Abstract
August–Copenhagen–Irish (ACI) rats are unique in that the ovary-intact females develop high incidence of mammary cancers induced solely by hormones upon prolonged exposure to high levels of estrogen alone. Studies have also shown that such prolonged exposure to high-dose estrogen results in human-like aneuploid mammary cancers in ovary-intact ACI rats. To determine the role of progesterone in mammary carcinogenesis, six-week-old intact and ovariectomized ACI rats were continuously exposed to low- and high-dose estrogen alone, progesterone alone, low-dose estrogen plus progesterone, and ovariectomized ACI rats with high-dose estrogen plus progesterone. Also, ovariectomized ACI rats were treated with high-dose estrogen plus progesterone plus testosterone to determine the role of the androgen, testosterone, if any, in hormonal mammary carcinogenesis. The results indicate that continuous exposure to high, but not low, concentrations of estrogen alone can induce mammary carcinogenesis in intact but not in ovariectomized rats. Mammary carcinogenesis in ovariectomized ACI rats requires continuous exposure to high concentrations of estrogen and progesterone. The addition of testosterone propionate does not affect tumor incidence in such rats. These results suggest that both ovarian hormones estrogen and progesterone are necessary for mammary carcinogenesis induced solely by hormones in ovariectomized ACI rats. Our results are in agreement with the Women's Health Initiative studies, where treatment of postmenopausal women with estrogen (ERT) alone did not increase the risk of breast cancer, but estrogen and progesterone (HRT) did.
In women, breast cancer is the most prevailing endocrine-related malignancy (1), and epidemiological studies argue that estrogens (E) are central to its etiology (2). Most of what we know regarding the role of ovarian hormones in human breast carcinogenesis is based primarily on epidemiological (2) and animal studies with mice carrying mammary tumor virus or treated with chemical carcinogen (see ref. 3) and/or rats treated with chemical carcinogens (4–6). The Women's Health Initiative studies showed increased breast cancer risk in postmenopausal women treated with estrogen and progesterone (HRT) (7) but not in postmenopausal women treated with estrogen (ERT) alone (8). Although E has historically been the focus of breast cancer etiology, it is clear that this E effect must be viewed in context of the entire woman or animal. However, the possible role of progesterone (P), if any, as the other main ovarian hormone in mammary carcinogenesis is essential for the understanding of breast cancer biology and its prevention. Progesterone receptor (PR) depends on the presence of E for its expression (9–11). E has been regarded as an initiator (12) and promoter (13, 14) of breast cancer. P has also been thought as a promoter (15) of breast cancer. E has also been thought to induce mammary carcinogenesis via the mitogenesis-mutagenesis-carcinogenesis route (16–18) through centrosome disturbance via aurora kinase overexpression (19) or through its carcinogenic metabolites (20).
Our goal was to determine the role of ovarian hormones in mammary carcinogenesis in rats by using carcinogenesis induced solely by hormones. Recent studies by Shull et al. (21) and Li et al. (22) have shown that when a 27.5-mg silastic capsule or 3-mg pellet of E, respectively, is implanted into intact 8- to 9-week-old female August–Copenhagen–Irish (ACI) rats, 100% develop mammary cancers by 6–7 months (21, 22) after hormone treatment. This hormone-induced carcinogenesis was shown by Li et al. to be preventable if the rats were also provided with the antiestrogen tamoxifen (22). Shull et al. (21) also noted that implantation of E capsules failed to induce mammary cancers in ovariectomized (OVX) ACI rats. This finding suggests that E alone might not be able to cause cancer and that the ovarian hormone, P, also may be required for mammary carcinogenesis in ACI rats. Hormonal carcinogenesis in ACI rats is highly relevant as a model for human breast cancer, because, as shown by Li et al. (22) and Shull et al. (23), these E-induced cancers exhibit aneuploidy, as do invasive ductal mammary carcinomas in women. Mammary cancers that were chemically induced in the Brown Lewis Norway and Sprague–Dawley rats presented aneuploidy in 10–15% of the mammary cancers, whereas E-induced mammary tumors in ACI and Noble rats showed 85% occurrence in carcinoma in situ and 90% in frank tumors (24). Li et al. (25) also found that the sequence of events that is believed to occur in women is recapitulated in E-treated rats, where, with time, small focal dysplasias are seen, followed by large focal dysplasias with atypical ductal hyperplasia, ductal carcinoma in situ, and finally frank mammary cancers. ACI appears to be a strain of rat highly suitable for analyzing the role ovarian hormones play in hormonal mammary carcinogenesis.
In this study of hormonal mammary carcinogenesis, we describe the effects of low and high doses of E and low doses of E with P in ovary-intact ACI rats. In OVX rats, in addition to these similarly treated groups, we will also investigate the effect of high doses of E with P in ovarian hormone-induced mammary carcinogenesis. These studies are intended to clarify whether E and/or P are necessary for mammary carcinogenesis induced solely by hormones. In addition, in the Noble rat strain, treatment with E in silastic capsules produced a 22% mammary cancer incidence. Treatment with silastic capsules of testosterone propionate (TP) alone produced a 17% incidence. However, the combination of E plus TP produced a 53% incidence (26). Moreover, epidemiological studies have shown that high circulating levels of endogenous estrogen and testosterone increase the risk of breast cancer in both premenopausal (27) and postmenopausal women (28–31). Therefore, here, we also study the combined influence of E with P and testosterone propionate (TP) on mammary carcinogenesis in ACI rats. Our results have implications for the role of ovarian hormones in breast carcinogenesis and for both HRT and ERT treatment of postmenopausal women.
