Abstract
Background
Research indicates that the etiology of autism has a strong genetic component, yet so far the search for genes that contribute to the disorder, including several whole genome scans, has led to few consistent findings. However, three studies indicate that the complement C4B gene null allele (i.e. the missing or nonfunctional C4B gene) is significantly more frequent in individuals with autism. Due to the close proximity of the CYP21A2 gene to the C4B locus (3 kb) it was decided to examine samples from autistic subjects, including many with known C4B null alleles for common CYP21A2 mutations.
Methods
Samples from subjects diagnosed with autism and non-autistic controls (controls) previously typed for C4B null alleles were studied. Allele specific polymerase chain reaction (PCR) methods were used to determine 8 of the most common CYP21A2 genetic mutations, known to completely or partially inhibit 21-hydroxylase, the enzyme encoded by the CYP21A2 gene.
Results
Although the combined autism and control study subjects had 50 C4B null alleles only 15 CYP21A2 mutations were detected in over 2250 genotypes. Eight mutations were detected in the autistic samples and 7 in the controls. The frequency of CYP21A2 mutations was similar between the autism and control samples. Only one individual (autistic) carried a chromosome containing both C4B null allele and CYP21A2 mutations.
Background
Autism is a severe neurodevelopmental disorder that is approximately four times more common in males than females. The current prevalence for the disorder is approximately 1 in 152 children [1]. Although there are various mechanisms that can lead to this behaviorally defined condition, in most cases the etiology remains unknown. A strong genetic component clearly exists [2]; however, consistent detection of disease associated genetic variants has rarely been reported. Whole genome scans using microarray technology may better detect the genetic contributions to autism susceptibility [3].
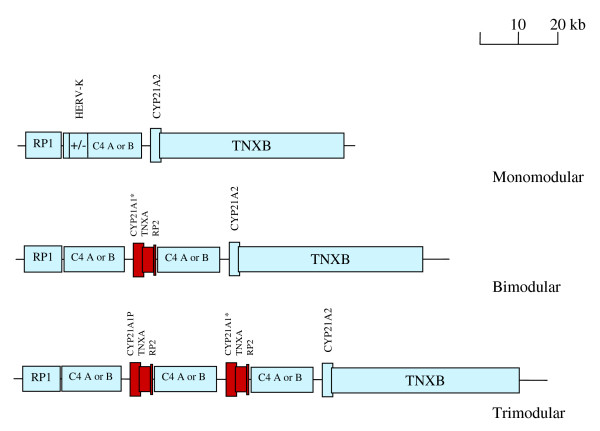
Genes located in the RCCX module found on chromosome 6 in the human leukocyte antigen (HLA) locus are associated with various disease states [4,5]. This module contains the genes RP, C4, CYP21, and TNX (abbreviated RCCX) in a contiguous sequence. Different variants of these four genes can exist in the RCCX module including RP1 or RP2, C4A or C4B (long or short), CYP21A2 or CYP21A1P, and TNXA or TNXB. RP2 and TNXA are gene fragments while CYP21A1P is a pseudogene. A single chromosome usually contains one, two, three RCCX modules in tandem, but rare cases can have four modules.
Because of the diversity in the number and size of the RCCX modules misalignments and unequal crossovers occur during meiosis resulting frequently in deletions, conversions, duplications along with the acquisition of mutations from nearby pseudogenes or gene segments [6]. One such mutation in this module is the missing/nonfunctional C4A or C4B gene (C4 null allele). Therefore, the C4 containing complex is an extraordinarily complex region of the human genome [7] (Figure 1).
Figure 1.
Three common arrangements of the RCCX module. Monomodular, bimodular, and trimodular are present in about 17, 69 and 14% of chromosomes, respectively [7]. The C4 gene which is either C4A or C4B can be either a long or short variant depending upon the presence of a 6.36 kb endogenous retrovirus, HERV-K(C4). Pseudogenes or gene fragments are red. CYP21A1* indicates either a CYP21A1P pseudogene or the CYP21A2 gene. In the present study 40 chromosomes from autistic individuals had a C4B null allele. Of these chromosomes 19 were monomodular and 21 were bimodular. In the control subjects with C4B null alleles 10 chromosomes were monomodular and 1 was bimodular. Bimodular C4B null alleles were significantly more frequent in autistic subjects compared to controls (P = 0.0001). No C4B null alleles or CYP21A2 mutations were detected in the subjects (2 autistic, 4 control) with trimodular RCCX modules, determined by protein immunofixation electrophoresis [10].
