Abstract
Insulin resistance, a hallmark of type 2 diabetes and obesity, is associated with increased activity of MAP and stress-activated protein (SAP) kinases, which results in decreased insulin signaling. Our goal was to investigate the role of MAP kinase phosphatase-4 (MKP-4) in modulating this process. We found that MKP-4 expression is up-regulated during adipocyte and myocyte differentiation in vitro and up-regulated during fasting in white adipose tissue in vivo. Overexpression of MKP-4 in 3T3-L1 cells inhibited ERK and JNK phosphorylation and, to a lesser extent, p38MAPK phosphorylation. As a result, the phosphorylation of IRS-1 serine 307 induced by anisomycin was abolished, leading to a sensitization of insulin signaling with recovery of insulin-stimulated IRS-1 tyrosine phosphorylation, IRS-1 docking with phosphatidylinositol 3-kinase, and Akt phosphorylation. MKP-4 also reversed the effect of TNF-α to inhibit insulin signaling; alter IL-6, Glut1 and Glut4 expression; and inhibit insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Overexpression of MKP-4 in the liver of ob/ob mice decreased ERK and JNK phosphorylation, leading to a reduction in fed and fasted glycemia, improved glucose intolerance, decreased expression of gluconeogenic and lipogenic genes, and reduced hepatic steatosis. Thus, MKP-4 has a protective effect against the development of insulin resistance through its ability to dephosphorylate and inactivate crucial mediators of stress-induced insulin resistance, such as ERK and JNK, and increasing MKP-4 activity might provide a therapy for insulin-resistant disorders.
Keywords: cytokines, diabetes, kinases, obesity
Insulin resistance is a central defect in obesity, type 2 diabetes, and the metabolic syndrome. These disorders have been associated with a chronic inflammatory state and an activation of cellular stress responses (1). Elevated levels of free fatty acids and cytokines, such as TNF-α, IL-6, or IL-1 β, are involved in this process by activating stress pathways that impede insulin signaling and lead to a decreased insulin response (1). Among these pathways, activation of the MAP kinases (ERK 1 and 2) (2) and SAP kinases (JNK and p38MAPK) are crucial in the insulin resistance (3–5). One target of the MAP and SAP kinases is insulin receptor substrate (IRS)-1, which is phosphorylated on serine residues (3). This impairs the ability of the insulin receptor to tyrosine phosphorylate IRS-1 and thus reduces its ability to interact with the downstream components of the insulin-signaling network (6). Blocking these stress kinases has the potential to prevent development of insulin resistance, and thus these serve as targets for the development of pharmacological inhibitors (7, 8).
Several negative regulators modulate the intensity and the duration of the stress response. The most direct regulators are MAPK phosphatases (MKP), a class of phosphatases with dual-specificity activity toward threonine and tyrosine residues that dephosphorylate and inactivate the MAP and SAP kinases. Although the MAP and SAP kinases have been extensively studied in the regulation of insulin action, the role of MKPs remains largely unknown. The MKPs are regulated by a number of factors (9). The N-terminal domain of MKPs increases their substrate specificity, and this interaction results in activation of the enzymatic activity (9). The MKPs are also induced by a variety of stimuli (10). In addition, localization determines the compartment in which they find their targets (10). This is important because the MAP and SAP kinases can translocate from the cytoplasm to the nucleus and phosphorylate a broad range of proteins (11). Finally, MKP activity can be regulated by phosphorylation and by oxidation of the catalytic site (12, 13).
Among the MKPs, MKP-4, also known as dual-specificity phosphatase-9 (DUSP-9), is an interesting candidate for the regulation of the stress responses involved in insulin resistance. MKP-4 has been shown to be expressed in insulin-sensitive tissues, and its expression is modulated in insulin-resistant states (14). MKP-4 has also been shown to inhibit adipocyte differentiation and modify some insulin actions (14). However, the molecular targets and the mechanism of action of MKP-4 in insulin resistance are unclear. In this article, we study in detail the regulation of MKP-4 expression and the effect of MKP-4 on insulin signaling and insulin action in vitro and in vivo, especially in situations with increased stress kinase activity.
Results
Expression and Localization of MKP-4 in Insulin-Sensitive Cell Lines and Tissues.