Results
Effect of Hormonal Treatment with Estrogen (E), Progesterone (P), and Testosterone Propionate (TP) on Body Weights of Female ACI Rats.
The initial mean body weight of female ACI rats was ≈105 g before treatment starting at 6 weeks (32) (Table 1). Twenty-two weeks after the initial implantation of 30 mg of E (high E), female ACI rats from group 3 showed statistically significant weight loss (P < 0.0005) compared with the untreated intact control, group 1 (Table 1). Similarly, compared with the untreated OVX control (group 6), statistically significant weight losses were seen in group 8, which was treated with 30 mg of E (P < 0.0005); group 11, which was treated with 30 mg of E plus 30 mg of P (P < 0.0005); and group 12, which was treated with 30 mg of E plus 30 mg of P plus 30 mg of TP (P < 0.0005) (Table 1). Therefore, at 28 weeks of age and 22 weeks after initial treatment, all of the implanted 30-mg E capsules were exchanged with 3-mg E capsules to reduce weight loss. At the termination of the experiment, group 1 (the intact control group) showed no statistical weight differences when compared with group 3, which was treated with high E (30 mg → 3 mg). In the OVX rats, group 8, which was treated with high E (P < 0.005), and group 11, which was treated with high E plus 30 mg of P (P < 0.005), showed statistically significant weight loss compared with group 6 (the OVX control). However, group 12, which was treated with high E plus 30 mg of P plus 30 mg of TP, showed no statistical differences in body weight compared with group 6, the OVX control. As a result of high estrogen treatment, survival was affected in groups 3 (7/15), 8 (8/14), and 11 (11/14).
Table 1.
Experimental groups, body weights, and survival of ACI rats at 28 and 45 weeks of age
| Group | Treatment | Mean body weight ± SEM, g |
Surviving rats | |
|---|---|---|---|---|
| 28 weeks of age | 45 weeks of age | |||
| Intact | ||||
| 1 | Untreated control | 177.2 ± 2.9 (10) | 182.5 ± 4.4 (10) | 10/10 |
| 2 | Low E | 174.6 ± 1.4 (15) | 185.3 ± 1.6 (15) | 15/15 |
| 3 | High E* | 157.8 ± 3.6 (14) | 172.8 ± 4.2 (7) | 7/15 |
| 4 | 30 mg P | 194.7 ± 1.8 (15) | 208.0 ± 2.3 (15) | 15/15 |
| 5 | Low E plus 30 mg of P | 191.3 ± 2.8 (15) | 202.5 ± 3.0 (15) | 15/15 |
| Ovariectomized | ||||
| 6 | Untreated control | 190.4 ± 2.9 (10) | 196.0 ± 3.5 (10) | 10/10 |
| 7 | Low E | 193.7 ± 3.4 (15) | 210.3 ± 3.9 (15) | 15/15 |
| 8 | High E | 150.4 ± 3.3 (13) | 178.9 ± 3.2 (8) | 8/14 |
| 9 | 30 mg P | 201.4 ± 3.9 (15) | 207.1 ± 4.9 (15) | 15/15 |
| 10 | Low E plus 30 mg of P | 198.2 ± 2.8 (14) | 207.3 ± 3.0 (14) | 14/14 |
| 11 | High E plus 30 mg of P | 165.4 ± 2.9 (12) | 172.7 ± 5.2 (11) | 11/14 |
| 12 | High E plus 30 mg of P plus 30 mg of TP | 177.3 ± 2.8 (15) | 184.6 ± 5.6 (14) | 14/15 |
Body weight at initiation of hormone treatment at 6 weeks was ≈105 g. The number of rats used to calculate body weight is in parentheses. The number of surviving rats indicates premature deaths occurring before the termination of the experiment and not because of tumor growth. E, estrogen; P, progesterone (P); TP, testosterone propionate.
*At 28 weeks of age (22 weeks of treatment), 30 mg estrogen capsules were exchanged with 3 mg estrogen for the remainder of the experiment.
Effect of Various Doses and Combinations of E and P in Ovary Intact and E, P, and TP in OVX Female ACI Rats on Mammary Lobulo-Alveolar Development at Termination (45 weeks of age).
