Abstract
T helper 1 (Th1) cells mediate powerful cellular immune responses. However, if unbalanced, Th1 immunity eventually may cause pathology. Recently, it has been shown that IL-10, an antiinflammatory cytokine strongly antagonizing Th1-mediated effects, can be produced by Th1 cells and is indeed essential for self-regulation of Th1 immunity. Here, we show that Notch induces IL-10 production in newly developing and already established Th1 cells via a signal transducer and activator of transcription 4 (STAT4)-dependent process. Notch signaling in the presence of the cytokines IL-12 or IL-27 induces Th1 cells to produce large amounts of IL-10 without diminishing IFN-γ production. Notch-modified Th1 cells completely lose their inflammatory capacity and instead are able to actively suppress a Th1 cell-induced delayed-type hypersensitivity (DTH) reaction in an IL-10-dependent fashion. IL-10 production can be elicited by active forms of all four mammalian Notch receptors but was found to be specific for the Delta-like family of Notch ligands. Dendritic cells (DC) selectively acquire Delta-like 4 expression upon stimulation with various Toll-like receptor (TLR) ligands and concomitantly induce IL-10 production by Th1 cells in vitro and in vivo. This effect can be selectively reversed by pharmacological inhibitors of Notch signaling (γ-secretase inhibitor). Our data suggest that Notch regulates IL-10 production in Th1 cells by a STAT4-dependent process that converts proinflammatory Th1 cells into T cells with regulatory activity. This pathway may provide unique opportunities for therapeutic intervention in Th1-driven immune diseases and for Th1-associated vaccination strategies.
Keywords: immunoregulation, inflammation, Notch signaling, T helper differentiation, T regulatory 1 cells
T helper 1 (Th1) cells, characterized by the production of large amounts of IFN-γ, control inflammatory immune responses and mediate the defense against intracellular pathogens. To prevent pathology associated with uncontrolled activation, the immune system also employs various negative regulatory circuits. One such strategy is the release of immunosuppressive cytokines. First of all, IL-10 has the capacity to potently suppress Th1 effector functions, mainly by preventing the activation of antigen-presenting cells (APCs) (1). Among CD4+ T cells, IL-10 originally has been described as a Th2-associated cytokine that antagonizes Th1 responses (1, 2). Subsequently, IL-10 has been shown to be produced also by specialized subsets of regulatory T cells (Treg), such as CD25+FoxP3+ Treg (3, 4), and T regulatory (Tr) 1 (5) cells, which are thought to be essential for an effective negative regulation of immune responses.
Interestingly, Th1 cells themselves were found to secrete IL-10 (6, 7). Thus, Th1-derived IL-10 recently has been shown to be critical for the prevention of immune pathology in the course of infection with the intracellular pathogens Toxoplasma gondii and Leishmania major (8, 9). These results revealed a self-limiting property of Th1 cells to contain immune reactions independently of regulatory T cells.
The molecular pathways leading to IL-10 expression in Th1 cells are only beginning to be understood. In Th2 cells, IL-10 is induced by the main Th2-polarizing cytokine IL-4 by the transcription factor GATA-3 as part of the Th2 differentiation program (10, 11). Repeated stimulation under suboptimal conditions induces IL-10, producing T cells with regulatory capacity. However, these cells express no or reduced levels of IFN-γ compared with Th1 cells (5, 12, 13). In contrast, Th1 cells generated in vitro contain only a few IL-10-secreting cells. The frequency of these cells can be increased by repeated stimulation in the presence of IL-12 (11, 14, 15). Recently, another member of the IL-12 family of cytokines, IL-27, has been described to induce IL-10 production both in naïve and effector T cells (16–18).
The Notch pathway previously has been implicated in the regulation of peripheral T cell activation (19–21) and the differentiation into various effector T cell populations (22). However, the complexity of the Notch system, which is a direct consequence of the numerous potential receptor–ligand interactions of four Notch receptors expressed on T cells and five Notch ligands [Jagged-1, Jagged-2, Delta-like (Dll)-1, Dll-2, and Dll-4] expressed on APCs, so far has prevented the establishment of a clear-cut picture of the role of Notch in peripheral T cell differentiation. Recent data convincingly show that Notch signaling is required for Th2 differentiation in vivo (23–27). However, the proposed involvement of Notch in Th1 (23, 28, 29) or likewise regulatory T cell development (30, 31) is less clear. These partially contradictory results strongly suggest that the outcome of Notch-directed T cell differentiation depends on additional, as-yet-uncharacterized, costimulatory signals or certain environmental niches.
