Abstract
The Drosophila Akt (dAkt) serine/threonine kinase is a component of the insulin receptor/PI3K signaling pathway that regulates cell growth. Here, we show that this kinase is expressed during Drosophila oogenesis and is required for egg chamber development. Loss of dAkt function in follicle cells causes a cell-autonomous reduction of cell size while expression of the constitutively active myristylated form of this kinase (dAktmyr) causes increased cell size. Accordingly, expression of the antagonist dPTEN in the same follicular domains causes reduced follicle cell size. Perturbations of dAkt function do not affect follicle cell proliferation or cell death. Of interest, expression of dAktmyr in the posterior domain of the follicular epithelium causes a delay in the posterior movement of follicular epithelium and dumpless-like egg chambers. It appears that dAkt is required for maintaining the continuity of cell size within the follicular epithelium, which in turn is necessary for its proper morphogenesis.
Keywords: cell growth, dAkt, follicle cells, oogenesis, Drosophila
INTRODUCTION
Drosophila oogenesis is a well-characterized model for studying basic questions in developmental and cell biology. The ovary consists of a relatively small number of cell types that are involved in several complex processes. The follicular epithelium that surrounds the Drosophila egg chamber is a powerful model system in which to study mechanisms underlying relevant biological processes such as cell proliferation, cell growth, and cell death relevant to epithelial morphogenesis. This epithelium is composed of the descendants of two somatic stem cells located in the region 2 of the germarium (Margolis and Spradling, 1995), the anterior tip of the ovariole. The stage 1 egg chamber that arises from the germarium is composed of 15 germ-line nurse cells and the oocyte organized in a syncytium, surrounded by a monolayer of somatic follicle cells. This egg chamber proceeds with a continuous growth and differentiation program from stage 1 through stage 14 of oogenesis (for a review, see Spradling, 1993). During early stages of oogenesis the nurse cells become highly polyploid. The follicle cells divide mitotically four to five times until stage 6 to reach a number of approximately 1,000, and starting from stage 7 to stage 9, they undergo three rounds of an endoreplication cycle that give rise to 16 copies of genomic DNA (Lilly and Spradling, 1996; Calvi et al., 1998). During stage 8, vitellogenesis begins and the oocyte starts to grow in size. By stage 14, the egg is mature and ready to be laid. During oogenesis, an elaborate process of communication that involves exchanges of information between the oocyte and the surrounding epithelial cells, as well as paracrine signaling within follicle cells, patterns the follicular epithelium (reviewed in Nilson and Schüpbach, 1999; van Eeden and St. Johnston, 1999; Dobens and Raftery, 2000).
The dAkt kinase is a critical mediator of the evolutionary conserved InR/PI3K (insulin receptor/phosphatidylinositol 3-kinase) signaling pathway that recently has emerged as one of the main signaling routes used by cells to control their increase in size (Stocker and Hafen, 2000; Garofalo, 2002; Goberdhan and Wilson, 2003). It has been shown that the dAkt kinase has three different roles during Drosophila development. During early embryogenesis dAkt acts as a cell survival gene, and embryos that lack the maternal contribution of functional dAkt undergo ectopic apoptosis (Scanga et al., 2000). Early embryos overexpressing dPTEN (Drosophila Phosphatase and Tensin homologue deleted on chromosome 10), a negative regulator of dAkt activity, undergo similar fates (Staveley et al., 1998; Cho et al., 2001). In mid-embryogenesis, dAkt is involved in tracheal cell fate determination and migration (Jin et al., 2001; Lavenburg et al., 2003). Later in development, the dAkt kinase has a role in regulating cell size and loss of dAkt reduces cell size in the wings and eyes of the fly (Verdu et al., 1999; Scanga et al., 2000). Moreover, cell-autonomous effects on cell size were observed after knockout or overexpression of dAkt in isolated clones of wing cells. The expression of a constitutively activated membrane-anchored dAkt during eye development results in eyes of increased size due to enlarged ommatidia (Stocker et al., 2002).
These distinct functions, thus, appear to be tissue specific: in pluripotent early embryonic cells, dAkt prevents apoptosis, whereas in differentiated epithelium, dAkt participates in cell motility or growth control. To examine if this simple model can extend to other tissues, we analyzed the dAkt function in ovarian follicle cells. A possible role of dAkt during oogenesis in follicle cells has been suggested from recent data showing that perturbation of InR/PI3K signaling could block oogenesis and could interfere with follicle cell functions. It has been shown that, in ovaries from inr mutant females, the oogenesis is blocked before the onset of vitellogenesis (Böhni et al., 1999) and that the ecdysone synthesis, mainly performed by the follicle cells in wild-type ovaries (Delbecque et al., 1990), is reduced (Tu et al., 2002). Moreover, chico (insulin receptor substrate) mutants exhibit female sterility (Chen et al., 1996), and it has been reported that chico mutation impairs the ability of the follicle cells to respond to nutritional changes and to enter vitellogenesis (Drummond-Barbosa and Spradling, 2001). Our results show that the dAkt function is required in the follicular epithelium to control cell growth and that perturbation of its function in follicle cells causes altered cell size without affecting cell proliferation or cell death and resulted in morphological defects in the epithelium.
RESULTS
dAkt Kinase Is Expressed in Follicle Cells During Oogenesis
We have noted lacZ expression in ovaries of heterozygous flies carrying the semilethal dAkt4226 mutation, generated by the insertion of the lacZ reporter gene in the upstream regulatory region of dAkt (Spradling et al., 1999; Gao et al., 2000). We found expression of the reporter gene in germ line cells and in follicle cells, suggesting that the dAkt gene is transcriptionally active in these epithelial cells (data not shown).
To confirm the expression of dAkt in the ovary, we first examined the expression pattern of the dAkt protein by using a rabbit polyclonal antiserum raised against the whole dAkt protein. In Western blot, this antibody recognizes a cluster of bands between ~60 and 120 kDa in both ovarian and embryonic extracts (Fig. 1A), similar to the pattern described previously (Andjelkovic et al., 1995). The spread of multiple bands may reflect the association of activated Akt with various membrane components that remain attached in sodium dodecyl sulfate (SDS) gel (Andjelkovic et al., 1997). No other bands were detected in the ovarian samples, whereas a couple of smaller species were seen in the embryonic sample, possibly degradation products. By using this antibody, we performed whole-mount immunostaining of the ovaries. Confocal microscope analysis showed that the dAkt kinase is expressed in both germ-line and follicle cell line in early oogenesis (Fig. 1B). In mid-oogenesis, as exemplified by stage 9 (Fig. 1C,E), dAkt expression is much more abundant in the follicle cells. Interestingly, within the follicle cells, dAkt is more concentrated on the basal side (Fig. 1D). This finding may indicate that extracellular signal that activates dAkt is provided on the basal side of the follicular epithelium. This finding is consistent with the notion that the nutrients that sustain the egg chambers are supplied from the outside (basal side). Also, dAkt is much more abundant in the cell periphery than in the nucleus (Fig. 1F). dAkt expression persists through the completion of oogenesis (data not shown).
