Abstract
The potent neuroprotective activities of neurotrophic factors, including insulin-like growth factor 1 (IGF-1), make them promising candidates for treatment of amyotrophic lateral sclerosis (ALS). In an effort to maximize rate of motor neuron transduction, achieve high levels of spinal IGF-1, and thus enhance therapeutic benefit, we injected an adeno-associated virus 2 (AAV2)-based vector encoding human IGF-1 (CERE-130) into lumbar spinal cord parenchyma of SOD1G93A mice. We observed robust and long-term intraspinal IGF-1 expression and partial rescue of lumbar spinal cord motor neurons, as well as sex-specific delayed disease onset, weight loss, decline in hindlimb grip strength and increased animal survival.
Keywords: Adeno, associated virus, insulin, like growth factor 1, gene therapy, neurodegeneration, amyotrophic lateral sclerosis, neuroprotection
1. Introduction
ALS is characterized by relatively rapid degeneration of upper and lower motor neurons, with death normally occurring 2–5 years following diagnosis [15]. Most cases are of a sporadic nature, while ~10% are familial. Of this familial portion, 20% are linked to various point mutations in the Cu/Zn superoxide dismutase 1 (SOD1) gene on chromosome 21 [50]. Transgenic mice [14,27,62] and rats [32,41] carrying mutant human SOD1(G93A, G37R, G86R, G85R) genes have been generated, and, despite the existence of other animal models of motor neuron loss, are currently the most widely used ALS models. The cause of the relatively selective death of motor neurons is unknown; however, a number of mechanisms have been shown to at least contribute to ALS pathogenesis [15].
Gene delivery of neurotrophic molecules is a possible therapeutic strategy for ALS treatment [3,9,23]. While alterations in CNS trophic factor signaling are likely not the primary cause of ALS, changes in levels of neurotrophic factors and their receptors, as well as dysfunction of associated intracellular signal transduction pathways, are associated with ALS and could contribute to disease progression [7,60].
IGF-1 is a pleitropic neurotrophic factor [54]. Liver-derived IGF-1 crosses the blood brain barrier, and IGF-1, IGF-1 receptors, IGF-1 binding proteins (IGFBPs) and intracellular IGF-1 associated signaling factors (IRS-1, PI3 kinase) are all expressed in CNS, particularly in spinal cord ventral gray matter (including motor neurons) [11]. IGF-1 is myotrophic and a prototypical neuronal survival factor that exerts pro-survival effects specifically on motor neurons [22]. While adverse side effects were not observed, clinical trials in Europe [12] and North America [36] showed little to no benefit of systemically administered recombinant IGF-1 in ALS patients. This may have been due to lack of sustained delivery, sequestration of exogenous IGF-1 by high levels of systemic and/or CNS IGFBPs, and low efficiency of protein delivery to motor neurons via systemic injections.
Viral vector delivery offers an attractive means for achieving sustained long-term expression of neurotrophic factor in a specific region of interest, while avoiding unwanted side effects and peripheral IGF-1 sequestration. Direct intraparenchymal spinal cord injection of gene delivery vectors may prove to be the most efficient means for providing IGF-1 to degenerating motor neurons because of reliable high titer delivery of virus directly to regions of cell loss, lack of dependency on retrograde transport along large, dysfunctional motor axons, and the potential ability to target delivery to more abundant glia and interneurons (depending on tropism of AAV serotype). For these reasons, we have tested the efficacy of multi-segmental intraparenchymal delivery of an adeno-associated virus 2 (AAV2) gene delivery vector encoding human IGF-1 to the lumbar spinal cord of SOD1G93A mice.
2. Results
Long-term expression of IGF-1 and GFP in lumbar spinal cord of SOD1G93A mice following AAV2 delivery
GFP (Fig 1D) and IGF-1 (Fig 1A, C) were expressed in SOD1G93A lumbar cord at high levels even at disease end-stage, up to 90 days post-injection. Similar to GFP, IGF-1 expression was observed in continuous distinct regions in gray matter, likely related to each individual injection. GFP expression was observed in distinct individual cells, the neuropil, in neuronal cell bodies of gray matter at the level of injection (including motor neurons: Fig 1D-inset), and in neuritic processes in white matter both at the level of injection and in regions rostral and caudal to injection sites. The rostrocaudal extent of GFP expression for each injection was 0.6–1.2 mm, and the estimated volume of distribution of GFP product in gray matter was 0.04–0.21 mm3. Expression of GFP was not seen in IGF-1 injected cords, and similar long-term GFP expression was found following AAV2 GFP injections into lumbar spinal cord of wild-type mice (not shown). IGF-1 expression was found in neuronal cell bodies and neuritic processes of gray matter at injection sites, was specifically noted in ventral horn motor neurons (Fig 1C - arrows), but was seen to a lesser extent in surrounding white matter. The rostrocaudal extent for each IGF-1 injection site was 2.4–2.6 mm, and the estimated volume of distribution of IGF-1 product in gray matter was 0.13–0.21 mm3 for each site. Expression of human IGF-1 was not seen in formulation buffer-injected cords (Fig 1B). Unfortunately, tissue was unavailable to measure total IGF-1 levels in lumbar spinal cord.
Figure 1. AAV2 IGF-1 delivery results in long-term IGF-1 expression and rescues motor neurons in the lumbar spinal cord of SOD1G93A mice.