The RCCX module may play a significant role in the genetic underpinnings of autism. Several studies have shown that the frequency of C4B null alleles is increased in individuals with autism [8-10], the most recent of which found that 42.4% of autistic subjects carry a C4B null allele compared to 14.5% of controls [10]. The CYP21A2 gene is located approximately 3 kb downstream of C4 and the concurrent deletion of C4B with portions of the CYP21A2 has been described [11,12]. Therefore, the aim of the present research was to determine if the C4B null allele, found frequently in subjects with autism, is associated with CYP21A2 mutations. As well, the overall frequency of CYP21A2 mutations in autistic verses control subjects was determined.
C4 genes encode innate immune C4 proteins that are important in the complement cascade. CYP21A2 encodes an enzyme, 21-hydroxylase, which is important in the synthesis of cortisol and in maintaining proper androgen levels.
Over 2,250 genetic typings of CYP21A2 mutations were completed in 80 autistic and 60 controls subjects that had previously been typed for C4B null alleles [10]. Fifteen total CYP21A2 mutations where detected; however, only one individual (autistic) carried a chromosome containing both a C4B null allele and CYP21A2 mutations. Therefore, in these subjects it does not appear that C4B null alleles are associated with the CYP21A2 mutations studied.
Methods
Subjects
This study utilized samples previously characterize for C4A and C4B null alleles in an autism case-control study [10]. Autistic subjects and controls were Caucasian of Northern European descent and IRB (Utah State University) approval was obtained for this study. As reported, the subjects were diagnosed with autism using DSM-IV criteria by pediatric psychiatrists and psychologists expert in the evaluation of autism. The Autism Diagnostic Observation Schedule (ADOS) [13] and the Autism Diagnostic Inventory (ADI) [14] confirmed the Diagnosis. Various CYP21A2 genetic determinations were completed in 80 individuals with autism (8 female, 72 male) and 60 control subjects (15 female, 45 male). Parents of the particular subjects were typed if their child was positive for a mutation.
DNA preparation
DNA samples were extracted from peripheral blood mononuclear cells as previously described [15]. To genotype samples with limited amounts of DNA, whole genome amplification was performed using multiple displacement amplification (MDA) based on the method of Dean et al.[16]. MDA was performed using RepliPHI™ Phi 29 Reagent Sets (Epicentre®Technologies, Madison, Wisconsin).
PicoGreen® quantitation of amplified DNA was performed using a Quant-iT™ DNA Assay Kit from Molecular Probes™ (Eugene, Oregon) according to kit protocol. Fluorescence was measured with a Synergy HT microplate reader (BIO-TEK®, Winooski, Vermont).
Polymerase chain reaction
Seven mutations were determined by allele-specific polymerase chain reaction (PCR) based on the method of Wilson et al.[17]. This method is as accurate as the dot blot procedure [17]; therefore, it is sensitive enough to detect a mutation in only one CYP21A2 gene if more than two copies of the gene are present. The mutations analyzed included amino acid substitutions (P30L, I172N, V281L, R356W, exon 6 cluster mutation (L236N, V237Q, M239K)), a splicing mutation (intron 2 (656) A/C to G), and a deletion (exon 3, 8 base pair deletion). Each reaction contained a primer specific for either the common or rare genetic variant in conjunction with a primer that amplified only the CYP21A2 gene and not the pseudogene (Table 1). A PCR based assay for detection of a 30 kb deletion/conversion affecting both C4B and CYP21A2 was performed based upon the method described in Keen-Kim et al.[18].
Table 1.
