Abstract
Objective
Converging evidence suggests that the subgenual cingulate (SGC) is implicated in regulation of mood and in the pathophysiology of mood disorders. Our objective was to carry out the first meta-analysis of SGC volumes in patients with mood disorders.
Methods
We reviewed 10 volumetric magnetic resonance imaging studies of SGC volumes in patients with unipolar depression and bipolar disorders. For meta-analysis, we used standardized differences between means (SDMs) and random effects models. In the search for sources of heterogeneity, we subdivided the studies on the basis of diagnosis and presence of family history.
Results
The volumes of left and right SGC in patients with mood disorders were significantly reduced relative to healthy control subjects (SDM –0.38, 95% confidence interval [CI] –0.67 to –0.1 and SDM –0.2, 95% CI –0.4 to –0.007, respectively). There were significant SGC volume reductions in patients with unipolar (left SGC SDM –0.5, 95% CI –0.92 to –0.07; right SGC SDM –0.33, 95% CI –0.64 to –0.02,), but not bipolar, disorder. Patients with a positive family history of mood disorders showed significant left SGC volume decrease (SDM –0.52, 95% CI –0.96 to –0.07), which was not present among subjects without family history of mood disorders. There was no association between age and SGC volumes.
Conclusion
The available evidence suggests the existence of left and less robust right SGC volumetric reductions in patients with mood disorders, predominantly in those with unipolar depression. The effect size of this difference was moderate and increased in more homogeneous subgroups of patients with a positive family history. The clustering of SGC abnormalities in patients with a family history, their presence early in the illness course and their lack of progression with age make SGC a candidate for a primary vulnerability marker, although studies in unaffected high-risk subjects are missing.
Medical subject headings: magnetic resonance imaging, bipolar disorder, depressive disorder, gyrus cinguli
Abstract
Objectif
Des données convergentes indiquent que le cortex cingulaire subgénual (CCS) joue un rôle dans la régulation de l'humeur et dans la pathophysiologie des troubles de l'humeur. Nous voulions procéder à la première méta-analyse du volume du CCS chez des patients atteints de troubles de l'humeur.
Méthodes
Nous avons passé en revue dix études volumétriques fondées sur l'imagerie par résonance magnétique portant sur le volume des CCS chez des patients atteints de dépression unipolaire et de troubles bipolaires. Pour la méta-analyse, nous avons utilisé les différences normalisées entre moyennes (DNM) et des modèles à effets aléatoires. Pour trouver des sources d'hétérogénéité, nous avons subdivisé les études en fonction du diagnostic et de la présence d'antécédents familiaux.
Résultats
Les volumes des CCS gauche et droit chez les patients atteints de troubles de l'humeur avaient diminué considérablement par rapport à ceux de sujets témoins en bonne santé (DNM –0,38 et intervalle de confiance [IC] à 95 %, –0,67 à –0,1, et DNM, –0,2 et IC à 95 %, –0,4 à –0,007, respectivement). On a constaté des réductions importantes du volume des CCS chez les patients atteints de dépression unipolaire (DNM CCS gauche –0,5 et IC à 95 %, –0,92 à –0,07; DNM CCS droit –0,33 et IC à 95 %, –0,64 à –0,02), mais non chez les patients atteints de troubles bipolaires. Chez les patients qui avaient des antécédents familiaux positifs de troubles de l'humeur, on a constaté une augmentation significative du volume du DSS gauche seulement (DNM –0,52, IC à 95 %, –0,96 à –0,07), cette augmentation étant absente chez les patients qui n'avaient pas d'antécédents familiaux de trouble de l'humeur. Il n'y avait aucun lien entre l'âge et le volume des CCS.
Conclusion
Les données disponibles indiquent l'existence de réductions volumétriques du CCS gauche et, moins importantes, du CCS droit, chez les patients qui ont des troubles de l'humeur, et principalement chez ceux qui ont une dépression unipolaire. L'ampleur de l'effet de cette différence était modérée et a augmenté chez les sous-groupes plus homogènes de patients qui avaient des antécédents familiaux positifs. Le regroupement des anomalies du CCS chez les patients qui avaient des antécédents familiaux, leur présence dès le début de l'évolution de la maladie et leur absence d'évolution avec l'âge font du CCS un candidat pour un marqueur de vulnérabilité primaire, même s'il n'y a pas d'études sur des sujets à risque élevé non atteints.
