Abstract
The purpose of this study was to determine whether biocompatible and biodegradable vasoactive intestinal peptide-grafted sterically stabilized phospholipid mixed nanomicelles (VIP-SSMM; size, ~15 nm), a novel nanosized actively-targeted drug delivery platform for breast cancer, accumulate in human MCF-7 breast cancer cells. Using hydrophobic CdSe/ZnS quantum dots (QD), we found that QD-loaded VIP-SSMM accumulated significantly faster and in greater quantity in MCF-7 cells than did QD-loaded SSMM alone (p<0.05). This process was mediated, in part, by VIP receptors because excess human VIP, but not PACAP6–38 or galanin, significantly attenuated this response (p<0.05). Taken together, these data indicate that VIP-SSMM are actively targeted to human breast cancer cells through VIP receptors. We suggest that VIP-SSMM could be used as an actively-targeted nanosized drug delivery platform for breast cancer cells over-expressing VIP receptors.
Keywords: nanomedicine, nanobiotechnology, drug delivery, quantum dots, PACAP, galanin, DSPE-PEG2000, phosphatidylcholine
INTRODUCTION
Rational design of actively-targeted nanomedicines to treat breast cancer is an innovative approach to improve the therapeutic index of chemotherapeutic drugs [1]. This modality harnesses the inherent pathophysiological features of breast cancer, such as over-expression of vasoactive intestinal peptide (VIP) receptors [2, 3], and distinct physicochemical properties of the nanocarrier, such as biocompatibility and prolonged circulation time [4, 5].
To this end, we have recently developed and tested actively-targeted human VIP-grafted sterically stabilized phospholipid mixed micelles (VIP-SSMM) as long-circulating, biocompatible and biodegradable nanocarriers for water-insoluble chemotherapeutic drugs such as paclitaxel [6]. These structures have been extensively characterized using quasi-elastic light scattering (QELS), circular dichroism (CD), small angle neutron scattering (SANS) and small angle x-ray scattering (SAXS) [7–9]. Previous in vivo biodistribution studies in our lab using MNU-induced breast cancer in rats have shown that paclitaxel loaded VIP-SSMM accumulated significantly more at the tumor site compared to non-targeted paclitaxel loaded SSMM (AUC0–24hr: 87.8 ± 9.5µg.h/g vs. 45.81 ± 2.28µg.h/g). In vivo efficacy studies showed that paclitaxel encapsulated in VIP-targeted nanocarriers produced significant tumor reduction in comparison to non-targeted carriers (79.7 ± 3% vs. 39.98 ± 5.9%) [10].
However, the mechanisms whereby these nanocarriers are internalized into target cells are uncertain. VIP receptor mediated internalization of SSMM may also play an important role in overcoming multi-drug resistance by overloading cellular efflux pumps with large amounts of free drug delivered intracellularly. The purpose of this study was to begin to address this issue by incorporating hydrophobic quantum dots (QD) - nanosized colloidal semiconductor crystals [11–13], into the core of SSMM and VIP-SSMM and optically tracking their accumulation in human MCF-7 breast cancer cells. Compared to traditional organic dyes, quantum dots provide superior brightness and immunity to photobleaching [11], which facilitates the ability to track micelles during extended periods of time using laser scanning confocal microscopy. Our goal was to use SSMM encapsulated QD as a model system, and duplicate, as closely as possible, the formulation methods we have successfully used to encapsulate hydrophobic anticancer agents in order to determine their intracellular fate.
MATERIALS AND METHODS
Egg-phosphatidylcholine (EPC) was purchased from Lipoid GmbH (Ludwigshafen, Germany). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine–N-[methoxy (polyethylene glycol)-2000] (DSPE-PEG2000) was obtained from Northern Lipids (Vancouver, BC). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine–N-[methoxy (polyethylene glycol)-3400]-succinimidyl propionate (DSPE-PEG3400-SPA) was obtained from Nektar (Huntsville, AL). PACAP6–38 was obtained from American Peptide Company (Sunnyvale, CA). Vasoactive Intestinal Peptide (human/rat) and galanin were synthesized using solid-phase synthesis by the Protein Research Laboratory at the Research Resources Center, University of Illinois at Chicago. Eagle’s minimum essential media, sodium bicarbonate, non-essential amino acids, sodium pyruvate and fetal bovine serum were all from Cellgro (Herndon, VA). Bovine insulin was obtained from Sigma (St Louis, MO). Red emitting CdSe/ZnS core shell quantum dots (λem=620 nm, diameter~5 nm) were obtained from Evident Technologies (Troy, NY).
