Abstract
As the interface with the outside world, the airway epithelial barrier is critical to lung defense. Because of respiratory efforts, the airways are exposed to shear stress; however, little is known regarding the effects of shear on epithelial function. We report that low-level shear stress enhances epithelial barrier function, an effect that requires serial activation of the transient receptor potential vanilloid (TRPV) 4 and L-type voltage-gated calcium channel (VGCC) and an increase in intracellular calcium. These changes lead to a selective decrease in aquaporin-5 (AQP5) abundance because of protein internalization and degradation. To determine whether AQP5 plays a role in mediating the shear effects on paracellular permeability, we overexpressed hAQP5 in 16HBE cells, an airway epithelial cell line without endogenous AQP5. We found that AQP5 expression was needed for shear-induced barrier enhancement. These findings have direct relevance to the regulation of epithelial barrier function, membrane permeability, and water homeostasis in the respiratory epithelia.
Keywords: permeability, epithelium, transient receptor potential vanilloid, voltage-gated calcium channel, cytoskeleton
Mechanical forces elicit biologically relevant signals, best demonstrated in the cardiovascular and musculoskeletal systems, where stretch and shear alter differentiation and proliferation (1–5). The airway wall exists in a mechanically dynamic environment, and each breath causes circumferential and longitudinal expansion and contraction (6). Airway epithelial cells are exposed to luminal shear stress (frictional force per surface area) generated by airflow, which at rest breathing is 0.5–3 dynes/cm2. Shear forces may be a log higher with exercise (7, 8) and may increase to nearly 1,700 dynes/cm2 with cough and bronchospasm (8). In asthma, smooth muscle constriction produces airway folding, reducing luminal caliber producing heterogeneous shear at different parts along the airway (9).
Endothelial shear stress alters cytoskeletal organization, cell shape, and gene expression (10–20). Endothelial shear increases intracellular calcium concentration [Ca2+]i, triggering nitric oxide production (20), changes in actin stress fiber formation, and altered paracellular permeability (21) in part due to activation of transient receptor potential vanilloid (TRPV) 4, a nonselective cation channel (22). Mechanical stimulation alters airway epithelial EGF signaling (23), surface liquid height (8), and ciliary beat frequency (24). TRPV4 is also present in airway epithelial cells and is activated by temperature, hypotonicity (25–28), and shear stress (22, 29–31). TRPV4 activation has been implicated in alveolar septal barrier function (32), but its role in airway epithelia remains undefined.
We find that low levels of shear stress enhance airway epithelial barrier function, an effect mediated through increases in [Ca2+]i by sequential activation of TRPV4 and voltage-gated calcium channel (VGCC). Although VGCC has been implicated in lung vascular tone, its role in airway epithelia is unclear. Calcium influx triggers selective degradation of aquaporin-5 (AQP5), an apical channel with high selectivity for water (33). In salivary gland cells, AQP5 also may participate in the regulation of paracellular permeability (34). Similarly, we find that altered AQP5 expression modulates paracellular permeability in response to shear stress in airway epithelial cells. These studies provide insight into a homeostatic mechanism likely central to airway epithelial function.
Results
Shear Stress Alters Epithelial Paracellular Permeability.
To examine the effects of shear on epithelial barrier function, we differentiated primary human bronchial epithelial cells (NHBE) at an air–liquid interface, exposed them to shear for 1–2 h (see Materials and Methods), and then transferred the membrane to an intact transwell chamber for measurement of FITC-dextran permeability. Shear stress decreased paracellular permeability (Fig. 1A).
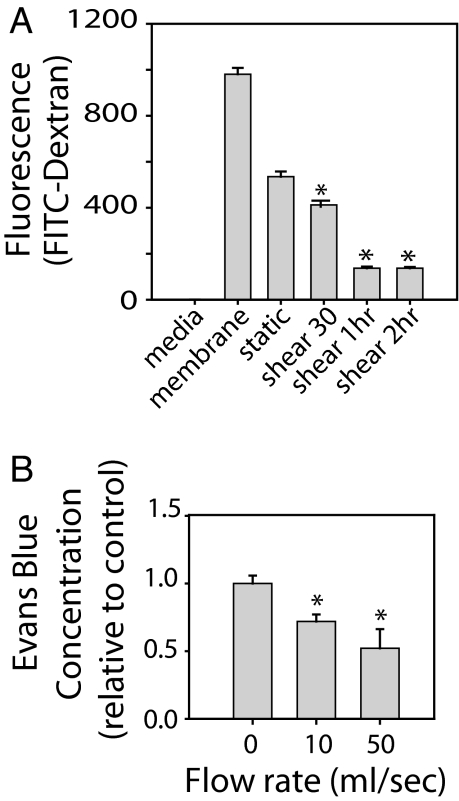
Fig. 1.
