Abstract
Objective
To index the extent to which treatment response in posttraumatic stress disorder (PTSD) is predicted by rostral anterior cingulate cortex (rACC) volume.
Method
We used structural magnetic resonance imaging in a 1.5 T scanner to examine subjects with PTSD (n = 13), traumatized control subjects (n = 13) and nontraumatized control subjects (n = 13). Subjects with PTSD then participated in 8 sessions of cognitive-behavioural therapy, after which we reassessed them for PTSD.
Results
According to voxel-based morphometry, treatment responders had larger rACC volume than nonresponders. Further, symptom reduction was associated with larger rACC volume.
Conclusion
Consistent with evidence for the neural bases of extinction learning, PTSD patients with larger rACC volume may be better able to regulate fear during cognitive-behavioural therapy and thus achieve greater treatment gains.
Medical subject headings: stress disorders, post-traumatic; magnetic resonance imaging; cognitive therapy
Abstract
Objectif
Indexer la mesure dans laquelle la réponse au traitement du trouble du stress posttraumatique (TSPT) est prédite par le volume du cortex cingulaire antérieur rostral (CCAr).
Méthode
Nous avons utilisé l'imagerie par résonance magnétique structurelle dans un appareil de 1,5 T pour examiner des sujets atteints de TSPT (n = 13), des sujets témoins traumatisés (n = 13) et des sujets témoins non traumatisés (n = 13). Les sujets atteints de TSPT ont participé ensuite à huit séances de thérapie cognitivocomportementale à la suite desquelles nous avons réévalué leur TSPT.
Résultats
Selon la morphométrie voxel, les sujets qui ont répondu au traitement avaient un CCAr plus volumineux que ceux qui n'ont pas réagi. De plus, on a associé la réduction des symptômes à un CCAr plus volumineux.
Conclusion
Conformément aux données probantes relatives au fondements neuraux de l'apprentissage par extinction, les patients atteints de TSPT qui ont un CCAr plus volumineux sont peut-être mieux en mesure de contrôler la peur pendant une thérapie cognitivocomportementale et de tirer ainsi de plus grands avantages du traitement.
Introduction
There is an outstanding need to understand the neural mechanisms underpinning treatment response in patients with posttraumatic stress disorder (PTSD). Although cognitive-behavioural therapy (CBT) is the treatment of choice for PTSD, many patients do not benefit from this therapy.1 Understanding neural factors associated with successful response to CBT may lead to better treatments. CBT involves repeated exposure to trauma reminders or memories until the patient masters his or her anxiety.2 CBT maybe a form of extinction learning in which conditioned fear responses are inhibited by new learning of safe associations,3 and common neural mechanisms may underpin both extinction learning and response to CBT.4
Animal5–7 and human8,9 studies have found that the ventromedial prefrontal cortex (vmPFC) is implicated in extinction learning. Further, cortical thickness of the vmPFC is positively correlated with extinction learning in humans.10 These findings accord with models positing that PTSD is maintained by elevated amygdala reactivity that is inadequately regulated by vmPFC.11 This model is supported by evidence that PTSD patients display reduced activation of the rostral anterior cingulate cortex (rACC) during fear processing.12–15 A range of studies have investigated the volume of the ACC in PTSD patients,16,17 and meta-analysis of these studies indicates that PTSD patients have smaller ACC volume than trauma-exposed people without PTSD.18
Using structural magnetic resonance imaging (SMRI), 2 studies have investigated whether successful treatment of PTSD will reverse the documented pattern of small hippocampal volume; whereas brief psychotherapy did not alter hippocampal volume,19 long-term use of paroxetine did increase hippocampal volume.20 Consistent with extinction models of PTSD, a recent functional MRI study found that successful response to CBT for PTSD was associated with increased ACC activation during viewing of fearful faces assessed before and after treatment.21 To date, no studies have investigated the capacity of brain structure to predict treatment response in PTSD. We hypothesized that positive response to CBT would be associated with larger rACC volume because this region is associated with extinction learning in humans, impaired in PTSD patients during fear processing and central to prevailing models of PTSD recovery.