Fig. 1.
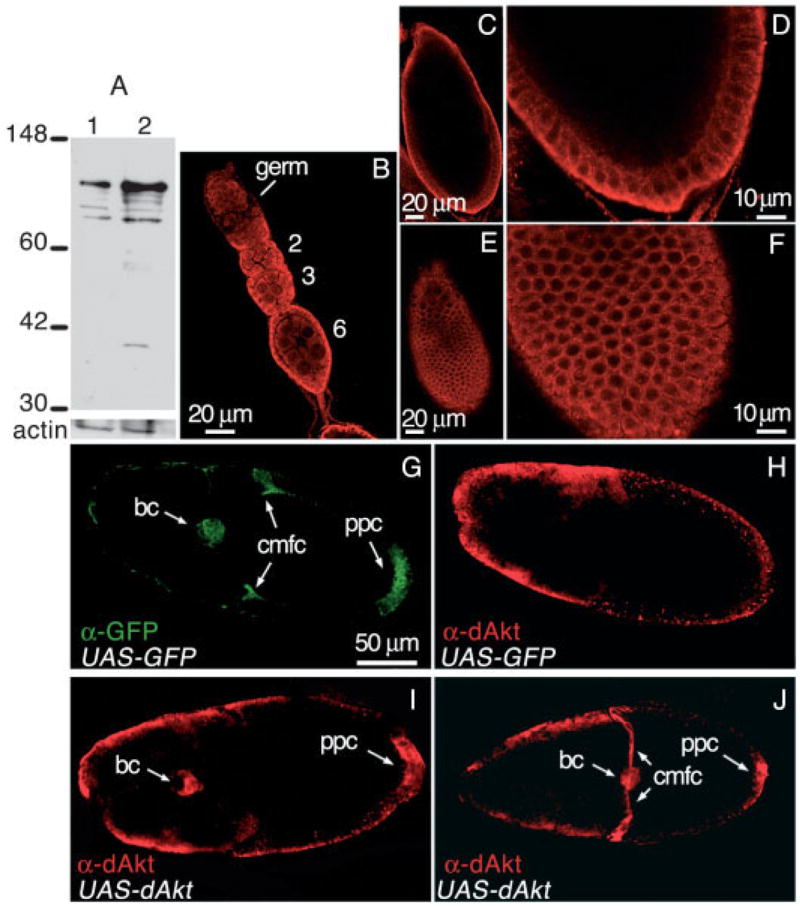
dAkt expression during oogenesis. A: Western blot analysis of protein extracts from whole ovaries (lane1) and 0–16 hr embryos (lane 2) with the anti-dAkt antibody. Actin serves as a loading control. Molecular size markers in kilodaltons are shown. B–F: Wild-type ovaries stained with the anti-dAkt antibody and analyzed using confocal microscopy. B: During oogenesis, dAkt is detected throughout the egg chambers. Numbers indicate the oogenic stage of the egg chambers. C: Optical cross-section of a stage 9 egg chamber. dAkt is mostly in the follicle cells. D: In this enlarged view, dAkt appears to be localized more to the basal side. E: Surface view of the same stage 9 egg chamber as in C, showing dAkt expression in the follicular epithelium. F: In this enlarged view, dAkt appears to be more concentrated in the cell periphery. Anterior is up in all panels. G–J: Flies carrying Gal4 driven by slow border cell enhancer (slbo>Gal4) and UAS-GFP were crossed with flies carrying UAS-dAkt (wild-type dAkt), and the egg chambers were immunostained with indicated antibodies (anti– green fluorescent protein [GFP] or anti-dAkt). Note that the gains of these confocal images were reduced compared with those in B–F, to show clearly the overexpressed dAkt. G,H: For the slbo>Gal4, UAS-GFP strain, the same egg chamber was double-stained with anti-GFP and anti-dAkt antibodies. G: GFP expression shows the tissue-specific expression pattern directed by slbo-Gal4 (arrows). bc: border cells; cmfc: centripetally migrating follicle cells; pfc: posterior follicle cells. H: dAkt staining of the same egg shows the endogenous levels of dAkt. There is no endogenous expression of dAkt in bc, and no elevated levels in cmfc and pfc. I,J: For the slbo>Gal4, UAS-GFP; UAS-dAkt flies, the egg chambers were stained with anti-dAkt antibody, which detected high levels of dAkt expression in slbo-specific cells; stage 9 (I) and stage 10B (J) eggs are shown. Migration of bc and cmfc is not affected. Anterior is to the left.
Because none of the dAkt alleles used in this study are protein-null, the specificity of the antibody cannot be assessed by differentiating wild-type and mutant cells. To this end, we used the antiserum to stain egg chambers overexpressing wild-type dAkt in defined cell types. A slow border cell enhancer-driven Gal4 (slbo>Gal4; Rørth et al., 1998) was used to ectopically express UAS-GFP alone or together with the UAS-dAkt transgene. As shown in Figure 1G, anti– green fluorescent protein (GFP) staining of a slbo>Gal4; UAS-GFP egg chamber marks the most prominent slbo-specific cell types: border cells (bc), centripetally migrating follicle cells (cmfc), and a group of posterior follicle cells (pfc). Note that the slbo enhancer also drives expression in a cluster of anterior follicle cells, which are only partially visible in the confocal sections shown here. The same egg chamber double-stained with the anti-dAkt antibody showed no endogenous dAkt expression in the border cells and no elevated expression in cmfc and pfc (Fig. 1H), indicating that the antibody is not prone to false-positive staining. By contrast, when the UAS-dAkt transgene was driven by slbo>Gal4, the three cell types clearly displayed high levels of dAkt expression (Fig. 1I,J). We are confident, therefore, that this antibody is specific. We also note that ectopic expression of dAkt in border cells did not result in size change or migratory defects (Fig. 1I,J), indicating that other signaling components required for activating dAkt are also absent in these cells.