Shear stress reduces paracellular permeability. (A) NHBE cells exposed to shear, followed by assay of FITC-dextran (70 kDa) movement from the upper to lower chamber determined in the absence of cells (membrane) or under static or shear conditions. Low-level shear stress decreases paracellular permeability. (B) Mouse trachea was exposed to phasic shear or static conditions; paracellular permeability was assessed with Evans blue-4% albumin. Similar to in vitro findings, low levels of phasic air shear decreased paracellular permeability (n = 3). *, P < 0.05 with ANOVA one-way Bonferroni correction.
To examine the effects of shear on epithelial permeability in intact trachea, mouse trachea was resected and attached to a ventilator (see Materials and Methods) to create phasic airflow. After 1 h of ventilation, Evans blue dye-4% albumin, a commonly used tracer that, because of its size, is restricted to paracellular movement, was instilled in the trachea for 20 min. Then the trachea was flushed with PBS to eliminate residual dye, which was confirmed by visual inspection. Flows of 10 and 50 μl/s (1 and 5 dynes/cm2) decreased Evans blue permeation in tissue homogenates compared with static conditions (Fig. 1B), indicating that low levels of shear enhance barrier function in both human airway epithelial cells and mouse trachea.
Shear-Induced Changes in Permeability Are Mediated by TRPV4 and L-Type Calcium Channels.
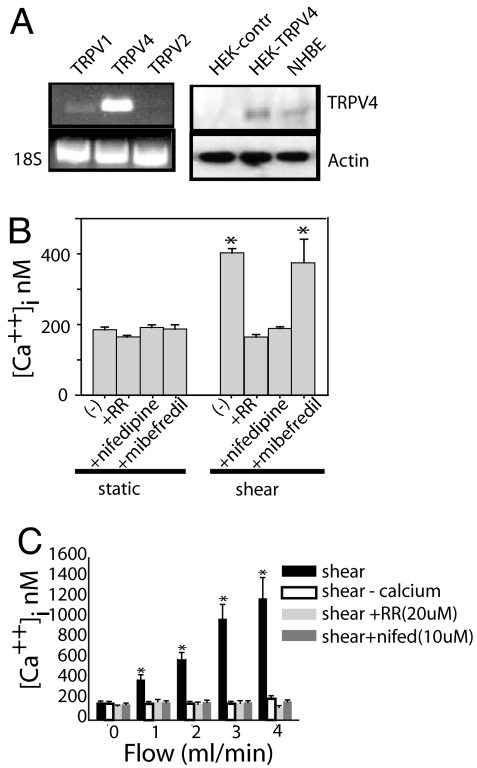
Endothelial shear increases [Ca2+]i (35, 36), with subsequent actin reorganization and stress fiber formation (21). Similarly, fura-2-loaded NHBE cells exposed to flow (0.5 ml/min; 1.5 dynes/cm2) exhibited increased [Ca2+]i (Fig. 2B). NHBE cells express both TRPV4 and TRPV1 (Fig. 2A). Addition of ruthenium red (RR), a nonselective TRPV inhibitor, blocked the increase in [Ca2+]i. Neither capsazepine, a TRPV1 inhibitor (data not shown), nor the T-type channel inhibitor, mibefredil (Fig. 2B), block the shear-induced increase in [Ca2+]i. In contrast, the VGCC inhibitor, nifedipine, blocked the shear-induced increases in [Ca2+]i. Increasing flow increased [Ca2+]i (Fig. 2C). This response was blocked by perfusion with calcium-free buffer, RR, or the VGCC inhibitors (Fig. 2C).
Fig. 2.
Shear stress increases intracellular calcium. (A) Analysis by RT-PCR of TRPV1, TRPV2, and TRPV4 in NHBE cells (Left) and 18S mRNA control. NHBE cells express TRPV1 and TRPV4. TRPV4 expression is shown by RT-PCR and immunoblotting (Right). (B) NHBE cells were loaded with Fura-2, and [Ca2+]i was measured before and after shear stress. Shear-induced increases in [Ca2+]i were blocked by 20 μM RR and 10 μM nifedipine but not 10 μM mibefredil. (C) Increase in shear stress (increasing flow) across NHBE cells led to a corresponding increase in [Ca2+]i. Calcium-free perfusate, RR, or nifedipine blocked the shear-induced increase in [Ca2+]i. (n = 3; six cells measured per experiment). *, P < 0.05 with ANOVA one-way Bonferroni correction.
To examine the relation between calcium and barrier function, we exposed NHBE cells to shear in the presence or absence of calcium-free medium, nifedipine, and RR: All three agents blocked the shear-induced changes in paracellular permeability (Fig. 3A). Similarly, treatment of trachea with nifedipine blocked the shear-induced enhancement of barrier function (reduction in Evans blue uptake) seen in untreated trachea (Fig. 3B). In static conditions, TRPV4-null trachea had significantly greater tissue Evan's blue uptake than wild type; however, shear did not enhance barrier function in TRPV4-null mice (Fig. 3B). These findings indicate that both VGCC and TRPV4 activation are required for shear-induced reduction in epithelial permeability.