Introduction
The first full gyrus beneath the genu of corpus callosum (subgenual cingulate [SGC]) is an important cortical region. As a part of the ventromedial prefrontal cortex, it receives afferents from the orbitofrontal cortex and sends direct or indirect efferents to subcortical and limbic areas including the hypothalamus, periaqueductal grey, striatum, nucleus accumbens, thalamus, amygdala, hippocampus and entorhinal cortex.1 Studies of healthy subjects suggest that the SGC and parts of the orbitofrontal cortex form a nexus for sensory integration, modulation of autonomic responses and mediation of emotional and reward-related behaviours.2,3 Humans with lesions including the SGC show impaired emotional processing in the absence of marked cognitive impairment.4,5 These mechanisms could play a role in both depression and mania.
Converging lines of evidence suggest that the ventromedial prefrontal cortex and anterior cingulate are indeed implicated in the pathophysiology of mood disorders. Changes in these regions have been detected in structural neuroimaging,6–8 neuropathological postmortem,9–11 functional neuroimaging6,12 and proton magnetic resonance spectroscopy (MRS) studies13–16 of patients with bipolar disorder or unipolar depression. Modulation of the activity of this area was implicated in the resolution of depressive symptoms.17,18 Further, both leucotomy and, recently, deep brain stimulation decrease activity within the SGC and successfully alleviate depression in patients refractory to previous treatments.19
Neuroanatomical studies of the SGC are also important for correct interpretation of functional neuroimaging data. Studies have shown increased perfusion of the SGC after the induction of sadness in healthy subjects.20 Interestingly, patients with unipolar depression and bipolar disorder have been found to have decreased resting-state glucose uptake and perfusion in this region.21 Computer simulations performed to correct the positron emission tomography measures for the effects of a reduced cortical volume concluded that glucose uptake in the remaining SGC tissue is actually abnormally increased in depressive subjects relative to control subjects.22
Studies measuring SGC volumes are remarkable for their high level of methodological consistency in anatomical definitions of region of interests, which is an exception among volumetric magnetic resonance imaging (MRI) studies. Comparability of methods makes studies of the SGC ideal for meta-analysis. Here we review neuroanatomical studies measuring the SGC volume in patients with unipolar depression and bipolar disorder. To address potential sources of heterogeneity, we subdivided the studies by diagnosis and presence of a family history of mood disorders. Our a priori hypotheses were that SGC volumes would be decreased in both bipolar and unipolar patients and that the volumetric reductions would be more prominent in patients with a family history of mood disorders.
Methods
Study ascertainment
Studies were considered for inclusion 1) if they were published by April 30, 2007, 2) if they compared a group of subjects with bipolar disorder or recurrent major depression and a psychiatrically healthy control group, and 3) if they reported volumetric brain measurements of left or right subgenual cingulate. We included studies reporting results from a mixture of unipolar and bipolar patients, even when the results for each diagnosis were not separated. We analyzed the left and right SGC separately because most studies did not provide mean and variance estimates for the whole SGC volume. Further, the functional significance of lesions in the left and right hemisphere differs, and abnormalities lateralized only to 1 hemisphere have also been reported for other pair structures. When a study reported means and standard deviations (SDs) of a structure adjusted for confounds, we used these in the meta-analysis in place of raw means.
We excluded studies 1) if only overall SGC volumes rather than separate results for left and right sides were provided, 2) if grey matter density (voxel-based morphometry) or relative volumes rather than actual volumes were reported, 3) if the structure measurement was an area and not a volume, 4) if noncontiguous slices were used for the measurements, 5) if the mean or SD of SGC volumes could not be extracted from the manuscript, or 6) if tracings included not only subgenual anterior cingulate but also grey matter from adjacent gyri. Two reviewers (T.H. and M.K.) assessed each study to ensure that all inclusion and exclusion criteria were met and that all data were correctly transcribed.
We carried out a systematic search of the MEDLINE, EMBASE and SCOPUS databases for articles published in any language between 1997 (the first report about SGC abnormalities in mood disorders21) and April 30, 2007. The following medical subject headings were used: magnetic resonance imaging and bipolar affective disorder or depressive disorder. We also searched review articles of neuroimaging studies in mood disorders and reference lists of included papers for additional published studies.
Statistical analyses
Meta-analyses were performed with Microsoft Excel 2000 and Comprehensive Meta Analysis, Version 2 (Borenstein et al, Biostat, Englewood, NJ, 2005). As a measure of effect size, we used standardized difference between means (SDM), which is identical to Cohen's d measure of effect size. Because we cannot expect constant population effect size across studies (fixed effects), we decided to use the random effects model, with “study” as the random effect. This assumes that the population of studies has variable true effects that are normally distributed. We calculated effect sizes for the combined as well as separate unipolar and bipolar samples. The joint analyses of bipolar and unipolar subjects maximize statistical power as well as allowing for analyses of change in heterogeneity when diagnostic groups are analyzed separately. Further, SGC volume decrease is a candidate for endophenotype and, as such, may underlie a set of symptoms overlapping in both unipolar depression and bipolar disorders. Third, studies done with young subjects with unipolar depression23,24 cannot rule out the development of manic episodes later in life because depression is the most frequent initial manifestation of bipolar disorders. Last but not least, some studies analyzed a mixed sample of subjects with unipolar depression and bipolar disorder.7,23
We calculated I2, to provide an easily interpretable measure of consistency between studies.25 The I2 is an estimate of the percentage of total variation across studies that is due to true heterogeneity rather than chance. I2 is placed between 0% and 100%. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity.
