Abstract
Vaccination with plasmid DNA encoding Ag85A from M. bovis BCG can partially protect C57BL/6 mice against a subsequent footpad challenge with M. ulcerans. Unfortunately, this cross-reactive protection is insufficient to completely control the infection. Although genes encoding Ag85A from M. bovis BCG (identical to genes from M. tuberculosis) and from M. ulcerans are highly conserved, minor sequence differences exist, and use of the specific gene of M. ulcerans could possibly result in a more potent vaccine. Here we report on a comparison of immunogenicity and protective efficacy in C57BL/6 mice of Ag85A from M. tuberculosis and M. ulcerans, administered as a plasmid DNA vaccine, as a recombinant protein vaccine in adjuvant or as a combined DNA prime-protein boost vaccine. All three vaccination formulations induced cross-reactive humoral and cell-mediated immune responses, although species-specific Th1 type T cell epitopes could be identified in both the NH2-terminal region and the COOH-terminal region of the antigens. This partial species-specificity was reflected in a higher—albeit not sustained—protective efficacy of the M. ulcerans than of the M. tuberculosis vaccine, particularly when administered using the DNA prime-protein boost protocol.
Author Summary
Buruli ulcer (BU) is an infectious disease characterized by deep, ulcerating skin lesions, particularly on arms and legs, that are provoked by a toxin. BU is caused by a microbe belonging to the same family that also causes tuberculosis and leprosy. The disease is emerging as a serious health problem, especially in West Africa. Vaccines are considered to be the most cost-effective strategy to control and eventually eradicate an infectious disease. For the moment, however, there is no good vaccine against BU, and it is still not fully understood which immune defence mechanisms are needed to control the infection. The identification of microbial components that are involved in the immune control is an essential step in the development of an effective vaccine. In this paper, we describe the identification of one of these microbial components, i.e., antigen 85A, a protein involved in the integrity of the cell wall of the microbe. Our findings obtained in a mouse model now need to be extended to other experimental animals and later to humans. Combination with a vaccine targeting the toxin may be a way to strengthen the effectiveness of the vaccine.
Introduction
Buruli ulcer (BU), also known as Bairnsdale ulcer, is an infectious, necrotizing skin disease caused by Mycobacterium ulcerans (M. ulcerans) occurring mostly in tropical and subtropical areas. Cases have been reported in several countries in West and Central Africa, in Central and South America, in Southeast Asia and in Australia. BU is emerging as a serious health problem, especially in West Africa, where it is the third leading cause of mycobacterial disease in immunocompetent people, after tuberculosis and leprosy. In some countries in Africa, thousands of cases occur annually and in these areas BU has supplanted leprosy to become the second most important human mycobacterial disease. The natural history of M. ulcerans infection and subsequent development of BU is not completely elucidated. M. ulcerans bacteria have been found in endemic areas in stagnant water or slowly moving water sources and in aquatic snails and carnivorous insects [1],[2]. So far, person to person transmission has not been reported. The infection causes initially a painless nodular swelling which can eventually develop into an extensive necrotizing lesion. M. ulcerans has the particularity to produce a family of toxin molecules, the so-called mycolactone (ML), polyketides that can suppress the immune system and destroy skin, underlying tissue and bone, causing severe deformities [3]–[5]. ML suppresses the in vitro TNF-α production by murine macrophages infected with M. ulcerans (4) and it strongly affects the maturation and the migratory properties of DC [5]. On the other hand, ML does not seem to affect the production of the inflammatory cytokine MIP-2, involved in the recruitment of neutrophils (4). M. ulcerans has an initial intracellular infection stage but virulent ML producing strains induce apoptosis of the infected cells and can subsequently be found extracellularly [3],[6]. Only few Mycobacterium species produce mycolactone toxins [7]. M. ulcerans isolates from different geographical areas produce different types of mycolactone, i.e. mycolactone A/B, C, D, E and F [8],[9].