Loss of dAkt Function in Follicle Cells Causes Reduced Cell Growth
To identify which specific cellular processes are regulated by dAkt in the ovary, we analyzed the loss-of-function phenotype using the embryonic lethal allele dAkt1q. This allele encodes an inactive form of the dAkt kinase carrying the amino acid replacement F327I in a core residue of the kinase catalytic domain (Staveley et al., 1998). dAkt1q mutant is homozygous lethal. To examine the mutational effects in the follicle cells, an adult tissue, genetic mosaic analysis was used. Homozygous dAkt1q clones, genetically marked by the absence of GFP, were obtained through somatic recombination using the FLP/FRT system (Golic, 1991; Xu and Rubin, 1993) and were induced by driving FLP under the control of a heat shock promoter (Xu and Harrison, 1994). To induce mutant clones during oogenesis, heat shocks were applied to adult females that were dissected 48–72 hours later. It should be noted that the follicular mutant clones are most likely generated early because follicular mitotic cycle ends at stage 6. To analyze follicle cell morphology, we performed immunostaining of mosaic ovaries for alpha-spectrin, which localizes to the apicolateral follicle cell contacts (Lee et al., 1997) and participates in the organization and formation of the membrane cytoskeleton (Hudson and Cooley, 2002). Nuclei are marked by propidium iodide staining. Figure 2 shows the confocal analysis of stage 8 (Fig. 2A–H) and stage 9 (Fig. 2I–P) egg chambers each containing two mutant clones (Fig. 2B,J, boxed areas). The follicle cells homozygous for the dAkt1q mutation, marked by the absence of GFP, are greatly reduced in size compared with both the parental heterozygous cells and the sister wild-type clones (Fig. 2C,F,K,N in which asterisks mark sister wild-type cells that can be distinguished from heterozygous cells by higher levels of GFP). Follicle cell nuclei in mosaic clones are also reduced in size compared with wild-type and heterozygous cells, as assessed by propidium iodide staining (Fig. 2D,G,L,O) and 4′,6-diamidine-2-phenylidole-di-hydrochloride (DAPI) staining (not shown). In addition, the propidium iodide and DAPI staining show no chromatin condensation in dAkt1q clones, indicating that loss of dAkt function do not cause apoptosis. Thus, loss of dAkt function causes a cell-autonomous effect on follicle cell growth, because only mutant follicle cells show reduced cell size (Fig. 2E,H,M,P).
Fig. 2.
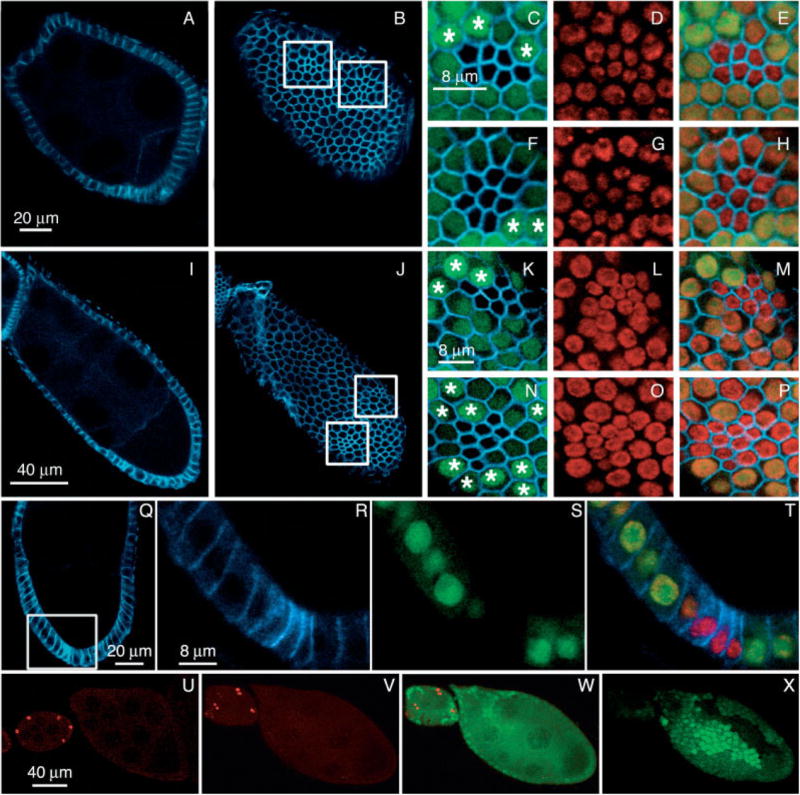
Analysis of dAkt loss of function in follicle cells. A–P: Stage 8 (A–H) and stage 9 (I–P) dAkt1q mosaic egg chambers labeled for alpha-spectrin (blue) and propidium iodide (red). Absence of green fluorescent protein (GFP) expression (green) marks the cells that are homozygous for dAkt1q, and the asterisks indicate sister wild-type cells, which show stronger GFP staining than the neighboring heterozygous counterparts. A,B,I,J: A and I are cross-sections and B and J are surface sections. Mutant follicle cell clones are marked by the boxes in B and J. C–E,F–H: Higher magnification views of the left and right boxed area in B, respectively. K–M,N–P: Higher magnification views of the left and right boxed area in J, respectively. C,F,K,N: Merged images of the GFP and alpha-spectrin signals. D,G,L,O: Propidium iodide staining of nuclei. E,H,M,P: Merged GFP, alpha-spectrin, and propidium iodide signals. Q–T: Cross-section of a stage 9 egg chamber stained for alpha-spectrin (blue), propidium iodide (red), and GFP (green). R–T: Enlarged view of the boxed area in Q; mutant cells (lack of GFP in S) are narrower, but the height remains the same as the wild-type neighbors. U–X: Egg chambers labeled for anti-PH3 (red). U: Wild-type egg chambers. V–X: The same dAkt1q mosaic egg chambers are shown in anti-PH3 alone (V) or merged images of anti-PH3 and GFP (W,X), and in optical cross-section (V,W) or surface section (X). U,W,X: In wild-type egg chambers (U) and dAkt1q follicular clones (W,X), no PH3 staining can be seen after stage 6. Anterior is up in panels Q–T and is toward the left in all other panels.
To ensure that the observed reduced surface area of the dAkt mutant cells is not due to cell elongation and constriction, cross-sections of the mutant clones were analyzed (Fig. 2Q–T). The basal–apical length of the mutant cells remains constant, suggesting that the smaller cell size results from reduced cell diameter.