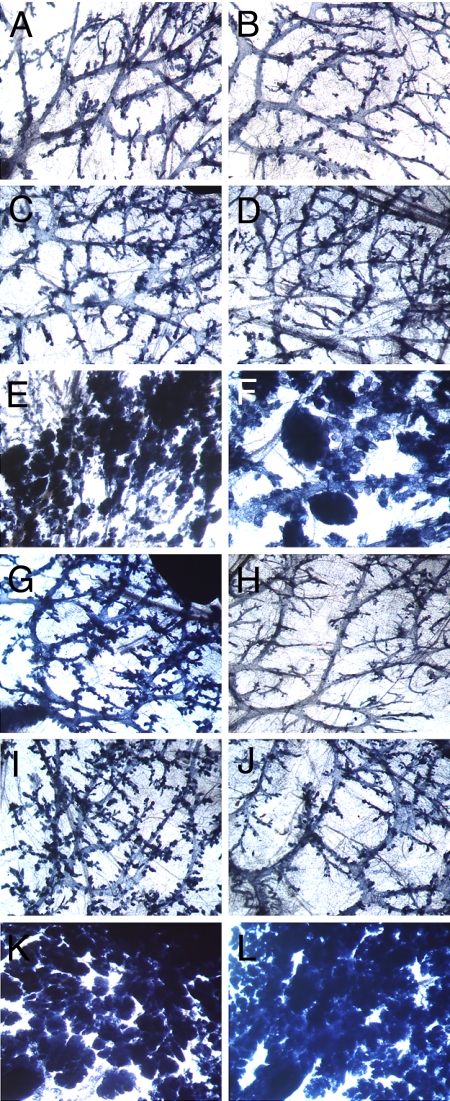
Group 1, the untreated intact controls (Fig. 1A), and group 6, the untreated OVX controls (Fig. 1B), showed very little lobulo-alveolar development (Table 2). However, although in group 1 (intact control) ductal growth filled the entire mammary gland fat pad at 45 weeks of age, in group 6 (OVX untreated control), ductal growth filled an average of only 70% of the mammary gland fat pad. Group 2 (intact) and group 7 (OVX) treated with low E (10 μg of E) showed a slight increase in ductal growth (Fig. 1 C and D, respectively), and ductal growth filled the mammary gland fat pad in both cases. High E alone caused extensive lobulo-alveolar growth and filled the mammary gland fat pad in the intact group 3 (Fig. 1E). In contrast, OVX group 8 (Fig. 1F), which was treated with high E, also demonstrated extensive lobulo-alveolar development, which filled on average only 75% of the mammary gland fat pad. However, upon termination, groups 3 and 8 exhibited milky secretions in their mammary glands. Treatment with 30 mg of P showed ductal growth in intact group 4 (Fig. 1G) but much less ductal branching in OVX group 9 (Fig. 1H), and ductal growth filled the mammary gland fat pad in both cases. Intact group 5, which was treated with low-dose E (10 μg) plus 30 mg of P (Fig. 1I), showed ductal growth that was more extensive than in groups treated with either 10 μg of E or 30 mg of P, and the ductal growth filled the mammary gland fat pad. OVX group 10 (Fig. 1J), which was treated with 10 μg of E plus 30 mg of P, showed ductal growth but reduced branching versus the similarly treated intact group 5. The most extensive lobulo-alveolar development occurred in the OVX groups 11 (Fig. 1K), which was treated with high E plus 30 mg of P, and 12 (Fig. 1L), which was treated with high E plus 30 mg of P plus 30 mg of TP. Both OVX groups 11 and 12 showed mammary gland development characteristic of late pregnancy with milky secretions, although, in group 12, mammary gland development was the most extensive.
Fig. 1.
Whole-mount morphology of mammary glands demonstrating the effect of hormonal treatments in intact and ovariectomized ACI rats after 39 weeks of treatment with various hormone combinations (X20). Shown are number 4 mammary glands of ACI rats at 45 weeks of age from the following groups: intact untreated (A), OVX untreated (B), intact treated with 10 μg of estrogen (low E) (C), OVX treated with low E (D), intact treated with (30→ 3 mg of E) (high E) (E), OVX treated with high E (F), intact treated with 30 mg of progesterone (P) (G), OVX treated with 30 mg of P (H) intact treated with low E plus 30 mg of P, (I), OVX treated with low E plus 30 mg of P (J), OVX treated with high E plus 30 mg of P (K), and OVX treated with high E plus 30 mg of P plus 30 mg of testosterone propionate (L).
Table 2.
Effect of continuous hormonal treatments for 39 weeks on lobulo-alveolar differentiation (LA Diff) and mammary carcinoma (MC) in ovary intact and ovariectomized ACI rats
| Treatment | Intact |
Ovariectomized |
||||
|---|---|---|---|---|---|---|
| Group | LA Diff | MC* | Group | LA Diff | MC* | |
| Untreated control | 1 | No | 0/10 | 6 | No | 0/10 |
| Low E | 2 | Slight | 0/15 | 7 | Slight | 0/15 |
| High E† | 3 | Extensive | 5/7 | 8 | Extensive | 0/8 |
| 30 mg P | 4 | Slight | 0/15 | 9 | No | 0/15 |
| Low E plus 30 mg of P | 5 | Some | 0/15 | 10 | Slight | 0/14 |
| High E plus 30 mg of P | — | ND | ND | 11 | Most Extensive | 11/11 |
| High E plus 30 mg of P plus 30 mg of TP | — | ND | ND | 12 | Most Extensive | 14/14 |
Hormone treatment was initiated at 6 weeks of age and terminated at 45 weeks of age after 39 weeks of treatment. E, estrogen; P, progesterone; TP, testosterone propionate; ND, not done.
*Number of rats with mammary carcinoma/total rats in group.
†At 28 weeks of age (22 weeks of treatment), 30-mg E capsules were exchanged with 3-mg E capsules for the remainder of the experiment.
Development of Mammary Cancers in Ovary Intact and OVX Female ACI Rats upon Treatment with Various Doses and Combinations of E, P, and TP.