We used Egger's regression test to measure funnel plot asymmetry and examine a risk of publication bias.26 To test for association between age versus SDM and year of publication versus SDM, we performed metaregression, using nonparametric rank correlation (Kendall τ). We used χ2 to test differences in the proportion of female subjects between studies.
We adopted a significance level of p = 0.05, 2-tailed for all above-mentioned analyses. We also tested whether the SGC volume reductions would remain significant in replication studies, that is, after exclusion of the first published positive study.21 For this unidirectional hypothesis, we used a significance level of p = 0.05, 1-tailed.
Finally, we calculated the proportions of studies with at least 1 statistically significant finding. This allowed us to include 2 studies that did not separate left and right SGC volumes and were therefore excluded from the meta-analyses.
Results
Results of the systematic search
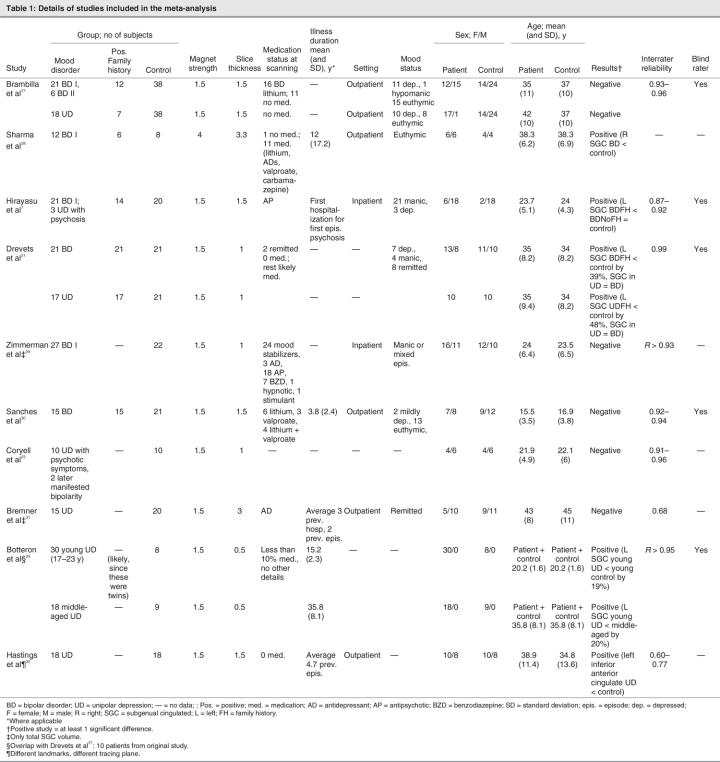
Out of 785 studies initially found by the systematic search, 115 focused on the anterior cingulate, using various neuroimaging techniques: 17 used voxel-based morphometry, 44 used functional MRI, 10 used MRS, 10 used another MRI application and 13 used positron emission tomography or single photon emission computer tomography coregistration, while 8 were review papers and 13 performed region of interest volumetric analyses of the anterior cingulate, with 10 specifically measuring SGC volumes7,21,23,24,27–32 (Table 1). Of these latter studies, 2 contained information only on total SGC volumes.29,31 Therefore, we included 8 studies with 210 patients (99 with bipolar disorder and 111 with unipolar depression) in this meta-analysis. All studies used DSM-III-R33 or DSM-IV34 diagnostic criteria. Most investigated patients were treated at the time of scanning with mood stabilizers, antidepressants, antipsychotics or their combinations. In the studies by Brambilla and colleagues27 and Hastings and colleagues,32 the groups with unipolar disorder were untreated, and only 10% of patients in the study by Botteron and colleagues24 received medication at the time of scanning. Five studies provided separate results for patients with a family history of mood disorders.7,21,27,28,30 Most studies included both men and women, with the exception of a purely female sample in a study by Botteron and colleagues.24 There were significant between-study differences in the proportions of male and female patients (χ29 = 24.91, p < 0.01). Two studies included data from a mixture of patients with bipolar disorder and unipolar depression that had not been separated for analysis. One study included 21 patients with bipolar I disorder and 3 patients with unipolar depression with psychotic symptoms.7 We analyzed this study together with other studies of bipolar disorder patients. The second study included 10 patients with unipolar disorder and psychosis, 2 of whom later developed bipolarity,23 and we analyzed this study together with studies of unipolar depression patients. With the exception of 2 studies with inpatients,7,29 all the studies included outpatients. Slice thickness, a potential source of heterogeneity, ranged from 0.5 to 3.3 mm. Except for 1 study using a 4T magnet,28 all remaining investigations used 1.5T magnets. All studies except 1 used the same anatomical definitions of SGC.21 The means in studies using the same landmarks ranged from 118 mm to 445 mm3. A study by Hastings and colleagues32 used a slightly different method (tracings predominantly in the axial plane) and different anatomical landmarks (posterior boundary defined by posterior surface of the genu of corpus callosum rather than anteriormost appearance of internal capsule). The means in this study ranged between 636 mm3 and 827 mm3.
