diamonds) over a 2-week period. Mice were subsequently grafted with male skin and monitored for graft rejection. (B) B6 control as well as B6 female mice that were supplied with 10 μg/day of HY peptide as described in A (n = 5, each group) were injected with a mixture of CFSElow male and CFSEhi female B6 spleen cells. The ratio of male to female injected hematopoietic cells among PBLs was determined by FACS. Each symbol represents an individual mouse. (C) Two hundred rad-irradiated control (n = 8) or peptide-infused (n = 7) B6 female mice received 5 × 106 B6 Thy1.1 BM cells. PBLs collected when indicated were stained for Thy1.1 and Thy1.2 surface markers, and the ratio of male to female cells was determined by FACS. Each symbol represents an individual mouse. Data are representative of at least four independent experiments.
To test whether tolerance to male leucocytes also could be achieved, peptide-infused or control female mice were injected with an equal mixture of male and female spleen cells, each labeled with different 5,6-carboxyfluorescein diacetate-succinimidyl ester (CFSE) intensity to enable in vivo tracing. Peripheral blood lymphocytes (PBLs) of recipient mice were subsequently monitored in 7-day intervals. In control mice, the ratio of male to female CFSE-labeled cells declined rapidly, resulting in complete elimination of male cells after 3 weeks, whereas in peptide-infused mice the ratio remained constant, indicating that mice had become tolerant to HY-expressing cells (Fig. 1B).
To test whether this procedure was suited to establish stable mixed hematopoietic chimerism after bone marrow (BM) transplantation, we injected male Thy1.1 B6 BM cells into sublethally irradiated HY peptide-infused or control B6 (Thy1.2) female mice. In the blood of control mice, the ratio of male to female cells was low (Fig. 1C). In contrast, stable mixed hematopoietic chimerism was observed in peptide-infused recipients (Fig. 1C).
Reduced Frequency of HY-Specific T Cells and Male-Specific Cytokine Response in Female Mice Tolerized to Male Tissue.
Tracking of antigen-specific T cells is required to identify mechanisms regulating antigen-specific immune responses. Studies in transgenic models have provided significant advances in this regard. Yet, findings made in transgenic animals cannot always be reproduced in WT animals. Indeed, WT animals contain only a tiny proportion of T cells with a given antigen specificity, which may respond differently than the more frequent cells with transgenic TCRs.
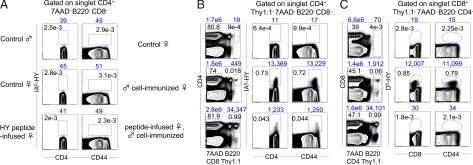
To identify HY peptide-specific CD4 T cells, we generated HY peptide-loaded I-Ab tetramers (HY-IAb tetramers) [for details, see supporting information (SI) Materials and Methods]. In naïve female and male mice, very few, if any, tetramer+ CD4+ cells could be detected (Fig. 2 A and B), reflecting the low frequency of HY-specific CD4 T cells in WT female mice and the expected negative selection of such cells in male mice. HY-IAb tetramer+ cells also could not be detected in peptide-infused female mice, indicating that peptide infusion resulted in limited expansion of HY-specific cells (Fig. 2A), which is in agreement with a previous study using transgenic mice (17). The picture was different when peptide-infused and naïve B6 female mice were immunized with 15 × 106 male Thy1.1 B6 spleen cells (peptide infused, immunized and immunized control, respectively) and analyzed 1–8 weeks after immunization. When CD4-enriched splenocytes were stained with CD4 and CD44 antibodies and HY-IAb tetramers, excluding 7-aminoactinomycin D (7AAD)+, B220+, CD8+, Thy1.1+ cells, CD4+CD44hiHY-IAb+ cells were now discernible, but their frequency and number were reduced by 4- to 20-fold in peptide-infused, immunized versus immunized control mice regardless of the time point of observation (Fig. 2B) (data not shown). With regard to the kinetics of the HY-specific CD4 response, in both groups of mice, HY-IAb+ CD4 cells were first visualized 5 days after immunization, underwent expansion (reaching a peak at 3 weeks), and declined progressively thereafter. In addition, in both groups, HY peptide-specific CD4 T cells displayed the phenotype of activated/memory T cells (i.e., they were CD62LlowCD45RBlowCD69−) (data not shown). Because the brightness of Thy1.1 staining permitted its distinction from other exclusion markers with the same emission wavelength, it also became evident that peptide-infused, immunized mice contained 30- to 90-fold more male Thy1.1+ cells than immunized control mice (Fig. 2B), again indicating male cell rejection in the latter, but not the former, recipients.