Sterically stabilized phospholipid mixed micelles (SSMM, size, 15 nm ± 3 nm) were prepared as previously described in our laboratory with modifications [6]. Briefly, DSPE-PEG2000 and egg PC (total lipid concentration, 5 mM) were dissolved in chloroform in a 90:10 molar ratio. Quantum dots dissolved in toluene (80 µg/ml) were added to the mixture, vortexed and evaporated under argon and 600 mm Hg vacuum. The resulting film was dried under vacuum overnight. Thereafter, the film was hydrated with HEPES buffer (10 mM, pH 7.4), vortexed, sonicated and allowed to equilibrate in the dark for 2 h at 25°C to form SSMM-QD. Activated DSPE-PEG3400-SPA was conjugated to human VIP (0.3 mM) as previously described [14]. The VIP-DSPE-PEG3400 constructs were then inserted into previously formed SSMM-QD by incubating for 30 minutes at 25°C to form quantum dots self-associated with VIP-SSMM (VIP-SSMM-QD). In preparation for incubation with cells, culture media was added to the VIP-SSMM-QD and SSMM-QD solutions for final in vitro exposure concentrations of 50 µM for phospholipids and 3 µM for VIP. The molar ratios used throughout were based upon extensive previous optimization studies using isothermal titration calorimetry (ITC) and circular dichroism (CD) [15].
Adherent MCF-7 human breast cancer cells (ATCC, Manassas, VA) were incubated in complete growth medium consisting of Eagle’s minimum essential media (EMEM) with 2 mM L-glutamine and Earle’s BSS adjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids,1 mM sodium pyruvate and supplemented with 0.01 mg/mL bovine insulin and 10% fetal bovine serum in humidified air with 5% CO2 at 37°C.
MCF-7 cells were seeded onto 12 mm glass cover slips in 24-well tissue culture plates at a density of 15,000 cells/well for 3 days. On the day of the experiment, the media was replaced with culture media containing SSMM-QD or VIP-SSMM-QD and incubated at 37°C for various time intervals from 30 minutes to 16 hours. In another series of experiments, cells were pre-treated with culture media containing 30 µM human VIP, PACAP6–38 or galanin for 30 min at 37°C prior to being replaced with culture media containing SSMM-QD or VIP-SSMM-QD and incubated as above. Assuming 100,000 VIP receptors per cell, 30 µM represents a ratio of approximately 7.5 × 105 peptide molecules per VIP-R.
At the conclusion of the required incubation period, cells were washed with serum-free media and PBS. To aid in determining whether the quantum dots were inside or outside the cell membrane, the cell membrane was outlined using a green (λem=519 nm) fluorescent wheat germ agglutinin (WGA) probe (Molecular Probes, Eugene, OR), washed with PBS, fixed in 4% paraformaldehyde and washed again with PBS. The cover slips were then mounted onto glass slides using Vectashield antifade mounting media containing 4′,6-Diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingham, CA) to counterstain cell nuclei in blue.
Images of cells were obtained using an Olympus IX70 inverted fluorescence microscope coupled with a QImaging RETIGA 1300 cooled-CCD digital camera. Digital images were analyzed using IPLab software (Scanalytics, Rockville, MD). A normalized fluorescence signal value was determined by comparing the ratio of the red fluorescent signal from quantum dots in an image frame to the number of cells visible in that frame. All images were taken under identical exposure conditions. Five images were used to compute a mean for each individual time point. To localize quantum dots within cells, optical z-stack sections were obtained using a Zeiss LSM 510 Axiovert 1000 laser scanning confocal microscope with argon UV laser excitation wavelengths set to 364 nm (for QD and DAPI) and 488 nm (for WGA).
Data are expressed as means ± standard deviation. Statistical analysis was performed using ANOVA, Tukey’s post-hoc test and X2 test where appropriate. P<0.05 was considered statistically significant.