Sequences of oligonucleotide primers for allele-specific PCR
| CYP21A2 Mutations [ref] | rs # | Primer | 5'-Sequence-3' |
| 30 kb deletion [18] | common forward | gcttcttgatgggtgatcaat | |
| rare forward | tccccaatccttactttttgtc | ||
| reverse | cctcaatcctctgcagcg | ||
| V281L [17] | rs6471 | common reverse | tccactgcagccatgtgcac |
| rare reverse | tccactgcagccatgtgcaa | ||
| forward | gagggatcacatcgtcgtggagatg | ||
| I172N [17] | rs34607927 | common forward | tcctcacctgcagcatcat |
| rare forward | ctctcctcacctgcagcatcaa | ||
| reverse | agctgcatctccacgatgtga | ||
| R356W [17] | common reverse | ctaagggcacaacgggccg | |
| rare reverse | ctaagggcacaacgggcca | ||
| forward | gagggatcacatcgtcgtggagatg | ||
| P30L [17] | common forward | tccggagcctccacctccc | |
| rare forward | tccggagcctccacctcct | ||
| reverse | agctgcatctccacgatgtga | ||
| IN2 (656) A/C to G [17] | common forward (A) | ttcccaccctccagcccccaa | |
| common forward (C) | ttcccaccctccagcccccac | ||
| rare forward | ttcccaccctccagcccccag | ||
| reverse | agctgcatctccacgatgtga | ||
| Ex 3 (8 bp deletion) [17] | common forward | cggacctgtccttgggagactac | |
| rare forward | actacccggacctgtccttggtc | ||
| reverse | agctgcatctccacgatgtga | ||
| Ex 6 cluster | (see reference 17 for further description) | ||
| L236N | common reverse | agctgcatctccacgatgtga | |
| V237Q | rs12530380 | rare reverse | tcagctgcttctcctcgttgtgg |
| M239K | rs6476 | forward | cggacctgtccttgggagactac |
Statistics
Chi-square analysis along with the Fisher's Exact Test where performed using SPSS 14 (SPSS Inc., Chicago, Illinois). A two-tailed test with a P-value of < 0.05 was considered significant after Bonferoni corrections for multiple comparisons.
Results and discussion
Although the 80 autistic individuals studied had 40 C4B null alleles and the 60 control individuals had 10 C4B null alleles only 15 total CYP21A2 mutations were detected in over 2250 genotypes. Eight CYP21A2 mutations were detected in the autistic subjects and 7 in the controls (Table 2). Only one individual (autistic) had a chromosome carrying both a C4B null allele and a CYP21A2 mutation. Therefore, no association was determined between C4B null alleles and CYP21A2 mutations in the study subjects.
Table 2.
Frequencies of the C4B null allele and CYP21A2 mutations
| Autism | Control | P-value | |
| C4B null allele | 40/160 | 10/120 | P = 0.0003 |
| 30 kb deletion/conversion | 1/160 | 2/120 | Not significant (NS) |
| CYP21A2 mutations | |||
| V281L | 3/160 | 1/120 | NS |
| I172N | 1/160 | 2/120 | NS |
| R356W | 1/160 | 0/120 | NS |
| P30L | 2/160 | 1/120 | NS |
| IN2 | 0/160 | 0/120 | NS |
| Ex 3 Del | 0/160 | 1/120 | NS |
| Ex 6 cluster | 0/160 | 0/120 | NS |
Overall, the number of CYP21A2 mutations did not differ between the autism and control groups and no group differences were found in frequencies of individual CYP21A2 mutations. The frequency of CYP21A2 mutations seen in this research is similar to general population frequencies reported by others [19]. Five individuals with autism (6.25%) and five controls (8.33%) carried a CYP21A2 mutation. Two individuals with autism and one control subject carried two or three mutations. In these cases the mutations all typed to individual parents. Thus, the multiple mutations were found on single chromosomes. When a mutation was found in the child's DNA it was also present in DNA from one of the parents, thus confirming both accurate typing and absence of de novo mutations in the study children.