Fig. 2.
Frequency of HY-specific T cells in B6 mice. (A) CD4-enriched splenocytes from B6 control male, female, and peptide-infused female mice were stained with 7AAD as well as B220, and CD8 PerCP-labeled mAbs for exclusion and assayed for IAb-HY tetramer binding and CD4/CD44 expression. (B) CD4-enriched splenocytes from control, male Thy1.1 cell-immunized control as well as peptide-infused B6 female mice were stained as in A with the addition of Thy1.1 PerCP mAb. Immunized mice were analyzed 3 weeks after male cell injection. (C) CD8-enriched splenocytes from control, immunized control as well as peptide-infused B6 female mice were stained with 7AAD, B220, CD4, Thy1.1, as well as with Db-HY tetramers and CD8/CD44 mAbs. Immunized mice were analyzed 4 weeks after male cell injection. Percentage and absolute number of gated cells are depicted inside and above plots, respectively. Data are representative of >15 independent experiments.
To determine whether infusion of the MHC class II-restricted HY peptide also can affect HY-specific CD8 responses, CD8+ splenocytes from naïve, immunized control and HY peptide-infused female mice were analyzed for binding of Db tetramers loaded with the immunodominant MHC class I (Db)-restricted, Uty-encoded HY peptide, WMHHNMDLI (HY-Db tetramers). No tetramer-specific CD8 cells could be detected in naïve female mice, whereas an HY-Db tetramer+ CD8 T cell population was readily observed in immunized control mice (Fig. 2C). In the latter mice, HY-Db tetramer+ CD8 T cells became detectable 2 weeks after immunization, peaked at 4–5 weeks, and contracted thereafter. Yet small numbers of tetramer+ CD8 cells could still be found 16 weeks after immunization (data not shown). In contrast, HY-Db tetramer+ CD8 T cells were completely absent in peptide-infused, immunized female mice over a period of 2–16 weeks after immunization (Fig. 2C) (data not shown). Thus, infusion of an I-Ab-presented peptide prevented an HY-Db-specific CD8 response.
To examine whether HY-IAb+ cells in peptide-infused, immunized female mice were functionally active, we compared HY-IAb+ cells from immunized control and peptide-infused female mice for their capacity to produce IL-2. When cultured in the absence of HY peptide, HY-IAb+, but not HY-IAb−, CD4 T cells from immunized control mice secreted IL-2 (SI Fig. 6). This secretion likely reflected the fact that male antigen was presented by splenic antigen-presenting cells. In contrast, only a very small proportion of HY-IAb+ CD4 T cells from peptide-infused, immunized mice was found to produce IL-2 (SI Fig. 6). Furthermore, in response to exogenously added peptide, 60% of HY-IAb+ CD4 T cells from immunized control mice, but only 15% of HY-IAb+ CD4 T cells from peptide-infused, immunized mice, produced IL-2 (SI Fig. 6).
Induction of HY Peptide-Specific, Foxp3+ Tregs in HY Peptide-Infused Female Mice.