Here, we identify Notch as a potent inducer of IL-10 both in developing and in already established IFN-γ-secreting Th1 cells. This effect requires costimulation by the cytokines IL-12 or IL-27 that depends on signal transducer and activator of transcription 4 (STAT4) signaling. Our data suggest that Notch regulates IL-10 production by Th1 cells under inflammatory conditions and thereby facilitates the self-limitation of Th1 immune responses.
Results
Notch Influences Th Cell Differentiation.
To determine the influence of T cell-polarizing cytokines on Notch-induced T cell differentiation, we stimulated naïve CD4+ T cells under Th1, Th2, or nonpolarizing conditions and retrovirally transduced the cells with constitutively active Notch (N3IC). At day 5 of culture, the transduced T cells were isolated according to their GFP expression, restimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin, and analyzed for cytokine production.
Notch did not grossly alter the cytokine profile under nonpolarizing or Th2 conditions. As reported earlier (23, 32), IL-4 production was slightly increased by Notch. No changes were observed in IL-2, IL-5, IL-13, IL-17, or IFN-γ expression. Notch slightly induced IL-10 under nonpolarizing conditions and further increased the already high levels of IL-10 in Th2 cells. Similarly, under Th1 conditions, Notch only minimally affected expression of the Th1 cytokines IL-2 and IFN-γ. The most striking observation, however, was a strong IL-10 production by Notch-transduced Th1 cells (Fig. 1A), whereas IL-10 was virtually absent in control Th1 cultures.
Fig. 1.
Notch induces IL-10 in developing Th1 cells. (A) Cytokine profile after PMA/ionomycin restimulation of naïve CD4+CD25−CD62L+ T cells from C57BL/6 mice that had been cultured for 5 days under nonpolarizing, Th1-polarizing, or Th2-polarizing conditions and retrovirally transduced with constitutively active Notch or GFP alone (control). (B) Intracellular cytokine staining of Notch- or control-transduced T cells cultured under Th1-polarizing conditions for 5 days and PMA/ionomycin restimulation. Percentages indicate frequency of cytokine-producing cells among GFP-positive or GFP-negative cells (gated on CD4+ T cells). (C) Intracellular staining for IFN-γ and IL-10 on PMA/ionomycin-restimulated T cells (gated on CD4+ T cells). (D) IL-10 mRNA levels after PMA/ionomycin restimulation of Notch (N)- or control (C)-transduced T cells sorted for GFP expression. (E) IL-10 expression analyzed by intracellular staining after retroviral transduction with Notch-1 to Notch-4 (N-1 to N-4) of naïve T cells cultured under Th1 conditions and PMA/ionomycin restimulation. C, control transfected. (F) Frequency of IL-10-producing cells analyzed by intracellular staining after PMA/ionomycin restimulation of naïve T cells from BALB/c mice that had been cultured under Th1-polarizing conditions and retrovirally transduced with a cytosolic (NICcyt) or a membrane-bound (NICmem) form of an active Notch or GFP alone. GSI had been added during culture as indicated. (Data are representative of at least three independent experiments each.)
Notch Induces IL-10 in Newly Developing and Established Th1 Cells.
To confirm the de novo expression of IL-10 in Th1 cells, we performed intracellular cytokine staining (Fig. 1B). Strikingly, Notch induced IL-10 in ≈40% of the transduced Th1 cells, whereas <1% of control cells produced IL-10. Importantly, IL-10 expression was restricted to GFP-positive, i.e., Notch-transduced T cells, indicating a cell-intrinsic effect (Fig. 1B). The frequency of IFN-γ-expressing cells was up-regulated by Notch from ≈50% to 95%. Moreover, intracellular cytokine staining unveiled a strong correlation between IFN-γ and IL-10. Indeed, all IL-10-producing T cells exhibited an IFN-γ/IL-10 double-positive phenotype (Fig. 1C). Similarly, IL-10 mRNA was up-regulated only in Notch-transduced T cells (Fig. 1D). Active forms of all of the Notch receptors (Notch-1 to Notch-4) were capable of up-regulating IL-10 expression (Fig. 1E). The effect could be reversed by a specific inhibitor of the γ-secretase complex (GSI) in cells overexpressing a membrane-bound active Notch (Fig. 1F, NICmem) but not in cells transduced with a cytosolic active Notch (NICcyt) that is no longer sensitive to the inhibitor. These data show that the Notch-mediated induction of IL-10 expression under Th1-polarizing conditions involves the classical γ-secretase-dependent Notch pathway.