HLA extended haplotyes known to contain C4B null alleles are increased in frequency in autistic individuals (i.e. HLA extended haplotypes 35.2 (n = 4), 35.3 (n = 2), 44.1 (n = 8), and 58.1 (n = 1)). Extended haplotypes 35.3 and 58.1 have monomodular RCCX structures (C4A: C4B null allele) and extended haplotypes 35.2 and 44.1 are bimodular (C4A, C4A:C4B null allele). No CYP21A2 mutations were observed in these haplotypes.
The HLA 8.1 is present in 10% of the Caucasian population and represents the most common extended haplotype. It has a monomodular RCCX structure with a C4A null allele and a normal C4B gene (C4A null allele: C4B). This extended haplotype, referred to as COX in the MHC Haplotype Project [20], has been completely sequenced [21]. Of the eighteen subjects with an 8.1 extended haplotype one had the rare SNP creating the TTG codon that encodes for leucine at position 281 while 17 subjects had the common codon GTG that encodes for valine at position 281. This observation is in agreement with DNA sequencing data that shows low level SNP diversity in 8.1 extended haplotypes from different individuals [22].
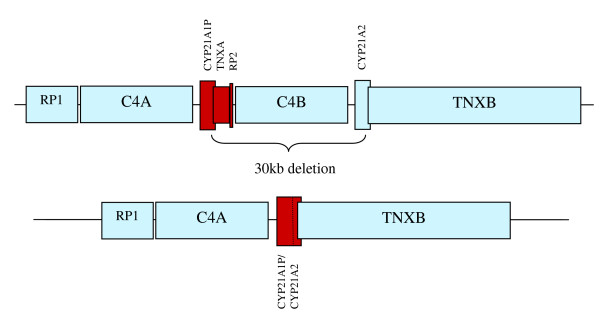
The specific CYP21A2 mutations were chosen because they are the most common mutations that result in significant decreases in 21-hydroxylase activity. One of the mutations investigated was a 30 kb deletion/conversion that deletes C4B and converts CYP21A2 into a nonfunctional CYP21A1P/CYP21A2 hybrid with its pseudogene (Figure 2). This mutation was of particular interest because it provides a direct link between a C4B null allele and a CYP21A2 mutation. One autistic subject and two controls carried this mutation. Again, these three polymorphisms were inherited and did not involve de novo mutations.
Figure 2.
The 30 kb deletion/conversion of C4B and CYP21A2. This diagram depicts the most common arrangement of the RP, C4, CYP21, and TNX (RCCX) gene module. Pseudogenes or gene fragments are red. The 30 kb deletion removes part of CYP21A1P, TNXA, RP2, C4B and part of CYP21A2 leaving a non-protein encoding CYP21A1P/CYP21A2 region.
It has been reported that two of the mutations, P30L and V281L, partially inhibit 21-hydroxylase activity (30–60% of normal), whereas the other mutations analyzed cause either complete or nearly complete enzyme inhibition [23]. Four subjects with autism (5%) carried a partially inhibiting mutation (P30L or V281L) compared to two control subjects (3.33%). This difference is not statistically significant.
21-hydroxylase deficiency is the most common cause of congenital adrenal hyperplasia. Some evidence supports the idea of 21-hydroxylase being involved in autism [24], which could result in the excessive androgen production seen in some cases [25] and thereby contribute to disease etiology [26,27]. The present data does not provide genetic support for 21-hydroxylase involvement in autism.
Conclusion
This study examined both mono and bimodular RCCX genetic modules that contain C4B null alleles for mutations in the adjoining CYP21A2 gene. The C4B null alleles seen in autism are not associated with the CYP21A2 genetic mutations examined in this study. The frequency of CYP21A2 mutations was similar between the autism and control groups. Based on family typings no de novo mutations of C4B or CYP21A2 were apparent in study subjects. Therefore, the CYP21A2 mutations studied do not appear to contribute to the etiology of autism. However, a role for CYP21A2 in autism cannot be ruled out as other factors affecting CYP21A2 gene expression such as promoter polymorphisms or epigenetic variation were not studied and may be relevant [28]. As well, a weak association may be beyond the statistical power of the present study to detect.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
DWO aided in the molecular genetic studies. JDO assigned the C4 typing. ART aided in the study design and assays, coordination the research and helped to draft the manuscript. TLS conceived of the study, carried out the molecular genetic studies, participated in the design, performed the statistical analysis and drafted the manuscript. All authors have read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This research project was supported with funding from The Jonty Foundation and the Center for Persons with Disabilities (Utah State University). We would like to thank Dr. Dave Ward (Nevada Cancer Institute) and Dr. Jonna Westover (CPD) for their suggestions and aid in preparing the manuscript.