Reduction of the follicle cell size could arise from an extension of the proliferative program of these cells beyond stage 6 of oogenesis without accompanying cell growth. However, by counting the number of cells in mutant and wild-type twin spots of stage 10 egg chambers it appears that follicle cell proliferation is not affected by the loss of dAkt kinase. We calculated the ratio of the cell number within the dAkt1q mutant clones per cell number within the sister wild-type clones (number of clones examined = 18) and obtained a mean value of 0.94 (± 0.17 standard deviation). Furthermore, the mosaic ovaries were stained with antibodies against phosphohistone H3 (PH3) that are specific for the phosphorylated form of histone H3 present only in mitotic nuclei (anti-PH3; Hendzel et al., 1997). In mosaic dAkt1q egg chambers (Fig. 2V–X) as in wild-type ovaries (Fig. 2U) no PH3-positive cells were detected beyond stage 6, indicating that loss of dAkt function in follicular clones does not induce detectable extension of the proliferative program of the follicle cell population. In addition, considering that the number of cells in dAkt1q mutant and sister clones is the same, it is possible to rule out the possibility that inappropriate cytokinesis without DNA replication causes reduced cell size in dAkt1q mutant clones.
Alternatively, the reduced cell size could be due to perturbation of the three endoreplication cell cycles (Lilly and Spradling, 1996) through which the follicle cells become polyploid and increase their size by the end of stage 10B (Calvi et al., 1998). We note that the intensity of propidium iodide staining per unit area of the nuclei is not altered in the mutant clones (Fig. 2D,G,L,O). Because the overall size of the mutant nucleus is smaller, the total DNA content within the mutant cell is proportionally reduced. This in turn indicates that the number of endoreplication cycles is reduced in mutant cells.
The results described above define for the first time a role for the dAkt kinase in egg chambers development. This kinase is required for proper growth of follicle cells, and loss of its function alters follicle cell development without altering proliferation and death of these cells.
Expression of Constitutively Active dAkt Kinase Increases Follicle Cell Size
To examine if activated AKT would have the opposite effect as loss of function, we specifically expressed a myristylated form of dAkt (dAktmyr) in the follicle cells. dAktmyr includes a N-terminal Src myristylation signal that targets it to the membrane. This form of dAkt is constitutively active (Kennedy et al., 1997). We took advantage of the Gal4/UAS enhancer trap system (Brand and Perrimon, 1993) that is widely used to drive tissue-specific expression of cloned genes in Drosophila. The expression of this transgene was induced in two spatially restricted follicular domains using the 55B and the E4 enhancer trap Gal4 lines. As previously reported, the Gal4 line 55B (Brand and Perrimon, 1994; Queenan et al., 1997) activates expression in the anterior population of the follicle cells, beginning at stage 8 (Fig. 3A). At stage 10B, the expression is mainly confined to the anterior columnar follicle cells (Fig. 3B). The Gal4 line E4 (Queenan et al., 1997) drives expression in a posterior patch of follicle cells in stage 9 (Fig. 3C) and stage 10 (Fig. 3D) egg chambers.
Fig. 3.

55B and E4 enhancer trap patterns in the follicular epithelium. A,B: Nomarski views of the UAS-lacZ reporter expression (X-gal staining) driven by the 55B Gal4 enhancer trap line; the 55B insertion directs expression of beta-galactosidase in the anterior domain of follicular epithelium in stage 8 (A) and stage 10B (B) egg chambers. C,D: Nomarski views of the UAS-lacZ reporter expression (X-gal staining) driven by the E4 Gal4 enhancer trap line; the E4 insertion activates expression of beta-galactosidase in follicle cells at the posterior of stage 9 (C) and stage 10A (D) egg chambers. The egg chambers are oriented with the anterior region toward the left. The oocytes are outlined with a dotted line.
In a stage 8 egg chamber (Fig. 4A,B), overexpressing UAS-dAktmyr driven by 55B, the follicle cells anterior to the oocyte are enlarged (Fig. 4B), in the region where 55B driver specifies (see Fig. 3A). Similarly, in a stage 9 egg chamber (Fig. 4C,D) overexpressing UAS-dAktmyr driven by E4, the follicle cells at the posterior become enlarged (Fig. 4D), in the region where E4 specifies (see Fig. 3D). The follicle cells could reach the size more than twice that of the neighboring cells (Fig. 4Ba,Da). Propidium iodide staining also shows that the size of the nuclei of these follicle cells is almost twice that of the nuclei of the neighboring cells (Fig. 4Bb,Db).
Fig. 4.
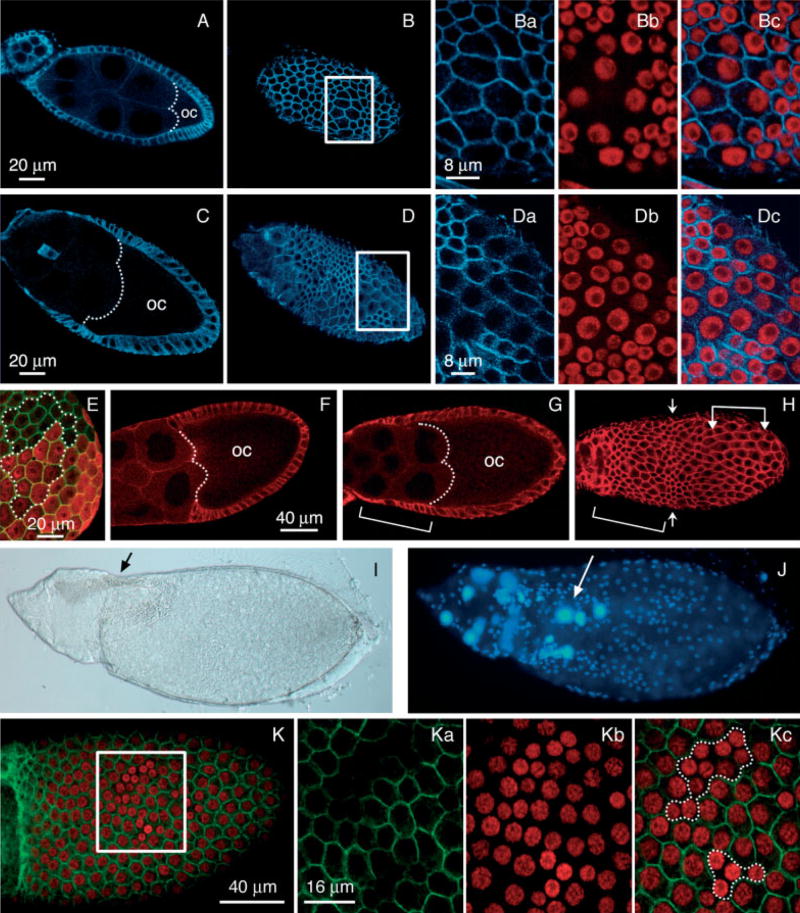
Follicle cell size and epithelial phenotypes induced by dysregulation of dAkt activity. A–D: Egg chambers labeled for alpha-spectrin (blue) and propidium iodide (red). A,B: Cross-section (A) and surface section (B) of 55B/UAS-dAktmyr late stage 8 egg chamber. Boxed area is enlarged in Ba–c. Ba: Alpha-spectrin. Bb: Propidium iodide. Bc: Merged. C,D: Cross-section (C) and surface section (D) of E4/UAS-dAktmyr stage 9 egg chamber. Boxed area is enlarged in Da–c. Da: Alpha-spectrin. Db: Propidium iodide. Dc: Merged. The dotted lines in A and C mark the anterior end of the oocytes (oc). E: Surface section of the posterior region of stage 10 egg chamber obtained from UAS-lacZ; E4/UAS-dAktmyr females and labeled for alpha-spectrin (green) and beta-galactosidase (red). The outlined areas containing 13 wild-type (lacZ-negative) and 11 lacZ-expressing (red) cells are used for measuring the average surface area per cell by NIH-Image (see text). These two populations are chosen because they do not fall into the region with strong curvature