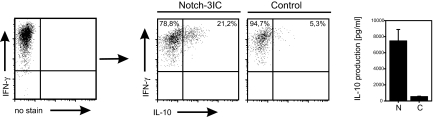
To test whether IL-10 also can be induced in already established Th1 cells, we Th1-polarized naïve T cells for 1 week and purified IFN-γ-producing cells by IFN-γ capture assay. Then, these cells were transduced with Notch and cultured for additional 5 days under Th1 conditions. As shown in Fig. 2, all cells still expressed IFN-γ. However, although only 5% of the cells coexpressed IL-10 in the control sample, >20% of the Notch-transduced Th1 cells produced IL-10. This difference was even more pronounced when the secreted cytokines were measured in the culture supernatants.
Fig. 2.
Notch induces the immunosuppressive cytokine IL-10 in established IFN-γ-secreting Th1 cells. IFN-γ producers that had been isolated from 1-week-old Th1 cells were subjected to an additional week of Th1 culture and retroviral transduction with Notch (N) or GFP alone as a control (C). After PMA/ionomycin restimulation, IFN-γ and IL-10 production was analyzed by intracellular staining and in culture supernatants. (Data are representative of two independent experiments.)
STAT4 Activation by IL-12 or IL-27 Is Critical for IL-10 Induction by Notch.
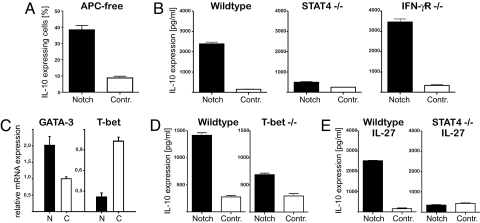
Next, we determined which additional factors are required for the Notch-mediated IL-10 induction. To exclude a role for further costimulatory signals provided by APCs, highly purified T cells were stimulated in the presence of IL-12 by using plate-bound anti-CD3/anti-CD28. Under these conditions, we still observed comparable levels of IL-10 in Notch-transduced T cells (Fig. 3A). In contrast, IL-10 production was abrogated in STAT4- but not in IFN-γR-deficient cells (Fig. 3B), supporting the idea that direct signaling by STAT4 is required for IL-10 induction.
Fig. 3.
Notch-mediated IL-10 induction strictly depends on STAT4 activation. (A) Analysis of IL-10 production by intracellular staining after PMA/ionomycin restimulation of naïve T cells transduced with active Notch or GFP alone as control and cultured for 5 days cultured under Th1 conditions and plate-bound anti-CD3/anti-CD28 stimulation. (B) IL-10 levels in culture supernatants of PMA/ionomycin-restimulated isolated Notch- or control-transduced T cells from wild-type, STAT4−/−, or IFN-γR−/− mice that had been cultured for 5 days under Th1 conditions. (C) Expression of GATA-3 and T-bet in isolated Notch (N)- or control (C)-transduced T cells after 5 days of culture under Th1 conditions. (D and E) IL-10 levels in culture supernatants of PMA/ionomycin-restimulated isolated Notch- or control-transduced T cells from wild-type and T-bet−/− mice that had been cultured for 5 days under Th1 conditions (D) or from wild-type and STAT4−/− mice that had been cultured for 5 days in the presence of IL-27 (E). (Data are representative of at least two independent experiments.)
Expression of the Th1-specific transcription factor T-bet was down-regulated in Notch-transduced cells at day 5 of the culture (Fig. 3C), indicating that T-bet is not critical for IL-10 up-regulation. In accordance with this observation, IL-10 still was observed in T-bet-deficient cells although at a slightly reduced level (Fig. 3D). The expression of the Th2 transcription factor GATA3 was slightly increased by Notch (Fig. 3C). However, no Th2 cytokines, such as IL-4, IL-5, or IL-13, were expressed in Notch-transduced cells under Th1-polarizing conditions (Fig. 1 A and B). FoxP3 was not up-regulated by Notch (data not shown).
IL-27 recently has been described as inducing IL-10 in T cells (16–18). Indeed, when IL-27 was added to naïve T cell cultures, we observed an expression of IL-10, which was comparable to that induced by IL-12 alone (Fig. 3E). However, upon transduction with Notch, the amount of IL-10 was drastically increased. Again, the up-regulation of IL-10 was completely blocked in T cells from STAT4 knockout mice. Thus, our data indicate that STAT4 activation by IL-12 or IL-27 is sufficient for Notch-mediated up-regulation of IL-10 production in Th1 cells.