IGF-1 (A, C) and GFP (D) were expressed in SOD1G93A lumbar cord at high levels even at disease end-stage. IGF-1 (C - arrows: enlargement of box in A) and GFP (D - inset) expression was specifically noted in ventral horn motor neurons. Expression of human IGF-1 was not seen in formulation buffer-injected cords (B: Insert is higher magnification enlargement of box). Co-localization of GFP with GFAP+ astrocytes was not observed (E). AAV2 GFP and AAV2 IGF-1 SOD1G93A mice had greatly reduced numbers of motor neurons compared to wild-type age-matched mice at 110 days of age (F). Compared to AAV2 GFP injected controls, AAV2 IGF-1 mice had a significantly greater number of motor neurons at 110 days of age (F). No differences in the response of Iba1+ microglia was noted between lumbar spinal cord of AAV2 GFP and AAV2 IGF-1 mice (G–H)
To determine the identity of AAV2-infected spinal cord cells, double-immuno labeling of GFP with specific phenotypic markers was employed. GFP co-localized with NeuN+ neurons (not shown); however, no co-localization of GFP with GFAP+ astrocytes was observed (Fig 1E).
AAV2 IGF-1 delivery partially rescued motor neuron loss in SOD1G93A mice
AAV2 GFP (14.7 ± 0.7 motor neurons/section; n = 4) and AAV2 IGF-1 (18.7 ± 1.6/section; n = 5) SOD1G93A mice had greatly reduced numbers of motor neurons compared to wild-type age-matched mice (30.9 ± 1.3/section; n = 4) at 110 days of age (Fig 1F). Compared to AAV2 GFP injected controls, AAV2 IGF-1 animals had a significantly greater number of motor neurons at 110 days of age (p < 0.01; Fig 1F), demonstrating that IGF-1 delivery partially rescued motor neuron loss in SOD1G93A mice. While the number of mice for sex-specific analysis of motor neuron survival was small, no gender differences were noted within the AAV GFP (males: 14.8 ± 0.8 motor neurons/section; n = 2; females: 14.4 ± 0.5 motor neurons/section; n = 2) or AAV IGF-1 (males: 18.7 ± 2.2 motor neurons/section; n = 2; females: 18.8 ± 0.4 motor neurons/section; n = 3) treatment groups.
To access a potential mechanism of neuroprotection, the host microglial response was examined. No differences in the response of Iba1+ microglia was noted between lumbar spinal cord of AAV2 GFP and AAV2 IGF-1 mice (Fig 1G–H), suggesting that therapeutic effects of IGF-1 were not mediated by reduction in the host immune response.
AAV2 IGF-1 and AAV2 GFP delivery had no adverse behavioral effects
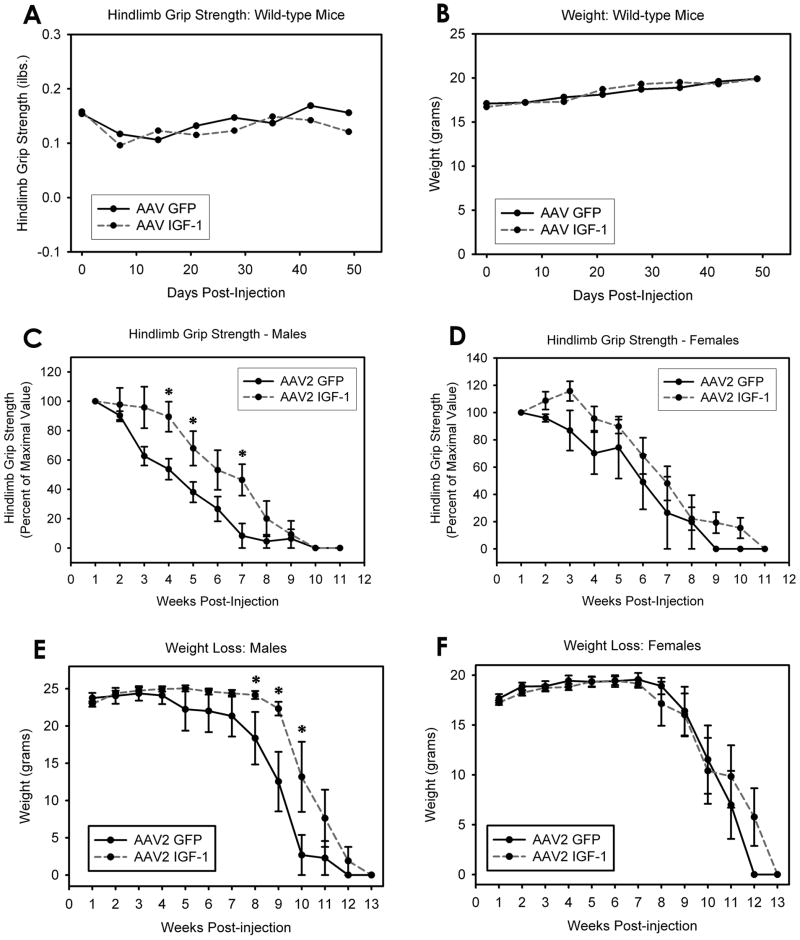
Injections of either AAV2 GFP or AAV2 IGF-1 did not result in weight loss or decline in hindlimb grip strength in wild-type mice (Fig 2A–B; p > 0.05 for all comparisons). Considering that multiple intraparenchymal spinal cord injections is a relatively invasive technique, these results are important in demonstrating that the injection procedure itself had no apparent adverse behavioral effects. Furthermore, there were no differences in weight or hindlimb grip strength values between AAV2 GFP and AAV2 IGF-1 mice (Fig 2A–B; p > 0.05 for all comparisons), suggesting that IGF-1 exerted no adverse behavioral effects on normal animals.
Figure 2. AAV2 IGF-1 delivery delayed hindlimb grip strength decline and weight loss selectively in male SOD1G93A mice.
Injections of either AAV2 GFP or AAV2 IGF-1 did not result in weight loss or decline in hindlimb grip strength in wild-type mice (A–B). Furthermore, there were no differences in weight or hindlimb grip strength values between AAV2 GFP and AAV2 IGF-1 wild-type mice (A–B). No change in hindlimb grip strength decline was noted for female SOD1G93A mice (D), while a significant delay in grip strength decline was seen in male SOD1G93A mice (C). In male SOD1G93A mice, AAV2 IGF-1 significantly delayed weight decline compared to AAV2 GFP controls at 8, 9 and 10 weeks post-injection (E). Compared to AAV2 GFP controls, AAV2 IGF-1 injections had no effect on weight decline in female SOD1G93A mice (F).