Table 1
Results of the meta-analysis
Combined groups
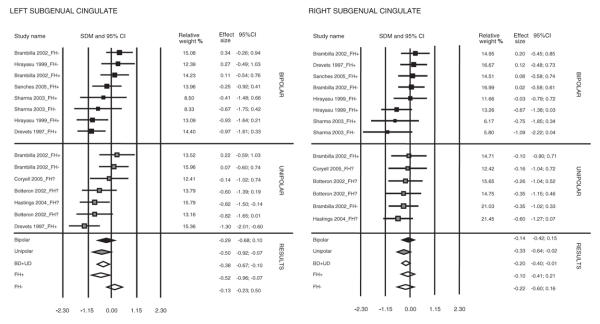
Both the left and right SGC volumes were significantly smaller in patients with mood disorders versus control subjects (SDM –0.38, standard error [SE] 0.145, 95% confidence interval [CI] –0.67 to –0.1, z = –2.65, p = 0.008; SDM –0.2, SE 0.1, 95% CI –0.4 to –0.007, z = –2.03, p = 0.04, respectively) (Fig. 1). Excluding the single study with differing anatomical landmarks32 resulted in significant SGC volume reduction in the left, but not the right, SGC volumes in patients versus control subjects (SDM –0.35, SE 0.15, 95% CI –0.65 to –0.05, z = –2.3, p = 0.02; SDM –0.17, SE 0.11, 95% CI –0.37 to 0.04, z = –1.58, p = 0.11, respectively). There continued to be a significant left and right SGC volume reduction in the studies remaining after exclusion of the first positive study,21 (SDM –0.24, SE 0.13, 95% CI –0.50 to 0.02, z = –1.85, p = 0.03, 1-tailed; SDM –0.24, SE 0.11, 95% CI –0.45 to –0.03, z = –2.28, p = 0.01, 1-tailed).
Fig. 1: Left and right subgenual cingulate SDMs, 95% CIs and relative weights for studies included in the meta-analyses. Results are presented for combined groups, unipolar, bipolar, family history positive and family history negative subjects. SDM = standard difference between means; CI = confidence interval; FH- = family history negative; FH+ = family history positive; FH? = family history status not provided; BD = bipolar disorder; UD = unipolar depression.
In categorical terms, including also the 2 studies that reported only the whole SGC volumes, a significant difference between patients and control subjects was detected in 5/10 studies.
There was a moderate heterogeneity among studies for the left SGC (I2 = 53.46%) but not for the right SGC (I2 = 0%). The heterogeneity for the left SGC among studies was I2 = 54.03% after exclusion of the single methodologically different study.32 In a search for the sources of heterogeneity, we subdivided the studies according to diagnosis and family history.
Groups divided by diagnosis
Of the entire sample, 99 patients were diagnosed with bipolar disorder and 111 with unipolar depression. Both the left and right SGC volumes were significantly smaller in unipolar depression patients versus healthy control subjects (SDM –0.5, SE 0.22, 95% CI –0.92 to –0.07, z = –2.3, p = 0.02; SDM –0.33, SE 0.16, 95% CI –0.64 to –0.02, z = –2.07, p = 0.04, respectively), even after exclusion of the first positive study21 (left SGC SDM –0.35, SE 0.19, 95% CI –0.73 to –0.02, z = –1.84, p = 0.03, 1-tailed; right SGC SDM –0.33, SE 0.16, 95% CI –0.64 to –0.02, z = –2.07, p = 0.04, 1-tailed). Excluding the single study with differing anatomical landmarks resulted in a trend for left SGC volume decrease among unipolar depression patients, with no significant difference between unipolar patients and healthy control subjects in right SGC volume (SDM –0.44, SE 0.25, 95% CI –0.93 to 0.06, z = –1.74, p = 0.08; SDM –0.25, SE 0.18, 95% CI –0.60 to 0.1, z = –1.41, p = 0.16, respectively).