RESULTS
Quantum dots self-associated with VIP-SSMM accumulated faster and in greater quantity in human MCF-7 cells than did quantum dots in SSMM alone over the 16-h observation period (Figure 1; each group, n=5; p<0.05). Pre-treatment of cells with excess VIP (30 µM) for 30 min significantly attenuated accumulation of quantum dots in MCF-7 cells exposed to VIP-SSMM-QD but not in cells exposed to SSMM-QD (Figure 1; each group, n=5; p<0.05). This effect lasted less than 3 h due, most likely, to VIP receptors no longer being affected by the pre-treatment with free VIP (Figure 1). Accumulation of VIP-SSMM-QD in human MCF-7 cells was specific because pretreatment with PACAP6–38, a PAC1 receptor antagonist, or galanin, an unrelated peptide with similar molecular mass as VIP, had no significant effects on this process (Figures 2a and 2b, respectively; each group, n=5; p>0.5).
Figure 1.
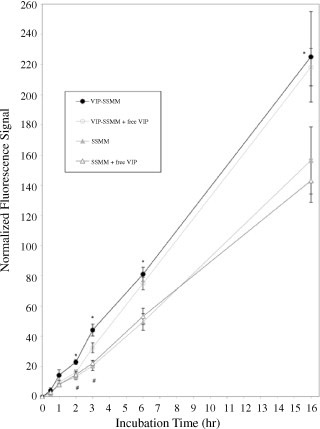
Normalized fluorescence signal of vasoactive intestinal peptide-grafted, quantum dots-encapsulated sterically stabilized mixed phospholipid micelles (VIP-SSMM-QD) and SSMM-QD in human MCF-7 cells in the absence and presence of excess free VIP (30 µM). Each group, n=5; * p<0.05 for VIP-SSMM-QD in comparison to SSMM-QD, and SSMM-QD with excess free VIP; # p<0.05 for VIP-SSMM-QD in comparison to VIP-SSMM with excess free VIP. Total number of cells counted for each time point: 0.5 h, 3477; 1 h, 5310; 2 h, 4405; 3 h, 6419; 6 h, 3970; 16 h, 2266.
Figure 2.
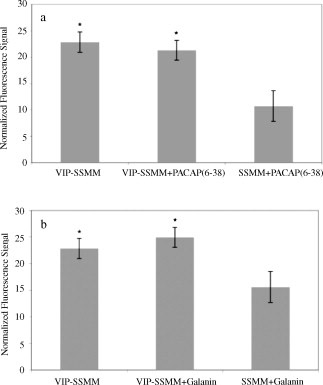
Normalized fluorescence signal in human MCF-7 cells after 2 h incubation with vasoactive intestinal peptide-grafted, quantum dots-encapsulated sterically stabilized mixed phospholipid micelles (VIP-SSMM-QD) or SSMM-QD in the absence (VIP-SSMM) and presence of excess of PACAP(6–38) (a) and galanin (b) (each, 30 µM). * p<0.05 in comparison to SSMM-QD to excess PACAP(6–38) and galanin, respectively.
Next, we examined the cellular distribution of quantum dots self-associated with VIP-SSMM or SSMM using laser scanning confocal microscopy (Figure 3). With incubation time <1 h, quantum dots localized primarily on the cell surface or within the plasma membrane. However, with longer incubation, quantum dots accumulated intracellularly in a time-dependent fashion. Quantum dots were not observed within the nucleus. After 16 h, quantum dots could be found in the cytoplasm in 77% of MCF-7 cells incubated with targeted VIP-SSMM-QD, while 17% of MCF-7 cells incubated with non-targeted SSMM-QD displayed quantum dots within the cytoplasm (n=68, p<0.05; Figure 3).
Figure 3.
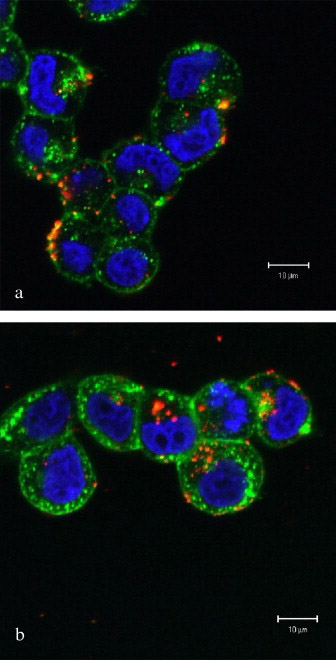
Representative laser scanning confocal microscopy sections of human MCF-7 cells after 16 h incubation with SSMM-QD (a) and VIP-SSMM-QD (b). Red, CdSe/ZnS quantum dots. Blue, 4′,6-Diamidino-2-phenylindole labeled cell nuclei. Green, fluorescent wheat germ agglutinin labeled cell membrane
DISCUSSION
The new finding of this study is that active targeting of sterically stabilized phospholipid mixed micelles to the cytoplasm of human MCF-7 cells can be accomplished by engaging VIP receptors on the surface of these cells. This process is specific and time-dependent. The VIP receptor sub-type(s) mediating this response remains to be determined.