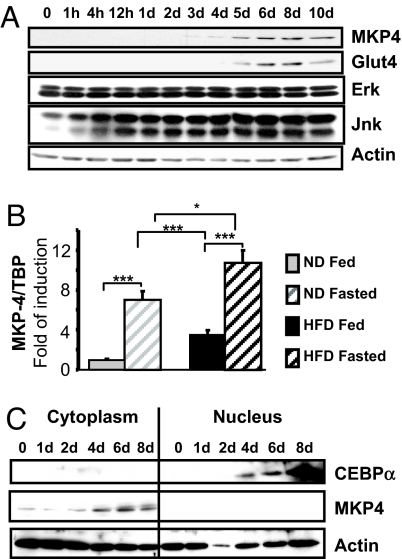
The expression of MKP-4 was assessed during differentiation of several insulin-sensitive cell lines. In 3T3-L1 cells, whereas ERK and JNK expression remained stable, MKP-4 expression was detected at day 3 after the induction and then gradually increased until it reached a plateau by day 6, when the cells become adipocytes (Fig. 1A). A similar pattern was observed during brown adipocyte differentiation [supporting information (SI) Fig. 6A]. In C2C12 myoblasts, both MKP-4 and MKP-1 expression was detected as early as day 1 and increased to the end of the differentiation process (SI Fig. 6B), whereas MKP-7 expression was detectable in the undifferentiated cells, rose slightly, and then returned to basal levels by day 6. Thus, MKP-4 expression increased in all of the insulin-sensitive cell lines in parallel with differentiation, as the cells acquired their metabolic function.
Fig. 1.
MKP-4 protein expression and localization in insulin-sensitive cells and tissues. (A) As specified in Experimental Methods, 3T3-L1 cells were induced to differentiate. Cell lysates were prepared at different time points after induction of differentiation and analyzed by immunoblots. Typical autoradiographs from at least three independent experiments are shown. (B) Mice were fed with either regular chow (10% fat, gray bars) or a high-fat diet (60% fat, black bars) for 11 weeks and then killed either in the early morning in the fed state (hatched bars) or after an overnight fast (gray and black bars). Expression of MKP-4 in epididymal adipose tissue was assessed by real-time PCR, normalized to TBP, and presented as the mean ± SEM. n ≥ 8 mice for each group. *, P < 0.05; **, P < 0.005; ***, P < 0.001. (C) Nuclear and cytoplasmic extracts were prepared at various times after induction of differentiation in 3T3-L1 cells and immunoblotted as specified. The first six lanes correspond to the cytoplasmic extracts, and the last six lanes correspond to the nuclear extracts.
To determine whether MKP-4 expression is modulated in vivo under physiological and pathological conditions, we analyzed MKP-4 expression in insulin-sensitive tissues of lean and obese mice in various feeding conditions. Two groups of mice were studied: obese mice fed a high-fat diet (HFD) and lean control mice on a regular chow diet. In the normal-chow group, fasting induced a 7-fold increase in MKP-4 mRNA in adipose tissue compared with control-fed mice (Fig. 1B). Chronic exposure to HFD also increased MKP-4 levels by 3-fold in the fed state, and levels of MKP-4 expression further increased by 3-fold upon overnight fasting. Expression of the MKP-1 and MKP-3 were modestly or not changed in these conditions (SI Fig. 6 C and D). By contrast, there was no modulation of MKP-4 by fasting in the liver and skeletal muscle of lean mice. However, HFD increased MKP-4 expression by 2.5-fold in the fed state in the liver (SI Fig. 6E) and by 3-fold in the fasted state in skeletal muscle (SI Fig. 6F).
In addition to expression, the activity of the MKPs is also regulated by intracellular localization (10). For example, MKP-3 is primarily cytoplasmic (15), MKP-1 is primarily nuclear (16), and MKP-7 is able to shuttle from one compartment to another (17). Cell fractionation followed by Western blotting of cytoplasmic and nuclear extracts obtained from differentiating 3T3-L1 cells revealed that MKP-4 was located in the cytoplasmic compartment at all time points during differentiation and was not detected in the nuclear compartment (Fig. 1C), suggesting that MKP-4 dephosphorylates its targets in the cytoplasm of the cell.
MKP-4 Specificity in Cells and Protective Effect Against Stress-Induced Insulin Resistance.