The nature of immune protection against M. ulcerans infection remains unclear. In general, resistance to intracellular bacteria is primarily mediated by T cells with pivotal roles of Th1 type cytokines IFN-γ and TNF-α and this apparently is the case for M. ulcerans infection as well [10]. Progression of active Buruli ulcer is characterized by gradual down regulation of systemic and local Th1 type immune responses. Peripheral blood mononuclear cells from Buruli ulcer patients show reduced lymphoproliferation and IFN-γ production in response to specific stimulation with M. ulcerans [11]–[13]. Reduced IFN-γ response does not seem to be caused by decreased interleukin-12 production [14]. Also, semi-quantitative RT-PCR analysis demonstrated high IFN-γ and low IL-10 levels in early, nodular lesions whereas low IFN-γ and high IL-10 mRNA levels are observed in late ulcerative lesions [13]. Using a similar RT-PCR comparison of granulomatous versus non-granulomatous lesions, Phillips et al demonstrated higher expression of IL-12p35, IL-12p40, Il-15, IL-1β and TNF-α in patients from the former group and higher expression levels of IL-8 (human homologue of MIP-2) in the latter group [15]. Finally, Kiszewski et al have also confirmed that in ulcerative lesions without granuloma, there is increased expression of IL-10 and higher bacillary counts. [16].
It is not yet clear whether antibodies play a protective role against BU but the humoral immune response during M. ulcerans infection may be useful for serodiagnosis of BU. In contrast to tuberculosis and leprosy, immunoglobulin IgG antibody production against M ulcerans can be found even in early stages of infection [17]. IgG antibodies cannot be used to readily discern between patients and family controls, but primary IgM antibody responses against M. ulcerans culture filtrate proteins can be detected in sera from 85% of confirmed BD patients and only in a small proportion in sera from healthy family controls [18]. Antibody responses against the M. ulcerans homologue of the M. leprae 18-kDa small heat shock protein -that has no homologues in M. bovis and M. tuberculosis- can be used as serological marker for exposure to M. ulcerans [19].
BU results in considerable morbidity. Because of the late detection of the disease, treatment is principally by excision of the lesion, sometimes necessitating skin grafting [20]. WHO is currently recommending combined rifampicin and streptomycin treatment of nodules for eight weeks in the hope of reducing the need for surgery [21],[22]. Unfortunately, there is no specific vaccine against BU for the moment [23]. M. bovis BCG (Bacille Calmette et Guérin) vaccine, used for the prevention of tuberculosis, has been reported to offer a short-lived protection against the development of skin ulcers [24]–[26] and to confer significant protection against disseminated cases of BU, e.g. osteomyelitis, both in children and in adults [27],[28]. The precise M. ulcerans antigens that induce a protective immune response are poorly defined. The complete genome sequence of M. ulcerans has recently been published and will hopefully help to advance research and identification of relevant genes [29]. The 65 kD heat shock protein is expressed in considerable amounts by M. ulcerans bacilli in vitro and in vivo, and is immunogenic for both B and T cells in mice. Nevertheless, vaccination of mice with plasmid DNA encoding Hsp65 from M. leprae, having 96% sequence identity with Hsp65 from M. ulcerans, limited only weakly the progression of experimental M. ulcerans infection in tail [30]. We have previously reported that vaccination with BCG or with plasmid DNA encoding Ag85A from M. bovis BCG can partially protect B6 mice against footpad challenge with M. ulcerans [31]. Antigen 85 is a major secreted component in the culture filtrate of many mycobacteria such as M. bovis BCG, M. tuberculosis and M. avium subsp. paratuberculosis [32]. The antigen 85 complex (Ag85) of M. tuberculosis is a family of three proteins, Ag85A, Ag85B and Ag85C, which are encoded by three distinct but highly paralogous genes and that display an enzymatic mycolyl-transferase activity, involved in cell wall synthesis [33],[34]. Members of the Ag85 family rank among the most promising tuberculosis vaccine candidates, and are actually being tested in clinical trials, formulated as Hybrid-1 fusion protein of Ag85B with ESAT-6 or as recombinant Modified Vaccina Ankara virus encoding Ag85A booster vaccine of BCG [35],[36]. We have previously sequenced the gene encoding Ag85A from M. ulcerans and reported that it shares 84.1% amino acid sequence identity and 91% conserved residues with the gene encoding Ag85A from M. tuberculosis (which is identical to the Ag85A gene of M. bovis BCG) [31]. The genes encoding Ag85B and Ag85C of M. ulcerans have recently been sequenced as well and – as for M. tuberculosis- were localized on different loci in the genome [29].