AAV2 IGF-1 delivery partially slowed hindlimb grip strength decline selectively in male SOD1G93A mice
Compared to AAV2 GFP controls, AAV2 IGF-1 SOD1G93A mice injected with AAV2 IGF-1 showed a significant delay in the decline in hindlimb grip strength when all animals from both sexes were analyzed together (p < 0.05 at 3, 4, 5 and 7 weeks post-injection; data not shown). No change in hindlimb grip strength decline was noted for female mice (p > 0.05 for all comparisons; Fig 2D), while a significant delay in grip strength decline was seen in male mice (p < 0.05 at 4, 5 and 7 weeks post-injection; Fig 2C).
AAV2 IGF-1 delivery partially slowed weight decline selectively in male SOD1G93A mice
AAV2 IGF-1 significantly delayed weight decline compared to AAV2 GFP controls at 8, 9 and 10 weeks post-injection in male mice (Fig 2E; p < 0.05), but no effect was found in female mice (Fig 2F; p > 0.05 for all comparisons). Motor neuron rescue by AAV2 IGF-1 likely slowed muscle atrophy and consequent weight loss. In addition, because AAV2 IGF-1 mice maintained greater hindlimb motor function than AAV2 GFP controls, weight was likely maintained in AAV-2 IGF-1 mice because they were better able to feed.
AAV2 IGF-1 delivery delayed disease onset selectively in male SOD1G93A mice
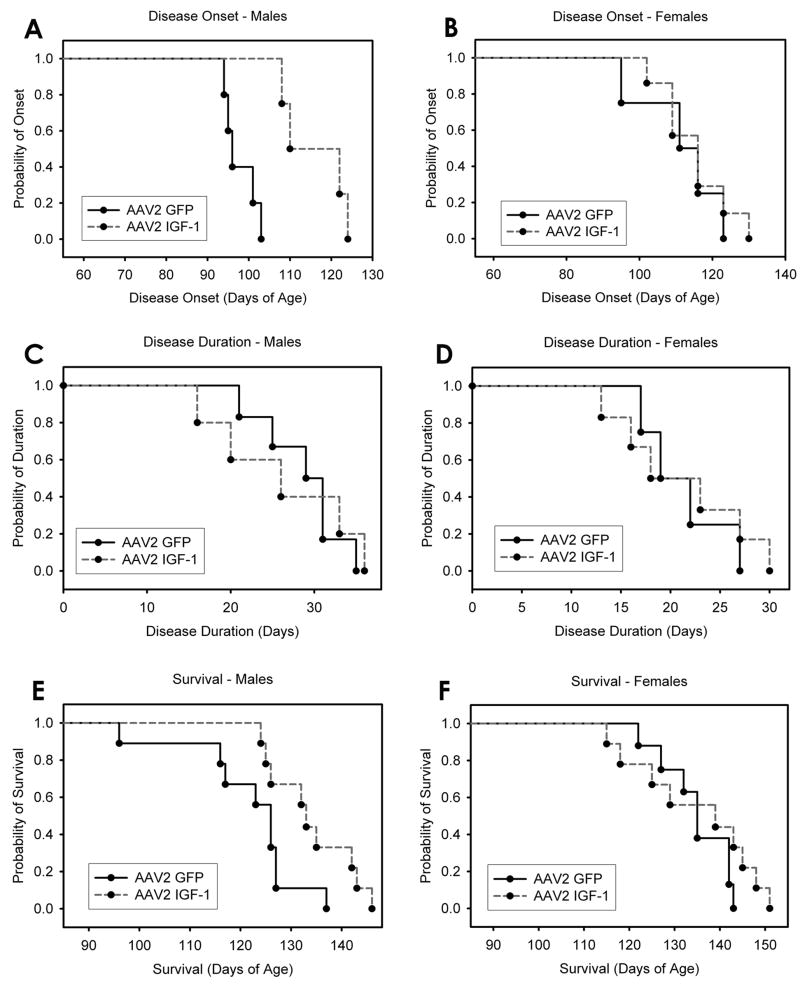
Compared to AAV2 GFP controls, AAV2 IGF-1 mice showed a significant delay in disease onset of 11.9 days (102.9 ± 10.9 days vs 114.7 ± 8.2 days; p < 0.05; data not shown) when all animals from both sexes were analyzed together. No change in onset was noted for female mice (111.3 days ± 14.6 vs 115.0 ± 9.4 days; p > 0.05; Fig 3B), while a significant increase in onset of 19.0 days was seen in male mice (97.8 days ± 3.8 vs 116.8 ± 8.1 days; p < 0.05; Fig 3A).
Figure 3. AAV2 IGF-1 delivery delayed disease onset and extended survival selectively in male SOD1G93A mice.
No change in disease onset was noted for female SOD1G93A mice (B), while a significant increase in onset of 19.0 days was seen in male SOD1G93A mice (A). However, no change was observed in disease duration, the time between disease onset and end-stage, in male (C) or female (D) SOD1G93A mice. AAV2 IGF-1 significantly increased survival in male SOD1G93A mice by 12.3 days compared to the AAV2 GFP group (E), while no effect was found in females (F).
AAV2 IGF-1 delivery did not extend disease duration in male and female SOD1G93A mice
No change was observed in disease duration, the time between disease onset and end-stage, in male mice (28.6 days ± 5.5 vs 26.3 ± 9.7 days; p > 0.05; Fig 3C), female mice (21.7 days ± 4.6 vs 22.2 ± 8.4 days; p > 0.05; Fig 3D) or when all animals from both sexes were analyzed together (26.0 ± 6.0 days vs 22.2 ± 9.8 days; p > 0.05; data not shown).