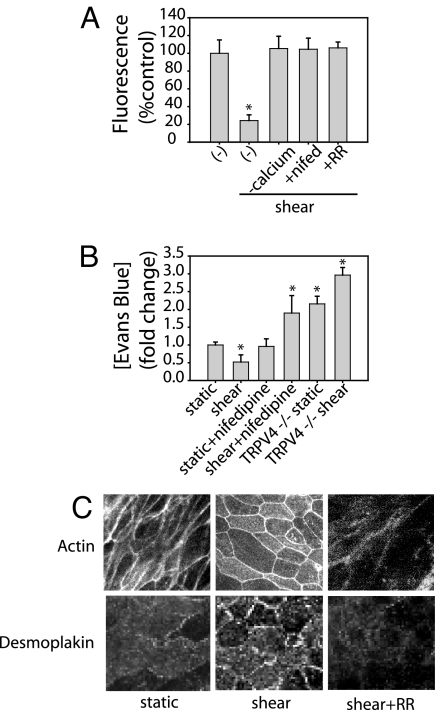
Fig. 3.
TRPV4 or VGCC inhibition block shear-induced barrier enhancement. (A) NHBE cells were exposed to shear in the presence or absence of calcium-free Krebs, 20 μM RR, or 10 μM nifedipine. FITC-dextran permeability was measured by fluorescence. All three blocked the shear-induced reduction in paracellular permeability. (B) Trachea incubated in 10 μM nifedipine or trachea isolated from TRPV4−/− mice showed no reduction in paracellular permeability in response to shear stress. (C) In NHBE cells, shear stress alters actin expression and causes redistribution of desmoplakin. Both are prevented by the addition of 20 μM RR to the perfusate (n = 3). *, P < 0.05 with ANOVA one-way Bonferroni correction.
Reduced paracellular permeability can result from cytoskeletal reorganization or altered cell–cell contact. To examine potential mechanisms of shear-reduced changes in barrier function, actin and desmosome organization was assessed under static and shear conditions. Shear stress altered actin distribution and cell shape, and it promoted the peripheral distribution of the desmosome-associated protein, desmoplakin. Both effects were blocked by RR (Fig. 3C).
Shear-Induced Increases in [Ca2+]i Mediate Changes in AQP5 Abundance and Distribution.
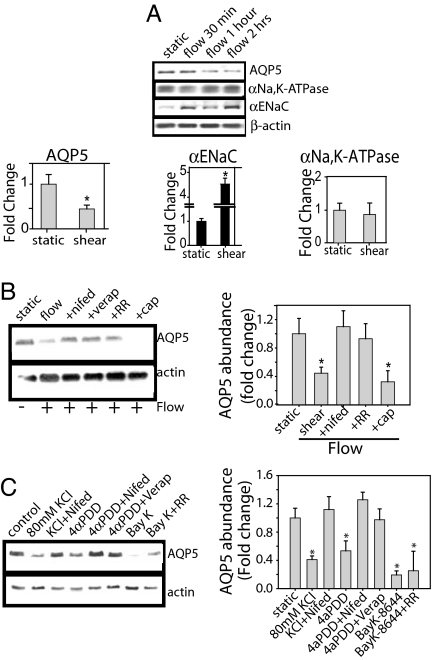
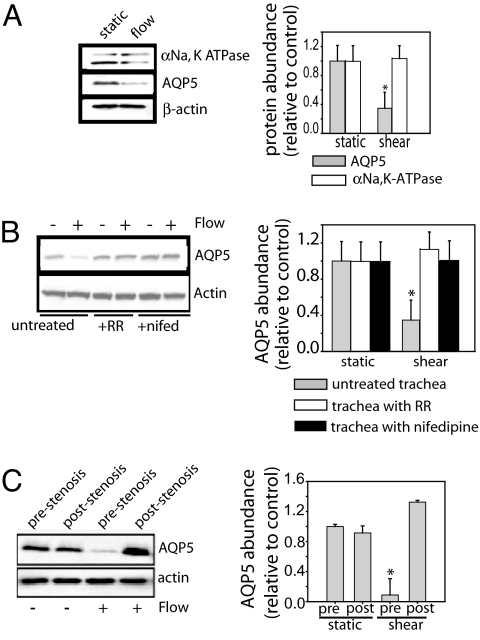
Because TRPV4 activation regulates AQP5 expression in response to hypotonic stress (26) and AQPs have been implicated in the regulation of cell volume and shape, we examined the effect of shear on AQP5 abundance. In shear-exposed NHBE, AQP5 abundance decreased, αENaC increased, and αNa,K-ATPase expression did not change (Fig. 4A). Treatment with RR, nifedipine, or verapamil inhibited the shear-induced reduction of AQP5 (Fig. 4B), with no effect on αENaC or αNa,K-ATPase (data not shown), suggesting that activation of both TRPV4 and VGCC participate in the down-regulation of AQP5. Although we have previously shown that cAMP regulates AQP5 (37), we found that PKA inhibition did not block the shear effects on AQP5.