There were no differences in volumes of the right or left SGC between bipolar disorder patients and healthy control subjects even after exclusion of the first positive study.21
In categorical terms, a significant difference between patients and comparison subjects was found in 3/6 studies of subjects with bipolar disorder and 3/6 studies of subjects with unipolar depression.
Subdivision by diagnosis did not markedly change the heterogeneity among left SGC findings (bipolar I2 = 54.91%, unipolar I2 = 53.8%, unipolar without methodologically different study32 I2 = 58.37%).
Groups divided by family history
Ninety-two patients (24 unipolar and 68 bipolar) in 5 studies had a family history of mood disorders . Patients with a family history of mood disorders had a significantly smaller left SGC than healthy control subjects (SDM –0.52, SE 0.22, 95% CI –0.96 to –0.07, z = –2.28, p = 0.02), without statistically significant differences in the right SGC volumes. For both left and right SGC, excluding the first positive study resulted in nonsignificant differences between patients with a positive family history and control subjects.
Forty-two patients were without a family history of mood disorders (11 unipolar and 31 bipolar). There were no differences in right or left SGC between patients with mood disorders without a family history and healthy control subjects.
In categorical terms, 3/5 studies of familial patients reported significant volume decrements in patients versus control subjects. None of the 3 studies of patients without a family history found significant differences between the groups.
Selecting subjects on the basis of a positive family history increased the heterogeneity among the left SGC findings (I2 = 61.1%). Subdivision by the absence of a family history markedly decreased the heterogeneity among the left SGC findings (I2 = 0%).
Separate analysis of 5 studies comprising only patients with bipolar disorder and a positive family history (n = 68) yielded a significant left SGC volume reduction (SDM –0.49, SE 0.22, 95% CI –0.93 to –0.05, z = –2.18, p = 0.03) in the absence of right SGC volumetric abnormalities. Excluding the first positive study yielded only a trend for SGC volume decrease (SDM –0.34, SE 0.23, 95% CI –0.80 to 0.11, z = –1.47, p = 0.07, 1-tailed). Three studies of 31 patients with bipolar disorder without a family history showed no significant differences in right or left SGC volumes between patients and control subjects. Two studies assessed only unipolar familial subjects. The low number of studies did not warrant separate analyses.
Metaregression
There was no statistically significant association between age or year of publication and SDMs for either the left or right SGC.
Publication bias
There was no evidence for publication bias in any of the meta-analyses showing significant between-group differences (e.g., left SGC volumes in the whole sample, left and right SGC volumes in patients with unipolar depression, left SGC volumes in patients with familial bipolar disorder). The Egger regression intercept suggested evidence for funnel plot asymmetry for right SGC volumes in the overall sample, with smaller studies showing more pronounced right SGC decreases (Intercept –3, SE 1.18, t12 = 2.56, p = 0.01).
Discussion
The results of this study demonstrate that left and right subgenual cingulate volumes are decreased in patients with mood disorders. These findings do not seem to be due to type I error because significant SGC volume decreases were independently replicated by 4 research groups. Further, structural abnormalities in the SGC have also been reported in postmortem9–11 and voxel-based morphometry studies35 of patients with mood disorders. The results did not markedly change, with exception of analyses of patients with a family history, after exclusion of the first positive study, which also reported the largest effect sizes. Also corroborating the strength of these findings is the lack of association between SDM and year of publication.
In keeping with our a priori hypotheses, the SGC volumetric abnormalities were predominantly manifested among familial patients, whereas all comparisons among patients without a family history of mood disorders were negative. The significant differences among familial patients were largely driven by the seminal study of Drevets and colleagues21; after exclusion of this study, they became nonsignificant, even though 4/5 studies among familial patients reported SGC volumetric reductions. Clustering of findings among familial subjects suggests that SGC volumetric abnormalities might be associated with genetic risk. In keeping with this, SGC volume decreases were already found early in the illness course (first-episode, early-onset unipolar depression). Further, SGC abnormalities do not seem to be secondary to burden of illness because no studies found correlations between the SGC volumes and number of previous episodes, age at first episode or length of illness. Similarly, none of the 2 studies with a prospective arm21,23 found changes in the SGC during intervals of up to 8 years between scans. Clustering of SGC abnormalities among patients with a genetic liability to mood disorders, their presence early in the course of illness and lack of worsening with progression of the disease make SGC abnormalities a candidate for a vulnerability marker. Studies of unaffected relatives of patients with mood disorders would be necessary to corroborate this hypothesis.