AAV2 IGF-1 delivery extended survival selectively in male SOD1G93A mice
When male and female SOD1G93A mice were analyzed together, AAV2 IGF-1 increased survival by 6.6 days (127.8 ± 11.7 days vs 134.4 ± 10.8 days; p > 0.05; data not shown) compared to AAV2 GFP; however, this increase was not significant. AAV2 IGF-1 significantly increased survival in male mice by 12.3 days (121.7 ± 11.4 days vs 134.0 ± 7.7 days; p < 0.05) compared to the AAV2 GFP group (Fig 3E), while no effect was found in females (134.8 ± 7.6 days vs 134.8 ± 13.4 days; p > 0.05; Fig 3F). Interestingly, AAV2 IGF-1 delivery was only able to extend male survival to the level of AAV2 GFP female controls.
3. Discussion
We have demonstrated that AAV2-based delivery of IGF-1 directly to lumbar spinal cord has therapeutic benefit (albeit gender-specific) in a well-established mouse model of ALS. We also observed sustained IGF-1 expression, despite ongoing disease processes. Expression was not confined to sites of injection, but was distributed in neuritic processes over a relatively broad region of lumbar spinal cord. This is of particular relevance because of the spatially diffuse nature of ALS and the desire to widely distribute IGF-1.
Viral Vector Delivery as ALS Therapy
Viral vector delivery of neurotrophic factors represents a useful approach for ALS and other neurodegenerative CNS disorders. Previous studies in animal models of motor neuron disease (SOD1, wobbler, progressive motor neuronopathy, spinal muscle atrophy) have shown that therapeutic benefits can be achieved via adenovirus-, lentivirus-, herpes simplex virus- and adeno-associated virus (AAV)-based delivery of genes such as mutant SOD1-targeted RNAi [48,49], VEGF [6], NT-3 [28], CNTF [2], GDNF [1,58], IGF-1 [34], Bcl-2 [5] and cardiotrophin-1 [13]. Clinical translation with some of these proteins has not yielded similar efficacy in human ALS patients [12,25,26,36]; however, trials have mostly involved systemic injection of protein, not viral vector-based gene delivery.
Methods of AAV IGF-1 Delivery
Previous studies of intramuscular AAV2 IGF-1 delivery resulted in robust efficacy in mutant-SOD1 mice [34]. This approach, however, is complicated by dependency on intact, functional and muscle-innervating motor axons and low efficiency of vector retrograde transport, even in an intact, healthy system. Human ALS cases are mostly diagnosed after extensive motor neuron loss and/or denervation, at a point when capacity for retrograde transport may be diminished. Large muscle masses call for large amounts of highly purified vector; however, “large-scale” production of AAV serotypes other than AAV2 has not been resolved [24]. Retrograde transport delivers vector only to motor neurons, while an extensive additional pool of glia and interneurons exists for trophic production.
As shown in the present study, intraparenchymal delivery to ventral spinal cord provides a way to directly introduce high titers of virus to regions of dieing motor neurons. However, effects observed in this study were not as robust as those attained with intramuscular AAV2 IGF-1. Furthermore, direct injection is associated with a number of practical disadvantages such as risk of further damage because of surgical invasiveness (though no apparent behavioral effects are reported in this study), costliness, difficulty of procedure standardization, and the diffuse nature of ALS, potentially requiring injections at multiple levels of spinal cord, brainstem and cortex. While we have demonstrated the efficacy of multi-segmental deliver only in the lumbar region, Dodge et al. [21] reported that injection of AAV1 or AAV2 IGF-1 into deep cerebellar nuclei (DCN) promoted significant benefit in SOD1 mice. Because of the anatomy of axonal connections between DCN and all levels of spinal cord, this represents a technique to target multiple spinal cord levels with minimal invasiveness.
Injections of vector into cerebrospinal fluid via lumbar puncture (spinal tap) or via intracerebroventricular injection are excellent alternatives for delivering vector extensively throughout the neuraxis. Preliminary studies both in mutant-SOD1 rodents [42,45] and in ALS patients [43] have shown the efficacy of intrathecal delivery of IGF-1 protein; nevertheless, efficiency relative to other delivery means has not been addressed. Further work will be needed to optimize the regimen, including timing, dose, and region(s) of injection.
Benefits of AAV-based Vectors
AAV, a non-pathogenic single-stranded DNA parvovirus, is a promising viral candidate for gene delivery to ALS patients. Benefits include [9]: rare vector sequence integration into host genome, efficient transduction of both dividing and non-dividing cells, long-term gene expression, the existence of at least 8 serotypes allowing for differential targeting of specific CNS cell populations, lack of toxicity, and minimal stimulation of the host immune response.
Temporal Considerations
Because of the ongoing nature of ALS, sustained gene expression is thought to be necessary; however continuous, unregulated expression of high levels of neurotrophins may be associated with side effects, such as the motor disturbances observed with GDNF delivery in the Parkinson’s disease rat model [35]. Temporal regulation via rapamycin [59] and tetracycline [18] -based expression systems may provide useful tools.
Timing of vector introduction is also important. Should IGF- 1 be given as early as possible, and are repeated injections over some as yet to be determined interval needed? Work in organotypic cultures demonstrated that motor neuron rescue from excitotoxicity was reduced when IGF-1administration was delayed [8]. Unfortunately, many neurons have already been lost or have undergone denervation from muscle by the time of ALS diagnosis. In the present study, we delivered vector at 60 days of age, a time before onset of motor neuron loss and behavioral deficits. Kaspar et al [34] reported benefit when AAV2 IGF-1 was introduced at symptom onset, suggesting that even late delivery is promising, provided that the delivery regimen is designed properly.