Notch-Modified Th1 Cells Lose Proinflammatory Capacity and Become Suppressive in Vivo in an IL-10-Dependent Manner.
To investigate the proinflammatory or antiinflammatory capacity of IL-10-producing Notch-modified Th1 cells in vivo, Notch- or control-transduced ovalbumin-specific T cell receptor (OVA-TCR) transgenic Th1 cells were adoptively transferred into BALB/c recipient mice. After 24 h, a delayed-type hypersensitivity (DTH) reaction was induced by footpad injection of OVA peptide.
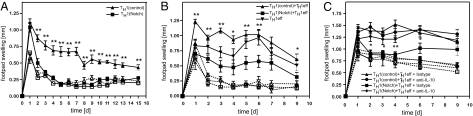
Control-transduced Th1 cells elicited a strong footpad swelling that peaked at day 1 and then slowly declined but remained clearly detectable until day 14. In sharp contrast, after a comparable acute response on day 1, swelling rapidly declined in mice that had received Notch-transduced Th1 cells and had ceased completely within 72 h (Fig. 4A). These results indicate that Notch-modified Th1 cells have lost their potential to maintain inflammatory responses for prolonged periods of time.
Fig. 4.
Notch-modified Th1 cells lose their proinflammatory capacity and become suppressive in a DTH response. OVA-TCR transgenic Notch- or control-transduced Th1 cells were adoptively transferred into BALB/c mice. After 24 h, a DTH reaction was induced by footpad injection of OVA peptide (filled symbols). Feet of control mice were injected with PBS (open symbols). (A and B) Notch- or control-transduced Th1 cells were transferred either alone (A) or cotransferred with conventional Th1 cells (B). (C) Mice received an anti-IL-10 antibody or isotype control before cotransfer of Notch- or control-transduced Th1 cells with conventional Th1 effector cells (Th1eff). Statistical significance was estimated by two-tailed Mann–Whitney test (*, P < 0.05; **, P < 0.01). (Data are representative of two independent experiments, six animals per group.)
To test whether Notch-modified Th1 cells become actively suppressive, we cotransferred Notch- or control-transduced OVA-specific Th1 cells together with the same number of conventional OVA-specific Th1 effector cells (Th1eff). Th1 effector-induced footpad swelling was further increased by cotransfer of control-transduced Th1 cells, probably because of the increased number of proinflammatory cells. In contrast, the presence of Notch-transduced Th1 cells significantly reduced footpad swelling, even when compared with Th1 cells transferred alone (Fig. 4B). This suppression was almost abrogated by a neutralizing anti-IL10 antibody (Fig. 4C).
These data show that Notch induces a functional switch in Th1 cells from a proinflammatory into an antiinflammatory phenotype with the potential to actively suppress inflammatory reactions via an IL-10-dependent mechanism.
Expression of Notch Receptors and Notch Ligands on T Cells and Dendritic Cells (DCs).
We then wanted to identify Notch receptors and Notch ligands that could play a role during physiologic T cell–APC interactions. To this end, we analyzed the endogenous expression pattern of these molecules on the mRNA level. Only Notch-1 and Notch-2, but not Notch-3 or Notch-4, were expressed by naïve and activated T cells [supporting information (SI) Fig. 7A].
Ex vivo-isolated splenic and lymph node CD11c+ DCs expressed low levels of Jagged-1, and Dll-1 and Dll-4 were not detectable (SI Fig. 7B). Because it had been described that Toll-like receptor (TLR) activation can induce Notch ligand expression (23, 33), we also analyzed the effects of various TLR ligands. Strikingly, LPS (TLR2/4), Flagellin (TLR5), or CpG (TLR9) stimulation strongly induced Dll-4 on DCs, whereas the expression of Dll-1 and Jagged-1 was largely unaffected (SI Fig. 7B).
CpG Induces Notch-Dependent IL-10 Production in Th1 Cells in Vitro and in Vivo.