that can skew the surface area measurement. F–J: Egg chambers expressing UAS-dAktmyr in the posterior domain exhibit altered morphogenesis of the follicular epithelium. F–H: Wild-type (F) and (G,H) E4/UAS-dAktmyr egg chambers were labeled for alpha-spectrin (red). F: The dotted line marks the boundary between the columnar and the squamous cells, which demarcates the anterior end of the oocyte (oc). G,H: The brackets mark the follicle cells that have not moved completely to cover the oocyte. G: The dotted line marks the anterior end of the oocyte. H: Surface view of the same egg chamber as in G, showing the irregularity of follicle cell sizes. A band of small cells is indicated by arrows. A group of enlarged cells in the posterior is indicated by arrowhead brackets. I,J: Nomarski images of dumpless egg chambers with developing respiratory appendages (arrow in I), a stage 12–13 morphogenic marker. J: The 4′,6-diamidine-2-phenylidole-dihydrochloride (DAPI) staining (blue) shows large nuclei inside the oocyte proper (arrow). K: Surface section of a stage 10 egg chamber labeled for CD2 (green) and propidium iodide (red) showing follicle cell clones overexpressing UAS-dPTEN (boxed area). K: The boxed area is shown at higher magnification in Ka–c. Two follicle cell clones overexpressing dPTEN (dotted area in Kc) are marked by the absence of the CD2 marker (Ka). Nuclear size of these follicle cells (Kb) is reduced compared with the follicle cells that do not overexpress dPTEN. Anterior is to the left in all panels.
Correlation between dAktmyr expression and increased cell size was further verified by coexpressing UAS-dAktmyr and UAS-lacZ driven by E4. As shown in Figure 4E, the follicle cells expressing dAktmyr, marked by beta-galactosidase expression, exhibit also increased cell size. Note that beta-galactosidase and, by extension, dAktmyr are expressed variably among individual cells, which may explain the variable sizes observed of these cells. Nonetheless, surface area measured by the NIH-Image software indicates that the lacZ-expressing cells are on average ~43% larger than the wild-type cells (30.1 μm2 vs. 21 μm2).
Interestingly, dAktmyr overexpression driven by E4 (posterior) but not 55B (anterior) resulted in an additional morphogenic defect. In wild-type ovaries at stage 9, the follicle cells undergo rearrangement resulting in posterior movement of the epithelial sheet, such that only approximately 30–50 anterior follicle cells remain over the nurse cells to form a squamous epithelium, whereas most (~95%) of the follicle cells migrate posteriorly to form a columnar epithelium over the oocyte at stage 10 (Fig. 4F; Spradling, 1993). At stage 10, which is evidenced when the oocyte reaches 50% of the length of the egg chamber, the columnar follicle cell should exactly aligned with the oocyte. A significant delay of this rearrangement was detected in many stage 10 egg chambers from E4/UAS-dAktmyr females. In these egg chambers, the follicular epithelium shows strong irregularity in cell size (Fig. 4H) and a great number of columnar follicle cells yet remain associated with the nurse cells (Fig. 4G, bracket). This delay of follicular sheet migration is not due to an increased number of follicle cells in the posterior domain, because the proliferative program of follicle cells is not extended as assessed by anti-PH3 staining of egg chambers from E4/UAS-dAktmyr females (data not shown). We note that perturbation of the dAkt activity does not alter the epithelial characteristics of the follicle cells, as the apically localized alpha-spectrin expression is not changed (Fig. 4G,H); nor is the apical–lateral localization of beta-catenin (data not shown). It, therefore, appears that planar continuity of cell size in the follicular layer is important for the morphogenesis of this epithelium.
In addition, the distinct “dumpless” phenotype was observed in stage 14 egg chambers (13.3% dumpless: 105/682 wild-type) in which the persistence of the nurse cell compartment was detected in the presence of the respiratory appendages (Fig. 4I,J).
Expression of UAS-dPTEN Reproduces the dAkt1q Mutant Phenotype
We analyzed the effect on follicle cell growth resulting from expression of the lipid phosphatase dPTEN, a negative regulator of the Inr/PI3K signaling pathway. It has been shown that dAkt activity is negatively regulated by the lipid phosphatase dPTEN by means of degradation of 3-phosphoinositides (Scanga et al., 2000). Therefore, overexpression of dPTEN is expected to phenocopy dAkt mutants. We generated clones of cells overexpressing the UAS-dPTEN transgene (Scanga et al., 2000) using the Flp/Gal4 technique (Pignoni and Zipursky, 1997; Neufeld et al., 1998). The UAS-dPTEN transgene was expressed under the actin promoter in clonal patches of cells by using the Flp-out cassette Act5C>CD2>Gal4 and a heat-shock Flp recombinase. To induce clones, adult females were heat-shocked at 37°C for 1 hr and incubated for 2 days at 25°C before having their ovaries dissected. The locations of the dPTEN-expressing clones were mapped by the absence of the membrane-targeted CD2 marker. Figure 4K shows the confocal analysis of a stage 10 egg chamber containing two clones of follicle cells overexpressing dPTEN (boxed area). These follicle cells (dotted outlines in Fig. 4Kc), marked by the absence of the CD2 marker (Fig. 4Ka), exhibit reduced nuclear sizes, as assessed by propidium iodide staining of DNA (Fig. 4Kb), as well as reduced cell diameter. No defects on follicular epithelium morphogenesis were detected in these egg chambers. We also found that expression of the UAS-dPTEN transgene, promoted by the 55B or the E4 Gal4 drivers, causes reduced follicle cell growth in many anterior or posterior follicle cells, respectively, without altering follicular epithelium morphogenesis (data not shown).