There is more robust evidence for the left-sided SGC volume abnormalities, as suggested by the larger (albeit nonsignificantly so) effect sizes for the left side, compared with the right side, in all analyses; the decrease only in left, not right, SGC volumes in some groups of subjects (familial patients); and the fact that, with 1 exception, all positive studies reported a significant decrease in left SGC only. The more robust left volumetric decrease is unlikely to be due to differences in laterality between subjects because most of the study participants were right-handed. The overall right SGC volume decrease is interesting; only 1 study reported significantly smaller right SGC volume in patients compared with control subjects. However most of the available comparisons found some level of nonsignificant right SGC volume decreases in patients compared with control subjects. The findings for the right SGC volume need to be interpreted with caution because there was evidence for funnel plot asymmetry, with more pronounced right SGC decreases found in smaller precision studies.
It is a question whether more robust left volumetric decreases have any pathophysiological significance. It has been hypothesized that left frontal regions mediate approach behaviours and positive affects.36 Lesions of the left frontal lobes most often lead to depression.37 Left SGC abnormalities thus might underlie a propensity for depression. Indeed, the effect size increased in studies of unipolar patients (–0.50) and decreased to nonsignificant in studies of bipolar patients (–0.29), relative to the overall sample (–0.38). We cannot directly compare SGC volumes among unipolar and bipolar patients because only 2 studies evaluated both of these diagnostic categories. Drevets and colleagues found decreased SGC volumes in both unipolar depression and bipolar disorder patients.21 Conversely, Brambilla and colleagues found no differences in subjects with either bipolar disorder or unipolar depression.27 Notably, however, the same proportions (50%) of studies found SGC abnormalities in subjects with either unipolar depression or bipolar disorder.
Absence of overall differences in the presence of abnormalities in individual studies may be due to the dilution of overall results by heterogeneity. Indeed, there was a moderate heterogeneity among studies for the left SGC measurements. In searching for sources of this heterogeneity, we considered methodological, demographic and clinical variables.
The heterogeneity was not due to differences in MRI volumetry because the methods used for SGC tracing were remarkably similar. All but 1 study32 used the same anatomical definitions and traced the SGC on the coronal plane. Exclusion of the 1 methodologically slightly different study did not change the heterogeneity. Slice thickness was also unlikely to play a role because all but 1 study used 1.5-mm and thinner slices. A single study28 used a suboptimal slice thickness of 3.3 mm; this was, however, partially offset by the use of a 4T magnet and a grey–white matter contrast-enhancing algorithm. The MRI protocols in all studies allowed for complete coverage of the SGC with contiguous slices. Interrater reliabilities (IR) of studies included in the meta-analysis were sufficient, with the exception of 2 positive studies — the first28 did not include information about IR, and the second32 had an IR of 0.6 and 0.77 for the right and left SGC, respectively. It is of concern that 2 positive28,32 and 2 negative23,31 studies did not specifically state that all volumetric measures had been done blinded to the diagnostic status of patients.
With regard to demographic factors, age was unlikely to play a role because metaregression showed no association between standardized differences in volumes and age. In keeping with this finding, Hirayasu and colleagues7 found significant differences in SGC volumes already present in first-episode subjects at the relatively low age of 23.4 years. Another study of unipolar depression patients found a similar extent of abnormalities in both young (mean age 20.2, SD 1.6 y) and middle aged (mean age 35.8, SD 8.1 y) subjects, without a correlation between the extent of SGC changes and age in the range of 17–55 years.24 Further, similar proportions of positive and negative studies assessed patients with an average age of less than 25 years (3/5 negative studies and 2/4 positive studies). Notably, however, the only study performed in pediatric subjects with bipolar disorder was negative.30
Sex might have contributed to differences between studies because there were significant differences in the proportions of female subjects in the studies. One study found abnormalities only in men but not women.32 Another study, however, found SGC abnormalities in a purely female sample.24 To complicate matters further, a third small study found a trend for interaction between sex and family history.28
With regard to clinical sources of variation, subdividing the studies according to family history or diagnosis did not significantly decrease the heterogeneity. Out of 5 negative studies, 4 assessed patients treated on an outpatient basis at the time of scanning. The differences among the studies, however, cannot be readily attributed to the severity of illness. In 2 of the negative studies, all or most of the patients had a history of previous hospitalizations,31 some for depression with symptoms of psychosis.23 Further, 1 negative study used currently hospitalized patients,29 and 1 positive study used never-hospitalized outpatients.24 Medication status was also unlikely to play a role because significant changes in SGC volumes between patients and control subjects were found in unmedicated as well as in medicated subjects. Both medicated and unmedicated patients were also present in negative studies. Further, no SGC differences were noted between patients with or without current or lifetime treatment with antipsychotics, benzodiazepines or lithium.27,29,30
None of the variables available for systematic study (diagnostic category, presence of family history) markedly reduced the heterogeneity in left SGC values detected in this meta-analysis. There seem to be unknown confounding variables that contribute to differences between studies. One such confounder could be the proportion of depressive and manic episodes. As discussed above, SGC volumetric abnormalities may be a hallmark of depression. High heterogeneity and smaller effect sizes in bipolar disorder patients relative to unipolar depression patients might thus be due to the presence of bipolar disorder patients with a preponderance of manic episodes. Alternatively, medial prefrontal cortex and anterior cingulate contain high levels of glucocorticoid receptors and have been implicated in regulation of hypothalamic-pituitary-adrenal (HPA) axis activity.38–41 Differences in HPA axis reactivity or regulation between patient populations might underlie some of the inconsistencies among studies. It would be interesting to study SGC abnormalities together with measures of HPA axis activity or responsiveness.