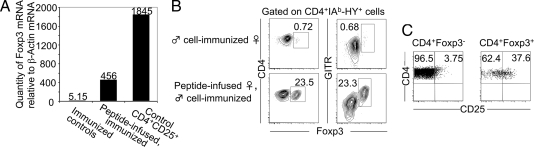
Because antigen-specific Tregs could be induced by peptide infusion in TCR-transgenic mice (17), we quantified by real-time PCR the levels of Foxp3 mRNA relative to that of β-actin in sorted HY-IAb+ cells from immunized control and peptide-infused WT female mice. Total HY-IAb+ cells from peptide-infused, immunized mice contained ≈100-fold more Foxp3 mRNA than HY-IAb+ cells from immunized control mice and ≈25% of that found in polyclonal CD4+CD25+ cells from WT mice (Fig. 3A). This finding suggested that HY peptide-specific CD4 T cells from peptide-infused, immunized mice were composed of Foxp3+ and Foxp3− cells, which was verified by GITR extracellular and Foxp3 intracellular staining. Because HY-IAb tetramer staining of CD4 T cells is severely disturbed by cell-fixation procedures, B220−7AAD−CD8−Thy1.1−CD4+tetramer+ cells from male Thy1.1 immunized control and peptide-infused female mice were sorted to 99% purity, mixed with sorted Thy1.1+ carrier cells, fixed, and stained for Foxp3. CD4+Thy1.1− cells from immunized control mice presented with rare Foxp3+ cells, whereas cells from peptide-infused, immunized mice consistently segregated into a larger Foxp3−GITRlow population and a smaller (10–30%) Foxp3+GITRhi population (Fig. 3B). Foxp3− HY-IAb+ CD4 T cells were CD25−, whereas Foxp3+CD4+HY-IAb+ cells expressed CD25, albeit at levels insufficient to distinguish all Foxp3+ from Foxp3− cells (Fig. 3C). Thus, peptide infusion followed by immunization yields a much higher frequency of HY-specific Foxp3+ CD4 T cells than immunization alone in WT mice. These results do not entirely rule out the possibility that in WT mice the frequency of Tregs increased by preferential expansion of preexisting Tregs after peptide infusion, rather than conversion of naïve T cells into Tregs. If this assumption were true, Foxp3+ cells should expand better in response to peptide infusion than naïve T cells.
Fig. 3.
Induction of HY-specific Foxp3+ Tregs in WT female mice. (A) IAb-HY tetramer+ CD4+ cells from male cell-immunized control and peptide-infused B6 female mice as well as CD4+CD25+ cells from B6 female mice were FACS purified. Foxp3-specific mRNA levels of each sample were quantified by Taqman quantitative PCR and normalized to β actin-mRNA levels by using fluorescent probes. (B) IAb-HY tetramer+ CD4+ cells from immunized, control and peptide-infused B6 female mice were FACS purified and assayed for extracellular GITR and intracellular Foxp3 expression levels. The percentage of Foxp3+ cells is shown. (C) Dot plots show extracellular CD25 expression levels by Foxp3+ and Foxp3− IAb-HY tetramer+ CD4+ cells of peptide-infused, immunized B6 female mice. Numbers show percentage of cells. FACS data are representative of at least 10 independent experiments.
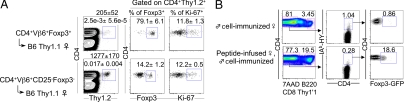
This possibility was tested by using RAG-2−/− HY peptide-specific TCR-transgenic (Marilyn) female mice, in which immunization is not required because of the high frequency of transgenic cells. In HY peptide-infused Marilyn female mice, ≈20% of CD4+TCRβ+ cells expressed intracellular Foxp3 and high levels of GITR (SI Fig. 7A). Cells with that phenotype were not found in control Marilyn mice (SI Fig. 7A), in which all CD4+TCRβ+ cells stained with HY-IAb tetramers (SI Fig. 7C). Foxp3+CD4+ cells from peptide-infused Marilyn mice expressed CD25 at levels similar to those observed in peptide-infused, immunized WT mice (Fig. 3C and SI Fig. 7B). Thus, peptide infusion can convert naïve T cells with an HY-specific transgenic TCR into Tregs. To compare the proliferation of naïve T cells versus preexisting Tregs upon peptide infusion, the same number of Foxp3-GFP+ and Foxp3-GFP− Vβ6+ CD4 T cells from RAG-2 competent Marilyn mice with a Foxp3-GFP reporter transgene were transferred into Thy1-mismatched B6 hosts. As shown in Fig. 4A, peptide infusion resulted in a reduced recovery of donor cells in recipients injected with Foxp3-GFP+ cells versus Foxp3-GFP− cells. In the latter, some of the Foxp3-GFP− cells had become Foxp3-GFP+ as expected from the conversion observed in peptide-infused RAG-2−/− Marilyn mice. In both groups, the proportion of donor cells stained with Ki-67, a nuclear protein expressed in cycling cells, was about the same (Fig. 4A). Thus, these data indicate that, with cells expressing the same TCR, Foxp3+ cells have no proliferative advantage over Foxp3− cells in response to peptide infusion.