Method
Participants
The study comprised 13 patients diagnosed with PTSD, 13 traumatized control subjects and 13 nontraumatized control subjects, who participated in collaboration with the Brain Resource International Database (BRID). Diagnoses of PTSD were made by clinical psychologists using the Clinician Administered PTSD Scale (CAPS).22 Participants with PTSD included survivors of motor vehicle collisions (n = 6) and assault (n = 7); the mean time elapsed since trauma was 75.7 (standard deviation [SD] 86.5) months, and the mean CAPS total score was 75.2 (SD 20.1). Participants with PTSD were recruited from the Traumatic Stress Clinic, Westmead Hospital, and trauma-exposed and nontraumatized control subjects were recruited from community centres. Trauma-exposed and nontrauma-exposed control subjects were screened for psychiatric morbidity by the SPHERE screening tool.23 Trauma-exposed control subjects had experienced a criterion A stressor but had not developed PTSD in their lifetime (meeting no more than 1 lifetime PTSD cluster criterion). Nontraumatized control subjects had never experienced a criterion A stressor and had no psychiatric history. Exclusion criteria included a history of psychosis or substance abuse, brain injury, neurologic disorder or serious medical conditions related to thyroid or heart. Comorbidity in the PTSD population was diagnosed with the Structured Clinical Interview for DSM-IV.24 Of the PTSD patients, 5 had comorbid depression, 1 had comorbid panic disorder and 5 were taking SSRI medications for depression. Participants also completed the Depression Anxiety Stress Scale.25 Written informed consent was obtained and ethical approval was obtained from the Sydney West Area Health Service Human Ethics Board.
Treatment
Participants received 8 once-weekly sessions of CBT in a randomized controlled trial.26 All participants in this study received education, imaginal exposure, in vivo exposure, cognitive restructuring and relapse prevention. At the completion of treatment, patients were reassessed for PTSD by independent clinical psychologists using the CAPS-2 .
MRI acquisition
Before treatment, all subjects underwent a single T1-weighted volumetric magnetization prepared rapid gradient echo SMRI scan on a Siemens 1.5 Tesla Vision Plus system (Siemens, Erlangen, Germany) at Westmead Hospital, Sydney, Australia. Images were obtained in the sagittal plane, with scan parameters as follows: repetition time = 9.7 milliseconds, echo time = 4 milliseconds, inversion time = 200 milliseconds, flip angle = 12°. We acquired a total of 180 contiguous 1-mm slices with a 256 × 256 matrix with an in-plane resolution of 1 × 1 mm, resulting in isotropic voxels.
Image preprocessing
Images were processed with the use of SPM2 (Wellcome Department of Cognitive Neurology, London, UK) running on MATLAB 6.5 (MathWorks, Natick, Mass.). Details of the voxel-based morphometry (VBM) protocol are presented elsewhere.27 Brain images were spatially normalized by transforming each brain into a standardized stereotactic space based on the International Consortium for Brain Mapping 152 template (Montreal Neurological Institute [MNI]). A custom T1-image template with 333 brain images from the BRID was used. The first step in spatial normalization involved estimating the optimum 12-parameter affine transformation (3 translations, 3 rotations, 3 zooms and 3 shears) for matching the participant's image to the template. The second step accounted for global nonlinear shape differences, which were modelled by a linear combination (7 × 8 × 7) of smooth spatial basis functions.28 The normalized images were resliced into 1.5-mm3 voxels, segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) probability maps, stripped of extracerebral voxels, modulated with the Jacobian determinants from the spatial normalization and smoothed with a 12-mm Gaussian kernel.