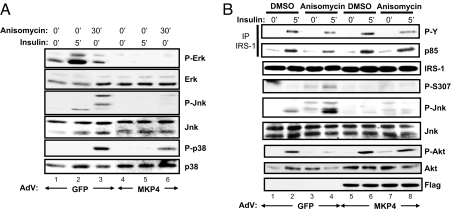
Members of the MKP family have different specificity for different MAP and SAP kinases (9, 18). To identify the MKP-4 substrates in insulin-sensitive cells, we analyzed the phosphorylation status of different members of the MAPK family in 3T3-L1ΔCAR fibroblasts infected with adenoviruses mediating GFP (AdV-GFP) as control or MKP-4 (AdV-MKP-4) expression. Two days after infection, cells were stimulated with insulin (5 min) or treated with anisomycin (30 min). In control cells, insulin stimulated ERK and JNK phosphorylation, whereas anisomycin produced strong phosphorylation of JNK and p38MAPK (Fig. 2A, lanes 1–3). By contrast, in cells overexpressing MKP-4, the ERK and JNK phosphorylation responses to insulin and anisomycin were decreases >80% (Fig. 2A, lanes 4–6, and SI Fig. 7A), and p38MAPK phosphorylation was reduced by 50% (Fig. 2A, lanes 4–6 and SI Fig. 7A).
Fig. 2.
MKP-4 inhibits ERK and JNK more than p38MAPK and protects against anisomycin-induced insulin resistance. The 3T3-L1ΔCAR cells were infected with equivalent amounts of AdV-GFP or AdV-MKP-4. (A) Forty-eight hours after infection, some wells were treated with anisomycin (5 μg/ml, 30 min) or with insulin (10 nM, 5 min). Levels of expression of total and serine-phosphorylated ERK, JNK, and p38MAPK were assessed by immunoblot analysis. (B) Forty-eight hours after infection, some wells were treated with anisomycin (5 μg/ml, 30 min) and then with insulin (10 nM, 5 min). Proteins from cell lysates were immunoprecipitated by using anti-IRS-1 antibodies, resolved by SDS/PAGE, and immunoblotted with anti-phosphotyrosine and anti-p85 antibodies or directly resolved by SDS/PAGE and then immunoblotted with antibodies as indicated.
Oxidative stress, inducers of endoplasmic reticulum (ER) stress, and proinflammatory cytokines, like TNF-α, have been shown to induce insulin resistance by activating MAP and SAP kinases, such as JNK (3–5). The ability of MKP-4 to dephosphorylate and deactivate these kinases prompted us to investigate whether MKP-4 could modulate insulin resistance engendered by stress inducers. By using undifferentiated and differentiated 3T3-L1ΔCAR cells infected with adenoviruses overexpressing GFP, 30 min of anisomycin treatment increased JNK activity and IRS-1 Ser-307 phosphorylation (Fig. 2B, lanes 1–4, Fig. 3A, lanes 1–6, and SI Fig. 7B), resulting in a 50% reduction of IRS-1 tyrosine phosphorylation and parallel decreases in the association of PI3-kinase with IRS-1 and insulin-stimulated phosphorylation of Akt (Fig. 2B, lanes 1–4, and SI Fig. 7B). By contrast, in cells overexpressing MKP-4, anisomycin-induced JNK phosphorylation and IRS-1 serine 307 phosphorylation was reduced by up to 75% (Fig. 2B, Fig. 3A, lanes 10–15, and SI Fig. 7B), and tyrosine phosphorylation of IRS-1, association with p85, and activation of Akt were rescued (Fig. 2B, lanes 5–8, and SI Fig. 7B).
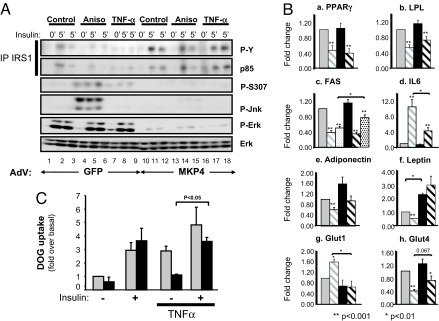
Fig. 3.
MKP-4 protects against TNF-α-induced inhibition of insulin signaling and changes on gene expression. Differentiated 3T3-L1ΔCAR adipocytes were infected with equivalent amounts of control AdV-GFP or AdV-MKP-4. (A) Some wells were subsequently pretreated with anisomycin (5 μg/ml, 30 min) (lanes 4–6 and 13–15) or with TNF-α (25 ng/ml, 5 h) (lanes 7–9 and 16–18) and then stimulated with insulin (10 nM, 5 min). Proteins from cell lysates were immunoprecipitated by using anti-IRS-1 antibodies, resolved by SDS/PAGE, and immunoblotted with anti-phosphotyrosine and anti-p85 antibodies or directly resolved by SDS/PAGE and then immunoblotted with antibodies as indicated. (B) The 3T3-L1 adipocytes infected with AdV-GFP (gray bars) or AdV-MKP-4 (black bars) were treated (hatched bars) or not (gray and black bars) with TNF-α (25 ng/ml, 24 h) and with insulin (10 nM) during the last 8 h (dotted bars). mRNA expression was assessed by using real-time quantitative PCR in three independent experiments. Results are expressed as fold of stimulation and presented as the means ± SEM. (C) For 24 h before insulin stimulation (10 min at 100 nM), 3T3-L1 adipocytes infected with AdV-GFP (gray bars) or AdV-MKP-4 (black bars) were pretreated or not with TNF-α. At 5 min after insulin stimulation, 2-deoxyglucose uptake was measured. Means ± SEM of three independent experiments are shown.