Fig. 4.
Induction of HY-specific Foxp3+ cells from naïve Foxp3− CD4 T cells. (A) Foxp3-GFP+ and Foxp3-GFP− Vβ6+CD4+ cells from RAG-2+/− Marilyn mice with a Foxp3-GFP reporter were enumerated and examined for Foxp3 and intracellular Ki-67 expression by FACS 14 days after adoptive transfer into Thy1.1 B6 female mice and HY peptide infusion. Percentage and absolute number (average ± SD of four mice per group) of gated cells are depicted inside and above plots, respectively. (B) Sorted CD4+Foxp3-GFP− cells from Foxp3-GFP reporter, Thy1.2 female mice, and CD4+CD25+ cells from Thy1.1 B6 female mice were coinjected into Thy1.2 B6 nude female mice. Recipient mice were either immunized with male Thy1.1 B6 spleen cells or HY peptide-infused for 2 weeks and subsequently immunized. Then, 3 weeks after male cell immunization, 7AAD−B220−CD8−Thy1.1−, HY-IAb tetramer+Thy1.2+CD4+ cells were examined for the expression of Foxp3-GFP.
Further experiments directly addressed the conversion of polyclonal HY-specific cells. Foxp3-GFP− cells from H-2b, Thy1.2+ female mice expressing a Foxp3-GFP reporter were transferred into B6 nude mice (H-2b, Thy1.2+), together with CD4+CD25+ T cells from Thy1.1 B6 mice (H-2b, Thy1.1+), to have a combination of distinguishable non-Tregs and Tregs in the host. Mice were then either immunized with Thy1.1 B6 male spleen cells (controls) or peptide infused and then immunized. Three weeks after immunization with male cells, control mice had fewer Thy1.1+ cells than HY peptide-infused mice, indicating rejection of male cells (Fig. 4B). When HY-IAb+Thy1.2+ female cells were analyzed for the expression of Foxp3, 18% of peptide-infused but <1% of control mice exhibited GFP expression (Fig. 4B). Collectively, the data presented in this section show that antigen-specific polyclonal naïve T cells that exist with a low frequency in WT mice can be converted into Tregs by peptide infusion and, subsequently, expanded by immunization.
In Vitro Suppressive Activity of HY-Specific, Foxp3+ Tregs in HY Peptide-Infused Marilyn Female Mice.
The proliferative and suppressive activity of HY-specific CD4 T cells from peptide-infused as well as control RAG-2−/− Marilyn female mice was assessed in vitro because insufficient numbers for adoptive transfer experiments were obtained from peptide-infused WT mice (for details, see SI Materials and Methods). In the absence of peptide, neither CFSE-labeled naïve CD4+TCRβ+ cells nor CFSE-labeled CD4+TCRβ+GITRhi (Foxp3+; see SI Fig. 7A) or CD4+TCRβ+GITRlow (Foxp3−; see SI Fig. 7A) cells from control and peptide-infused Marilyn mice, respectively, divided in vitro (SI Fig. 8A). In the presence of the peptide, 80% of naïve CD4+TCRβ+ cells underwent proliferation (SI Fig. 8A). In contrast, CD4+TCRβ+GITRhi and CD4+TCRβ+GITRlow cells from peptide-infused mice remained either undivided or exhibited marginal proliferation, respectively (SI Fig. 8A). To distinguish naïve cells from those obtained after peptide infusion in coculture experiments, CD4+TCRβ+ cells of control Marilyn mice were labeled with CFSE (green dye), whereas GITRhi and GITRlow CD4+TCRβ+ cells of peptide-infused mice were labeled with 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMT-MR; orange dye). Addition of the GITRhi subset to CFSE+ naïve T cells abolished the proliferation of the latter, indicating that CD4+TCRβ+GITRhi cells were able to suppress proliferation of naïve T cells in vitro (SI Fig. 8B). GITRlow cells also exhibited some suppressive function in vitro, which may reflect the ongoing differentiation of these cells into Foxp3+ Tregs (SI Fig. 8B).