Statistical analysis
A voxel-wise analysis of covariance (ANCOVA) was employed in SPM2 to determine whether there were group (PTSD responders v. nonresponders v. trauma-exposed control subjects) volumetric GM differences in the ACC gyrus (as defined by the automatic anatomical labelling mask).29 We included the entire ACC to determine whether regions directly associated with the rACC were implicated in treatment response. Following from our a priori hypotheses, planned contrasts were then conducted between PTSD treatment responders and treatment nonresponders and between each of these groups and trauma-exposed control subjects. In line with previous studies of the ACC in PTSD,30 the rACC was defined as the portion of the ACC that lies anterior and superior to the genu of the corpus callosum. The posterior boundary was operationally defined as y = +30 mm.31 The dorsal ACC was defined as ACC superior to the corpus callosum between y = 0 mm and +30 mm.32 The ventral ACC was defined as cingulate gyrus inferior to the corpus callosum genu, and operationally defined as ACC below z = 0.31 Depression score and global GM volume were statistically controlled for in the analysis. Output was in the form of statistical parametric maps (SPMs). Given the small number of subjects, we statistically corrected (using Gaussian random fields theory33) for the total number of GM voxels in the brain, and the criterion for significance was set to p < 0.05 (the “height threshold”) with an extent threshold of 5 contiguous significant voxels. As a result of the Jacobian modulation in the preprocessing, these analyses can be considered as tests of voxel-level GM volume.34 All suprathreshold voxels were reported in terms of MNI coordinates.
To investigate the relation between patients' treatment outcome and voxel-wise GM volume in the ACC gyrus, we performed linear regression analyses at each GM voxel in the ACC. GM volume of voxels in the ACC gyrus was entered as the predictor variable, and change in total CAPS score from pre-to posttreatment was the dependant variable. Depression score and global GM volume were entered as covariates; a height threshold of pcorrected < 0.05 and an extent threshold of 5 contiguous voxels was employed. We conducted confirmatory correlational analyses in SPSS by deriving modulated voxel values of GM probability maps from SPM and correlating these with change in PTSD severity from pre-to posttreatment. To control for initial severity of pretreatment PTSD symptoms, we calculated residual change in PTSD severity from a regression of pretreatment total CAPS scores on posttreatment total CAPS scores. These residual values were then correlated in SPSS with modulated voxel values of GM probability maps.
Results
Clinical data
Treatment responders (n = 7) did not meet PTSD criteria after treatment and nonresponders (n = 6) did meet PTSD criteria. Groups did not differ in age or sex (age, F3,35 = 0.51, p > 0.05; sex, χ2 = 0.28, p > 0.05). There were group differences in depression (F3,35 = 9, p < 0.001); Tukey post hoc tests revealed that PTSD nonresponders had greater depression than either control group but did not differ from PTSD responders. There was no difference in the time posttrauma for the 2 PTSD groups or for trauma-exposed control subjects (F2,23 = 1.2, p > 0.05).
MRI data
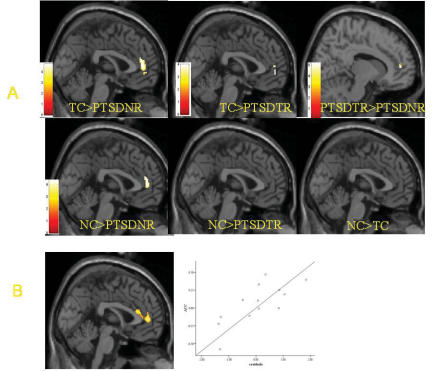
The structural volume of ACC across groups is presented in Figure 1A. The overall group effect in the ANCOVA was significant for the left ACC (MNI coordinates –8 26 30, z = 3.92, p < 0.001, voxels = 6). Planned t tests revealed that there were significant reductions in rACC volume for nonresponders, compared with both the trauma-exposed control subjects (MNI 0 46 8, z = 4.13, p < 0.001, voxels = 63) and nontrauma-exposed control subjects (MNI 0 46 8, z = 3.77, p < 0.001, voxels = 34). The nonresponders did not show greater volume in any region of the ACC relative to either control group.
Fig. 1: (A) Summary of group differences in ACC volume between control subjects not exposed to trauma (NC, n = 13), control subjects exposed to trauma (TC, n = 13), PTSD treatment responders (PTSD-TR, n = 7) and PTSD treatment nonresponders (PTSD-NR, n = 6). (B) summary of the correlational analysis showing the rACC region that is correlated with treatment outcome (change in total CAPS score from pre to posttreatment) and the scatterplot of this correlation (r = 0.85, p < 0.001, r2 = 0.73). 152 × 130 mm (150 × 150 DPI). ACC = anterior cingulate cortex; PTSD = posttraumatic stress disorder; rACC = rostral anterior cingulate cortex; CAPS = Clinician Administered PTSD Scale.