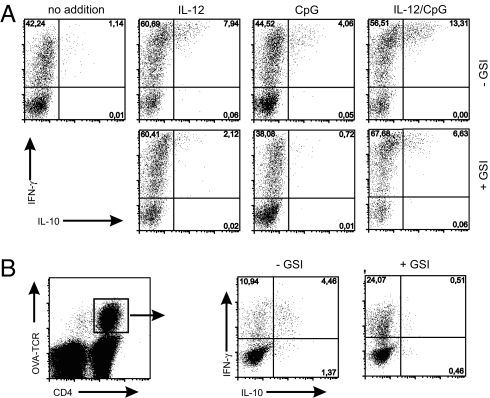
To test whether the expression of Dll-4 by activated DCs may have functional relevance, we cocultured naïve OVA-TCR transgenic T cells with purified DCs in the presence of CpG, IL-12, or both. After 5 days, IFN-γ and IL-10 production was analyzed by intracellular staining after PMA/ionomycin restimulation. Without further additions, only ≈1% of the T cells expressed IL-10. CpG or IL-12 alone increased the frequency of IL-10-producing cells to 5% and 8%, respectively. After the combined addition of IL-12 and CpG, ≈14% of the T cells produced IL-10, which was strictly coexpressed with IFN-γ. Importantly, under all three culture conditions, GSI clearly reduced IL-10 production by 50–80%, whereas the expression of IFN-γ remained largely unaffected (Fig. 5A).
Fig. 5.
CpG induces Notch-dependent IL-10 production in Th1 cells in vitro and in vivo. (A) IL-10 and IFN-γ expression analyzed by intracellular staining of naïve T cells after 5 days of coculture with CD11c+ DCs from spleen and lymph nodes and addition of IL-12, CpG, or IL-12/CpG in the presence or absence of GSI (gated on CD4+ T cells). (Data are representative of at least three independent experiments.) (B) IL-10 and IFN-γ expression analyzed by intracellular staining after PMA/ionomycin restimulation of OVA-TCR transgenic T cells that had been isolated from draining lymph nodes after adoptive transfer and s.c. immunization with OVA/CpG in the presence or absence of GSI. (Data are representative of three independent experiments, three animals per group.)
Next, we determined whether the Notch-mediated IL-10 induction also can be observed in vivo. To this end, we transferred naïve OVA-TCR transgenic T cells into BALB/c recipients and immunized them with OVA peptide/CpG in the absence or presence of GSI. On day 5, the coexpression of IFN-γ and IL-10 was investigated by intracellular staining (Fig. 5B). After CpG/OVA administration, ≈15% of the OVA-specific T cells produced IFN-γ. Approximately 30% of these cells coexpressed IL-10. Strikingly, the production of IL-10 was completely blocked in mice that had received GSI, whereas IFN-γ production was enhanced. These data suggest that Notch signaling triggered by Dll-4 expressed on activated DCs can induce IL-10 production by Th1 cells in a physiological immune response.
Notch Ligands of the Dll Family Mediate IL-10 Induction.
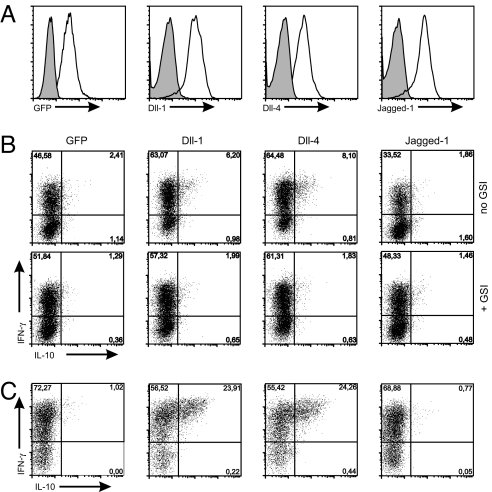
To confirm the importance of Dll-4 for Notch-mediated IL-10 up-regulation during T cell–APC interactions, we generated A20 B cell lines overexpressing the Notch ligands Jagged-1, Dll-1, and Dll-4 (Fig. 6A). Naïve OVA-TCR transgenic T cells were cultured with the ligand-expressing A20 cells for 1 week under Th1-polarizing conditions. Approximately 6% and 8% of the T cells expressed IL-10 after coculture with Dll-1- and Dll-4-transduced A20 cells, respectively, whereas Jagged-1- and GFP-transduced A20 cells did not induce significant IL-10 production. In accordance with published data (23), Jagged-1-expressing A20 cells reduced IFN-γ production by ≈20% compared with control A20 cells, whereas Dll-1 and Dll-4 slightly increased the frequency of IFN-γ-positive cells. Both IL-10 induction by Dll-1 or Dll-4 and suppression of IFN-γ by Jagged-1 were completely reversed by GSI (Fig. 6B). After a second week of coculture under Th1 conditions, Dll-4 or Dll-1 expressing A20 induced ≈24% of IL-10-producing Th1 cells. Again, Jagged-1 was not able to elicit IL-10 expression (Fig. 6C). These data show that Notch signaling triggered by ligands of the Dll family induces IL-10 production in Th1 cells, especially on repeated stimulation.