Fig. 4.
Shear stress alters epithelial transporter expression. (A) In NHBE cells, shear stress decreased AQP5 abundance and increased αENaC expression with no change in αNa, K-ATPase expression. (B) NHBE cells treated with inhibitor were exposed to shear stress for 2 h. TRPV4 inhibition with 20 μM RR and VGCC inhibition with 10 μM verapamil or 10 μM nifedipine blocked shear-induced decreases in AQP5; the TRPV1 inhibitor, capsazepine (50 μM), had no effect. (C) In NHBE cells, under static conditions, TRPV4 activation with 4αPDD decreases AQP5, which was blocked by VGCC inhibition with nifedipine. VGCC activation with BayK-8644 decreased AQP5; the TRPV4 inhibitor, RR, did not prevent the decrease (n = 3). *, P < 0.05 with ANOVA one-way Bonferroni correction.
Using down-regulation of AQP5 as the readout, we assessed whether TRPV4 and VGCC were activated in series or in parallel (Fig. 4C). Under static conditions, TRPV4 activation with 4αPDD decreased AQP5 abundance, an effect blocked by VGCC inhibition with nifedipine. Activation of VGCC with BayK-8644 also decreased AQP5 abundance, but RR did not prevent the decrease (Fig. 4C), indicating TRPV4 activation followed by VGCC.
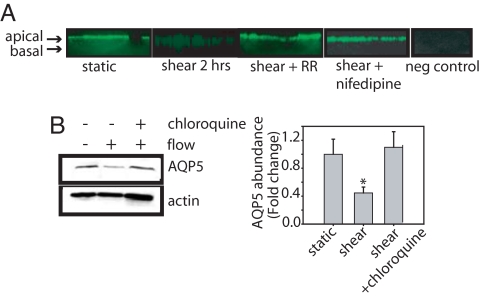
To further examine the AQP5 response, we assessed AQP5 localization. In untreated NHBE cells, apical AQP5 labeling is evident (Fig. 5A). After exposure to shear, apical signal was lost and increased intracellular AQP5 was seen. Treatment with either RR or nifedipine blocked the shear-induced AQP5 internalization (Fig. 4A).
Fig. 5.
Shear-induced AQP5 internalization and degradation. (A) Immunofluorescence reveals that in NHBE cells, 2 h of shear stress leads to AQP5 internalization, which was blocked by TRPV4 inhibitor, 20 μM RR, or VGCC inhibitor, nifedipine (10 μM), with preimmune serum control. (B) NHBE cells were exposed to shear stress in the presence of absence of the lysosome inhibitor, chloroquine (100 μM), and harvested after 2 h. Chloroquine blocked the shear-induced reduction in AQP5 abundance (n = 3). *, P < 0.05 with ANOVA one-way Bonferroni correction.
We previously demonstrated lysosomal degradation of AQP5 (37). To determine whether shear reduces AQP5 via lysosomal degradation, NHBE cells were pretreated with the lysosomal inhibitor, chloroquine (100 μM), for 30 min and exposed to shear. Chloroquine prevented the shear-induced reduction in AQP5 abundance (Fig. 4B). Real-time PCR revealed that AQP5 mRNA was not reduced by shear [ΔCt = 0.99 (static) and 0.96 (shear)], indicating no decrease in transcriptional activity.
Compared with static, exposure of mouse trachea to shear also decreased AQP5, with no change in αNa,K-ATPase abundance (Fig. 6A). Treatment with either RR or nifedipine blocked the AQP5 reduction (Fig. 6B). To further examine shear effects, we introduced a midtracheal stenosis to produce turbulent flow and therefore lower shear distal to the obstruction. Absent flow, the midtracheal stenosis did not affect AQP5 abundance in up- or downstream segments (Fig. 6C). With flow, AQP5 was decreased proximal to the stenosis. AQP5 abundance did not change in the downstream segment with reduced shear (Fig. 6C). Exposure in the opposite direction (from distal to proximal trachea) produced similar changes (prestenosis reduction in AQP5, poststenosis no change), indicating a primary effect of flow, rather than tracheal orientation (data not shown).
Fig. 6.