Contributor Information
Thayne L Sweeten, Email: thayne@cpd2.usu.edu.
Daniel W Odell, Email: dan@cpd2.usu.edu.
J Dennis Odell, Email: dennis@cpd2.usu.edu.
Anthony R Torres, Email: rtorres@cpd2.usu.edu.
References
- Autism and Developmental Disabilities Monitoring Network Surveillance year 2002 Principal Investigators; Centers for Disease Control and Prevention Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:12–28. [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Jacquemont ML, Sanlaville D, Redon R, Raoul O, Cormier-Daire V, Lyonnet S, Amiel J, Le Merrer M, Heron D, de Blois MC, Prieur M, Vekemans M, Carter NP, Munnich A, Colleaux L, Philippe A. Array-based comparative genomic hybridisation identifies high frequency of cryptic chromosomal rearrangements in patients with syndromic autism spectrum disorders. J Med Genet. 2006;43:843–849. doi: 10.1136/jmg.2006.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chung EK, Zhou B, Lhotta K, Hebert LA, Birmingham DJ, Rovin BH, Yu CY. The intricate role of complement componenet C4 in human systemic lupus erythematosus. In: Tsokos GC, editor. Complement in Autoimmunity. Basel, Karger; 2004. pp. 98–132. [DOI] [PubMed] [Google Scholar]
- White PC, New MI, Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci USA. 1984;81:7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Mendoza AR, Welch TR, Zipf WB, Yu CY. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module) J Biol Chem. 1999;274:12147–12156. doi: 10.1074/jbc.274.17.12147. [DOI] [PubMed] [Google Scholar]
- Blanchong CA, Zhou B, Pupert KL, Chung EK, Jones KN, Sotos JF, William BZ, Rennebohm RM, Yu CY. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) nodules in Caucasians: the load of RCCX genetic diversity on major histocompatibility complex-associated disease. J Exp Med. 2000;191:2183–2196. doi: 10.1084/jem.191.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RP, Singh VK, Cole P, Odell JD, Pingree CB, Warren WL. Increased frequency of the null allele at the complement C4b locus in autism. Clin Exp Immunol. 1991;83:438–440. doi: 10.1111/j.1365-2249.1991.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RP, Singh VK, Averett RE, Odell JD, Maciulis A, Burger RA, Daniels WW, Warren WL. Immunogenetic studies in autism and related disorders. Mol Chem Neuropathol. 1996;28:77–81. doi: 10.1007/BF02815207. [DOI] [PubMed] [Google Scholar]
- Odell D, Maciulis A, Cutler A, Warren L, McMahon W, Coon H, Stubbs G, Henley K, Torres A. Confirmation of the association of the C4B null allele in autism. Hum Immunol. 2005;66:140–145. doi: 10.1016/j.humimm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Carroll MC, Palsdottir A, Belt KT, Porter RR. Deletion of complement C4 and steroid 21-hydroxylase genes in the HLA class III region. EMBO J. 1985;4:2547–2552. doi: 10.1002/j.1460-2075.1985.tb03969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider PM, Carroll MC, Alper CA, Rittner C, Whitehead AS, Yunis EJ, Colten HR. Polymorphism of the human complement C4 and steroid 21-hydroxylase genes: restriction fragment length polymorphisms revealing structural deletions, homoduplications, and size variants. J Clin Invest. 1986;78:650–657. doi: 10.1172/JCI112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. The autism diagnostic observation schedule: A standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- LeCouteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan JD. Autism Diagnostic Interview: A semistructured interview for parents and caregivers of autistic persons. J Autism Dev Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Torres AR, Maciulis A, Stubbs EG, Cutler A, Odell D. The transmission disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism spectrum disorder. Hum Immunol. 2002;63:311–316. doi: 10.1016/S0198-8859(02)00374-9. [DOI] [PubMed] [Google Scholar]