Fig. 6.
Notch ligands of the Dll family induce IL-10 production by Th1 cells. (A) Staining for Notch ligands on A20 B cells that had been lentivirally transduced with Notch ligands Dll-4, Dll-1, Jagged-1, or GFP alone as control. (B and C) Analysis of IL-10 and IFN-γ production after PMA/ionomycin restimulation of purified naïve OVA-TCR transgenic T cells after coculture with Notch ligand-expressing A20 B cells and stimulation with OVA peptide under Th1-polarizing conditions for 1 week (B) or 2 weeks (C). In some samples, GSI had been added (gated on CD4+ T cells). (Data are representative of three independent experiments.)
Discussion
Our study identifies a unique role for Notch in effector T cell differentiation. In addition to its previously described potential to directly instruct Th1 or Th2 cell differentiation, our data suggest that Notch also modifies the way T cells respond to the proinflammatory cytokines IL-12 or IL-27 by selectively enhancing IL-10 production and thereby conveying antiinflammatory capacity to otherwise proinflammatory Th1 cells.
So far, Notch has been described to instruct the differentiation of naïve T cells into various effector T cell lineages. An essential role for Notch in Th2 differentiation has been demonstrated convincingly in vitro and in vivo. Th2 responses are severely impaired in the absence of Notch (25, 32), and both IL-4 and GATA-3 have been identified as being direct transcriptional targets of Notch (23, 24, 27). Although we do not see drastic changes in the overall cytokine profile upon overexpression of constitutively active Notch in naïve T cells, the slight increase in Th2 cytokine expression we observe under nonpolarizing and under Th2 conditions is in accordance with these data.
In contrast, the precise function of Notch in Th1 differentiation is more obscure. Notch can induce or enhance Th1 differentiation in vitro (23, 28), and the Th1 transcription factor T-bet has been identified as a Notch target gene (29). Although some studies report Notch to be important for Th1 differentiation in vivo (29, 34), others find it to be dispensable (25) or even observe enhanced IFN-γ levels after abrogation of Notch signaling (32). We found increased IFN-γ production upon Notch activation only under Th1-polarizing conditions. In contrast, in vivo inhibition of Notch signaling by GSI resulted in enhanced IFN-γ production. Thus, the impact of Notch on Th1 differentiation, as analyzed by the expression of Th1 cytokines, varies drastically between different experimental systems. It can only be speculated that it is influenced by further costimulatory signals or the cytokine environment.
However, our data demonstrate that the drastic enhancement of IL-10 expression in IFN-γ-producing T cells represents the most striking effect of Notch on T cell differentiation, which we consistently observed under various experimental conditions in vitro and in vivo. The induction of these IFN-γ/IL-10 double producers is not restricted to naïve T cells but is equally effective in already established Th1 cells.
Importantly, IL-10 induction by Notch strictly depends on simultaneous STAT4 activation either by IL-12 or IL-27. Both cytokines, but especially IL-27, recently have been implicated in the regulation of IL-10 expression by Th1 cells (11, 14–18). In agreement with previous reports, we find basal IL-10 expression in the absence of Notch signaling when naïve T cells are cultured with either IL-12 or IL-27 alone. However, in either case, IL-10 production is drastically enhanced by Notch. This difference is best exemplified by the fact that Notch-modified Th1 cells completely lose their proinflammatory capacity and instead suppress Th1-mediated immune reactions in vivo via an IL-10-dependent process.
The strong synergistic effect of Notch on IL-12/IL-27-mediated IL-10 induction suggests that it acts as a master regulator that sensitizes proinflammatory Th1 cells to develop into IFN-γ+IL-10+ T cells with antiinflammatory potential in response to canonical inflammatory cytokine signals.
The IL-27-mediated IL-10 induction has been described as depending on STAT1 and/or STAT3 (17, 18) but to be independent of STAT4 (18). In contrast, the Notch-dependent up-regulation of IL-10 by IL-27 is abolished in STAT4-deficient T cells, suggesting that different molecular pathways are triggered when IL-27 acts alone or in combination with Notch. Apart from the STAT4 activation, we have no indication for the involvement of other major Th1-associated factors because Notch induced comparable levels of IL-10 in T cells from IFN-γ receptor-deficient and T-bet-deficient mice.