Phasic shear stress created by airflow decreased AQP5 abundance in mouse trachea. (A) Ex vivo mouse trachea was exposed to either static or shear conditions for 1 h. Shear stress decreased AQP5 abundance without a change in α1Na, K-ATPase. (B) Nifedipine (10 μM) or RR (20 μM) prevented the shear-induced decrease in AQP5 abundance. (C) Flow disruption by a midtracheal stenosis altered AQP5 expression. Laminar flow created greater shear stress proximal to the obstruction, whereas turbulent flow led to lower shear stress distally. Higher shear proximal to the obstruction caused less AQP5 expression compared with the distal, lower shear region (n = 3). *, P < 0.05 with ANOVA one-way Bonferroni correction.
AQP5 and Paracellular Permeability.
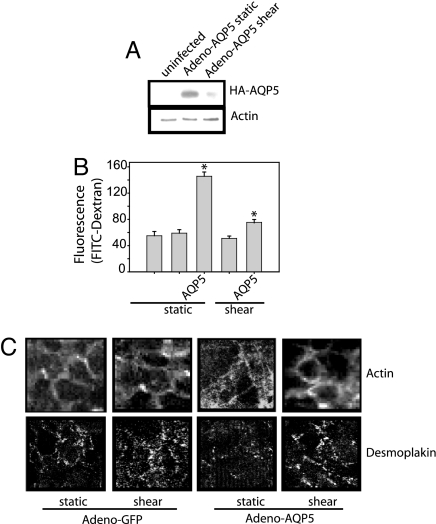
Because TRPV4 and VGCC mediated increases in [Ca2+]i lead to both decreased AQP5 and decreased paracellular permeability, we examined the relation of AQP5 to paracellular permeability.16HBE cells, which endogenously express TRPV4 but not AQP5 (data not shown), were grown on inserts to confluence and infected with either Adeno-GFP or Adeno-AQP5 (85–90% infection efficiency). As with NHBE, shear decreased AQP5 abundance in adeno-hAQP5 infected 16HBE cells (Fig. 7A). 16HBE cells expressing hAQP5 had increased baseline paracellular permeability, compared with either GFP-expressing cells or uninfected 16-HBE cells (Fig. 7B), and less cortical actin (Fig. 7C). In GFP-expressing cells, shear did not alter paracellular permeability (Fig. 7B). In AQP5-expressing cells, shear reduced paracellular permeability. In AQP5-expressing cells, shear stress induced a peripheral redistribution of both actin and desmoplakin (Fig. 7C).
Fig. 7.
The presence of AQP5 alters shear-induced changes in paracellular permeability. (A) 16HBE cells expressing Adeno-hAQP5 were exposed to static or shear conditions. AQP5 expression decreased after 2 h of shear stress. (B) 16HBE cells infected with Adeno-GFP virus or Adeno-hAQP5 were exposed to static or shear. Presence of Adeno-hAQP5 caused increased paracellular permeability, compared with either infection with a GFP control or an uninfected control. After the application of shear stress, Adeno-hAQP5-expressing cells had significantly decreased paracellular permeability, compared with static conditions (n = 3). *, P < 0.05 with ANOVA one-way Bonferroni correction. (C) 16HBE cells were infected with Adeno-GFP virus or Adeno-hAQP5 under static and shear condition. Actin fibers were assessed by using Texas-red phalloidin. Only hAQP5-expressing cells had altered actin expression and desmoplakin redistribution in response to shear stress.
Discussion
The airway epithelial barrier serves several functions. In addition to protecting against infectious and noninfectious respirable particles, the epithelium segregates signals initiated in the apical and basal compartments (38, 39). Disruption of the epithelial barrier as occurs during infection, inflammation, or asthma is believed to contribute to disease expression (39–42). Our findings indicate that shear forces can regulate barrier function and provide insight into an important homeostatic mechanism.
Using both cell culture and intact trachea, we show that shear stress enhances epithelial barrier function. Although transepithelial electrical resistance is often used as a surrogate for permeability, it measures instantaneous open probability of pores within tight junction strands, rather than the passage of solute and may diverge from solute permeability, highlighted when chicken occludin was expressed in MDCK cells, causing increased electrical resistance and solute permeability (43, 44). In our study, calcium influx followed sequential activation of TRPV4 and VGCC, and solute permeability was blocked by either channel. TRPV4 functions as a molecular integrator of biophysical stimuli relevant to the airway luminal environment, such as temperature, hypotonicity, and shear stress (28). In mammary epithelial cells (45) and in the lung vasculature (46), TRPV4 activation modulates paracellular permeability. It appears to play a similar role in the airways.
AQP5 abundance was altered by shear, and shear-induced modulation of paracellular permeability did not occur in the absence of AQP5. Kawedia et al. (34) demonstrated decreased paracellular permeability in salivary gland cells from AQP5-null mice, suggesting a role for AQP5 in cell–cell interactions. Our findings are consistent with those observations. Shear provoked changes in cell-shape change, as well as actin and desmoplakin redistribution. Desmoplakin, a component of desmosomes, binds to intermediate filaments within the cells and thereby contributes to the barrier function the monolayer. Shear effects on actin and desmoplakin were not seen in the absence of AQP5. Other AQPs also are linked to cytoskeletal changes. AQP2 binds actin (47) and AQP0 binds to intermediate filament proteins to alter cells shape and morphology (48).