The results of this study support and extend previous reports in the literature. Conjugating PEG directly to quantum dots significantly reduced nonspecific binding and peptide-conjugated quantum dots have been targeted to the angiotensin I receptor [16, 17]. Using non-targeted quantum dot phospholipid micelles, Fan et al. showed that these micelles accumulate in primary rat hippocampal neurons within 16 h [18]. Internalization of nanoparticles into target cells increases with decreased particle size and is mediated by diverse pathways, including phagocytosis, macropinocytosis, clathrin-mediated endocytosis and caveolae-mediated endocytosis [19–22].
The mechanism(s) whereby VIP-grafted SSMM-QD are internalized into MCF-7 cells remains to be elucidated. Conceivably, VIP-receptor interactions on MCF-7 cells could anchor micelles to the cell membrane with similar mechansims (subsequent internalization and intracellular delivery of the nanocarrier) as reported in the literature [23–26]. Importantly, VIP-receptor interactions with subsequent intracellular delivery may also mitigate extrusion of SSMM-encapsulated drug molecules from cells, including multi-drug resistant cells, thereby prolonging intracellular retention as shown in this study (Figure 3). Clearly, additional studies are warranted to support or refute these hypotheses.
In summary, we found that hydrophobic quantum dots loaded into VIP targeted SSMM (VIP-SSMM-QD) accumulated significantly faster and in greater quantity in MCF-7 cells than did quantum dots loaded into non-targeted SSMM (SSMM-QD). This process was mediated, in part, by VIP receptors because excess human VIP, but not PACAP6–38 or galanin, significantly attenuated this response. Taken together, these data indicate that VIP-SSMM are actively targeted to human breast cancer cells through VIP receptors. We suggest that VIP-SSMM could be used as an actively-targeted nanosized drug delivery platform for breast cancer cells over-expressing VIP receptors.
ACKNOWLEDGEMENTS
We thank Mei Ling Chen for assistance with confocal microscopy imaging and Ernest Gemeinhart for helpful discussions during the course of these studies. This study was supported, in part, by DOD grant BCRP, #DAMD 17-02-1-0415, VA Merit Review and NIH grants R01 AG024026, R01 HL72323 and C06RR15482.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Koo O, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Gespach C, Bawab W, de Cremoux P, Calvo F. Pharmacology, molecular identification and functional characteristics of vasoactive intestinal peptide receptors in human breast cancer cells. Cancer Res. 1988;48:5079–5083. [PubMed] [Google Scholar]
- 3.Dagar S, Krishnadas A, Rubinstein I, Blend MJ, Onyuksel H. VIP grafted sterically stabilized liposomes for targeted imaging of breast cancer: in vivo studies. J Control Release. 2003;91:123–133. doi: 10.1016/s0168-3659(03)00242-6. [DOI] [PubMed] [Google Scholar]
- 4.Allen C, Dos Santos N, Gallagher R, Chiu GNC, Shu Y, Li WM, Johnstone SA, Janoff AS, Mayer LD, Webb MS, Bally MB. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly(ethylene glycol) Biosci Rep. 2002;22:225–250. doi: 10.1023/a:1020186505848. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 6.Krishnadas A, Rubinstein I, Onyuksel H. Sterically stabilized phospholipid mixed micelles: in vitro evaluation as a novel carrier for water-insoluble drugs. Pharm Res. 2003;20:297–302. doi: 10.1023/a:1022243709003. [DOI] [PubMed] [Google Scholar]
- 7.Ashok B, Arleth L, Hjelm RP, Rubinstein I, Onyuksel H. In vitro characterization of PEGylated phospholipid micelles for improved drug solubilization: effects of PEG chain length and PC incorporation. J Pharm Sci. 2004;93:2476–2487. doi: 10.1002/jps.20150. [DOI] [PubMed] [Google Scholar]
- 8.Arleth L, Ashok B, Onyuksel H, Thiyagarajan P, Jacob J, Hjelm RP. Detailed structure of hairy mixed micelles formed by phosphatidylcholine and PEGylated phospholipids in aqueous media. Langmuir. 2005;21:3279–3290. doi: 10.1021/la047588y. [DOI] [PubMed] [Google Scholar]