We demonstrate here that IFN-γ+IL-10+ T cells also are induced in vivo during immune responses after CpG/OVA vaccination. Importantly, IL-10 but not IFN-γ production is completely blocked by GSI, clearly indicating a Notch-dependent process. Our data are consistent with the previously described occurrence of IFN-γ+IL-10+ T cells in vivo (7, 35, 36). Recently, it has been demonstrated that, indeed, IL-10 produced by Th1 cells is critical in protecting the host from Th1-induced pathology (8, 9). It is tempting to speculate that Notch also may play a role in these situations. In support for this idea, we and others find that ligands of the Dll family, in particular Dll4, are strongly induced on DCs by several proinflammatory microbial stimuli known to drive Th1 immunity (23, 33, 34). The selective induction of Dll-4 on activated DCs in response to CpG stimulation and other microbial stimuli identifies Dll-4 as the presumptive physiologic ligand triggering Notch-dependent IL-10 production. In contrast, Jagged-1 is similarly expressed by resting and activated DCs, indicating a lesser functional importance under these circumstances. Indeed, we were able to confirm functional differences between Jagged and Dll ligands by their respective overexpression in an APC line. Jagged-1 decreased IFN-γ production in a GSI-sensitive manner as reported earlier (23), whereas Dll-1 and Dll-4 elicited IL-10 expression and even enhanced IFN-γ in Th1 cells. Overall, our observations support the emerging view that Jagged-1 drives Th2 development, whereas Dll ligands are more important for Th1 cell differentiation (23, 34). However, more refined experimental systems, such as DC-specific conditional knockout mice of the various Notch ligands, are required to identify the partner(s) mediating the critical physiological interaction.
In summary, our data suggest that the Notch pathway plays an important role for the autoregulation of Th1 immunity. Notch instructs T cells to respond to the canonical proinflammatory cytokines IL-12 and IL-27 by expressing the antiinflammatory mediator IL-10, which allows self-limitation of Th1 immune reactions. This finding defines a role for Notch as a molecular switch between proinflammatory and antiinflammatory Th1 cell function, thus representing a promising target for therapeutic intervention in inflammatory diseases and for improved vaccination strategies.
Materials and Methods
Mice.
BALB/c, OVA-TCRtg/tg DO11.10, C57BL/6 (BgVV Berlin), T-bet−/− (kind gift from J. Penninger, Vienna, Austria), IFN-γR−/− (kind gift from T. Schüler, Berlin, Germany), and STAT4−/− (The Jackson Laboratory) mice were housed under specific pathogen-free (SPF) conditions and used at 8 to 12 weeks of age.
Cell Purification.
Naïve CD4+ T cells were isolated by CD4 preenrichment (AutoMACS, Miltenyi Biotec) and FACS sorting of CD4+CD62L+CD25− T cells (FACS Aria, BD Biosciences). CD11c+ DCs were isolated via FACS. APCs were sorted via MACS using MHC class II microbeads. For isolation of IFN-γ-producing T cells, the IFN-γ secretion assay (Miltenyi Biotec) was used according to the manufacturer's protocols and sorted by FACS.
T Cell Stimulation.
Naïve T cells were cultured with irradiated MHCII+ cells (30 Gy, 1:4 ratio), A20 cells (120 Gy, 2:1 ratio), or CD11c+ DC (15:1 ratio) as APCs and anti-CD3 (0.5 μg/ml)/CD28 (1 μg/ml) or OVA323–339 peptide (0.2 μg/ml) was added. rIL-12 (10 ng/ml; R&D Systems) and anti-IL4 (10 μg/ml) (for Th1), anti-IL-12/anti-IFN-γ (10 μg/ml) and rIL-4 (100 ng/ml) (for Th2), anti-IL-4 and rIL-27 (10 ng/ml) (for IL-27) were added. Where indicated, 500 nM of CpG (ODN1826, Invivogen) or 125 nM GSI (Insolution GSI X, Calbiochem) was added. For APC-free systems, plate-bound anti-CD3 (5 μg/ml) and soluble anti-CD28 (1 μg/ml) were used.
BioPlex Analysis of Cytokine Production.
Concentrations of IL-2, IL-4, IL-5, IL-10, IL-13, IL-17, and IFN-γ were measured in culture supernatants with the BioPlex system (Bio-Rad Laboratories) according to the manufacturer's protocol. For detection, phycoerythrin-conjugated streptavidin (SA-PE) (Invitrogen) was used.
Immunization.
BALB/c mice were transferred with 1 × 107 CD62L+ OVA-TCR transgenic T cells (DO11.10) and immunized s.c. with 50 μg of OVA peptide and 20 μg of CpG (Invivogen). GSI DBZ (synthesized by Syncom) was used at 10 μM/kg in 0.1% Tween 80/0.5% Methocel 60 HG (Sigma-Aldrich) injected daily i.p.