We have provided evidence that shear modulates barrier function in airway epithelia by serial activation of TRPV4 and L-type VGCC, and with our findings support the recent proposal that AQP5 may contribute to regulation of paracellular permeability (34). These observations provide insight into a potentially important homeostatic mechanism in the respiratory tract. In addition, our studies suggest cross-talk between transcellular and paracellular pathways that may be relevant in multiple tissues.
Materials and Methods
See supporting information (SI) Materials and Methods for details.
Cell Culture.
NHBE (Lonza) were grown on collagen-coated inserts (Falcon) at 37°C with 5% CO2 in specified media and maintained at an air–liquid interface for 6–9 weeks before study; transepithelial resistance was >400 ohms when used. Inhibitors were used as pretreatment and were included in the perfusate. All experiments were repeated a minimum of three times. In specified studies, immortalized human bronchial epithelial cells (16HBE; a gift of Gary Cutting, The Johns Hopkins University School of Medicine) were cultured on inserts and infected with either GFP- or AQP5-expressing adenovirus.
Shear Stress.
We calculated the rate of perfusate flow in an open-plate apparatus required to create shear stress comparable shear in vivo, assuming the perfusate is a Newtonian, noncompressible fluid with no-slip boundary conditions. Fluid flow rates of 0.5–1 ml/min provided a shear stress consistent with the magnitude for airway epithelial cells in vivo. Cells on inserts were placed on coverslips and perfused in a chamber (RH-2; Warner Instrument) at 0.5–1 ml/min with a Krebs solution (49). The perfusate was gassed with 16% O2/5% CO2 at 37°C. Chamber temperature was maintained with an inline heat exchanger, and heated platforms were controlled by a dual-channel servo controller (SF-28 and TC-344B; Warner Instrument). The cells were exposed to laminar shear stress (τ) calculated by τ = 6 μQ/wh2, where Q = the flow, at 0.5–1 ml/min. The chamber dimensions (w and h) are 12.5 mm and 1 mm; assuming that the Krebs solution has a similar viscosity (μ) to water at (temperature 37°C) μ = 0.00653 dynes/cm2, then shear stress is τ = 1.5–3 dynes/cm2.
Sample Processing, Immunoblotting, and PCR.
See SI Materials and Methods for details.
Animal Studies.
Briefly, 8- to 10-week-old C57BL/6 mice (20–30 g; Charles River Laboratory) (approved by the Johns Hopkins Animal Care and Use Committee) were killed by 135/1.5 mg/kg i.p. ketamine/acepromazine. The trachea was resected and placed in a physiological Krebs bath under a heat lamp. Both ends of the excised trachea were cannulated with 18-gauge angiocaths, and one end was attached to a ventilator [tidal volume 200 μl, rate 120/min MiniVent (Harvard Apparatus); Micro Flow meter 5–500 μl/s (Cole-Parmer); 20 psi compressed air tank]. Air was humidified and warmed to 37°C before entry into the tracheal lumen. Laminar flow can be assumed given that the entry length of the angiocath before the trachea is greater than five times the length of the trachea (50). Flow rates were 10, 50, and 100 μl/s, which corresponded to shear stresses of ≈1, 5, and 10 dynes/cm2 assuming laminar flow (τ = 4μQ/πr3, where μ is the viscosity, Q is the flow, and r is the radius of the tube). In some experiments, a midtracheal obstruction was created with hemoclips (M&I Medical Sales) to create stenosis. The trachea was ventilated for 1 h, and results were compared with nonventilated trachea similarly mounted.
TRPV4−/− mice (51) were generously provided by M. Suzuki and A. Mizuno (Jichi Medical School) and by Daiichi Pharmaceutical Company.
Calcium Imaging.
Cells were incubated with 7.5 μM Fura-2 AM (Molecular Probes) for 60 min at 37°C under an atmosphere of 5% CO2/95% air and mounted in a heated chamber on the stage of a Nikon TSE 100 Ellipse inverted microscope. Fura-2 fluorescence ratios (R = F340/F380) allows estimation of [Ca2+]i (see SI Materials and Methods).
Permeability Assay in Vitro.