Therapeutic Actions of IGF-1 in ALS
Disease onset (but not progression) is delayed when mutant SOD1 expression is knocked out selectively in motor neurons [10]. These findings coincide with our results in that AAV2 IGF-1 delays motor neuron loss and onset in SOD1G93A mice, but does not affect disease duration. The AAV2 used in this study targets neurons, and thus the neuroprotection observed may be intrinsic to effects of IGF-1 on motor neurons rather than other non-neuronal cells. AAV serotypes that infect more abundant astrocytes or ependymal cells may also be an option for optimizing effects of IGF-1 in the spinal cord [53].
Nevertheless, in vitro studies suggest that infected motor neurons likely also secrete IGF-1, and consequently may exert beneficial actions on surrounding bystander neuronal and even non-neuronal cells, in addition to motor neurons [56]. For example, astrocytes, oligodendrocytes and microglia all express IGF-1 receptors, and IGF-1 has been shown to promote proliferation, differentiation, and survival of glial cells [17]. IGF-1 regulates proliferation and GFAP expression in astrocytes, and may therefore play a role in controlling astrogliosis that occurs in the spinal cord of mutant SOD1 rodents and humans with ALS. IGF-1 may also function in an anti-inflammatory fashion by antagonizing activation of microglia mediated by IFN-γ and/or blocking release of interleukin (IL)-1β by activated microglia [39], effects that could indirectly promote motor neuron survival by preventing neuronal cell death associated with microglial over-activation.
Alterations in components of the IGF-1 signaling system occur in ALS patients and in rodent models of the disease. While changes in total IGF-1 levels have not been found, increases in IGF binding proteins 2, 5 and 6 are seen in ventral horn [61], resulting in decreased levels of “free” IGF-1 to exert neuroprotective actions. In addition, inflammatory events associated with ALS (possibly via TNF-alpha) are believed to decrease transduction sensitivity of IGF-1 receptors and intracellular signaling components [54]. Vulnerable motor neurons are deprived of pro-survival IGF-1 signaling in ALS, furthering contributing to cell loss.
The neuroprotective actions of IGF-1 in the context of decreased IGF-1 signaling in ALS therefore makes gene delivery of IGF-1 a potentially important candidate for therapy. High levels of expression achieved via AAV2 delivery most likely provides a sufficient level of local IGF-1 to overcome loss of IGF-1 signaling associated with disease. The present results coincide with previous studies that have shown that IGF-1 exerts pro-survival effects specifically on motor neurons: 1) in dissociated culture [57], 2) in the organotypic spinal cord model of excitotoxicity [19], 3) during developmental programmed cell death [37], 4) following spinal cord ischemia [44], spinal transaction [51], peripheral axotomy [38], crush injury [47] and root avulsion [29], 5) in the wobbler mouse [30] and in the SOD1 mouse [20,34].
Sex-specific Effects
Sex-specific differences in disease onset and progression [31,40,52,55] and response to therapeutic interventions [16] have been noted in CNS disorders, including ALS. For example, studies with untreated mutant SOD1 mice [31] and rats [52], including results from the present study, demonstrate that disease onset and overall survival occur earlier on average in males. In response to an exercise regimen therapy, delayed disease onset and survival was noted in only female mice [55], while AAV2 IGF-1 promoted benefit selectively in males in the present study. While the mechanism for the male-only effect seen in the present study is unknown, potential reasons include differences in disease progression, mutant SOD1 expression, sex hormones and/or levels and sensitivity of components of the IGF-1 system. Robust and long-lasting IGF-1 expression was seen in both males and females, suggesting that the effect was unlikely related to sex-specific differences in transgene expression.
Combinatorial Therapies
We report modest effects of AAV2 IGF-1 delivery. More widespread delivery of virus, as well as delivery to relevant motor neuron pools (i.e. cervical and/or respiratory motor neurons), could produce more robust neuroprotection. The complexity of ALS will likely require a multi-faceted approach that combines gene therapy with other promising strategies, including cell transplants, pharmacological agents and additional trophic factors. For example, Arakawa et al. [4] report a supra-additive effect of IGF-1 and CNTF on the survival of isolated chick embryonic spinal motor neurons. In the pmn mouse, combined adenoviral vector administration of NT-3 and CNTF has a greater effect than NT-3 alone [28]. Kaspar et al [33] demonstrated that the efficacy of IGF-1 delivery via intramuscular injections can be further increased when combined with an exercise regimen. These are just a few examples representing the important trend toward combination therapy and the integrated approach that builds on the exciting recent advances in gene therapy vectors and ALS biology, and is likely to be the most effective strategy for successful treatment of patients with ALS.
4. Experimental Procedure
Vector plasmid constructs and vector production
The CERE-130 vector genome contains the AAV2 inverted terminal repeats (ITRs) flanking a transgene expression cassette containing the chicken beta-actin (CAG) promoter, the human IGF-1 cDNA and the human growth hormone gene (hGH) polyadenylation signal (polyA) (Stratagene, La Jolla, CA). AAV2-GFP genome structure is identical to that of CERE-130 except that the GFP cDNA is found in place of that of IGF-1. The details of cloning procedures and primer sequences are available upon request. Plasmid clones identity was confirmed by restriction digestions and nucleotide sequence determination.
Vectors were produced by triple transfection and purified. They were formulated in PBS, 2mM MgCl2 [formulation buffer (FB)] and vector titer was determined by quantitative PCR using vector specific primers.
Viral vector delivery
The care and treatment of animals in all procedures was conducted in strict accordance with the guidelines set by the European Communities Counsel Directive (November 24th, 1986), the NIH Guide for the Care and Use of Laboratory Animals, the Guidelines for the Use of Animals in Neuroscience Research and the Johns Hopkins University IACUC, and measures were taken to minimize any potential pain or animal discomfort.