DISCUSSION
The dAkt kinase acts in the Inr/PI3K pathway that controls growth, body size, reproduction, and the biology of ageing (Stocker and Hafen, 2000; Garofalo, 2002; Goberdhan and Wilson, 2003). The control of growth and size has received great attention recently within the field of developmental biology. Studies focusing on the control of growth have been difficult to address because this process is under the control of different signaling pathways. In addition, cell or tissue growth involves complex interactions among nutrition, metabolism, and hormones. We investigated the role of dAkt kinase in the follicular epithelium and found that its function is required for proper follicle cell growth. This process, in wild-type egg chambers, starts at stage 7 of oogenesis when the follicle cells concluded their proliferative program and stopped dividing. The follicle cells grow during stages 7–9 and achieve their mature size through three endoreplication cell cycles, during which DNA synthesis takes place without cell division (Lilly and Spradling, 1996; Calvi et al., 1998).
Our results show that loss of dAkt function in mosaic follicle cells greatly reduces cell size in a cell-autonomous manner. Follicle cells lacking dAkt function do not express, after stage 6, the mitotic PH3 marker, indicating that they go through the normal developmental transition from mitotic cycle to the endocycle. In addition, the pronounced reduction of size also extends to the follicle cell nuclei, while the chromatin does not appear condensed, based on the extents of DAPI and propidium iodide stainings, suggesting that their total DNA content is proportionally lower than that of wild-type follicle cell nuclei. Conversely, overexpression of constitutively active dAkt in the follicle cells results in enlarged cell and nuclear sizes. The existence of a positive relationship between cell size and DNA content has been documented in Drosophila as well as in other organisms (Saucedo and Edgar, 2002). Therefore, changes in follicle cell size may result from direct impact of dAkt on DNA synthesis. Alternatively, reduction of DNA contents in dAkt mutant cells may be the result of a global reduction of cell metabolism and protein synthesis, mediated by the functions of Tor and eukaryotic initiation factor 4F (eIF4E) complex (Miron et al., 2003; Hafen, 2004).
Interestingly, we observed that overexpression of constitutively active dAkt in the posterior domain resulted in defects in additional aspects of the follicular morphogenesis, that is, the delay of posterior movement of the epithelium (Fig. 4G,H) and the failure of nurse cell dumping (Fig. 4I,J), a process in which the nurse cell contents are actively transferred into the oocyte after stage 10B. These two defects may be linked, however. The dumping phenotype could arise from the delayed follicle sheet movement. In wild-type egg chambers when posteriorly migration of the follicular sheet is complete and the columnar follicle cells exactly envelope the oocyte at stage 10B, the anterior-most of these follicle cells will lead a second phase of migration along the oocyte–nurse cell boundary, pulling the follicular sheet inward. This process is termed centripetal migration. Successful centripetal migration is critical for nurse cell dumping. Dumpless phenotypes have been observed in mutants that affect centripetal migration (Dobens and Raftery, 2000). During nurse cell dumping, the follicular sheet between the nurse cells and the oocyte likely forms a physical barrier against the force that squeezes the nurse cell contents through small intercellular channels into the oocyte. In agreement with this hypothesis, big nuclei reminiscent of nurse cell nuclei are detected inside the oocyte proper of dumpless-like egg chambers (Fig. 4J, arrow). These results indicate that discontinuity of cell size in an epithelium could interfere with its development and function, revealing that the control of cell growth and cell size has the added importance to allow proper epithelial morphogenesis.
We should note that dAkt mutant clones in the follicular epithelium did not result in migration defects. This finding may be because the size of the clones is not large enough to exert influence on the whole epithelium. Although the possibility that dAktmyr exhibits some neomorphic properties cannot be ruled out, it is notable that the increased cell size phenotype resulting from expression of UAS-dAktmyr do not differ from what was expected for a gain of function dAkt allele. It is also noteworthy that dAktmyr overexpression in the anterior follicular domain did not cause delay in posterior movement. This finding may indicate that the driving force required for pulling the epithelium posteriorly is provided by the posterior follicle cells in association with the oocyte. This interesting possibility warrants future studies.
EXPERIMENTAL PROCEDURES
Fly Strains
Stocks were raised on standard cornmeal/yeast/agar medium at 25°C, and crosses were made at the same temperature unless otherwise stated. yw67c23/yw67c23 was used as the wild-type stock in this study. The dAkt4226 and slbo>Gal4 stocks were obtained from the Bloomington Stock Center (stock 11627 and stock 6458) and have the genotypes ry506, P{ry+t7.2=PZ}-Akt104226/TM3, ryRK Sb1 Ser1 and w; P{w[+mC]=Gal4-slbo.2.6}1206, P{w[+mC]=UAS-GFP.S65T}T2/CyO, respectively. The stocks used for clonal analysis were P{ry+, hsFLP}, y1 w1118; DrMio/TM3, ry Sb1 (Bloomington stock 7), w1118; P{ry+t7.2=neoFRT}82B, P{w+mC=Ubi-GFP(S65T)nls}3R/TM6B, Tb1 (Bloomington stock 5628) and w; TM6, Tb, Hu/P{w+, neo FRT}82B, dAkt1q (Jin et al., 2001) kindly provided by Dr. A. Manoukian (University of Toronto).
The E4 Gal4 line was kindly provided by Dr. T. Schüpbach (Princeton University) and has the following genotype w/w; +/+; P{w+Gal4}/P{w+Gal4}. The 55B Gal4 line (Bloomington stock 1803) has the following genotype: w/w; +/+; P{w+ mW.hs=GAWB}55B/P{w+mW.hs=GAWB}55B. The UAS-lacZ stock (Bloomington stock 3955) has the following genotype: w1118/w1118; P{w+mC=UAS-lacZ.NZ}20b/P{w+mC=UASlacZ.NZ}20b. The stocks w/w; +/+; UAS-dPTEN/UAS-dPTEN (Scanga et al., 2000) and w/w; +/+; UAS-dAktmyr/UAS-dAkt myr were also kindly provided by Dr. A. Manoukian. The y, w; UAS-dAkt strain was generated in Hsu lab. The UAS-lacZ chromosome was recombined with the UAS-dAKTmyr chromosome to obtain the following stock: w/w; +/+; UAS-lacZ, UAS-dAKTmyr/TM6. The Act5C>CD2>Gal4 line (Bloomington stock 4779) has the genotype: y1, w, P{w+mC=Gal4-Act5C(FRT.CD2).P}D/y1, w, P{w+mC=Gal4-Act5C(FRT.CD2).P}D.