A similar result was observed with the proinflammatory cytokine TNF-α. Thus, TNF-α (25 ng/ml) treatment for 5 h triggered the phosphorylation of IRS-1 on Ser-307 (Fig. 3A and SI Fig. 7C), and this was associated with a 50% decrease in insulin-stimulated tyrosine phosphorylation of IRS-1 and its association with p85 (Fig. 3A and SI Fig. 7C). Again, these effects were reversed in cells overexpressing MKP-4, with an 80% reduction in IRS-1 Ser-307 phosphorylation and restoration of IRS-1 tyrosine phosphorylation (Fig. 3A and SI Fig. 7C).
Effect of MKP-4 on TNF-α-Modulated Gene Expression and Inhibition of Glucose Uptake.
In addition to inhibition of insulin signaling, TNF-α modulates the expression of genes involved in several adipocyte functions (19). In 3T3-L1 adipocytes overexpressing GFP, treatment with 25 ng/ml TNF-α for 24 h reduced the expression of PPARγ and lipoprotein lipase (LPL) by 45–50% in control cells, and these effects were not changed by overexpressing MKP-4 (Fig. 3B a and b). TNF-α also reduced FAS expression by 60% and blocked the ability of insulin to up-regulate FAS expression (Fig. 3Bc). In cells overexpressing MKP-4, the TNF-α effect to reduce FAS was comparable; however, insulin partially restored FAS expression in these cells, consistent with increased insulin sensitivity in the cells overexpressing MKP-4 (Fig. 3Bc).
Exposure of fat cells to TNF-α also produces changes in expression of a number of adipokines (20). There was a 10-fold increase in the mRNA for IL-6 and a 40% decrease in the expression of adiponectin (Fig. 3B d and e). Both effects were partially reversed in cells overexpressing MKP-4 (Fig. 3B d and e). TNF-α also induced a 50% decrease in leptin mRNA expression. In cells overexpressing MKP-4, basal leptin mRNA expression was increased 2-fold, and the effect of TNF-α was abolished (Fig. 3Bf). As reported (21, 22), TNF-α also increased the expression of Glut1 by 1.5-fold and reduced the expression of Glut4 by 60% in control cells (Fig. 3B g and h). Both of these effects were reversed or partially reversed in cells overexpressing MKP-4 (Fig. 3B g and h).
To determine whether the protective effect of MKP-4 against TNF-α-induced inhibition of insulin signaling might alter the insulin response in pathological states, we assessed glucose uptake in 3T3-L1 adipocytes. In control cells, insulin stimulated a 3-fold increase in glucose uptake. TNF-α treatment for 16 h caused an elevation in the basal uptake of glucose and impeded the ability of insulin to further stimulate glucose uptake, consistent with insulin resistance (Fig. 3C). In cells overexpressing MKP-4, insulin-stimulated glucose uptake was similar to that observed in controls; however, the increased basal glucose uptake induced by TNF-α treatment was abolished, thus reestablishing the effect of insulin to stimulate glucose uptake (Fig. 3C).
MKP-4 Overexpression in Liver of ob/ob Mice Reduces the Features of Insulin Resistance.
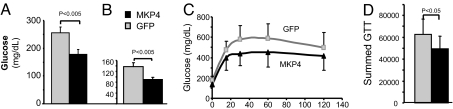
To investigate whether MKP-4 would correct insulin resistance in vivo, 10-week-old ob/ob mice were injected via the tail vein with adenoviruses mediating expression of GFP or MKP-4, resulting in overexpression of MKP-4 specifically in liver (SI Fig. 8). Signaling studies performed after acute injection of either saline solution or insulin (2 milliunits/g of body weight; see Experimental Procedures in SI Text) for 5 min revealed that MKP-4 overexpression reduced ERK and JNK phosphorylation and partially restored insulin-stimulated Akt phosphorylation in ob/ob mice compared with control (SI Fig. 8 and Results in SI Text). Blood glucose levels of MKP-4 expression were lower in both the fed and fasted states compared with GFP controls (Fig. 4 A and B). Glucose tolerance tests performed 5 days after adenovirus injection also revealed a significant 20% reduction in glucose excursions in mice overexpressing MKP-4 (Fig. 4 C and D).