DTH.
Th1 cells (5 × 105) were transferred alone or together with the same number of Notch- or control-transduced cells into naïve BALB/c mice. Neutralizing anti-IL-10 antibody (JES5–2A5–7) was injected i.p. Then, 24 h later, 250 ng of OVA323–339 peptide in incomplete Freund's adjuvant (IFA) (Sigma) was injected into the left footpad, and PBS/IFA was injected into the right footpad. Swelling was measured by an Oditest micrometer gauge in a blinded fashion.
Real-Time Quantitative RT-PCR.
Real-time PCR was performed with the LightCycler instrument and the FastStart DNA Master SYBR Green I kit (Roche Diagnostics). Cycling program (10 min at 95°C followed by 40 cycles of 15 s at 95°C, 15 s at 60°C (for Dll-1, IL-10, and FoxP3), 65°C (for UbcH5B, Jagged-1, Dll-4, Notch-1, Notch-3, T-bet, and GATA-3), or 67°C (for Notch-2 and Notch-4), and 15 s at 72°C). For primer sequences, see SI Materials and Methods. Data were evaluated with LightCycler software version 3.5.28 (Roche Diagnostics) and the second derivative maximum algorithm.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Katharina Raba and Toralf Kaiser for cell sorting and A. Hamann and M. Loehning for critical reading of the manuscript. S.R. was supported by a grant from the Boehringer Ingelheim Fonds. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 633 and SFB 650, and by European Community Grant MUGEN LSHG-CT-2005-005203.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712102105/DC1.
References
- 1.Moore KW, de Waal MR, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 5.Groux H, et al. A CD40+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 6.Assenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: Expression of interleukin-10 in interferon-gamma and in interleukin-4-expressing cells. Eur J Immunol. 1994;24:1097–1101. doi: 10.1002/eji.1830240513. [DOI] [PubMed] [Google Scholar]
- 7.Gerosa F, et al. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–234. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 8.Jankovic D, et al. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T (H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 11.Chang HD, et al. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur J Immunol. 2007;37:807–817. doi: 10.1002/eji.200636385. [DOI] [PubMed] [Google Scholar]
- 12.Barrat FJ, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerosa F, et al. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J Exp Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol. 1996;156:2776–2782. [PubMed] [Google Scholar]
- 16.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing antiinflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 18.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 19.Eagar TN, et al. Notch 1 signaling regulates peripheral T cell activation. Immunity. 2004;20:407–415. doi: 10.1016/s1074-7613(04)00081-0. [DOI] [PubMed] [Google Scholar]
- 20.Adler SH, et al. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J Immunol. 2003;171:2896–2903. doi: 10.4049/jimmunol.171.6.2896. [DOI] [PubMed] [Google Scholar]
- 21.Rutz S, Mordmuller B, Sakano S, Scheffold A. Notch ligands Delta-like1, Delta-like4 and Jagged1 differentially regulate activation of peripheral T helper cells. Eur J Immunol. 2005;35:2443–2451. doi: 10.1002/eji.200526294. [DOI] [PubMed] [Google Scholar]
- 22.Osborne BA, Minter LM. Notch signaling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 23.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 24.Amsen D, et al. Direct regulation of gata3 expression determines the T helper differentiation potential of notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu L, et al. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka S, et al. The interleukin-4 enhancer CNS-2 is regulated by Notch signals and controls initial expression in NKT cells and memory-type CD4 T cells. Immunity. 2006;24:689–701. doi: 10.1016/j.immuni.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Fang TC, Yashiro-Ohtani Y, Del BC, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maekawa Y, et al. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 29.Minter LM, et al. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol. 2005;6:680–688. [PubMed] [Google Scholar]
- 30.Hoyne GF, et al. Serrate1-induced notch signaling regulates the decision between immunity and tolerance made by peripheral CD4(+) T cells. Int Immunol. 2000;12:177–185. doi: 10.1093/intimm/12.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Yvon ES, et al. Over expression of the Notch ligand, Jagged-1 induces alloantigen-specific human regulatory T cells. Blood. 2003;102:3815–3821. doi: 10.1182/blood-2002-12-3826. [DOI] [PubMed] [Google Scholar]
- 32.Tanigaki K, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 33.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skokos D, Nussenzweig MC. CD8− DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204:1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 36.Shaw MH, et al. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J Immunol. 2006;176:7263–7271. doi: 10.4049/jimmunol.176.12.7263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.