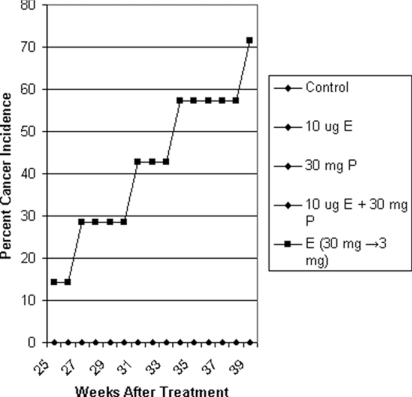
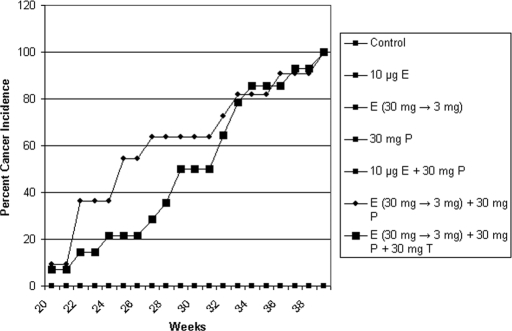
Continuous exposure to low doses of 10 μg of E, 30 mg of P alone, or 10 μg of E plus 30 mg of P in intact groups 2, 4, and 5 and OVX groups 7, 9, and 10 failed to induce mammary cancer in female ACI rats. In group 3, treatment with high-dose E alone (Fig. 2) caused mammary carcinogenesis beginning at 25 weeks after treatment in 71% of intact ACI rats (latency, 228 ± 25 days; multiplicity, 1.4 ± 0.8 cancers per rat) but not in OVX group 8 rats (0/8) (statistical significance, P = 0.007). Cancer incidence in intact group 3 treated with high E was not statistically different from OVX group 11 treated with high E and P and group 12 treated with high E, P, and TP. In intact group 3, which was treated with high E, four rats had palpable cancers, and one had ductal carcinoma in situ. The addition of 30 mg of P to high E in group 11 caused palpable mammary carcinogenesis in 100% of OVX ACI rats (latency 193 days, multiplicity 3.1 ± 0.6 cancers per rat). The addition of 30 mg of TP to high E plus 30 mg of P in group 12 also caused palpable mammary carcinogenesis in 100% of OVX ACI rats (latency, 208 ± 10 days, multiplicity 3.1 ± 0.7 cancers per rat). Groups 11 and 12 showed initial mammary cancer appearance at 20 weeks (Fig. 3). Compared with the OVX group 8, which was treated with high E, both group 11 (P = 1.32 × 10−5) and group 12 (P = 3.1 × 10−6) showed statistical significance in mammary cancer incidence. Mammary cancer types were cribriform and comedo. No mammary cancers were induced in any other intact group: control (0 of 10 rats), 10 μg of E (0 of 15 rats), 30 mg of P (0 of 15 rats), 10 μg of E plus P (0 of 15 rats) or the following OVX groups: control (0 of 10 rats), 10 μg of E (0 of 15 rats), 30 mg of P (0 of 15 rats), 10 μg of E plus P (0 of 14 rats).
Fig. 2.
Effect of various doses of estrogen (E), E plus progesterone (P), and P alone on mammary carcinogenesis in ovary-intact ACI rats. Tumors appeared only in the high E (30 mg → 3 mg) (filled squares) group. All other groups (filled diamonds) had no tumors.
Fig. 3.
Effect of various doses of estrogen (E), E plus progesterone (P), P alone, or E plus P plus testosterone propionate on mammary carcinogenesis in ovariectomized ACI rats. Tumors appeared only in high E (30 mg → 3 mg) plus 30 mg of P (filled squares) and high E (30 mg → 3 mg) plus 30 mg of P plus 30 mg of TP (filled triangles) groups. All other groups (filled diamonds) had no tumors.
Pituitary Adenomas.
Pituitary adenomas were found in both intact and OVX ACI rats (Groups 3, 8, 11, and 12), which were exposed to high estrogen, but not in any other group.
Discussion
Breast cancer incidence is high in developed countries, and breast cancer is increasing in developing countries as is longevity (33). No initiator of breast carcinogenesis is known except in the case of radiation exposure from either the atomic bombs in Japan (34) or radiation treatment for Hodgkin's lymphoma (35). At this time, a complete cure for all breast cancers is not possible. Prevention may be a possibility, and some BRCA-1/2 carrier women are opting for drastic measures, such as oophorectomy and/or total mastectomy at a relatively early age (36, 37). Anti-estrogen drugs are used in the prevention and therapy of human breast cancer (38, 39). All these drugs are based on the idea that estrogen is the primary ovarian hormone associated with breast carcinogenesis. Side effects with anti-estrogenic drugs can be quite severe (40). Early menarche (41) and late menopause (13) have been shown to produce increased risk of breast cancer because of increased exposure to estrogen. Also, estrogen has a mitogenic effect on breast tissue, resulting in greater breast density (42), which is considered a breast cancer risk factor. All of these studies have led to the idea that estrogen is a hormonal carcinogen and the possibility that the paradigm of mitogenesis—mutagenesis—carcinogenesis (16–18) may be the reason estrogen induces breast carcinogenesis. However, progesterone may also contribute to breast cancer risk, because it has been shown that breast epithelial cells present the highest level of mitogenesis during the luteal phase of the menstrual cycle when progesterone levels are at their highest (43, 44).