Fig. 4.
MKP-4 overexpression reduces hyperglycemia and improves glucose intolerance in ob/ob mice. Ten-week-old ob/ob mice were infected with AdV-GFP (n = 5, gray) or AdV-MKP-4 (n = 6, black) via tail vein injection. (A and B) Fed (A) and fasting (B) blood glucose levels were measured 2 days after adenovirus injection. (C) An i.p. glucose tolerance test (GTT) was performed on day 5 after a 6-h fast. (D) The area under the curve was estimated by summing the numerical integration values of successive linear segments of the glucose curve for 0–15, 15–30, 30–60, and 60–120 min.
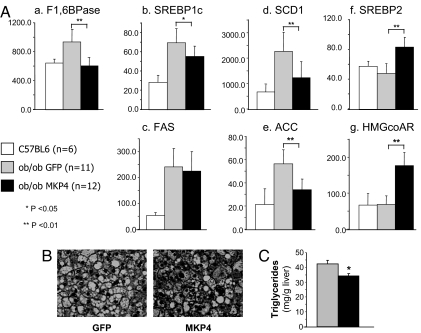
Finally, the expression of gluconeogenic and lipogenic enzymes was assessed. As a result of insulin resistance in the livers of ob/ob mice, the expression of gluconeogenic genes was increased, whereas insulin still stimulated the production of SREBP1c and lipogenic genes, e.g., fatty acid synthase (FAS), sterol CoA desaturase 1 (SCD1), and acetyl CoA carboxylase (ACC), leading to hepatic steatosis and more insulin resistance. MKP-4 overexpression in liver was able to restore the normal expression of fructose 1,6 bisphosphatase, one of the critical genes regulating gluconeogenesis (Fig. 5Aa) and produced increased SREBP2 and decreased SREBP1c expression. These changes were paralleled by an increase in HMGcoAR expression, a target gene of SREBP2, and a reduction in expression of genes involved in lipid synthesis, such as SCD1 and ACC, to levels close to those observed in controls (Fig. 5A b–g). As a result, hepatic steatosis was attenuated in ob/ob mice overexpressing MKP-4 in liver (Fig. 5B), and triglyceride content of the liver was diminished by 20% (Fig. 5C).
Fig. 5.
Effects of MKP-4 on gene expression and lipid accumulation in liver of ob/ob mice. (A) Gene-expression analysis was performed on liver extracts from 10-week-old control C57BL6 mice (n = 6, white bars) and ob/ob mice infected with AdV-GFP (n = 11, gray bars) or AdV-MKP-4 (n = 12, black bars). Hepatic expression of various genes was analyzed by real-time PCR. Expression levels were normalized to TBP and presented as the mean ± SEM. *, P < 0.05; **, P < 0.01. (B) Hematoxylin and eosin staining of liver section is shown at magnification ×40. (C) The triglyceride content in the liver of mice infected with AdV-GFP (n = 5, gray bars) or AdV-MKP-4 (n = 6, black bars) was measured as specified in Experimental Methods and presented as the means ± SEM. *, P < 0.05.
Discussion
Increased activity of ERK, JNK, and other stress kinases has been shown to induce insulin resistance and is a feature of obesity- and diabetes-related insulin resistance in mice and humans (4, 23, 24). Thus, one potential therapeutic strategy for these disorders and to improve insulin sensitivity would be to reduce the activity of these kinases (7, 8). In this study, we investigated the role of the dual specificity phosphatase MKP-4 (DUSP-9) in the process.
For this purpose, we used two in vitro models of stress-induced insulin resistance in 3T3-L1 cells: one produced by anisomycin and a second using TNF-α, a proinflammatory cytokine involved in obesity-induced insulin resistance. Our results show that MKP-4 can dephosphorylate ERK, JNK, and, to a lesser extent, p38MAPK in these cells and reverse both forms of stress-induced insulin resistance. The molecular basis for this protective effect resides primarily in the inhibition of phosphorylation of IRS-1 on serine 307. This restores IRS-1 tyrosine phosphorylation and reverses stress-induced insulin desensitization. In addition, MKP-4 overexpression reduces the increase in IL-6 after TNF-α treatment and partly reversed the changes in Glut1 and Glut4 expression. As a result, MKP-4 restores or partly restores insulin stimulation of glucose uptake and regulation of FAS expression.