Here, we report on a comparison of the immunogenicity and protective efficacy of vaccines encoding Ag85A from M. tuberculosis and from M. ulcerans. Vaccines were administered as plasmid DNA, purified protein in adjuvant or in a DNA prime-protein boost protocol. We and others have previously reported that DNA priming followed by protein boosting is an effective means to increase the potential of DNA vaccines [37]–[40].
Materials and Methods
Mice
C57BL/6 mice were bred in the Animal Facilities of the IPH-Pasteur Institute Brussels, from breeding couples originally obtained from Bantin & Kingman (UK). Mice were 8–10 weeks old at the start of the experiments. Female mice were used for immune analysis and male mice for the protection studies. This study has been reviewed and approved by the local Animal Ethics Committee (file number 030212/05).
Mycobacterial strains
Virulent M. ulcerans type 1 strain 04-855 from a Benin patient was isolated at the Institute for Tropical Medicine in Antwerp, Belgium. Bacteria grown on Löwenstein-Jensen medium were maintained and amplified in vivo in footpad of the mice. M. bovis BCG strain GL2 was grown for 2 weeks as a surface pellicle at 37°C on synthetic Sauton medium and homogenized by ball mill as described before [41].
Plasmid DNA constructions
Plasmid DNA encoding the mature 32 kD Ag85A from M. tuberculosis in V1J.ns-tPA vector was prepared as described before [31],[42]. The gene encoding Ag85A from M. ulcerans was amplified by PCR without its mycobacterial signal sequence using BglII restriction site containing primers and ligated into the same V1J.ns-tPA vector. The primers used were 5′-GGAAGATCTTGAGCGCTTGGTACTAGGC-3′ (forward) and 5′-GGAAGATCTTTTCGCGGCCGGGCCTGCCGGTGGA-3′ (reverse). In these plasmids the Ag 85A gene is expressed under the control of the promoter of IE1 antigen from cytomegalovirus, including intron A and it is preceded by the signal sequence of human tissue plasminogen activator.
Recombinant Ag85A proteins
Hexa-histidine tagged Ag85A protein from M. tuberculosis was purified from recombinant E. coli as described before [43]. The gene encoding the mature Ag85A protein from M. ulcerans was amplified by PCR from V1J.ns.tPA-85A vector. The primers used were 5′-CGCGGATCCGCGTTTTCGCGGCCGGGCCTGCCGTGGAA-3′ (forward) and 5′-CCCAAGCTTGGGCTAGGCGCCCTGGGTGTCACCG-3′ (reverse) with respectively BamHI and Hind III restriction sites. Ag85A gene was amplified without its mycobacterial signal sequence. Cloning in expression vector pQE-80L (QIAGEN), containing an NH2-terminal histidine-tag coding sequence, and purification were performed as described before [32]. Briefly, positives clones were screened on LB-ampicillin medium after ligation of the gene in the vector and transformation of E. coli DH5α cells. For expression, Top-10F' E. coli (Invitrogen) cells were transformed with plasmid encoding the 85A sequence. Recombinant protein was purified by immobilized metal affinity chromatography (IMAC) using gravity flow. The endotoxin level measured with the LAL kinetic chromogenic assay, was inferior to 10 EU/ml (endotoxin units per millilitre) or 0.03 EU/µg of purified protein (Cambrex Bioscience, New Jersey, America).
Peptide synthesis
Peptides spanning the entire mature 295 amino-acid Ag85A sequence of M. tuberculosis were synthesized as 20-mers, with the exception of the 18-mer spanning aa 35–53 and the 21 mer-peptide spanning amino acids 275–295 [44]. Peptides spanning the entire 294-amino acid Ag85A sequence of M. ulcerans were synthesized as 20-mers. All peptides were purchased from Ansynth Service B.V., The Netherlands.