Sixty-day-old wild-type and SOD1G93A mice (approximately 20–30 grams) received i.p. injections of anaesthetic cocktail [acepromazine maleate (0.7mg/kg; Fermenta Animal Health, Kansas City, MO), ketamine (95.0mg/kg; Fort Dodge Animal Health; Fort Dodge, IA), and xylazine (10.0mg/kg; Bayer, Shawnee Mission, KS)]. The back musculature was excised, and a laminectomy was performed above the L4 and L5 levels of the spinal cord. The dura was incised above the injection site using a 30-gauge needle. AAV2 IGF-1 or AAV2 GFP vectors were bilaterally injected into the ventral gray matter at four total sites in the lumbar L4-L5 region (bilaterally at L4 and L5; separated by 2.0 mm in the rostral-caudal axis). The injection pipette was secured to a manual micromanipulator (World Precision Instruments; Sarasota, FL) attached to an 80° tilting base. The tip was lowered to a depth of 1.3mm below the surface of the cord to target ventral horn, and was held in place for 2 minutes before and after cell injection. 1.5 μl of concentrated viral solutions (AAV2 IGF-1: 1.2×1010 vg/injection site; AAV2 GFP: 4.2×109 vg/injection site) was injected at each of the four sites. Vector was delivered under the control of a microsyringe pump controller (World Precision Instruments) at a rate of 0.75μL/minute. Dura was closed with 9-0 suture, and skin was closed with wound clips. The entire injection causes minimal disturbance to the spinal cord.
For weight, survival and grip strength analysis, 35 SOD1G93A mice (AAV2 IGF-1: n = 18 total, n = 9 males, n = 9 females; AAV2 GFP: n = 17 total, n = 9 males, n = 8 females) and 6 age-matched wild-type mice (AAV2 IGF-1: n = 6 total, n = 3 females; AAV2 GFP: n = 3 females) were used in total. For motor neuron counts, 9 SOD1G93A mice (AAV2 IGF-1: n = 5 total, n = 3 males, n = 2 females; AAV2 GFP: n = 4 total, n = 2 males, n = 2 females) and 4 age-matched wild-type mice (n = 4 total, n = 2 males, n = 2 females) were used in total. Each group consisted of an almost equal amount of males and females.
Animal model
Transgenic mice carrying the human SOD1 gene with the G93A mutation (SOD1 B6SJL-TgN[SOD1-G93A]1Gur) were used [27]. Mice were obtained from The Jackson Laboratory via in vitro fertilization/Speed Expansion program to provide one homogeneous heterozygous population. On average, this line of SOD1G93A mutants develop disease onset at approximately 90 days of age, and reach disease end-stage approximately 40 days later. In addition, wild-type B6SJL mice from Jackson were also used. All wild-type and SOD1G93A mice were housed at standard temperature (21°C) and in a light controlled environment with ad libitum access to the food and water. Mice were maintained in racks of ventilated cages located in the same room.
Behavioral analysis
Beginning one week before the injection of viral vectors, SOD1G93A and wild-type mice were monitored weekly for weight and for motor dysfunction by measuring hind limb muscle strength using a “Grip Strength Meter” (DFIS-2 Series Digital Force Gauge; Columbus Instruments, OH). Grip strength testing was performed by allowing the animals to grasp a thin bar attached to the force gauge. This was followed by pulling the animal away from the gauge until the hindlimbs released the bar. This provides a value for the force of maximal grip strength. The force measurements were recorded in three separate trials and the averages was used in analyses. In order to avoid dehydration, Aqua-Jel packs were provided when animals started to show disease symptoms. Disease onset was determined individually when an animal dropped to 80.0% of its maximal hindlimb grip strength [46]. Disease duration was measured as the time between disease onset and end-stage. To determine disease end-stage in a reliable and ethical fashion, an artificial end point was used for all SOD1G93A mutant mice. End-stage was defined by the inability of mice to right themselves within 30 seconds when placed on their sides. The moribund mice were scored as “dead”, and were subsequently euthanized.
Tissue processing
Animals were sacrificed at 110 days of age or at disease end-stage by transcardial perfusion with 0.3% saline, followed by ice-cold 4% paraformaldehyde (Fisher Scientific; Pittsburgh, PA). The entire spinal cord was removed. The lumbar region (L1-L5) was isolated and post-fixed in 4.0% paraformaldehyde.
Histology and Immunohistochemistry
For GFP and IGF-1 immunohistochemistry, spinal cords from end-stage mice were cryoprotected in 30.0% sucrose (Fisher)/.1 M phosphate buffer at 4°C for 3 days. The tissue was embedded in OCT (Fisher), fast frozen with dry ice, and stored at −80°C until processed. Spinal cord tissue blocks were cut in the sagittal or transverse plane at 20um thickness. Sections were collected on glass slides, or were collected in PBS for free-floating immunohistochemistry. For GFP, a 1-in-6 series of slides was counterstained with DAPI, and fluorescence was assessed microscopically. An image of each section with GFP fluorescence was collected, and the area of fluorescence circumscribed and measured on the image. The number of sections with GFP fluorescence was used to determine the rostrocaudal extent of product, and the areas measured on each section were used to determine the volume of distribution of product using Cavalieri’s formula. For IGF-1, immunohistochemistry was used to detect product on 1-in-12 series slides, and imaging and measuring were done as with GFP to determine rostrocaudal extent and volume of distribution of IGF1. To determine the phenotype of AAV2-infected cells, astrocytes were identified with GFAP and neurons were identified with NeuN. The host microglial reponse was examined with Iba1.
Motor neuron quantification
For motor neuron counts, tissue from L4 and L5 of 110 day old mice was dehydrated in a graded series of alcohol solutions, embedded in paraffin, and cut serially in the transverse plane. Serial cross-sections of the lumbar spinal cords (~2 mm total length) were made at 14 μm thickness, for a total of 140 sections. Sections were processed for cresyl violet staining. Motor neurons were counted in every 7th section at 20x magnification in order to avoid repetitive counting. Only motor neurons with a clearly identifiable nucleus and nucleolus, a cell soma over 100 μm2 and located within the ventral horn were counted [46].