Accumulation of Foxp3+ HY-Specific Tregs in Graft-Draining Lymph Nodes of Peptide-Infused WT Female Mice Transplanted with Male Skin.
Examination of HY-specific Tregs in spleen and draining lymph nodes of male skin-grafted peptide-infused female mice showed that the proportion of Foxp3+ HY-specific CD4 cells was 17% in the spleen, but 30% in the draining nodes, suggesting preferential accumulation of HY-specific Tregs either by specific homing and/or local expansion in antigen-draining nodes (SI Fig. 9). In contrast, the proportion of Foxp3+ HY-specific Tregs remained marginal in both tissues of male skin-grafted control mice (SI Fig. 9). Thus, the grafting of skin efficiently expanded HY-specific T cells as well as Tregs, and 30% of Tregs among HY-specific cells in graft-draining nodes were sufficient to prevent graft rejection.
Fate of HY-Specific CD4 and CD8 T Cells in the Context of Dominant Tolerance.
Because T cell deletion and/or anergy also could have contributed to the absence of HY-specific CD4 and CD8 T cell responses in peptide-infused WT female mice, we addressed whether induced Tregs can suppress these responses. Briefly, 30,000 CFSE+ naïve HY-specific Thy1.2+ CD4 or CD8 T cells were coinjected with 15 × 106 Thy1.1 B6 male cells into control or HY peptide-infused Thy1.1 B6 WT female mice. RAG-2−/− Marilyn female mice as well as RAG-2−/− female mice with a transgenic TCR specific for immunodominant Db-WMHHNMDLI complexes were used as a source of naïve CD4 and CD8 T cells, respectively.
When transferred into control mice, the number of HY-specific CD4 T cells increased ≈400-fold, reaching a peak 5 days after immunization, and declined thereafter (SI Fig. 10A). There was less increase in the number of HY-specific CD4 T cells in peptide-infused WT female mice (SI Fig. 10A), which could reflect reduced cell division and/or survival. With regard to expansion, a deficit in the proliferation of Thy1.2+CD4+ cells was noted in peptide-infused mice at days 5, 7, and 10, compared with Thy1.2+CD4+ cells in control hosts (SI Fig. 10B). With regard to activation markers, Thy1.2+ cells rapidly acquired the CD44hiCD62Llow phenotype of mature effector CD4 cells in control hosts, whereas CD44 up-regulation as well as CD62L down-regulation by Thy1.2+ cells were markedly delayed in peptide-infused hosts (SI Fig. 10C).
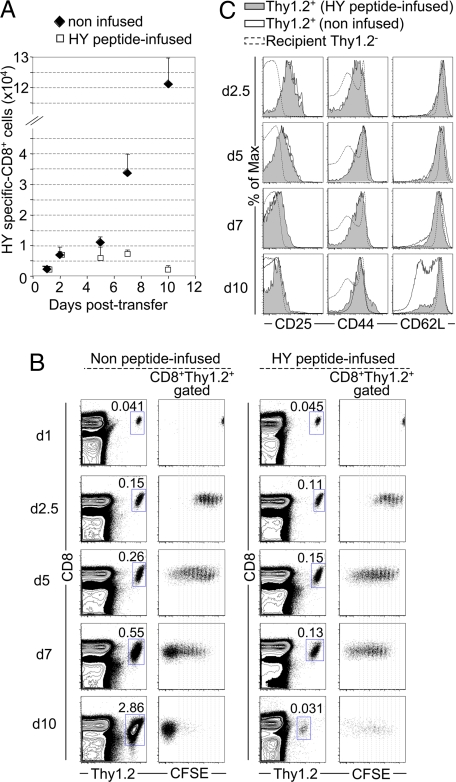
In similar experiments, HY-specific Thy1.2+ CD8 T cells expanded gradually, resulting in an ≈40-fold increase by day 10, in contrast to a 3-fold increase in peptide-infused WT female mice (Fig. 5A). At days 7 and 10, CD8+Thy1.2+ cells had divided extensively in control hosts and became mostly CFSE−, whereas CD8+Thy1.2+ cells in peptide-infused hosts showed proliferation arrest and a decline in number, suggesting cell death (Fig. 5 A and B). Furthermore, by day 10, about half of donor CD8 cells had differentiated into CD62LlowCD44hi effector cells in control hosts, whereas CD62L was not down-regulated in peptide-infused recipients (Fig. 5C).