Clonal Analysis
Site-directed mitotic recombination was catalyzed by the heat shock-inducible FLP yeast recombinase at a FRT target element (Golic, 1991; Xu and Harrison, 1994). Females of the genotype P{ry+, hsFLP}, y1 w1118; DrMio/TM3, ry Sb1 were crossed to males w1118; P{ry+t7.2=neoFRT}82B, P{w+mC=Ubi-GFP(S65T)nls}3R/TM6B, Tb1. F1 adult males of the genotype P{ry+, hsFLP}, y1 w1118/Y; TM3, ry Sb1/P{ry+t7.2=neoFRT}82B, P{w+mC=Ubi-GFP(S65T)nls}3R were crossed to females w; TM6, Tb, Hu/P{w+, neo FRT}82B, dAkt1q. Freshly eclosed females of the genotype w/P{ry+, hsFLP}, y1 w1118; P{w+, neo FRT}82B, dAkt1q/P{ry+t7.2=neoFRT}82B, P{w+mC=Ubi-GFP(S65T)nls}3R were collected and heat shocked for 1 hr at 37°C as follows: one time the first day, three times the second day, and one time the third day. After each heat shock, these females were transferred to fresh vials with yw67c23 males and incubated at 25°C. Before dissection, the flies were transferred to fresh, yeasted food daily at 25°C for 3 days.
Clonal overexpression of UAS-dPTEN transgene was obtained by the Flp/Gal4 technique (Pignoni and Zipursky, 1997; Neufeld et al., 1998). Females of the genotype P{ry+, hs-FLP}, y1 w1118; DrMio/TM3, ry Sb1 (Bloomington stock 7) were crossed to males w1118; UAS-dPTEN UAS-dPTEN. F1 adult males of the genotype P{ry+, hsFLP}, y1 w1118/Y; TM3, ry, Sb1/UAS-dPTEN were crossed to females y1, w, P{w+mC=Gal4-Act5C-(FRT.CD2).P}D (Bloomington stock 4779). Freshly eclosed females of the genotype P{ry+, hsFLP}, y1 w1118/y1, w, P{w+mC=Gal4-Act5C(FRT.CD2).P}D; UAS-dPTEN/+ were collected and heat shocked for 1 hr at 37°C. Before dissection, the flies were transferred to fresh, yeasted food daily at 25°C for 2 days.
Gal4 Driven Expression in Follicle Cells
Females E4 (or 55B); UAS-dAktmyr, E4 (or 55B); UAS-dPTEN, E4; UAS-lacZ, UAS-dAktmyr and slbo>Gal4, UAS-GFP/+; UAS-dAkt/+ were obtained by crossing the parental strains. The crosses were performed at 18°C, and the progeny was transferred to yeasted vials at 29°C for 5 days before dissection. The X-Gal assay for beta-galactosidase activity was performed as previously described (Margolis and Spradling, 1995), and the ovaries were dissected and mounted in 50% glycerol in phosphate buffered saline (PBS) and viewed with Nomarski optics on a Zeiss microscope.
Immunofluorescence Microscopy
Fixation and antibody staining of hand-dissected ovaries were carried out as previously described (Andrenacci et al., 2001). The rabbit polyclonal anti-dAkt antibody was raised against the His-tagged full-length dAkt produced in bacteria. The antiserum was protein A-purified and used at 1:500 dilution. Other primary antibodies were used at the following titers: mouse anti–alpha-spectrin (1: 10, Developmental Studies Hybridoma Bank), rabbit anti-PH3 (1:200, Upstate Biotechnology), mouse anti-rat CD2 (1:500, Serotec), and rabbit anti-GFP (1:100, AbCam). Secondary antibodies used were CY5-conjugated goat anti-mouse (1:100, Jackson ImmunoResearch), CY3-conjugated goat anti-mouse (1:100, Sigma), and Cy3 goat anti-rabbit (1:400 Sigma). For propidium iodide nuclear counter-staining, the ovaries were washed three times in PBS and treated with RNase A (400 μg/ml in PBS, Sigma) for 2 hr. After three 10-min washes in PBS, the ovaries were labeled for 30 min with propidium iodide (5 μg/ml in PBS, Sigma). Afterward, the egg chambers were washed three times in PBS and mounted in Fluoromount (Electron Microscopy Sciences). Stained egg chambers were analyzed with conventional epifluorescence and with a TCS SL Leica confocal microscope.
Protein Extracts and Western Blot Analysis
The 0–16 hr embryos and ovaries from yw67c23 females were quickly collected in cold 2× Laemmli sample buffer (Laemmli, 1970), homogenized by sonication, frozen in liquid nitrogen, and stored at −80°C. After centrifugation for 5 min at 15,000 × g, the soluble material was mixed with an equal volume of water and boiled for 5 min. After the removal of insoluble material by centrifugation (5 min at 15,000 × g), the soluble proteins were resolved on 10–15–20% step-gradient polyacrylamide gels by SDS polyacrylamide gel electrophoresis. A total of 75 μg of extracts were loaded on each lane. Protein transfer to membranes and Western blotting were performed as previously described (Andrenacci et al., 2001). The dAkt proteins were detected by using the rabbit anti-dAkt antibody as described above diluted 1:1,000. The primary antibody was detected by using horseradish peroxidase-conjugated horse antibody (1/500 dilution) and ABC detection kit (Vector ABC Universal kit number pk-6200).
Acknowledgments
We thank Armen Manoukian and Trudi Schüpbach for the generous gifts of Drosophila strains that were essential for this study. We also thank the Bloomington Stock Center for providing us with fly stocks. We are particularly grateful to the Developmental Studies Hybridoma Bank at the University of Iowa for antibody. We acknowledge Carlo Taddei for his helpful discussions and for advice on confocal analysis. We also thank Silvia Gigliotti and Franco Graziani for critical reading of the manuscript. A very special thanks goes to Marco Privitera for his skillful processing of images and graphic work.
Grant sponsor: University of Bologna in the framework of the projects “Biologia della riproduzione e dello sviluppo” and “Meccanismi e segnali molecolari della sopravvivenza cellulare”; Grant sponsor: NIH; Grant number: RO1GM57843; Grant number: CA095888.