- Dean FB, Hosono S, Fang L, Wu X, Faruqi AF, Bray-Ward P, Sun Z, Zong Q, Du Y, Du J, Driscoll M, Song W, Kingsmore SF, Egholm M, Lasken RS. Comprehensive human genome amplification using multiple displacement amplification. Proc Natl Acad Sci USA. 2002;99:5261–5266. doi: 10.1073/pnas.082089499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Wei J, Cheng KC, Mercado AB, New MI. Rapid deoxyribonucleic acid analysis by allele-specific polymerase chain reaction for detection of mutations in the steroid 21-hydroxylase gene. J Clin Endocrinol Metab. 1995;80:1635–1640. doi: 10.1210/jc.80.5.1635. [DOI] [PubMed] [Google Scholar]
- Keen-Kim D, Redman JB, Alanes RU, Eachus MM, Wilson RC, New MI, Nakamoto JM, Fenwick RG. Validation and clinical application of a locus-specific polymerase chain reaction- and minisequencing-based assay for congenital adrenal hyperplasia (21-hydroxylase deficiency) J Mol Diagn. 2005;7:236–246. doi: 10.1016/S1525-1578(10)60550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner-Parzer SM, Nowotny P, Heinze G, Wäldhausl W, Vierhapper H. Carrier frequency of congenital adrenal hyperplasia (21-hydroxylase deficiency) in a middle European population. J Clin Endocrinol Metab. 2005;90:775–778. doi: 10.1210/jc.2004-1728. [DOI] [PubMed] [Google Scholar]
- The MHC Haplotype Project http://www.sanger.ac.uk/HGP/Chr6/MHC/index.shtml
- Stewart CA, Horton R, Allcock RJ, Ashurst JL, Atrazhev AM, Coggill P, Dunham I, Forbes S, Halls K, Howson JM, Humphray SJ, Hunt S, Mungall AJ, Osoegawa K, Palmer S, Roberts AN, Rogers J, Sims S, Wang Y, Wilming LG, Elliott JF, de Jong PJ, Sawcer S, Todd JA, Trowsdale J, Beck S. Complete MHC haplotype sequencing for common disease gene mapping. Genome Res. 2004;14:1176–1187. doi: 10.1101/gr.2188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WP, Vu Q, Li SS, Hansen JA, Zhao LP, Geraghty DE. Toward understanding MHC disease associations: partial resequencing of 46 distinct HLA haplotypes. Genomics. 2006;87:561–71. doi: 10.1016/j.ygeno.2005.11.020. [DOI] [PubMed] [Google Scholar]
- White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 21:245–291. doi: 10.1210/er.21.3.245. [DOI] [PubMed] [Google Scholar]
- Curin JM, Terzic J, Petkovic ZB, Zekan L, Terzic IM, Susnjara IM. Lower cortisol and higher ACTH levels in individuals with autism. J Autism Dev Disord. 2003;33:443–448. doi: 10.1023/A:1025019030121. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Ferrari P, Sulmont V, Duyme M, Roubertoux P. Androgenic activity in autism. Am J Psychiatry. 1997;154:1626–1627. doi: 10.1176/ajp.154.11.1626-a. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Fane BA, Wheelwright S, Mathews GA, Conway GS, Brook CG, Hines M. Androgens and autistic traits: A study of individuals with congenital adrenal hyperplasia. Horm Behav. 2006;50:148–153. doi: 10.1016/j.yhbeh.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. J Child Psychol Psychiatry. 2005;46:198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Araújo RS, Mendonca BB, Barbosa AS, Lin CJ, Marcondes JA, Billerbeck AE, Bachega TA. Microconversion between CYP21A2 and CYP21A1P promoter regions causes the nonclassical form of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:4028–4034. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]