Fig. 5.
In vivo response of HY-specific TCR transgenic CD8 T cells in the presence of induced WT Tregs. (A) CFSE-labeled CD8+Thy1.2+ from B6 RAG-2−/−, HY-specific, Db-restricted, TCR-transgenic female mice were enumerated by FACS after coinjection with male Thy1.1 B6 cells into control (filled diamonds) or HY peptide-infused (open squares) Thy1.1 B6 female hosts. Data represent the average (± SD) of at least three independent experiments. CFSE-labeled cells that were injected into nonimmunized female hosts remained undivided and could be detected up to 10 days after injection. (B) CFSE dilution of CD8+Thy1.2+-gated cells is shown for the same recipients as in A. (C) CD25, CD44, and CD62L expression by transferred CD8+Thy1.2+ cells in male Thy1.1 cell-immunized peptide-infused (filled area, continuous line) or control (open area, continuous line) WT Thy1.1 hosts as well as by host CD4+Thy1.2− (naïve) cells (dashed line). FACS data are representative of at least three independent experiments for each time point.
These results were reproducible when HY-specific T cells were injected 2 weeks after removal of peptide-delivering pumps, indicating survival of the induced Tregs in the absence of peptide infusion, compatible with the notion of a considerable lifespan of antigen-induced Tregs.
Discussion
MHC class II tetramer staining proved crucial to obtain evidence that antigen-specific, Foxp3-expressing Tregs can be induced from naïve T cells in WT mice by infusion of subimmunogenic doses of a TCR agonist peptide ligand. The data show that HY peptide-infused female mice tolerate male skin grafts, spleen cells, and BM transplants and that converted HY-specific, Foxp3+ Treg can expand after immunization. HY-specific immune responses can amount to a severe complication in clinical transplantation. The results reported here demonstrate that such reactions are preventable by HY peptide infusion. In fact, the detailed studies show that this protocol generates dominant tolerance that diminishes the expansion of HY-specific CD4 T cells and largely prevents expansion and survival of HY-specific CD8 T cells that normally follow exposure to male transplantation antigens.
Peptide infusion is accompanied by the generation of HY peptide-specific, Foxp3+ Tregs, which are difficult to visualize after their induction because the conversion is not accompanied by extensive division (17, 18) and therefore must be expanded by immunization with male cells. The Foxp3+ HY-specific Tregs generated in WT mice exhibit limited CD25 up-regulation, which prevented the use of CD25 to distinguish Foxp3+ from Foxp3− HY-specific CD4 T cells. Nevertheless, these observations are consistent with the analysis of Foxp3-GFP reporter mice, which demonstrated the existence of a significant proportion of CD25lowFoxp3+ cells endowed with a suppressive activity as potent as that of CD25hiFoxp3+ cells (24). Peptide-induced GITRhi Tregs were anergic in vitro and suppressed proliferation of naïve T cells. The GITRlow subset exhibited both reduced proliferation and suppressive activity in vitro because some of the GITRlow cells do express Foxp3 and therefore will suppress the proliferation of other GITRlow Foxp3− cells. Alternatively, the peptide infusion may initially generate anergic cells that subsequently up-regulate Foxp3 to become Tregs, and the GITRlowCD25low and GITRhiCD25hi cells may represent distinct stages along this pathway. In any case, the result indicates that Foxp3+ cells converted from naïve HY-specific T cells are suppressive in vitro.
These observations raise important questions with regard to the mechanisms of tolerance in vivo [i.e., with regard to cell-intrinsic or recessive mechanisms (anergy, deletion) or cell-extrinsic or dominant mechanisms by Tregs]. Although it is difficult to determine after the fact (i.e., peptide infusion and immunization), it can be addressed by the transfer of naïve, male-specific CD4 or CD8 cells concomitantly with immunization by male antigens into WT female mice already harboring induced HY-specific Tregs. These results show that dominant tolerance mechanisms limit the expansion and accumulation of male-specific CD4 and CD8 T cells by lessening their proliferation, affecting the survival of expanded cells and their acquisition of a memory phenotype. Thus, transplantation tolerance in HY peptide-infused mice can be described as being mediated by Tregs, although it cannot be excluded that, during the process of peptide infusion in WT mice, cell-intrinsic mechanisms such as anergy, which initially contributes to Treg generation, and deletion of male-specific CD4 T cells may be equally involved.