References
- Andjelkovic M, Jones PF, Grossniklaus U, Cron P, Schier AF, Dick M, Bilbe G, Hemmings BA. Developmental regulation of expression and activity of multiple forms of the Drosophila RAC protein kinase. J Biol Chem. 1995;270:4066–4075. doi: 10.1074/jbc.270.8.4066. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Andrenacci D, Cernilogar FM, Taddei C, Rotoli D, Cavaliere V, Graziani F, Gargiulo G. Specific domains drive VM32E protein distribution and integration in Drosophila eggshell layers. J Cell Sci. 2001;114:2819–2829. doi: 10.1242/jcs.114.15.2819. [DOI] [PubMed] [Google Scholar]
- Böhni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97:865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes Dev. 1994;8:629–639. doi: 10.1101/gad.8.5.629. [DOI] [PubMed] [Google Scholar]
- Calvi BR, Lilly MA, Spradling AC. Cell cycle control of chorion gene amplification. Genes Dev. 1998;12:734–744. doi: 10.1101/gad.12.5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Jack J, Garofalo RS. The Drosophila insulin receptor is required for normal growth. Endocrinology. 1996;137:846–856. doi: 10.1210/endo.137.3.8603594. [DOI] [PubMed] [Google Scholar]
- Cho KS, Lee JH, Kim S, Kim D, Koh H, Lee J, Kim C, Kim J, Chung J. Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3 kinase-dependent signaling pathway. Proc Natl Acad Sci U S A. 2001;98:6144–6149. doi: 10.1073/pnas.101596998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbecque JP, Weidner K, Hoffmann KH. Alternative sites for ecdysteriod production in insects. Invert Reprod Dev. 1990;18:29–42. [Google Scholar]
- Dobens LL, Raftery LA. Integration of epithelial patterning and morphogenesis in Drosophila ovarian follicle cells. Dev Dyn. 2000;218:80–93. doi: 10.1002/(SICI)1097-0177(200005)218:1<80::AID-DVDY7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Gao X, Neufeld TP, Pan D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev Biol. 2000;221:404–418. doi: 10.1006/dbio.2000.9680. [DOI] [PubMed] [Google Scholar]
- Garofalo RS. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol Metab. 2002;13:156–162. doi: 10.1016/s1043-2760(01)00548-3. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Wilson C. The functions of insulin signaling: size isn’t everything, even in Drosophila. Differentiation. 2003;71:375–397. doi: 10.1046/j.1432-0436.2003.7107001.x. [DOI] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Hafen E. Interplay between growth factor and nutrient signaling: lessons from Drosophila TOR. Curr Top Microbiol Immunol. 2004;279:153–167. doi: 10.1007/978-3-642-18930-2_10. [DOI] [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hudson AM, Cooley L. Understanding the function of actin-binding proteins through genetic analysis of Drosophila oogenesis. Annu Rev Genet. 2002;36:455–488. doi: 10.1146/annurev.genet.36.052802.114101. [DOI] [PubMed] [Google Scholar]
- Jin J, Anthopoulos N, Wetsch B, Binari RC, Isaac DD, Andrew DJ, Woodgett JR, Manoukian AS. Regulation of Drosophila tracheal system development by protein kinase B. Dev Cell. 2001;1:817–827. doi: 10.1016/s1534-5807(01)00090-9. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavenburg KR, Ivey J, Hsu T, Muise-Helmericks R. Coordinated functions of Akt and Ets1 in tubulogenesis. FASEB J. 2003;17:2278–2280. doi: 10.1096/fj.03-0040fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Brandin E, Branton D, Goldstein LS. alpha-Spectrin is required for ovarian follicle monolayer integrity in Drosophila melanogaster. Development. 1997;124:353–362. doi: 10.1242/dev.124.2.353. [DOI] [PubMed] [Google Scholar]
- Lilly MA, Sprading AC. The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 1996;10:2514–2526. doi: 10.1101/gad.10.19.2514. [DOI] [PubMed] [Google Scholar]
- Margolis J, Spradling A. Identification and behaviour of epithelial stem cells in the Drosophila ovary. Development. 1995;121:3797–3807. doi: 10.1242/dev.121.11.3797. [DOI] [PubMed] [Google Scholar]
- Miron M, Lasko P, Sonenberg N. Signaling from Akt to FRAP/TOR targets both 4E-BP and S6K in Drosophila melanogaster. Mol Cell Biol. 2003;23:9117–9126. doi: 10.1128/MCB.23.24.9117-9126.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- Nilson LA, Schüpbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–243. doi: 10.1016/s0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by Decapentaplegic. Development. 1997;124:271–278. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Queenan AM, Ghabrial A, Schüpbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- Rørth P, Szabo K, Bailey A, Laverty T, Rehm J, Rubin GM, Weigmann K, Milan M, Benes V, Ansorge W, Cohen SM. Systematic gain-of-function geneticsin Drosophila. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- Saucedo LJ, Edgar BA. Why size matters: altering cell size. Curr Opin Genet Dev. 2002;12:565–571. doi: 10.1016/s0959-437x(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Scanga SE, Ruel L, Binari1 RC, Snow B, Stambolic V, Bouchard D, Peters M, Calvieri B, Mak TW, Woodgett JR, Manoukian AS. The conserved PI3′K/PTEN/Akt signaling pathway regulates both cell size and survival in Drosophila. Oncogene. 2000;19:3971–3977. doi: 10.1038/sj.onc.1203739. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM. The Berkeley Drosophila genome project gene disruption project. Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley BE, Ruel L, Jin J, Stambolic V, Mastronardi FG, Heitzler P, Woodgett JR, Manoukian AS. Genetic analysis of protein kinase B (AKT) in Drosophila. Curr Biol. 1998;8:599–602. doi: 10.1016/s0960-9822(98)70231-3. [DOI] [PubMed] [Google Scholar]
- Stocker H, Hafen E. Genetic control of cell size. Curr Opin Genet Dev. 2000;10:529–535. doi: 10.1016/s0959-437x(00)00123-4. [DOI] [PubMed] [Google Scholar]
- Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E. Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science. 2002;295:2088–2091. doi: 10.1126/science.1068094. [DOI] [PubMed] [Google Scholar]
- Tu MP, Yin CM, Tatar M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell. 2002;1:158–160. doi: 10.1046/j.1474-9728.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- van Eeden F, St Johnston D. The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis. Curr Opin Genet Dev. 1999;9:396–404. doi: 10.1016/S0959-437X(99)80060-4. [DOI] [PubMed] [Google Scholar]
- Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat Cell Biol. 1999;1:500–506. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]
- Xu T, Harrison SD. Mosaic analysis using FLP recombinase. Methods Cell Biol. 1994;44:655–681. doi: 10.1016/s0091-679x(08)60937-1. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]