After shear, the excised insert was placed on the base of an intact transwell chamber, allowing for separation of the apical and basal compartments, with the cells on the apical surface of the top insert. The edges were sealed with gel (Dow Corning), and a rubber washer and 500 μl of FITC-coupled dextran beads (4 or 70 kDa, 10 mg/ml; Calbiochem) were added to the upper well for 20 min. Then, 3 ml of medium was placed in the bottom well. The FITC concentration in 1 ml of the lower well was determined by using a fluorometer (excitation 490 nm, detection 530 nm) and compared with static and cell-free two insert controls. Ex vivo, after exposure to shear or static conditions for 1 h, we instilled 50 μl of Evans blue dye-4% albumin into the lumen of the tracheal segments for 20 min. The lumen was gently flushed with PBS, and the tracheal segments were homogenized in formamide and incubated in a 60°C water bath for 18 h. The Evans blue dye concentration was measured by using a spectrophotometer and quantified by using predetermined standards.
Statistics.
All statistical analyses described in figure legends were performed by using STATA 9 (Stata Corporation).
Supplementary Material
ACKNOWLEDGMENTS.
We thank Makoto Suzuki and Atsuko Mizuno for providing TRPV4−/− mice. This work was supported by a Johns Hopkins Clinician Scientist Award (to V.K.S.), a Flight Attendant Medical Research Institute Clinical Innovator Award (to L.S.K.), and National Heart, Lung, and Blood Institute Grant R01-HL67191 (to L.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712287105/DC1.
References
- 1.White CR, Stevens HY, Haidekker M, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate ERK1/2 activation in human endothelial cells. Am J Physiol. 2005;289:H2350–H2355. doi: 10.1152/ajpheart.01229.2004. [DOI] [PubMed] [Google Scholar]
- 2.White CR, Haidekker MA, Stevens HY, Frangos JA. Extracellular signal-regulated kinase activation and endothelin-1 production in human endothelial cells exposed to vibration. J Physiol. 2004;555:565–572. doi: 10.1113/jphysiol.2003.059899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haidekker MA, White CR, Frangos JA. Analysis of temporal shear stress gradients during the onset phase of flow over a backward-facing step. J Biomech Eng. 2001;123:455–463. doi: 10.1115/1.1389460. [DOI] [PubMed] [Google Scholar]
- 4.White CR, Haidekker M, Bao X, Frangos JA. Temporal gradients in shear, but not spatial gradients, stimulate endothelial cell proliferation. Circulation. 2001;103:2508–2513. doi: 10.1161/01.cir.103.20.2508. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32:241–251. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- 6.Savla U, Sporn PH, Waters CM. Cyclic stretch of airway epithelium inhibits prostanoid synthesis. Am J Physiol. 1997;273:L1013–L1019. doi: 10.1152/ajplung.1997.273.5.L1013. [DOI] [PubMed] [Google Scholar]
- 7.Cebral JR, Summers RM. Tracheal and central bronchial aerodynamics using virtual bronchoscopy and computational fluid dynamics. IEEE Trans Med Imaging. 2004;23:1021–1033. doi: 10.1109/TMI.2004.828680. [DOI] [PubMed] [Google Scholar]
- 8.Tarran R, et al. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem. 2005;280:35751–35759. doi: 10.1074/jbc.M505832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olman MA. Epithelial cell modulation of airway fibrosis in asthma. Am J Respir Cell Mol Biol. 2003;28:125–128. doi: 10.1165/rcmb.F257. [DOI] [PubMed] [Google Scholar]
- 10.Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- 11.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 12.Davies PF, Zilberberg J, Helmke BP. Spatial microstimuli in endothelial mechanosignaling. Circ Res. 2003;92:359–370. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- 13.Davies PF. Multiple signaling pathways in flow-mediated endothelial mechanotransduction: PYK-ing the right location. Arterioscler Thromb Vasc Biol. 2002;22:1755–1757. doi: 10.1161/01.atv.0000034391.00347.71. [DOI] [PubMed] [Google Scholar]
- 14.Davies PF, Polacek DC, Shi C, Helmke BP. The convergence of haemodynamics, genomics, and endothelial structure in studies of the focal origin of atherosclerosis. Biorheology. 2002;39:299–306. [PMC free article] [PubMed] [Google Scholar]
- 15.Helmke BP, Davies PF. The cytoskeleton under external fluid mechanical forces: Hemodynamic forces acting on the endothelium. Ann Biomed Eng. 2002;30:284–296. doi: 10.1114/1.1467926. [DOI] [PubMed] [Google Scholar]
- 16.Helmke BP, Thakker DB, Goldman RD, Davies PF. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys J. 2001;80:184–194. doi: 10.1016/S0006-3495(01)76006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh TT, Davies MG, Trovato MJ, Svendsen E, Hagen PO. Alterations in wall tension and shear stress modulate tyrosine kinase signaling and wall remodeling in experimental vein grafts. J Vasc Surg. 1999;29:334–344. doi: 10.1016/s0741-5214(99)70386-1. [DOI] [PubMed] [Google Scholar]
- 18.Davies PF, et al. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol. 1997;59:527–549. doi: 10.1146/annurev.physiol.59.1.527. [DOI] [PubMed] [Google Scholar]