Vaccination protocols
In experiment 1, B6 mice were anesthesized by intraperitoneal injection of ketamine-xylazine and injected three times intramuscularly (i.m) in both quadriceps muscles with 2×50 µg of control V1J.ns-tPA (empty vector), V1J.ns-tPA-Ag85A DNA from M. ulcerans or from M. tuberculosis (abbreviated as Ag85A-DNA Mu and Ag85A-DNA Mtb in the figures). For protein immunization, mice were injected three times subcutaneously (s.c) in the back with 10 µg of purified recombinant Ag85A (abbreviated as rec85A-Mu and rec85A-Mtb in the figures), emulsified in Gerbu adjuvant, i.e. water miscible, lipid cationic biodegradable nanoparticles, completed with immunomodulators and GMDP glycopeptide (GERBU Biochemicals). For the DNA prime-protein boost, mice were immunized twice i.m. with Ag85A DNA from M. ulcerans or from M. tuberculosis and boosted s.c. with 20 µg of recombinant Ag85A protein respectively from M. ulcerans or M. tuberculosis in Gerbu adjuvant (abbreviated as Ag85A-DNA/recMu and Ag85A-DNA/recMtb in the figures). All mice received the two first injections at 3 week intervals and the third injection was given two months later. For BCG vaccination, mice were injected intravenously, in a lateral tail vein, at the time of the first DNA injection with 0.2 mg (corresponding to 106 CFU) of freshly prepared live M. bovis BCG [41].
In experiment 2, B6 mice were injected intramuscularly (i.m) three times, at 3 weeks intervals, in both quadriceps with 2×50 µg of control V1Jns.tPA DNA or plasmid DNA encoding 85A from M. ulcerans or from M. tuberculosis. For protein immunization, mice were injected three times subcutaneously (s.c) in the back with 10 µg of purified recombinant Ag85A from M. ulcerans or from M. tuberculosis, emulsified in monophosphoryl lipid A (MPL-A) from Salmonella enterica serovar Minnesota (Ribi ImmunoChem Research, Hamilton, Mont)) solubilized in triethanolamine. For the DNA prime-protein boost, mice were immunized twice i.m. with Ag85A DNA from M. ulcerans or from M. tuberculosis and boosted s.c. with 20 µg of purified recombinant Ag85A protein respectively from M. ulcerans or from M. tuberculosis in MPL-A.
Infection
Naïve and vaccinated B6 mice were infected with M. ulcerans 3 months (Exp1) or 6 weeks (Exp2) after the last vaccination. 105 acid fast bacilli (AFB), obtained by in vivo passage in footpad, were injected in the right footpad of the vaccinated mice. The number of bacilli injected, suspended in Dubos Broth Base medium (Difco), was determined by counting under a microscope after Ziehl Neelsen staining. Viability of the M. ulcerans inoculum was checked by plating on 7H11 Middlebrook agar, supplemented with oleic-acid-albumin-dextrose-catalase enrichment medium. Yellow colonies were counted after 8 weeks of incubation at 32°C. The number of Colony Forming Units corresponded to the number of AFB.
Cytokine production
Vaccinated mice were sacrificed 3 weeks after the third immunization (Experiment 1). Spleens were removed aseptically and homogenized in a loosely fitting Dounce homogenizer. Leucocytes (4×106 WBC/ml) from four mice per group were cultivated at 37°C in a humidified CO2 incubator in round-bottom micro well plates individually or pooled (as indicated) and analyzed for Th1 type cytokine response to purified recombinant his-tagged Ag85A (5 µg/ml), and synthetic peptides from M. ulcerans or M. tuberculosis (10 µg/ml). Supernatants from at least three wells were pooled and stored frozen at −20°C. Cytokines were harvested after 24 h (IL-2) and 72 h (IFN-γ), when peak values of the respective cytokines can be measured.
IL-2 assay
Interleukin-2 (IL-2) activity was determined in duplicate on 24 h culture supernatants using a bio-assay with IL-2 dependent CTLL-2 cells as described before [45]. IL-2 levels are expressed as mean counts per minute (cpm). Assay sensitivity is 10 pg/ml. A typical international standard curve of this assay has been published before [46].
IFN-γ assay
Interferon-γ (IFN-γ) activity was quantified by sandwich ELISA using coating antibody R4-6A2 and biotinylated detection antibody XMG1.2 obtained from Pharmingen. The standard murine recombinant IFN-γ used was obtained from R&D. The sensitivity of the assay is 10 pg/ml.