Several important questions cannot be answered from the available data. It is not clear whether SGC abnormalities are specific to mood disorders. Direct comparisons found no abnormalities in schizophrenia patients in the presence of abnormalities among patients with mood disorders.7 In keeping with this, 1 histopathological study found abnormalities in familial unipolar depression and bipolar disorder patients but not in patients with schizophrenia.9 Conversely, 2 postmortem studies reported neuronal density decreases both in patients with bipolar disorders and in patients with schizophrenia,10,11 in the absence of abnormalities among unipolar depression patients in 1 of these studies.11 It is also not clear whether volumetric changes in mood disorders are specific to subgenual portions of anterior cingulate. Volumetric abnormalities in patients with mood disorders have been found in neighbouring areas within the anterior cingulate (Brodmann's areas 32 and 25)42,43 but also in adjacent cortical regions (gyrus rectus, orbitofrontal cortex), even in the absence of SGC abnormalities.23,31 One study measuring the subgenual portion of the anterior cingulate together with adjacent cortical regions (Brodmann's areas 33 and 32) failed to find any abnormalities in patients with unipolar depression.44 Future studies should attempt to address the specificity of abnormalities to subgenual portions of the anterior cingulate. Last but not least, the mechanisms underlying the volumetric reductions are not clear. The evidence from histopathological studies is conflicting, with some studies reporting predominantly neuronal abnormalities in the absence of glial pathology10,11 and other studies finding only glial pathology in the absence of neuronal changes.9
This study has several limitations. Meta-analysis depends on the quality of the primary data, control for known confounding variables and comparability of methods among studies. The methodological comparability with regard to MRI and diagnostic methods was relatively good. The presence of marked heterogeneity suggests the existence of currently unknown confounders. Another limitation of this meta-analysis might be the relatively small number of studies. With 8 studies and 210 subjects, we were, however, clearly above the cut-off of 3 studies with at least 50 subjects set up in previous meta-analysis.45 Meta-analytical techniques could be distorted by preferential publication of positive findings, but we found no evidence of publication bias in the reviewed studies, with the exception of the overall right-SGC differences. However, the capacity of tests to detect publication bias is limited when there are few trials.
Overall, the available evidence suggests the existence of left, and also less robust right, SGC volumetric reductions in patients with mood disorders. The effect size of this difference is moderate and increases when more homogeneous subgroups, in particular patients with familial mood disorders, are studied. The clustering of SGC abnormalities among patients with a family history, their presence early in the course of illness and their lack of worsening with age or progression of the disease makes the SGC a candidate for a primary vulnerability marker, although studies in unaffected high-risk subjects are missing. Significant SGC volume decreases among unipolar depression patients in the absence of an overall difference in bipolar disorder patients, together with more robust left-side changes, may suggest that SGC volumetric reduction is related to depression. Future studies addressing this issue, as well as studies measuring SGC volumes in unaffected offspring of parents with mood disorders, are needed.
Acknowledgments
This study was supported by grant NR8786, Internal Grant Agency of Ministry of Health, Czech Republic, and by the NARSAD Young Investigator Award to Dr. Hajek.