Using a unique strain of rat, ACI, several investigators have shown that continuous exposure to pregnancy level of estrogen alone causes high incidence of mammary cancer (19, 23) and that estrogen metabolites are not carcinogenic by themselves in vivo in ACI rats (45). It has been reported that the mechanism of mammary carcinogenesis induced solely by estrogen is overexpression of Aurora-A, a centrosome kinase, leading to centrosome amplification and chromosomal instability (19). Progesterone has also been shown to contribute to chromosome instability in mammary cancer of p53 null mice (46). Our current studies were undertaken to determine the endocrine basis for ovarian involvement in the estrogen-induced mammary cancer in intact ACI rats. As mentioned in the introduction, one study by Shull et al. (21) was unable to induce mammary cancer with high-dose estrogen in ovariectomized ACI rats. Furthermore, Harvell et al. (47) reported that high estrogen treatment in caloric-restricted ACI rats reduced tumor incidence to 59% compared with 100% for the normal diet control. This estrogen-induced mammary carcinogenesis did not depend on circulating prolactin levels but was thought to be possibly due to a reduction in circulating progesterone (47). High doses of estrogen in groups 3, 8, 11, and 12 produced pituitary adenomas, which has been shown to result in increased prolactin production (21). The luteotrophic action of prolactin results in higher circulating levels of progesterone (48), which further suggests a role for this hormone.
Therefore, based on these important findings, we designed our current experiments to determine the reasons for the protective effect of ovariectomy in preventing estrogen-induced mammary carcinogenesis in ovariectomized ACI rats. The distinguishing difference between our experiments and those experiments of Shull et al. (21) and Li et al. (22) with intact rats is that our rats received their initial implant at 6 weeks, and their rats received their initial implant at 8–9 weeks. At 6 weeks, ductal growth in the mammary fat pad of ACI rats is less extensive than in 8- to 9-week-old ACI rats (unpublished results). All of these experiments used continuous treatment with comparable high pregnancy levels of estrogen. Although the results of Shull et al. (21) and Li et al. (22, 45) have demonstrated that treatment with high estrogen alone produced mammary carcinogenesis in 100% of ovary-intact ACI rats without premature mortalities, our results showed a delayed appearance of mammary cancer and a reduced (71%) incidence with premature mortalities. This reduced cancer incidence in our experiment is likely because, in our rats, initial hormonal treatment was begun at 6 weeks, when the mammary fat pad contained fewer ducts, and because high estrogen can stunt mammary gland growth (49). Our experiment and those of Shull and Li demonstrated body weight loss upon treatment with pregnancy level estrogen. In the experiments of Shull et al., these estrogen treatments produced pregnancy levels of circulating estrogen between 120 and 185 pg/ml (21, 32), and, in the experiments of Li et al., between 96 and 150 pg/ml (22, 24, 25, 45). Our previous results showed that a 3-week treatment with 30 mg of estrogen plus 30 mg of progesterone in intact rats produced circulating levels of estrogen of 168.8 ± 22.1 pg/ml (50). Treatment with 30 mg of estrogen alone for 1 week to 6 months produced circulating levels of between 118 and 166 pg/ml (unpublished results). These results fall within the normal levels for estrogen in pregnant rats (55–630 pg/ml) (51). We have also found that silastic capsules produce a steady rate of release of estrogen. In a dose–response experiment, Turan et al. (45) demonstrated that pellets containing 3 mg of estrogen produced 100% incidence of mammary cancer in intact ACI rats, but 1 mg of estrogen produced only 50% incidence of mammary cancer after 36 weeks of continuous treatment. This demonstrates that doses of lower pregnancy levels of estrogen are insufficient to induce 100% mammary carcinogenesis. The levels of progesterone in the estrous cycle range from 5 to 50 ng/ml and between 45 and 130 ng/ml during pregnancy (51). Results have shown that 30 mg of progesterone capsules produce a non-pregnancy level of 25.8 ± 6.6 ng/ml in circulation (50). What distinguishes the levels produced by capsules is the constancy of hormone levels versus the cyclic nature of production of estrogen and progesterone during the normal estrous cycle.
We also chose to treat such rats with a low non-pregnancy dose of estrogen (10 μg) that has been shown to produce a continuous circulating level of estrogen of 21 pg/ml (unpublished results) compared with 18.3 pg/ml in untreated age matched rats (50). This low non-pregnancy dose of estrogen caused some ductal growth but no mammary cancers in both the ovary-intact and OVX ACI rats. Our hormonal mammary carcinogenesis experiment differed from the previous experiments of Shull and Li in that we used progesterone in addition to estrogen. When intact and OVX ACI rats were treated with progesterone alone, only the intact rats showed ductal growth. When 30 mg of progesterone treatment was added to 10 μg of estrogen, neither OVX nor ovary-intact rats displayed any mammary carcinogenesis, although both showed ductal growth that was greatest in the intact rats. All high estrogen groups in ovary-intact or OVX rats produced lobulo-alveolar proliferation. Ductal or lobular-alveolar growth filled the fat pad in all untreated and treated intact groups at the termination of the experiment at 45 weeks. However, in the OVX untreated (group 6) and OVX (group 8) groups, high E-treated ACI rats exhibited <100% filling of the mammary fat pad.