Statistical analysis
Kaplan-Meier survival analysis of the SOD1G93A mice was conducted using the statistical software Sigmaplot (SAS Software). In some cases, Student t-test was performed to compare data between groups of animals. All data are presented as mean ± S.E.M., and significance level was set at p ≤ 0.05.
Acknowledgments
Natalie Perez for assistance with grip strength measurements; Jean Brennan and Ariadne Bolton for assistance with histology; all members of the Rothstein and Maragakis labs for helpful discussion; The ALS Association, The Robert Packard Center for ALS Research and the NIH (grants NS33958; NS41680) for funding; Project ALS for providing SOD1G93A mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, Wang Y, Ianakova E, Mohammad S, Lewis RA, Shy ME. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13:1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- 2.Aebischer P, Schluep M, Deglon N, Joseph JM, Hirt L, Heyd B, Goddard M, Hammang JP, Zurn AD, Kato AC, Regli F, Baetge EE. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med. 1996;2:696–699. doi: 10.1038/nm0696-696. [DOI] [PubMed] [Google Scholar]
- 3.Alisky JM, Davidson BL. Gene therapy for amyotrophic lateral sclerosis and other motor neuron diseases. Hum Gene Ther. 2000;11:2315–2329. doi: 10.1089/104303400750038435. [DOI] [PubMed] [Google Scholar]
- 4.Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990;10:3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzouz M, Hottinger A, Paterna JC, Zurn AD, Aebischer P, Bueler H. Increased motoneuron survival and improved neuromuscular function in transgenic ALS mice after intraspinal injection of an adeno-associated virus encoding Bcl-2. Hum Mol Genet. 2000;9:803–811. doi: 10.1093/hmg/9.5.803. [DOI] [PubMed] [Google Scholar]
- 6.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 7.Beck M, Karch C, Wiese S, Sendtner M. Motoneuron cell death and neurotrophic factors: basic models for development of new therapeutic strategies in ALS. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2(Suppl 1):S55–68. [PubMed] [Google Scholar]
- 8.Bilak MM, Kuncl RW. Delayed application of IGF-I and GDNF can rescue already injured postnatal motor neurons. Neuroreport. 2001;12:2531–2535. doi: 10.1097/00001756-200108080-00048. [DOI] [PubMed] [Google Scholar]
- 9.Boillee S, Cleveland DW. Gene therapy for ALS delivers. Trends Neurosci. 2004;27:235–238. doi: 10.1016/j.tins.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 11.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, Silani V, Vos PE, Wokke JH, Dobbins T. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 1998;51:583–586. doi: 10.1212/wnl.51.2.583. [DOI] [PubMed] [Google Scholar]
- 13.Bordet T, Lesbordes JC, Rouhani S, Castelnau-Ptakhine L, Schmalbruch H, Haase G, Kahn A. Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum Mol Genet. 2001;10:1925–1933. doi: 10.1093/hmg/10.18.1925. [DOI] [PubMed] [Google Scholar]
- 14.Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 15.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 16.Bushnell CD, Griffin J, Newby LK, Goldstein LB, Mahaffey KW, Graffagnino CA, Harrington RA, White HD, Simes RJ, Califf RM, Topol EJ, Easton JD. Statin use and sex-specific stroke outcomes in patients with vascular disease. Stroke. 2006;37:1427–1431. doi: 10.1161/01.STR.0000221315.60282.ca. [DOI] [PubMed] [Google Scholar]
- 17.Chesik D, Wilczak N, De Keyser J. The Insulin-like Growth Factor System in Multiple Sclerosis. Int Rev Neurobiol. 2007;79:203–226. doi: 10.1016/S0074-7742(07)79009-8. [DOI] [PubMed] [Google Scholar]
- 18.Chtarto A, Bender HU, Hanemann CO, Kemp T, Lehtonen E, Levivier M, Brotchi J, Velu T, Tenenbaum L. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003;10:84–94. doi: 10.1038/sj.gt.3301838. [DOI] [PubMed] [Google Scholar]
- 19.Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis. 1999;6:335–346. doi: 10.1006/nbdi.1999.0253. [DOI] [PubMed] [Google Scholar]
- 20.Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, Molinaro M, Rosenthal N, Musaro A. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dodge JCPMA, Clarke J, Yang W, Grissett L, Kim S, Wen R, Cheng SH, Kaspar BK, Shihabuddin LS. Intracerebellar injection of AAV-IGF-1 improves motor function and extends survival in a mouse model of amyotrophic lateral sclerosis. Society for Neuroscience Abstracts. 2006 [Google Scholar]
- 22.Dore S, Kar S, Quirion R. Rediscovering an old friend, IGF-I: potential use in the treatment of neurodegenerative diseases. Trends Neurosci. 1997;20:326–331. doi: 10.1016/s0166-2236(96)01036-3. [DOI] [PubMed] [Google Scholar]
- 23.Federici T, Boulis NM. Gene-based treatment of motor neuron diseases. Muscle Nerve. 2006;33:302–323. doi: 10.1002/mus.20439. [DOI] [PubMed] [Google Scholar]
- 24.Grimm D, Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- 25.Group BS. A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 26.Group CS. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology. 1996;46:1244–1249. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- 27.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 28.Haase G, Kennel P, Pettmann B, Vigne E, Akli S, Revah F, Schmalbruch H, Kahn A. Gene therapy of murine motor neuron disease using adenoviral vectors for neurotrophic factors. Nat Med. 1997;3:429–436. doi: 10.1038/nm0497-429. [DOI] [PubMed] [Google Scholar]
- 29.Haninec P, Houst’ava L, Stejskal L, Dubovy P. Rescue of rat spinal motoneurons from avulsion-induced cell death by intrathecal administration of IGF-I and Cerebrolysin. Ann Anat. 2003;185:233–238. doi: 10.1016/S0940-9602(03)80030-4. [DOI] [PubMed] [Google Scholar]