Because complete inactivation of the MKP-4 gene is embryonic lethal (25), we investigated how MKP-4 modulates insulin sensitivity in vivo by overexpression in liver of ob/ob mice using adenoviral-mediated gene transduction. We find that this improves some features of insulin resistance, including hyperglycemia, glucose intolerance, hepatic steatosis, and insulin signaling in the liver. Thus, increasing MKP-4 can improve insulin sensitivity both in vivo and in vitro by counteracting stress-induced inhibition of insulin signaling and the effects of stress on gene expression.
Our data on the effects of MKP-4 are consistent with several reports showing that decreasing JNK or ERK activities sensitizes insulin metabolic actions (2, 4), but appear to be in contrast to a study by Xu et al. (14) showing that MKP-4 expression blocks insulin-induced adipogenesis and inhibits insulin-stimulated glucose uptake. MKP-1 is also able to block adipocyte differentiation if expressed ectopically in fibroblasts (26). We believe that the apparent differences in results are due to the different experimental conditions used. Thus, MKP-4 and MKP-1 can inhibit ERK, which is crucial in early adipogenesis (27), and under some conditions, p38MAPK, which collaborates with the PI-3 kinase pathway in the regulation of glucose transport activity (28, 29). However, MKP-4 had no effect on p38MAPK in vivo and actually improved the reduced glucose-transport activation seen in TNF-α-induced insulin resistance. Because insulin regulates a large number of physiological functions including proliferation, survival, differentiation, and metabolism, it is possible that MKP-4 inhibits some of insulin's actions, such as the MAPK-dependent processes of proliferation and differentiation, while enhancing others that are normally negatively regulated by ERK and JNK. The latter would be especially true in states where these stress kinases are activated.
MKP-4 expression has been shown to be increased in insulin-sensitive tissues in some mouse models of obesity, such as db/db, ob/ob, or HFD-fed mice (14). We find that feeding status can also modulate MKP-4, but not MKP-3 and only moderately MKP-1, expression in white adipose tissue (WAT), indicating that regulation of MKP-4 expression in WAT is regulated by both physiological and pathological conditions. Interestingly, the regulation of MKP-4 by HFD and fasting appear to be different in liver and skeletal muscle. These changes in MKP-4 expression might reflect a compensatory mechanism against excessive MAPK activity in the tissues, as part of a negative feedback loop (10). Other mechanisms are also involved in the regulation of MKP activity, including oxidation of the cysteine residue crucial for MKP catalytic activity, which might occur in response of TNF-α (13).
In conclusion, MKP-4 is expressed in insulin-sensitive tissues and is up-regulated during their differentiation. In addition, its expression is modulated in situations of insulin resistance and by feeding status. More importantly, MKP-4 can modulate insulin signaling and insulin action in vitro and in vivo and exert a protective effect against stress-induced insulin resistance. These data strongly support a role of MKP-4 in modulating insulin action and suggest that this protein may provide a therapeutic target for treatment of type 2 diabetes.
Experimental Methods
Materials.
Antibodies for immunoblotting were ERK, JNK, p38MAPK, Akt, and their phosphorylated forms (Cell Signaling Technology); MKP-4 (Santa Cruz Biotechnology), phosphotyrosine (Upstate Biotechnology); IRS-1 (BD Biosciences). Rabbit polyclonal sera against IRS-1 and IRS-2 used for immunoprecipitation were produced in our laboratory. All chemicals and anti-flag antibody were purchased from Sigma. Recombinant murine TNF-α was from R&D Systems, and recombinant human insulin for in vivo studies was from Lilly. MKP-4 was cloned by RT-PCR using the SuperScript II kit from Invitrogen with RNA isolated from mouse liver. The sequence of the cDNA obtained was identical to the one corresponding to accession number AY316312 on the National Center for Biotechnology Information. Control GFP and MKP-4-expressing adenoviruses were constructed by using the Adeasy adenoviral vector system (Stratagene) and were purified by CsCl gradient and dialyzed in PBS containing 10% glycerol.
Cell Culture and Infection.