- 19.Davies PF, Mundel T, Barbee KA. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J Biomech. 1995;28:1553–1560. doi: 10.1016/0021-9290(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 20.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita T, et al. Role of Ca2+ and protein kinase C in shear stress-induced actin depolymerization and endothelin 1 gene expression. Circ Res. 1994;75:630–636. doi: 10.1161/01.res.75.4.630. [DOI] [PubMed] [Google Scholar]
- 22.Kohler R, et al. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 23.Tschumperlin DJ, et al. Mechanotransduction through growth-factor shedding into the extracellular space. Nature. 2004;429:83–86. doi: 10.1038/nature02543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winters SL, Davis CW, Boucher RC. Mechanosensitivity of mouse tracheal ciliary beat frequency: Roles for Ca2+, purinergic signaling, tonicity, and viscosity. Am J Physiol. 2007;292:L614–L624. doi: 10.1152/ajplung.00288.2005. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, et al. A role for AQP5 in activation of TRPV4 by hypotonicity: Concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem. 2006;281:15485–15495. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 26.Sidhaye VK, et al. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc Natl Acad Sci USA. 2006;103:4747–4752. doi: 10.1073/pnas.0511211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilius B, Voets T. Diversity of TRP channel activation. Novartis Found Symp. 2004;258:140–149. discussion 149–159, 263–266. [PubMed] [Google Scholar]
- 28.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: A paradigm for gating diversity. Am J Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 29.Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: Activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–27137. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- 30.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 31.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: Solving the calcium entry puzzle? Endothelium. 2003;10:5–15. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez DF, et al. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ Res. 2006;99:988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King LS, Kozono D, Agre P. From structure to disease: The evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–698. doi: 10.1038/nrm1469. [DOI] [PubMed] [Google Scholar]
- 34.Kawedia JD, et al. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc Natl Acad Sci USA. 2007;104:3621–3626. doi: 10.1073/pnas.0608384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jen CJ, Jhiang SJ, Chen HI. Invited review: Effects of flow on vascular endothelial intracellular calcium signaling of rat aortas ex vivo. J Appl Physiol. 2000;89:1657–1662. doi: 10.1152/jappl.2000.89.4.1657. discussion 1656. [DOI] [PubMed] [Google Scholar]
- 36.Worthen LM, Nollert MU. Intracellular calcium response of endothelial cells exposed to flow in the presence of thrombin or histamine. J Vasc Surg. 2000;32:593–601. doi: 10.1067/mva.2000.106955. [DOI] [PubMed] [Google Scholar]
- 37.Sidhaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem. 2005;280:3590–3596. doi: 10.1074/jbc.M411038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humlicek AL, et al. Paracellular permeability restricts airway epithelial responses to selectively allow activation by mediators at the basolateral surface. J Immunol. 2007;178:6395–6403. doi: 10.4049/jimmunol.178.10.6395. [DOI] [PubMed] [Google Scholar]
- 39.Winter MC, Shasby SS, Ries DR, Shasby DM. PAR2 activation interrupts E-cadherin adhesion and compromises the airway epithelial barrier: Protective effect of beta-agonists. Am J Physiol. 2006;291:L628–L635. doi: 10.1152/ajplung.00046.2006. [DOI] [PubMed] [Google Scholar]
- 40.Coyne CB, et al. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–3234. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogg JC, Pare PD, Boucher RC. Bronchial mucosal permeability. Fed Proc. 1979;38:197–201. [PubMed] [Google Scholar]
- 42.Zabner J, et al. Histamine alters E-cadherin cell adhesion to increase human airway epithelial permeability. J Appl Physiol. 2003;95:394–401. doi: 10.1152/japplphysiol.01134.2002. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy KM, et al. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109(Pt 9):2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 44.Balda MS, et al. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiter B, et al. TRPV4-mediated regulation of epithelial permeability. Faseb J. 2006;20:1802–1812. doi: 10.1096/fj.06-5772com. [DOI] [PubMed] [Google Scholar]
- 46.Hamanaka K, et al. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol. 2007;293:L923–L932. doi: 10.1152/ajplung.00221.2007. [DOI] [PubMed] [Google Scholar]
- 47.Noda Y, Horikawa S, Katayama Y, Sasaki S. Water channel aquaporin-2 directly binds to actin. Biochem Biophys Res Commun. 2004;322:740–745. doi: 10.1016/j.bbrc.2004.07.195. [DOI] [PubMed] [Google Scholar]
- 48.Lindsey Rose KM, et al. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–1570. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol. 2004;286:L848–L858. doi: 10.1152/ajplung.00319.2003. [DOI] [PubMed] [Google Scholar]
- 50.Bird RB, WES, Lightfoot EN. Transport Phenomena. New York: Wiley; 1960. pp. 42–51. [Google Scholar]
- 51.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.