The treatment responders displayed a small reduction in rACC volume relative to the trauma-exposed control group (MNI 0 52 4, z = 3.60, p < 0.001, voxels = 8) but did not reveal reductions in rACC when compared with the nonexposed control subjects. Treatment nonresponders had significant reductions in rACC, compared with treatment responders (MNI 12 50 8, z = 4.42, p < 0.001, voxels = 12). Treatment responders displayed greater volume in the dorsal ACC (MNI 8 26 30, z = 4.33, p < 0.001, voxels = 23) and left rostral-dorsal ACC (MNI –10 42 16, z = 3.55, p < 0.001, voxels = 19) than did the trauma-exposed control subjects, as well as greater volumes in left dorsal ACC (MNI –8 26 30, z = 4.32, p < 0.001, voxels = 24) and right rostro-dorsal ACC (MNI 6 48 20, z = 3.46, p < 0.001, voxels = 18) than did nonexposed control subjects. There were no differences in ACC volume between the trauma-exposed and nonexposed control subjects.
Figure 1B summarizes the main correlational findings between pretreatment ACC volume and change in total CAPS score from pre-to posttreatment. A significant positive correlation was observed between bilateral rACC cortex and change in total CAPS score (right, MNI coordinates 6 48 14, z = 3.43, p < 0.001, voxels = 23; left, MNI coordinates –2 46 6, z = 2.85, p < 0.003, voxels = 13). This correlation was also significant when we analyzed using SPSS and controlling for pretreatment total CAPS scores (r = 0.7, p < 0.001). That is, larger rACC volumes pretreatment were associated with greater symptom improvement.
Discussion
Better response to CBT was associated with larger rACC volume in participants with PTSD. Whereas treatment responders were characterized by rACC volume that was comparable to that of control subjects without PTSD, nonresponders were distinguished by smaller rACC volume. This finding accords with previous reports in animals5–7 and humans8–10 that extinction learning is associated with the vmPFC and associated regions. Convergent evidence has shown that people with chronic PTSD have reduced rACC activation during a range of tasks that involve the provocation of fear responses.12–15 CBT involves repeated exposure to memories of trauma and presumes that one can regulate the fear response in a manner that allows new learning of safety. Across both the symptom-provocation paradigms and the CBT used in the current study, deficient recruitment of rACC may be implicated in impaired regulation of fear reactions in PTSD patients.
The ACC can be divided into subsections that involve more affective (ventral ACC) and cognitive (dorsal ACC) responses. The rACC is situated between the dorsal and ventral subsections of the ACC, and receives input from both subsections.35 The rACC may integrate affective and cognitive processes and may be implicated in cognitive control over emotional responses.36 It has been suggested that the rACC may detect conflict in the affective domain and recruits cognitive processes to resolve the emotional conflict.37 Consistent with this proposal, the rACC is recruited when people ignore emotional words in a word-counting task, compared with when they ignore neutral words.38 Accordingly, the rACC may be required to optimally utilize CBT as it manages the anxiety elicited during exposure-based therapy.37 The current finding converges with evidence of treatment prediction for depression, which has found that rACC activation predicts treatment response to a range of antidepressants.39–41 The finding that treatment responders had larger dorsal ACC volume than nonresponders is consistent with evidence that the dorsal ACC contributes to cognitive control and facilitates resolution of conflict by focusing attention on relevant stimuli.42,43The capacity to focus attention, especially when the patient is potentially threatened by distracting emotional stimuli during therapy, may contribute to better treatment response because the patient is able to use treatment strategies effectively.