D. Orlicky (University of Colorado, Denver) provided 3T3-L1ΔCAR cells (expressing the receptor for adenovirus) (30). The 3T3-L1 cells and C2C12 cells were obtained from American Type Culture Collection. Brown adipose tissue cell lines were generated in our laboratory (31). All cells were grown in DMEM supplemented with 10% FBS and induced to differentiate as described (31, 32). The 3T3-L1 fibroblasts or 3T3-L1 differentiated into adipocytes were cultured overnight in media containing adenovirus at a multiplicity of infection of 200. All experiments were performed 48 h after infection.
Cell Lysates, Nuclear Lysates, Immunoprecipitation, and Immunoblots.
Cells were stimulated as indicated, after which they were washed in ice-cold PBS and harvested in lysis buffer containing vanadate and proteases inhibitors (32). For immunoprecipitation, whole-cell lysates were mixed with various antibodies and protein A-coupled to agarose beads (GE Healthcare Biosciences). Proteins from whole-cell lysates and immunoprecipitates were separated by SDS/PAGE, transferred to Immobilon-P membranes (Millipore) and blotted with antibodies as specified. For the preparation of cytoplasmic and nuclear extracts, lysis buffer with 0.1% Triton X-100 was used to gently disrupt the plasma membrane by agitation at 4°C. Nuclei were pelleted by centrifugation and lysed in a buffer containing 1% SDS.
RNA Extraction and Gene Expression.
RNA extraction from cells and tissues was performed by using the RNeasy kit (Qiagen). Gene expression was assessed by quantitative real-time PCR. One microgram of total RNA was used for cDNA synthesis by using a kit from Applied Biosystems. Specific primers for ACC, adiponectin, FAS, fructose 1,6 bisphosphatase, Glut1, Glut4, HMG-CoA reductase, IL6, leptin, LPL, MKP-4, PPAR-γ, SREBP1c, SREBP2, SCD1, and TBP were used for the PCR using the ABI Prism 7900HT (Applied Biosystems), and analysis was done with the ABI Prism SDS 2.2.2 software.
Glucose Uptake.
Glucose uptake in 3T3-L1 cells was performed as described (33). Briefly, cells were pretreated with 25 ng/ml TNF-α or buffer and stimulated with 100 nM insulin in KRH containing 0.1% BSA buffer for 10 min. Cells were incubated with 0.25 μCi of [3H]2-deoxyglucose for 5 min. Transport was stopped by addition of cytochalasin B. Cells were harvested in 0.05% SDS, and the amount of [3H]2-deoxyglucose taken up was measured by liquid scintillation counting.
Animals.
C57/BL6 male mice (TaconicFarms) were maintained on HFD (60% fat, n = 17) or LFD (10% fat, n = 18) for 11 weeks starting at 6 weeks of age. Each group of mice was killed in the early morning in the postprandial state [n = 9 for low-fat diet (LFD); n = 8 for HFD] or after an overnight fast (n = 9 for LFD and HFD). Male ob/ob mice and control littermates were purchased from The Jackson Laboratories. All of the mice were maintained on a 12-h light/dark cycle. All protocols for animal use were reviewed and approved by the Animal Care Committee of the Joslin Diabetes Center and were in accordance with Institutional Animal Care and Use Committee guidelines.
Animal Studies.
Adenoviral injection (2 × 1011 viral particles) was given into the tail vein. Blood glucose values were determined 2 days later in the fed state or after a 6-h fast by using a glucose meter (Ascensia Elite; Bayer). Glucose tolerance tests were performed 5 days after adenovirus injection by using 2 g of glucose per kilogram of body weight i.p. after a 6-h fast. The triglyceride content of the liver was determined by enzymatic assay (GPQ-Trinder; Sigma) of glycerol.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Dr. Yazmin Macotela for technical assistance and critical reading of the manuscript. This work was supported by National Institutes of Health Grant DK33201, a mentor-based American Diabetes Association award (to B.E.), and a fellowship from the Fondation pour la Recherche Médicale (to D.E.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712275105/DC1.