- 30.Hantai D, Akaaboune M, Lagord C, Murawsky M, Houenou LJ, Festoff BW, Vaught JL, Rieger F, Blondet B. Beneficial effects of insulin-like growth factor-I on wobbler mouse motoneuron disease. J Neurol Sci. 1995;129(Suppl):122–126. doi: 10.1016/0022-510x(95)00081-c. [DOI] [PubMed] [Google Scholar]
- 31.Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci. 2005;236:1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 34.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 35.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, Murphy MF, Natter HM, Norris FH, Rudnicki SA. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. 1997;49:1621–1630. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 37.Lewis ME, Neff NT, Contreras PC, Stong DB, Oppenheim RW, Grebow PE, Vaught JL. Insulin-like growth factor-I: potential for treatment of motor neuronal disorders. Exp Neurol. 1993;124:73–88. doi: 10.1006/exnr.1993.1177. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25:759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- 39.Maher FO, Clarke RM, Kelly A, Nally RE, Lynch MA. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–1571. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto A, Okada Y, Nakamichi M, Nakamura M, Toyama Y, Sobue G, Nagai M, Aoki M, Itoyama Y, Okano H. Disease progression of human SOD1 (G93A) transgenic ALS model rats. J Neurosci Res. 2006;83:119–133. doi: 10.1002/jnr.20708. [DOI] [PubMed] [Google Scholar]
- 41.Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH, Jr, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001;21:9246–9254. doi: 10.1523/JNEUROSCI.21-23-09246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagano I, Ilieva H, Shiote M, Murakami T, Yokoyama M, Shoji M, Abe K. Therapeutic benefit of intrathecal injection of insulin-like growth factor-1 in a mouse model of Amyotrophic Lateral Sclerosis. J Neurol Sci. 2005;235:61–68. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 43.Nagano I, Shiote M, Murakami T, Kamada H, Hamakawa Y, Matsubara E, Yokoyama M, Moritaz K, Shoji M, Abe K. Beneficial effects of intrathecal IGF-1 administration in patients with amyotrophic lateral sclerosis. Neurol Res. 2005;27:768–772. doi: 10.1179/016164105X39860. [DOI] [PubMed] [Google Scholar]
- 44.Nakao Y, Otani H, Yamamura T, Hattori R, Osako M, Imamura H. Insulin-like growth factor 1 prevents neuronal cell death and paraplegia in the rabbit model of spinal cord ischemia. J Thorac Cardiovasc Surg. 2001;122:136–143. doi: 10.1067/mtc.2001.114101. [DOI] [PubMed] [Google Scholar]
- 45.Narai H, Nagano I, Ilieva H, Shiote M, Nagata T, Hayashi T, Shoji M, Abe K. Prevention of spinal motor neuron death by insulin-like growth factor-1 associating with the signal transduction systems in SODG93A transgenic mice. J Neurosci Res. 2005;82:452–457. doi: 10.1002/jnr.20668. [DOI] [PubMed] [Google Scholar]
- 46.Pardo AC, Wong V, Benson LM, Dykes M, Tanaka K, Rothstein JD, Maragakis NJ. Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1(G93A) mice. Exp Neurol. 2006;201:120–130. doi: 10.1016/j.expneurol.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 47.Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, Shenaq SM, Schwartz RJ. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. Faseb J. 2003;17:53–55. doi: 10.1096/fj.02-0183fje. [DOI] [PubMed] [Google Scholar]
- 48.Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, Wong LF, Bilsland LG, Greensmith L, Kingsman SM, Mitrophanous KA, Mazarakis ND, Azzouz M. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- 49.Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 50.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 51.Sharma HS, Nyberg F, Gordh T, Alm P, Westman J. Topical application of insulin like growth factor-1 reduces edema and upregulation of neuronal nitric oxide synthase following trauma to the rat spinal cord. Acta Neurochir Suppl. 1997;70:130–133. doi: 10.1007/978-3-7091-6837-0_40. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki M, Tork C, Shelley B, McHugh J, Wallace K, Klein SM, Lindstrom MJ, Svendsen CN. Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotroph Lateral Scler. 2007;8:20–25. doi: 10.1080/17482960600982447. [DOI] [PubMed] [Google Scholar]
- 53.Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med. 2004;6(Suppl 1):S212–222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- 54.Trejo JL, Carro E, Garcia-Galloway E, Torres-Aleman I. Role of insulin-like growth factor I signaling in neurodegenerative diseases. J Mol Med. 2004;82:156–162. doi: 10.1007/s00109-003-0499-7. [DOI] [PubMed] [Google Scholar]
- 55.Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 56.Vincent AM, Feldman EL, Song DK, Jung V, Schild A, Zhang W, Imperiale MJ, Boulis NM. Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuromolecular Med. 2004;6:79–85. doi: 10.1385/NMM:6:2-3:079. [DOI] [PubMed] [Google Scholar]
- 57.Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S, Petravicz J, Breakefield XO. Single HSV-amplicon vector mediates drug-induced gene expression via dimerizer system. Mol Ther. 2003;7:790–800. doi: 10.1016/s1525-0016(03)00094-7. [DOI] [PubMed] [Google Scholar]
- 60.Wilczak N, de Keyser J. Insulin-like growth factor system in amyotrophic lateral sclerosis. Endocr Dev. 2005;9:160–169. doi: 10.1159/000085764. [DOI] [PubMed] [Google Scholar]
- 61.Wilczak N, de Vos RA, De Keyser J. Free insulin-like growth factor (IGF)-I and IGF binding proteins 2, 5, and 6 in spinal motor neurons in amyotrophic lateral sclerosis. Lancet. 2003;361:1007–1011. doi: 10.1016/S0140-6736(03)12828-0. [DOI] [PubMed] [Google Scholar]
- 62.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]