ELISA
Sera from immunized mice were collected by tail bleeding 3 weeks after the third vaccination. Levels of M. ulcerans specific total anti-Ag85A Igκ antibodies (Abs) were determined by direct enzyme-linked immunosorbant assay (ELISA) in sera from individual mice (four/group). The concentration of Ab was expressed by the optical density at a dilution of 1/100 of the sera. For isotype analysis, peroxidase-labeled rat anti-mouse immunoglobulin G1 (IgG1) and IgG2b (Experimental Immunology Unit, Université Catholique de Louvain, Brussels, Belgium) were used. Isotype titers were expressed as dilution endpoints (last serum dilution with an optical density (OD) value higher than a cut-off OD value calculated from the OD value plus three standard deviations (SD) of the secondary antibody only [42].
Protection analysis
In experiment 1 (Gerbu adjuvant), protection was evaluated by enumeration of Acid Fast Bacilli (AFB) nine weeks after footpad infection. Briefly, the skin and bones were removed from infected foot pad. Tissues were homogenized in a Dounce homogenizer and suspended in 2 ml of Dubos broth based medium containing glass bead. The number of AFB in 20 fields (surface of 1 field: 0.037994 mm2×20 with the 22 mm ocular diameter used) was counted on microscope slides after Ziehl-Neelsen staining. In experiment 2 (MPL-A adjuvant), protection was evaluated by monitoring foot pad swelling after M. ulcerans infection. The swelling was measured with a calibrated Oditest apparatus with a resolution of 0.01 mm as described previously [47]. Animals were euthanized when footpad swelling exceeded 4mm according to the rules of the local ethical commission.
Statistical analysis
For cytokine production analysis, antibody production and AFB counting, statistical analysis was made according to one-way ANOVA test. Subsequent multiple comparison between the 7 different groups of animals and the antigens used was made by a Tukey's correction test. Statistical results are represented in the figure by *** (P<0.001), ** (P<0.01) and * (P<0.05). For the comparison of survival curves, logrank test was used.
Results
Partially species-specific Th1 type cytokine production in spleen cell cultures from B6 mice vaccinated with Ag85A-DNA, Ag85A protein or with a DNA prime-protein boost
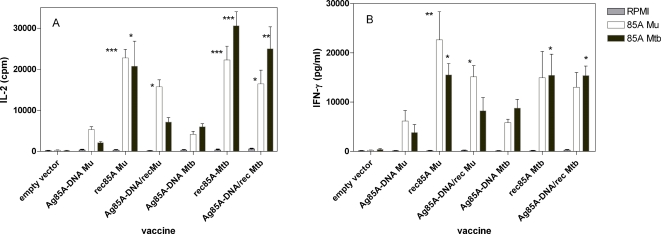
Spleen cells from mice vaccinated with the three different vaccine formulations produced significant levels of IL-2 (Figure 1A) and IFN-γ (Figure 1B) after in vitro stimulation with purified recombinant Ag85A from M. ulcerans or from M. tuberculosis. As expected from the 91% sequence similarity between both antigens, highly cross-reactive immune responses were observed, mice vaccinated with M. ulcerans vaccines recognizing the M. tuberculosis antigen and vice versa. Nevertheless, a certain level of species specificity was observed, particularly in the IL-2 responses. Confirming previous results obtained with a M. tuberculosis DNA vaccine [37], boosting plasmid DNA vaccinated mice with purified M. ulcerans protein increased significantly Ag 85A specific IL-2 and IFN-γ responses.
Figure 1. Spleen cell IL-2 (A) and IFN-γ (B) responses to RPMI medium (grey bars), recombinant Ag85A from M. ulcerans (white bars) and M. tuberculosis (black bars) in B6 mice vaccinated with Ag85A DNA, Ag85A protein or Ag85A DNA boosted with protein in Gerbu adjuvant, 3 months after the third immunization.
Cytokines levels tested on 24 h (IL-2) and 72 h (IFN-γ) culture supernatant of 4 mice tested individually/group. Data presented as means±SDs intra-assay. Statistically significant results as compared to the DNA vaccinated M. ulcerans or M. tuberculosis groups are represented in the figure by *** (P<0.001), ** (P<0.01) and * (P<0.05). The first group of comparison take all of the vaccinated mice compared to the DNA vaccinated mice in response to recombinant Ag85A from M. ulcerans. The second group of comparison take all of the vaccinated mice compared to the DNA vaccinated mice in response to recombinant Ag85A from M. tuberculosis.