Tolerance to male tissue also was achieved by intranasal infusion of HY peptide (25). However, the nature of the cells mediating tolerance in that system remained unknown. Our BM reconstitution experiments revealed an increase of alloengraftment in tolerized female mice. This observation is in agreement with previously published studies suggesting that BM engraftment and immune reconstitution can be enhanced in the presence of Tregs (7, 26, 27), although the antigen specificity of such Tregs was not known. One important conclusion of the present work is that the induction of male-specific Tregs by infusion with a single class II MHC-presented HY peptide induces long-lasting tolerance to all HY transplantation antigens represented by different Dby and Uty proteins encoded by different genes and recognized by CD4 and CD8 T cells, respectively. Thus, the induction of male-specific Tregs in female BM donors or female recipients of male transplants may have significant benefits in clinical transplantation.
Because successful transplantation tolerance by means of Treg-adoptive immunotherapy requires large numbers of cells that are difficult to obtain by expanding existing Tregs, alternative approaches must be considered to generate such cells. If a procedure as simple as peptide infusion, which permits de novo induction of Tregs from mature T cells, prevents transplant rejection or GVHD, it could offer a realistic opportunity to induce tolerance to a variety of antigens such as allergens, transplantation antigens, and antigens causing autoimmunity while minimizing undesirable side effects often associated with general immunosuppression.
Materials and Methods
Mice.
Thy1.2 and Thy1.1 B6 mice as well as B6 nude mice were from The Jackson Laboratory. C57BL/10 RAG-2−/− Marilyn mice (28) were a generous gift from P. Matzinger (National Institutes of Health, Bethesda, MD). RAG-2 competent, Foxp3-GFP-expressing Marilyn mice were obtained by crossing Marilyn with Foxp3-GFP reporter mice (24). Female B6.SJL RAG-2−/− HY-specific Db-restricted TCR-transgenic mice (29) were from Taconic Farms. Mice were maintained in a pathogen-free facility, and all procedures were in accordance with the guidelines of the Committee on Animals of Harvard Medical School.
Cell-Staining Procedures.
All staining reactions (for details, see SI Materials and Methods) were preceded by a 10-min incubation with a blockade mixture made of 2.4G2 supernatant (Fc block) and 10% rat and mouse sera (Jackson ImmunoResearch Laboratories ). Dead cells were systematically excluded by 7AAD or DAPI staining. For tetramer staining, spleen or lymph node cells were enriched either for CD4 or CD8 T cells by using SpinSep CD4 or CD8 T cell-enrichment kits (StemCell Technologies), respectively. Enriched populations were then stained with 20 μg/ml IAb-HY or 2.5 μg/ml Db-HY tetramer, respectively, for 30 min at room temperature. mAbs and viability dyes were added thereafter for 20 min on ice. For intracellular Foxp3 and Ki-67 stainings, eBiosciences fixation and permeabilization reagents were used. For the IL-2 secretion assay, total splenocytes were incubated with the blockade mixture and stained with IAb-HY tetramers prior in vitro culture. Subsequent stainings were conducted according to IL-2 Secretion Assay kit (Miltenyi Biotec) instructions. Enumeration of cells, acquisition, and cell sorting were performed by using a FACSAria and FACSDiva software (Becton Dickinson). Single-cell data analyses used the FlowJo software (Tree Star).
Adoptive Transfer Experiments.