Footnotes
Contributors: Drs. Hajek, Kozeny and Höschl designed the study. Drs. Hajek and Kopecek acquired the data, which Drs. Hajek, Kozeny and Alda analyzed. Dr. Hajek wrote the article, and all authors revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. T. Hajek, Department of Psychiatry, Dalhousie University, QEII HSC, A.J. Lane Bldg., Rm. 3093, 5909 Veteran's Memorial Lane, Halifax NS B3H 2E2; fax 902 473-1583; tomas.hajek@dal.ca
References
- 1.Price JL. Prefrontal cortical networks related to visceral function and mood. Ann N Y Acad Sci 1999;877:383-96. [DOI] [PubMed]
- 2.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005;6:691-702. [DOI] [PubMed]
- 3.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005;6:533-44. [DOI] [PMC free article] [PubMed]
- 4.Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res 1990;41:81-94. [DOI] [PubMed]
- 5.Bechara A, Tranel D, Damasio H, et al. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex 1996;6:215-25. [DOI] [PubMed]
- 6.Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry 1998;3:220-1. [DOI] [PubMed]
- 7.Hirayasu Y, Shenton ME, Salisbury DF, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry 1999; 156:1091-3. [DOI] [PMC free article] [PubMed]
- 8.Wilke M, Kowatch RA, DelBello MP, et al. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res 2004;131:57-69. [DOI] [PubMed]
- 9.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A 1998; 95: 13290-5. [DOI] [PMC free article] [PubMed]
- 10.Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry 2001;50:395-406. [DOI] [PubMed]
- 11.Bouras C, Kovari E, Hof PR, et al. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol 2001;102: 373-9. [DOI] [PubMed]
- 12.Kruger S, Alda M, Young LT, et al. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry 2006;163:257-64. [DOI] [PubMed]
- 13.Cecil KM, DelBello MP, Morey R, et al. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord 2002;4:357-65. [DOI] [PubMed]
- 14.Davanzo P, Yue K, Thomas MA, et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry 2003;160:1442-52. [DOI] [PubMed]
- 15.Davanzo P, Thomas MA, Yue K, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology 2001;24:359-69. [DOI] [PubMed]
- 16.Moore CM, Breeze JL, Gruber SA, et al. Choline, myo-inositol and mood in bipolar disorder: a proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disord 2000;2: 207-16. [DOI] [PubMed]
- 17.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000;48:830-43. [DOI] [PubMed]
- 18.Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004;61:34-41. [DOI] [PubMed]
- 19.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005;45:651-60. [DOI] [PubMed]
- 20.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999;156:675-82. [DOI] [PubMed]
- 21.Drevets WC, Price JL, Simpson JR Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997;386:824-7. [DOI] [PubMed]
- 22.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 1999;877:614-37. [DOI] [PubMed]
- 23.Coryell W, Nopoulos P, Drevets W, et al. Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry 2005;162:1706-12. [DOI] [PubMed]
- 24.Botteron KN, Raichle ME, Drevets WC, et al. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 2002;51:342-4. [DOI] [PubMed]
- 25.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed]
- 26.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed]
- 27.Brambilla P, Nicoletti MA, Harenski K, et al. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology 2002;27:792-9. [DOI] [PubMed]
- 28.Sharma V, Menon R, Carr TJ, et al. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. J Affect Disord 2003;77:167-71. [DOI] [PubMed]
- 29.Zimmerman ME, DelBello MP, Getz GE, et al. Anterior cingulate subregion volumes and executive function in bipolar disorder. Bipolar Disord 2006;8:281-8. [DOI] [PubMed]
- 30.Sanches M, Sassi RB, Axelson D, et al. Subgenual prefrontal cortex of child and adolescent bipolar patients: a morphometric magnetic resonance imaging study. Psychiatry Res 2005;138:43-9. [DOI] [PubMed]
- 31.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry 2002;51: 273-9. [DOI] [PubMed]
- 32.Hastings RS, Parsey RV, Oquendo MA, et al. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology 2004;29:952-9. [DOI] [PubMed]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed., revised. Washington: The Association; 1987.
- 34.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 35.Pizzagalli DA, Oakes TR, Fox AS et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry 2004;9:325, 393-405. [DOI] [PubMed]
- 36.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 1999;3:11-21. [DOI] [PubMed]
- 37.Starkstein SE, Robinson RG, Price TR. Comparison of cortical and subcortical lesions in the production of poststroke mood disorders. Brain 1987;110:1045-59. [DOI] [PubMed]
- 38.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 1993;13:3839-47. [DOI] [PMC free article] [PubMed]
- 39.Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci 1999;19:2834-40. [DOI] [PMC free article] [PubMed]
- 40.Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res 1985;328:176-80. [DOI] [PubMed]
- 41.Wolf OT, Convit A, de Leon MJ, et al. Basal hypothalamo-pituitary-adrenal axis activity and corticotropin feedback in young and older men: relationships to magnetic resonance imaging-derived hippocampus and cingulate gyrus volumes. Neuroendocrinology 2002;75:241-9. [DOI] [PubMed]
- 42.Kaur S, Sassi RB, Axelson D, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry 2005; 162:1637-43. [DOI] [PubMed]
- 43.Caetano SC, Kaur S, Brambilla P, et al. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry 2006;59:702-6. [DOI] [PubMed]
- 44.Kegeles LS, Malone KM, Slifstein M, et al. Response of cortical metabolic deficits to serotonergic challenge in familial mood disorders. Am J Psychiatry 2003;160:76-82. [DOI] [PubMed]
- 45.McDonald C, Zanelli J, Rabe-Hesketh S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 2004;56:411-7. [DOI] [PubMed]