M. ulcerans Ag85A specific antibody production in mice vaccinated with Ag85A-DNA, Ag85A protein or with a DNA prime-protein boost
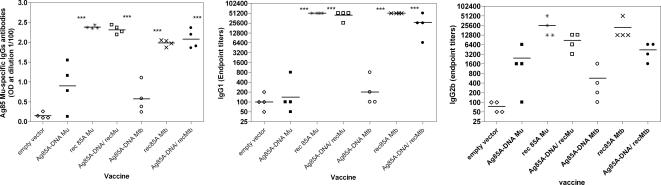
Significant cross-reactive antibody responses were induced against Ag85A from M. ulcerans (and from M. tuberculosis, data not shown) in mice vaccinated with the M. ulcerans and M. tuberculosis vaccines (Figure 2).
Figure 2. Ag85 M. ulcerans specific total IgG, IgG1 and IgG2b antibody levels in B6 mice.
Protein emulsified in Gerbu adjuvant. Data represent individual antibody levels (4 mice/group), mean value is indicated by the horizontal bars. * statistical analysis showing each group with a significant difference. For IgG1, groups c, d, f and g have a significative difference with the others groups. For IgG2b, only rec 85A Mu was significatively different from group a and e.
Antibody responses in DNA vaccinated mice demonstrated considerable individual variation, and were markedly increased by the protein boost. Vaccination with purified protein in Gerbu adjuvant was also very effective in inducing high level antibody production. DNA vaccination induced very little IgG1 isotype antibodies but biased predominantly an IgG2b isotype response, confirming the well known Th1 inducing properties of intramuscular plasmid DNA. In contrast, vaccination with protein emulsified in Gerbu adjuvant induced antibodies of both IgG1 and of IgG2b isotype. Confirming previous findings, DNA prime- protein boost vaccination resulted in increased and less variable antibody titers of both isotypes [37]. Vaccination with recombinant 85A protein or with the DNA prime /protein boost protocol induced significantly higher levels of total IgG and IgG1 antibodies as compared to plasmid DNA vaccination alone (P<0.001).
Identification of species-specific H-2b restricted Th1 T cell epitopes in M. ulcerans Ag85A
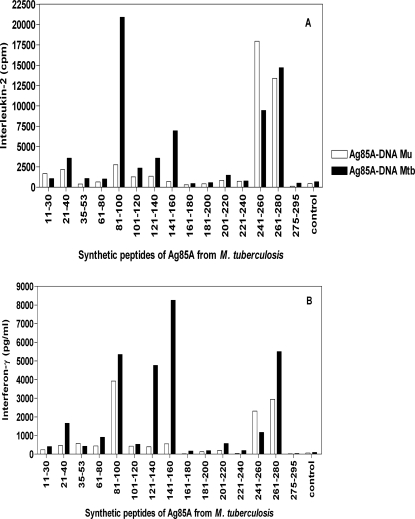
Despite the high level of sequence similarity (91%) between Ag85A from M. tuberculosis and M. ulcerans but in view of the partial species-specific Th1 type immune responses observed in the previous experiment, we decided to characterize the H-2b restricted immunodominant T cell epitopes, using synthetic 20-mer peptides spanning the entire mature sequence of Ag85A from M. ulcerans and from M. tuberculosis. Figure 3 shows the IL-2 and IFN-γ production induced in response to M. ulcerans peptides in mice vaccinated with M. ulcerans DNA (white bars) or M. tuberculosis DNA (black bars). Spleen cells from B6 mice vaccinated with M.ulcerans-Ag85A DNA produced significant levels of IL-2 (Figure 3A) and IFN-γ (Figure 3B) when stimulated with M. ulcerans peptides both from the NH2-terminal and COOH-terminal part of the protein, whereas IL-2 and IFN-γ responses of B6 mice vaccinated with the M. tuberculosis plasmid were almost exclusively directed against M. ulcerans peptide spanning aa 241–260. M. ulcerans DNA vaccinated mice also recognized this peptide very effectively. Responses against the NH2-terminal peptides spanning aa 61–80 and 81–100 of M. ulcerans-Ag85A were only observed in M. ulcerans DNA vaccinated mice, indicating that this NH2-terminal region was responsible for the partial species-specificity. This confirmed a previous finding (Inserts in Figures 3A and 3B) on species-specific T cell responses induced following in vitro stimulation with a purified, partial M. ulcerans Ag85A protein, spanning aa 17–150 in mice vaccinated with DNA encoding Ag85A from M. ulcerans or M. tuberculosis. IL-2 and IFN-γ responses against M. ulcerans peptide spanning aa 261–280 were also species-specific and only detected in mice immunized with the M. ulcerans vaccine.