Our results have confirmed the finding of Shull et al. (21), where female OVX ACI rats failed to develop mammary cancer when they were treated with high estrogen and have expanded the role of ovarian hormones, as follows, in mammary carcinogenesis in ACI rats (1). Neither treatment with low E, P, or low E plus P was sufficient to induce mammary carcinogenesis (2). Continuous exposure to high concentration of estrogen alone is sufficient for full lobulo-alveolar development in mammary glands of both intact and OVX rats, which was maintained for the duration of the experiment. However, such exposure results in mammary carcinogenesis in intact rats but fails to do so in OVX rats. These results also suggest that mitogenesis under special hormonal environment and not mitogenesis alone can result in mammary carcinogenesis.
OVX rats develop 100% of mammary cancer and intense lobulo-alveolar development only in the presence of high circulating levels of estrogen and relatively low non-pregnancy level of progesterone (4). OVX rats also develop 100% mammary cancer, when they are treated with testosterone propionate in addition to high estrogen and progesterone. In these rats, the intensity of lobulo-alveolar differentiation was increased over high estrogen and progesterone alone. Further studies, including the use of estrogen and testosterone without progesterone, will be required to elucidate the role of testosterone. We have found that high circulating pregnancy levels of estrogen along with relatively low non-pregnancy levels of progesterone are necessary for mammary carcinogenesis, which raises the possibility that it might be easier to antagonize progesterone levels with anti-progesterones rather than antagonize estrogen levels with anti-estrogens for preventive and therapeutic treatment of estrogen and progesterone receptor positive breast cancers.
To our knowledge, this is the first unequivocal demonstration that hormonal mammary carcinogenesis in the ACI rats requires prolonged exposure to both estrogen and progesterone. Further studies with various estrogen and progesterone doses will be necessary to determine the physiological roles of both pregnancy and non-pregnancy levels of ovarian hormones and their effects on mammary carcinogenesis. Finally, it has been more than 100 years since Beatson's discovery that ovary plays a major role in breast carcinogenesis in human females (52) and more than 70 years since Lacassagne's discovery that estrogen is the ovarian hormone involved in mouse mammary carcinogenesis (53). Later studies showed that these mice were infected with mouse mammary tumor virus (see ref. 3 for details). Our current study indicates that the other major ovarian hormone, progesterone, may also be necessary in mammary carcinogenesis induced solely by hormones in ovariectomized ACI rats. Further studies with anti-progesterones in intact ACI rats treated with high estrogen and ovariectomized ACI rats treated with high estrogen and progesterone will be necessary to determine whether progesterone, along with estrogen, is the ovarian cofactor for the hormonal induction of mammary carcinogenesis. Our results are in agreement with the results of the Women's Health Initiative studies, where estrogen alone given to women with hysterectomies produced no increase in breast cancer (8), but the combination of estrogen and progesterone increased relative risk for invasive breast cancer (7).
Materials and Methods
Animals.
ACI female rats were obtained from Harlan Sprague–Dawley at 4 weeks of age. Rats to be used intact were kept for 2 weeks before use, and the remaining rats were ovariectomized at 5 weeks. Treatment for intact and OVX rats was initiated for both groups at 6 weeks of age. They were fed Teklad 8640 and water ad libitum. Rats were kept in our vivarium in temperature-controlled rooms with a 12-h/12-h light/dark schedule. The University of California, Berkeley animal facility is accredited by the American Association for Accreditation of Laboratory Animal Care, and standards of the Guide for the Care and Use of Laboratory Animals were followed.
Hormonal Treatment.
At 6 weeks of age hormonal treatment was started for all groups of rats. All hormones were packed in individual silastic capsules [0.078 inch (1 inch = 2.54 cm) i.d. × 0.125 inch o.d.). Capsules to contain 30 milligrams of progesterone (P), 17β-estradiol (E), or testosterone propionate (TP) were packed without cellulose. Three milligrams of E or 10 μg of E were packed with cellulose (Sigma) to make up to 30 milligrams of material. Both 30-mg and 3-mg E capsules give rise to pregnancy level of E (measured at 55–630 pg/ml) in plasma (51). We define 10 μg of E, which gives 21.7 ± 6.6 pg/ml (unpublished results) and which is a level within the normal estrous cycle of 5–45 pg/ml (54), as low E. Control animals received empty silastic capsules. Silastic capsules were implanted dorsally s.c. Capsules were primed before implantation overnight by soaking at 37°C in medium 199 (Gibco).
Experimental Groups and ACI Rat Survival.
Due to body weight loss of rats implanted with 30-mg E capsules, all these capsules were exchanged for 3-mg E capsules at 22 weeks after the initiation of hormonal treatment. Thereafter, treatment was continued for an additional 17 weeks for a total of 39 weeks. See Table 1 for experimental groups. Each group began with 14–15 rats except group 1, the intact control, and group 6, OVX control, which had 10 rats each. Survival in groups treated with high estrogen was affected and is recorded in Table 1.
Tumor and Tumor Palpation.