We recognize that our study is limited by small sample size, yet our findings are robust because they were significant when we used familywise error correction. We also recognize that, although VBM has been used repeatedly in neuroimaging studies of PTSD,44,45 the technique has several limitations.46,47 VBM requires images to be coregistered into a common stereotactic space and to be of the same global shape, which may require the images to be warped. The normalization procedure remains sensitive to consistent morphological differences between images. The upshot of this is that morphological (i.e., shape) differences between groups can result in “activations” that can be mistakenly interpreted as evidence of between-group volumetric differences. There is evidence from 1 study on PTSD participants that the observed between-group VBM differences were due to shape and location abnormalities of the pregenual ACC in the PTSD group rather than to volumetric differences.48
This study requires replication using larger sample sizes and exploring further regions implicated in limbic hyperreactivity and cortical regulatory processes in PTSD. There is also a need to study the differential capacity of rACC to predict treatment response to pharmacotherapy as well as psychotherapy. Despite these limitations, this study represents the first demonstration of the rACC as a predictor of treatment response to CBT for PTSD. Future research needs to explore whether enhancing the capacity of rACC can facilitate treatment response for PTSD.
Acknowledgments
This study was funded by an ARC Linkage Grant (LP0212048) and a NHMRC Program Grant (300304).
Footnotes
Contributors: Drs. Bryant, Felmingham and Williams designed the study. Drs. Bryant, Felmingham, Kemp and Williams, and Mr. Hughes and Mr. Peduto acquired the data, which Drs. Bryant, Felmingham, Kemp and Williams and Mr. Whitford analyzed. All authors participated in the writing of the article and Drs. Bryant, Felmingham, Kemp and Williams, and Mr. Whitford and Mr. Peduto revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Prof. R.A. Bryant, School of Psychology, University of New South Wales, Sydney, Australia NSW 2052; fax 61 2 93853641; r.bryant@unsw.edu.au
References
- 1.Bradley R, Greene J, Russ E, et al. A multidimensional meta analysis of psychotherapy for PTSD. Am J Psychiatry 2005;162:214-27. [DOI] [PubMed]
- 2.Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry 2000;61(Suppl. 5):43-8. [PubMed]
- 3.Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry 2002;52:998-1007. [DOI] [PubMed]
- 4.Milad MR, Rauch SL, Pitman RK, et al. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol 2006;73:61-71. [DOI] [PubMed]
- 5.Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat Rev Neurosci 2004;5:844-52. [DOI] [PubMed]
- 6.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 2002;420:70-4. [DOI] [PubMed]
- 7.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 1993;163:109-13. [DOI] [PubMed]
- 8.Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci 2004;7:1144-52. [DOI] [PubMed]
- 9.Phelps EA, Delgado MR, Nearing KI, et al. Extinction learning in humans: role of the amygdale and vmPFC. Neuron 2004;43:897-905. [DOI] [PubMed]
- 10.Milad MR, Quinn BT, Pitman RK, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A 2005;102:10706-11. [DOI] [PMC free article] [PubMed]
- 11.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research – past, present, and future. Biol Psychiatry 2006;60:376-82. [DOI] [PubMed]
- 12.Bremner JD, Narayan M, Staib LH, et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999;156:1787-95. [DOI] [PMC free article] [PubMed]
- 13.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: A functional MRI investigation. Am J Psychiatry 2001;158:1920-2. [DOI] [PubMed]
- 14.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005;62:273-81. [DOI] [PubMed]
- 15.Williams LM, Kemp AH, Felmingham K, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 2006;29:347-57. [DOI] [PubMed]
- 16.Rauch SL, Shin LM, Segal E, et al. Selectively reduced regional cortical volumes in posttraumatic stress disorder. Neuroreport 2003;14: 913-6. [DOI] [PubMed]
- 17.Woodward SH, Kaloupek DG, Streeter CC, et al. Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry 2006;59:582-7. [DOI] [PubMed]
- 18.Karl A, Schaefer M, Malta LS, et al. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 2006;30:1004-31. [DOI] [PubMed]
- 19.Lindauer RJL, Vlieger E-J, Jalink M, et al. Majoie CBLMk Den Heeten G, Gersons, BPR. Effects of psychotherapy on hippocampal volume in out patients with post-traumatic stress disorder: a MRI investigation. Psychol Med 2005;35:1421-31. [DOI] [PubMed]
- 20.Vermetten E, Vythilingam M, Southwick SM, et al. Long term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 2003;54:693-702. [DOI] [PMC free article] [PubMed]
- 21.Felmingham K, Kemp A, Williams L, et al. Anterior cingulate and amygdala changes after cognitive behavior therapy of PTSD. Psychol Sci 2007;18:127-9. [DOI] [PubMed]
- 22.Blake DD, Weathers FW, Nagy LM, et al. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behavior Therapist 1990;13:187-8.