Figure 3. Spleen cell IL-2 (A) and IFN-γ (B) responses to whole Ag85A- M. ulcerans and its synthetic peptides, as tested on a pool of six B6 mice vaccinated 3 times at three weeks interval with Ag85A-DNA M. ulcerans (white bars) or Ag85A-DNA M. tuberculosis (black bars).
Graph in insert represents the cytokine production in response to partial Ag85A M. ulcerans aa 17–150. Unstimulated cells (grey bars). Data of insert represent mean±SD values of 3 mice tested individually.
Responses against M. tuberculosis peptides showed a reciprocal pattern (Figure 4). Confirming previous findings [37] M. tuberculosis peptide spanning aa 261–280 was very well recognized in M. tuberculosis DNA vaccinated mice (Figure 4A and 4B). It was also recognized by M. ulcerans vaccinated mice. Both DNA vaccinated groups also reacted against M. tuberculosis peptide spanning aa 241–260, previously found to contain the immunodominant H-2b restricted epitope recognized in BCG vaccinated and M. tuberculosis infected B6 mice (10). Responses against this M. tuberculosis peptide were even higher in M. ulcerans than in M. tuberculosis DNA vaccinated mice. IFN-γ responses against M. tuberculosis peptides spanning aa 121–140 and 141–160 were only observed in mice vaccinated with the M. tuberculosis DNA, whereas a cross-reactive immune responses was found against M. tuberculosis peptide spanning aa 81–100. A sequence comparison of identified immunodominant Th1 peptides of Ag85A from M. ulcerans and from M. tuberculosis, showing conserved and non-conserved amino acid changes is presented in Table 1.
Figure 4. Spleen cell IL-2 (A) and IFN-γ (B) responses to whole Ag85A-M. tuberculosis and its synthetic peptides in a pool of six B6 mice vaccinated, at three weeks interval with Ag85A-DNA M. ulcerans (white bars) or Ag85A-DNA M. tuberculosis (black bars).
Table 1. Immunodominant peptides of Ag 85 A from M. ulcerans and M. tuberculosis.
| Peptides : aa | Sequences of M. ulcerans * | Sequences of M. tuberculosis |
| 21–40 | NIKVQFQSGGANSPALYLLD | DIKVQFQSGGANSPALYLLD |
| 61–80 | yyqsgisvampvggqssfys | YDQSGLSVVMPVGGQSSFYS |
| 81–100 | dwynpacgkagcttykwetf | DWYQPACGKAGCQTYKWETF |
| 141–160 | dqfvysgslsalldpsqg ig | QQFVYAGAMSGLLDPSQA MG |
| 240–259 | FQAAYNAAGGHNAVWNFDDN | 85A :FQDAYNAGGGHNGVFDFPDS |
| 85B :FQDAYNAAGGHNAVFNFPPN | ||
| 261–280 | thsweywgaqlnamrpdlqh | THSWEYWGAQLNAMKPDLQR |
| 275–294 | rpdlqhtlgatpntgdtqga | KPDLQRALGATPNTGPAPQGA |
*: Bold Amino Acids are high conserved amino acid differences (determined according to the Needleman-Wunsh criterion) and Amino Acids underlined are the non-conserved aa difference in comparison to the Ag85A peptides of M. tuberculosis.