References
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelman JA, Berg AH, Lewis RY, Lisanti MP, Scherer PE. Tumor necrosis factor alpha-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK1/2 inhibitors in 3T3–L1 adipocytes. Mol Endocrinol. 2000;14:1557–1569. doi: 10.1210/mend.14.10.0542. [DOI] [PubMed] [Google Scholar]
- 3.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH (2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 4.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 5.Fujishiro M, et al. Three mitogen-activated protein kinases inhibit insulin signaling by different mechanisms in 3T3–L1 adipocytes. Mol Endocrinol. 2003;17:487–497. doi: 10.1210/me.2002-0131. [DOI] [PubMed] [Google Scholar]
- 6.Aguirre V, et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 7.Kaneto H, et al. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- 8.Manning AM, Davis RJ. Targeting JNK for therapeutic benefit: From junk to gold? Nat Rev Drug Discov. 2003;2:554–565. doi: 10.1038/nrd1132. [DOI] [PubMed] [Google Scholar]
- 9.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 10.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Pouyssegur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 12.Camps M, et al. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science. 1998;280:1262–1265. doi: 10.1126/science.280.5367.1262. [DOI] [PubMed] [Google Scholar]
- 13.Kamata H, et al. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 14.Xu H, et al. Dual specificity mitogen-activated protein (MAP) kinase phosphatase-4 plays a potential role in insulin resistance. J Biol Chem. 2003;278:30187–30192. doi: 10.1074/jbc.M302010200. [DOI] [PubMed] [Google Scholar]
- 15.Muda M, et al. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- 16.Wu JJ, Zhang L, Bennett AM. The noncatalytic amino terminus of mitogen-activated protein kinase phosphatase 1 directs nuclear targeting and serum response element transcriptional regulation. Mol Cell Biol. 2005;25:4792–4803. doi: 10.1128/MCB.25.11.4792-4803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda K, Shima H, Watanabe M, Kikuchi K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J Biol Chem. 2001;276:39002–39011. doi: 10.1074/jbc.M104600200. [DOI] [PubMed] [Google Scholar]
- 18.Muda M, et al. Molecular cloning and functional characterization of a novel mitogen-activated protein kinase phosphatase, MKP-4. J Biol Chem. 1997;272:5141–5151. doi: 10.1074/jbc.272.8.5141. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, et al. Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha. Mol Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 20.Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46:1594–1603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 21.Stephens JM, Pekala PH. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3–L1 adipocytes by tumor necrosis factor-alpha. J Biol Chem. 1991;266:21839–21845. [PubMed] [Google Scholar]
- 22.Stephens JM, Carter BZ, Pekala PH, Malter JS. Tumor necrosis factor alpha-induced glucose transporter (GLUT-1) mRNA stabilization in 3T3–L1 preadipocytes. Regulation by the adenosine-uridine binding factor. J Biol Chem. 1992;267:8336–8341. [PubMed] [Google Scholar]
- 23.Gum RJ, et al. Antisense protein tyrosine phosphatase 1B reverses activation of p38 mitogen-activated protein kinase in liver of ob/ob mice. Mol Endocrinol. 2003;17:1131–1143. doi: 10.1210/me.2002-0288. [DOI] [PubMed] [Google Scholar]
- 24.Carlson CJ, Koterski S, Sciotti RJ, Poccard GB, Rondinone CM. Enhanced basal activation of mitogen-activated protein kinases in adipocytes from type 2 diabetes: potential role of p38 in the downregulation of GLUT4 expression. Diabetes. 2003;52:634–641. doi: 10.2337/diabetes.52.3.634. [DOI] [PubMed] [Google Scholar]
- 25.Christie GR, et al. The dual-specificity protein phosphatase DUSP9/MKP-4 is essential for placental function but is not required for normal embryonic development. Mol Cell Biol. 2005;25:8323–8333. doi: 10.1128/MCB.25.18.8323-8333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaue H, et al. Role of MAPK phosphatase-1 (MKP-1) in adipocyte differentiation. J Biol Chem. 2004;279:39951–39957. doi: 10.1074/jbc.M407353200. [DOI] [PubMed] [Google Scholar]
- 27.Bost F, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- 28.Antonescu CN, et al. Reduction of insulin-stimulated glucose uptake in L6 myotubes by the protein kinase inhibitor SB203580 is independent of p38MAPK activity. Endocrinology. 2005;146:3773–3781. doi: 10.1210/en.2005-0404. [DOI] [PubMed] [Google Scholar]
- 29.Somwar R, et al. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3–L1 adipocytes without affecting GLUT4 translocation. J Biol Chem. 2002;277:50386–50395. doi: 10.1074/jbc.M205277200. [DOI] [PubMed] [Google Scholar]
- 30.Orlicky DJ, DeGregori J, Schaack J. Construction of stable coxsackievirus and adenovirus receptor-expressing 3T3–L1 cells. J Lipid Res. 2001;42:910–915. [PubMed] [Google Scholar]
- 31.Klein J, et al. Beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274:34795–34802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- 32.Emanuelli B, et al. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- 33.Moyers JS, Bilan PJ, Reynet C, Kahn CR. Overexpression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.