RAG-2 competent, Foxp3-GFP+, or Foxp3-GFP− Vβ6+CD4+ Marilyn T cells were FACS-sorted and injected i.v (1.8 × 105 cells per recipient) into Thy1.1 B6 female hosts infused with the HY peptide on the same day. Then 1 × 107 Foxp3-GFP−CD4+ from Foxp3-GFP reporter female mice were injected i.v. into B6 nude mice with 8.5 × 105 CD4+CD25+ cells from Thy1.1 B6 female mice. Recipient nude mice were either immunized with 1.25 × 107 male Thy1.1 B6 spleen cells or peptide-infused and subsequently immunized. CD4 or CD8 T cells from RAG2−/− Marilyn and RAG-2−/− HY-specific Db-restricted TCR-transgenic female mice were sorted and labeled in 10 μM CFSE (Molecular Probes). Thy1.1 B6 female hosts were injected i.v. with either CD4 or CD8 CFSE-labeled T cells (3 × 104 cells per recipient) and 1.5 × 107 B6 male Thy1.1 spleen cells. Splenocytes of recipient mice were analyzed by FACS when indicated.
Skin Grafting, BM Engraftment, and in Vivo Killing Assay.
B6 female mice were grafted with full-thickness tail skin (≈1.0 cm2) from male B6 and bandaged for 7 days. Grafts were monitored weekly and scored as rejected when <10% viable donor tissue remained. BM cells of B6 Thy1.1 male mice were injected i.v. (5 × 106 cells per mouse) into 200 rad-irradiated B6 Thy1.2 female mice. For the in vivo killing assay, splenocytes from female and male B6 mice were labeled in 10 μM and 1 μM CFSE, respectively. Cells were then mixed at an equal ratio and injected i.v. (2 × 107 cells per recipient) into B6 female mice.
Supplementary Material
ACKNOWLEDGMENTS.
We thank M. Sykes for providing demonstration of skin grafting; P. Matzinger (National Institutes of Health, Bethesda) for Marilyn mice; Melissa J. Call for DM-transfected cells (Dana–Farber Cancer Institute, Boston, MA); and the National Institute of Allergy and Infectious Diseases Tetramer Facility for providing Db-HY protein. This work was supported by National Institutes of Health Grants R37 53102 (to H.v.B.), PO1 AI045757, and RO1 AI057493 (to K.W.W.); and a Claudia Adams Barr grant (to I.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800149105/DC1.
References
- 1.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 4.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813–5816. [PubMed] [Google Scholar]
- 5.Karim M, Feng G, Wood KJ, Bushell AR. CD25+CD4+ regulatory T cells generated by exposure to a model protein antigen prevent allograft rejection: Antigen-specific reactivation in vivo is critical for bystander regulation. Blood. 2005;105:4871–4877. doi: 10.1182/blood-2004-10-3888. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-vs-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trenado A, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-vs-host disease while maintaining graft-vs-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeckel E, von Boehmer H, Manns MP. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2005;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edinger M, et al. CD4+CD25+ regulatory T cells preserve graft-vs-tumor activity while inhibiting graft-vs-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 12.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 13.Cobbold SP, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 14.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 15.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 16.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 17.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naïve T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 19.Chen TC, Cobbold SP, Fairchild PJ, Waldmann H. Generation of anergic and regulatory T cells following prolonged exposure to a harmless antigen. J Immunol. 2004;172:5900–5907. doi: 10.4049/jimmunol.172.10.5900. [DOI] [PubMed] [Google Scholar]
- 20.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–4869. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 21.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 22.Simpson E, et al. Minor H antigens: Genes and peptides. Transpl Immunol. 2002;10:115–123. doi: 10.1016/s0966-3274(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 23.Millrain M, et al. Examination of HY response: T cell expansion, immunodominance, and cross-priming revealed by HY tetramer analysis. J Immunol. 2001;167:3756–3764. doi: 10.4049/jimmunol.167.7.3756. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Chai JG, James E, Dewchand H, Simpson E, Scott D. Transplantation tolerance induced by intranasal administration of HY peptides. Blood. 2004;103:3951–3959. doi: 10.1182/blood-2003-11-3763. [DOI] [PubMed] [Google Scholar]
- 26.Joffre O, van Meerwijk JP. CD4+CD25+ regulatory T lymphocytes in bone marrow transplantation. Semin Immunol. 2006;18:128–135. doi: 10.1016/j.smim.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanash AM, Levy RB. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. [DOI] [PubMed] [Google Scholar]
- 28.Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naïve CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- 29.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.