- 23.Hickie IB, Davenport TA, Hadzi-Pavlovic D, et al. Development of a simple screening tool for common mental disorders in general practice. Med J Aust 2001;175(Suppl):S10-7. [DOI] [PubMed]
- 24.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis 1 Disorders – Clinician Version. New York: American Psychiatric Press; 1997.
- 25.Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 1995;33:335-43. [DOI] [PubMed]
- 26.Bryant RA, Moulds ML, Guthrie RM, et al. Imaginal exposure alone and imaginal exposure with cognitive restructuring in treatment of posttraumatic stress disorder. J Consult Clin Psychol 2003; 71:706-12. [DOI] [PubMed]
- 27.Whitford TJ, Farrow TF, Gomes L, et al. Grey matter deficits and symptom profile in first episode schizophrenia. Psychiatry Res 2005; 139: 229-38. [DOI] [PubMed]
- 28.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage 2000;11:805-21. [DOI] [PubMed]
- 29.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273-89. [DOI] [PubMed]
- 30.Bryant RA, Felmingham KL, Kemp AH, et al. Neural networks of information processing in posttraumatic stress disorder. A functional magnetic resonance imaging study. Biol Psychiatry 2005;58:111-8. [DOI] [PubMed]
- 31.Shin LM, Whalen PJ, Pitman RK, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001;50:932-42. [DOI] [PubMed]
- 32.Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc Natl Acad Sci U S A 2002; 99:523-8. [DOI] [PMC free article] [PubMed]
- 33.Worsley KJ, Marrett S, Neelin P, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1996;4:58-73. [DOI] [PubMed]
- 34.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-level morphometric study of aging in 465 normal adult human brains. Neuroimage 2001;14:21-36. [DOI] [PubMed]
- 35.Devinsky O, Morrrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995;118:279-310. [DOI] [PubMed]
- 36.Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 2000;4:215-22. [DOI] [PubMed]
- 37.Etkin A, Pittenger C, Polan HJ, et al. Toward a neurobiology of psychotherapy: Basic science and clinical applications. J Neuropsychiatry Clin Neurosci 2005;17:145-58. [DOI] [PubMed]
- 38.Whalen PJ, Bush G, McNally RJ, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 1998;44: 1219-28. [DOI] [PubMed]
- 39.Davidson RJ, Irwin W, Anderle MJ, et al. The neural substrates of affective processing in depressed patients treated with bentlafaxine. Am J Psychiatry 2003;160:64-75. [DOI] [PubMed]
- 40.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8:1057-61. [DOI] [PubMed]
- 41.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry 2001;158:405-15. [DOI] [PubMed]
- 42.Posner MI, DiGiroloam GJ. Executive attention: conflict, target detection, and cognitive control. In: Parasuraman R, editor. The attentive brain. Cambridge (MA): MIT Press; 1998. p. 401-23.
- 43.Weissman DH, Gopalakrishnan A, Hazlett CJ, et al. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex 2005;15:229-37. [DOI] [PubMed]
- 44.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulated gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A 2003;100:9039-43. [DOI] [PMC free article] [PubMed]
- 45.Jatzko A, Rothenhöfer S, Schmitt A, et al. Hippocampal volume in chronic posttraumatic stress disorder (PTSD): MRI study using two different evaluation methods. J Affect Disord 2006;94:121-6. [DOI] [PubMed]
- 46.Mechelli A, Price CJ, Friston KJ, et al. Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews 2005;1:1-9.
- 47.Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage 2001;14:1238-43. [DOI] [PubMed]
- 48.Corbo V, Clement M, Armony JL, et al. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biol Psychiatry 2005;58:119-24. [DOI] [PubMed]