Palpation began 6 weeks after initial hormonal capsule implantation. Rats were palpated weekly, and the length and width of tumors were measured with calipers. All surgeries were performed under continuous isoflurane anesthesia (Baxter Healthcare) delivered with oxygen push by equipment from VetEquip. Buprenex analgesic was given to each rat after surgery. When tumors reached 1 cm or larger, they were removed and fixed in phosphate buffered formalin for 18–24 h and then transferred to 70% ethanol before embedding in paraffin and sectioning. All tumors were then sectioned at 5 μm and stained with hematoxylin and eosin, and the histological type of mammary cancer was determined. Latency was calculated from appearance of the first palpable mammary cancer of each rat induced from the initiation of treatment. Rats were terminated when their third mammary tumor reached 1 cm as required by our University of California Institutional Animal Care and Use Committee or when they reached 45 weeks of age. All University of California Animal Care and Use Committee guidelines were followed.
Whole-Mount Analysis of Mammary Gland of Treated ACI Rats.
Mammary glands were excised and fixed in 10% formalin in PBS for 18–24 h. They were then defatted in acetone for 2 days and then transferred to 70% ethanol before staining with iron hemotoxylin for 2 days. Subsequently, they were dehydrated in a series of alcohols and then transferred and kept in Histo-Clear (National Diagnostics) before being microscopically examined for mammary growth, including lobulo-alveolar differentiation.
Statistics.
Among the treated groups, statistical significance of tumor incidence in rats was analyzed by Fisher's exact test, whereas the influence of hormonal treatment on body weights was determined by the Student's t test. Results are reported as mean ± SE. Statistical significance was determined at P < 0.05.
ACKNOWLEDGMENTS.
This work was supported by California Breast Cancer Program Grant 8BP-0132.
Footnotes
The authors declare no conflict of interest.
References
- 1.Smigal C, et al. Trends in breast cancer by race and ethnicity: Update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 2.Travis RC, Key TJ. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003;5:239–247. doi: 10.1186/bcr628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimkin MB. In: Moulton FR, editor. A Symposium on Mammary Tumors in Mice; Washington, DC: American Association for the Advancement of Science No. 22; 1945. pp. 85–122. [Google Scholar]
- 4.Dao TL. The role of ovarian hormones in initiating the induction of mammary cancer in rats by polynuclear hydrocarbons. Cancer Res. 1962;22:973–981. [PubMed] [Google Scholar]
- 5.Huggins C, Moon RC, Morii S. Extinction of experimental mammary cancer. I. Estradiol-17beta and progesterone. Proc Natl Acad Sci USA. 1962;48:379–386. doi: 10.1073/pnas.48.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grubbs CJ, Peckham JC, McDonough KD. Effect of ovarian hormones on the induction of 1-methyl-1-nitrosourea-induced mammary cancer. Carcinogenesis. 1983;4:495–497. doi: 10.1093/carcin/4.4.495. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, et al . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. J Am Med Assoc. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GL, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. J Am Med Assoc. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 9.Katzenellenbogen BS. Dynamics of steroid hormone receptor action. Annu Rev Physiol. 1980;42:17–35. doi: 10.1146/annurev.ph.42.030180.000313. [DOI] [PubMed] [Google Scholar]
- 10.Pasqualini JR, Nguyen BL. Progesterone receptors in the fetal uterus and ovary of the guinea pig: evolution during fetal development and induction and stimulation in estradiol- primed animals. Endocrinology. 1980;106:1160–1165. doi: 10.1210/endo-106-4-1160. [DOI] [PubMed] [Google Scholar]
- 11.Dix CJ, Jordan VC. Modulation of rat uterine steroid hormone receptors by estrogen and antiestrogen. Endocrinology. 1980;107:2011–2020. doi: 10.1210/endo-107-6-2011. [DOI] [PubMed] [Google Scholar]
- 12.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 13.Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. Anonymous. [PubMed] [Google Scholar]
- 14.Winchester DJ. Hormone replacement therapy: a promoter and modulator of breast cancer. Ann Surg Oncol. 2004;11:9–10. doi: 10.1007/BF02524338. [DOI] [PubMed] [Google Scholar]
- 15.Li CI, et al. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. J Am Med Assoc. 2003;289:3254–3263. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 16.Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–7421. [PubMed] [Google Scholar]
- 17.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 18.Henderson BE, Ross RK, Judd HL, Krailo MD, Pike MC. Do regular ovulatory cycles increase breast cancer risk? Cancer. 1985;56:1206–1208. doi: 10.1002/1097-0142(19850901)56:5<1206::aid-cncr2820560541>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Li JJ, et al. Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc Natl Acad Sci USA. 2004;101:18123–18128. doi: 10.1073/pnas.0408273101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueck AO, Seeger H. Breast cancer: Are oestrogen metabolites carcinogenic? Maturitas. 2007;57:42–46. doi: 10.1016/j.maturitas.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 22.Li SA, Weroha SJ, Tawfik O, Li JJ. Prevention of solely estrogen-induced mammary tumors in female aci rats by tamoxifen: Evidence for estrogen receptor mediation. J Endocrinol. 2002;175:297–305. doi: 10.1677/joe.0.1750297. [DOI] [PubMed] [Google Scholar]