- 9.Ashok B, Rubinstein I, Tsueshita T, Onyuksel H. Effects of peptide molecular mass and PEG chain length on the vasoreactivity of VIP and PACAP1-38 in PEGylated phospholipid micelles. Peptides. 2004;25:1253–1258. doi: 10.1016/j.peptides.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Krishnadas A, Rubinstein I, Sekosan M, Onyuksel H. Targeted delivery of paclitaxel to breast cancer by vasoactive intestinal peptide conjugated sterically stabilized phospholipid mixed micelles. AAPS J. 2004;6 Suppl 1:M1191. [Google Scholar]
- 11.Alivisatos P, Gu W, Larabell C. Quantum dots as cellular probes. Annu Rev Biomed Eng. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 12.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 14.Dagar S, Sekosan SM, Lee BS, Rubinstein I, Onyuksel H. VIP receptors as molecular targets of breast cancer: implications for targeted imaging and drug delivery. J Control Release. 2001;74:129–134. doi: 10.1016/s0168-3659(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 15.Krishnadas A, Rubinstein I, Onyuksel H. Vasoactive Intestinal Peptide (VIP) conjugated phospholipid nanocarrier for active targeted delivery of paclitaxel to breast cancer. Controlled Release Society 31st Annual Meeting; 2004. Transactions #497. [Google Scholar]
- 16.Bentzen EL, Tomlinson ID, Mason J, Gresch P, Warnement MR, Wright D, Sanders-Bush E, Blakely R, Rosenthal SJ. Surface modification to reduce nonspecific binding of quantum dots in live cell assays. Bioconjug Chem. 2005;16:1488–1494. doi: 10.1021/bc0502006. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson ID, Mason JN, Blakely RD, Rosenthal SJ. Peptide-conjugated quantum dots: imaging the angiotensin type 1 receptor in living cells. Methods Mol Biol. 2005;303:51–60. doi: 10.1385/1-59259-901-X:051. [DOI] [PubMed] [Google Scholar]
- 18.Fan H, Leve EW, Scullin C, Gabaldon J, Tallant D, Bunge S, Boyle T, Wilson MC, Brinker CJ. Surfactant-assisted synthesis of water-soluble and biocompatible semiconductor quantum dot micelles. Nano Lett. 2005;5:645–648. doi: 10.1021/nl050017l. [DOI] [PubMed] [Google Scholar]
- 19.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin and caveolae mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mousavi S, Malerod L, Berg T, Kjeken R. Clathrin-dependent endocytosis. Biochem J. 2004;377:1–16. doi: 10.1042/BJ20031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbell J. Enhancing drug function. Science. 2003;300:595–596. doi: 10.1126/science.1083625. [DOI] [PubMed] [Google Scholar]
- 22.Savic R, Luo L, Eisenberg A, Maysinger D. Micellar nanocontainers distribute to defined cytoplasmic organelles. Science. 2003;300:615–618. doi: 10.1126/science.1078192. [DOI] [PubMed] [Google Scholar]
- 23.Izzo RS, Scipione RA, Pellecchia C, Lokchander RS. Binding and internalization of VIP in rat intestinal epithelial cells. Regul Pept. 1991;33:21–30. doi: 10.1016/0167-0115(91)90011-5. [DOI] [PubMed] [Google Scholar]
- 24.McDonald TP, Dinnis DM, Morrison CF, Harmar AJ. Desensitization of the human vasoactive intestinal peptide receptor (hVIP2/PACAP R): evidence for agonist-induced receptor phosphorylation and internalization. Ann NY Acad Sci. 1998;865:64–72. doi: 10.1111/j.1749-6632.1998.tb11164.x. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen FC, Gammeltoft S, Westermark B, Fahrenkrug J. High affinity receptors for vasoactive intestinal peptide on a human glioma cell line. Peptides. 1990;11:1225–1231. doi: 10.1016/0196-9781(90)90156-y. [DOI] [PubMed] [Google Scholar]
- 26.Langlet C, Langer I, Vertongen P, Gaspard N, Vanderwinden JM, Robberecht P. Contribution of the carboxyl terminus of the VPAC1 receptor to agonist-induced receptor phosphorylation, internalization, and recycling. J Biol Chem. 2005;280:28034–28043. doi: 10.1074/jbc.M500449200. [DOI] [PubMed] [Google Scholar]