- 23.Harvell DM, et al. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc Natl Acad Sci USA. 2000;97:2779–2784. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JJ, et al. Ploidy differences between hormone- and chemical carcinogen-induced rat mammary neoplasms: Comparison to invasive human ductal breast cancer. Mol Carcinog. 2002;33:56–65. doi: 10.1002/mc.10022. [DOI] [PubMed] [Google Scholar]
- 25.Weroha SJ, Li SA, Tawfik O, Li JJ. Overexpression of cyclins D1 and D3 during estrogen-induced breast oncogenesis in female ACI rats. Carcinogenesis. 2006;27:491–498. doi: 10.1093/carcin/bgi278. [DOI] [PubMed] [Google Scholar]
- 26.Xie B, Tsao SW, Wong YC. Induction of high incidence of mammary tumour in female Noble rats with a combination of 17beta-oestradiol and testosterone. Carcinogenesis. 1999;20:1069–1078. doi: 10.1093/carcin/20.6.1069. [DOI] [PubMed] [Google Scholar]
- 27.Eliassen AH, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 28.Berrino F, et al. Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 1996;88:291–296. doi: 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- 29.Cauley JA, et al. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1999;130:270–277. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- 30.Dorgan JF, et al. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:533–539. [PubMed] [Google Scholar]
- 31.Hankinson SE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- 32.Harvell DM, et al. Rat strain-specific actions of 17beta-estradiol in the mammary gland: Correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Carcinogenesis. 2002;23:161–169. doi: 10.1073/pnas.050569097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol. 2005;34:405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 34.Land CE, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat Res. 2003;160:707–717. doi: 10.1667/rr3082. [DOI] [PubMed] [Google Scholar]
- 35.Travis LB, et al. Oestrogen exposure and breast cancer risk. J Natl Cancer Inst. 2005;97:1428–1437. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 36.Friedman LC, Kramer RM. Reproductive issues for women with BRCA mutations. J Natl Cancer Inst Monogr. 2005;(34):83–86. doi: 10.1093/jncimonographs/lgi012. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2. mutations: A decision analysis. J Clin Oncol. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 38.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plourde PV, Dyroff M, Dukes M. Arimidex: A potent and selective fourth-generation aromatase inhibitor. Breast Cancer Res Treat. 1994;30:103–111. doi: 10.1007/BF00682745. [DOI] [PubMed] [Google Scholar]
- 40.Wilking N, Isaksson E, von Schoultz E. Tamoxifen and secondary tumours. An update. Drug Saf. 1997;16:104–117. doi: 10.2165/00002018-199716020-00003. [DOI] [PubMed] [Google Scholar]
- 41.Hunter DJ, et al. Non-dietary factors as risk factors for breast cancer, and as effect modifiers of the association of fat intake and risk of breast cancer. Cancer Causes Control. 1997;8:49–56. doi: 10.1023/a:1018431104786. [DOI] [PubMed] [Google Scholar]
- 42.Aiello EJ, Buist DS, White E. Do breast cancer risk factors modify the association between hormone therapy and mammographic breast density? (United States). Cancer Causes Control. 2006;17:1227–1235. doi: 10.1007/s10552-006-0073-z. [DOI] [PubMed] [Google Scholar]
- 43.Ferguson DJ, Anderson TJ. Morphological evaluation of cell turnover in relation to the menstrual cycle in the “resting” human breast. Br J Cancer. 1981;44:177–181. doi: 10.1038/bjc.1981.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soderqvist G, et al. Proliferation of breast epithelial cells in healthy women during the menstrual cycle. Am J Obstet Gynecol. 1997;176:123–128. doi: 10.1016/s0002-9378(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 45.Turan VK, et al. The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17beta-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16alpha-hydroxyestradiol, and 4-hydroxyestrone. J Endocrinol. 2004;183:91–99. doi: 10.1677/joe.1.05802. [DOI] [PubMed] [Google Scholar]
- 46.Goepfert TM, et al. Progesterone facilitates chromosome instability (aneuploidy) in p53 null normal mammary epithelial cells. FASEB J. 2000;14:2221–2229. doi: 10.1096/fj.00-0165com. [DOI] [PubMed] [Google Scholar]
- 47.Harvell DM, et al. Diet-gene interactions in estrogen-induced mammary carcinogenesis in the ACI rat. J Nutr. 2001;131:3087S–3091S. doi: 10.1093/jn/131.11.3087S. [DOI] [PubMed] [Google Scholar]
- 48.Smith MS, McLean BK, Neill JD. Prolactin: the initial luteotropic stimulus of pseudopregnancy in the rat. Endocrinology. 1976;98:1370–1377. doi: 10.1210/endo-98-6-1370. [DOI] [PubMed] [Google Scholar]
- 49.Gardner WU. Inhibition of mammary growth by large amounts of estrogen. Endocrinology. 1941;28:53–61. [Google Scholar]
- 50.Guzman RC, et al. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci USA. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Numan M. In: The Physiology of Reproduction. Knobil E, Neill JD, editors. Vol 2. New York: Raven; 1994. pp. 221–302. [Google Scholar]
- 52.Beatson GT. On the treatment of the inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:104–107. [PMC free article] [PubMed] [Google Scholar]
- 53.Lacassagne A. Apparition de cancers de la mamelle chez la souris mâle, soumise à des injections de folliculine. Compt Rend Acad Sci. 1932;195:630–632. [Google Scholar]
- 54.Freeman ME. In: The Physiology of Reproduction. Knobil E, Neill JD, editors. Vol 2. New York: Raven; 1994. pp. 613–658. [Google Scholar]