Reduced M. ulcerans replication in footpad of mice vaccinated with Ag85A-DNA, Ag85A protein and with a DNA prime-protein boost
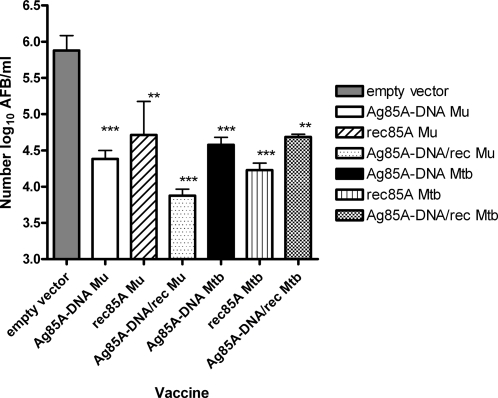
Mice were challenged three months after the third vaccination with 105 AFB of M. ulcerans in the footpad. Nine weeks later, when a significant swelling of the footpad appeared in the control mice vaccinated with empty vector, all animals were sacrificed and the number of AFB in the infected footpad was determined by Ziehl-Neelsen staining. As shown in Figure 5, a significant and strong reduction in the number of M. ulcerans AFB was observed in mice previously immunized with all three types of vaccine. Vaccination with specific M. ulcerans antigen using the DNA prime-protein boost protocol with Gerbu adjuvant conferred the highest protection with an almost one-hundred fold reduction in number of AFB as compared to the control group. This protection was comparable in magnitude to the protection conferred by the BCG vaccine. Difference between the vaccinated groups was not significant (ANOVA test; p>0.05).
Figure 5. Mycobacterial multiplication in mice infected with M. ulcerans strain 04-855.
Mice infected three months after the last vaccination (protocol 1 using Gerbu adjuvant). Results are mean±SD of AFB/ml of 4 individual mice, expressed in Log10. * p value as compared to mean log10 value obtained in control DNA mice infected with M. ulcerans.
Prolonged survival after M. ulcerans challenge in mice vaccinated with Ag85A-DNA or with a DNA prime-protein boost
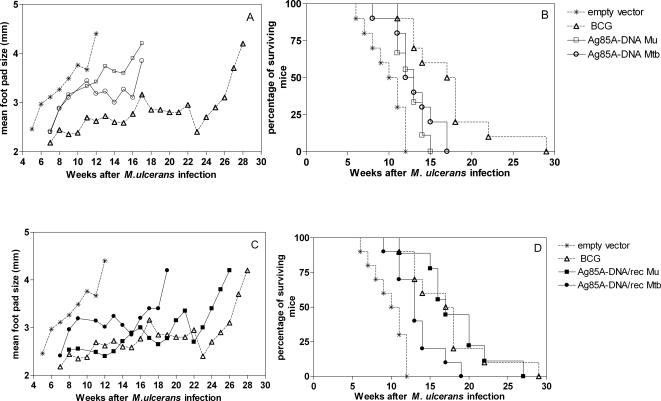
In a second experiment, protective efficacy of the vaccines was determined by weekly monitoring appearance and size of footpad swelling and survival as previously reported [47]. Mice were euthanized when footpad swelling was >4 mm. In this experiment mice were challenged with 105 AFB of M. ulcerans 04-855 at 6 weeks after the last immunization. The evolution of footpad swelling is shown in Figure 6A and 6C whereas the survival curves are represented in Figure 6B and 6D. In mice vaccinated with empty control vector, footpad size started to increase 5 weeks after M. ulcerans infection whereas in BCG vaccinated mice, footpad swelling was delayed for 7–8 weeks (Figures 6A and 6C). Vaccination with DNA encoding Ag85A from M. tuberculosis or from M. ulcerans delayed onset of foot pad swelling by only 2 to 3 weeks (Figure 6A). DNA prime/protein boost protocol using the M. tuberculosis Ag85A did not increase vaccine efficacy (Figure 6C) whereas vaccination with DNA encoding Ag85A from M. ulcerans boosted with the recombinant Ag85A-MPL-A protein from M. ulcerans delayed onset of foot pad swelling to the same extent as the BCG vaccine by 7 to 8 weeks (Figure 6C). Survival curves reflected the footpad swelling pattern. Median survival time of mice vaccinated with empty vector was 10.5 weeks, whereas BCG vaccination delayed significantly the moment when mice had to be euthanized, resulting in a median survival time of 17.5 weeks (Figures 6B and 6D) (p<0.001 compared to empty vector vaccinated mice; p<0.01 compared to 85A-DNA Mu vaccinated mice). M. ulcerans DNA vaccinated mice demonstrated a median survival time of 13 weeks (p<0.01 compared to empty vector vaccinated mice according to the log rank test). Similar results were observed in mice vaccinated with DNA encoding Ag85A from M. tuberculosis (median survival time 12.5 weeks) (Figure 6B). Boosting DNA vaccinated mice with protein from M. tuberculosis did not increase protective efficacy of the DNA vaccine but priming with DNA encoding Ag85A from M. ulcerans and boosting with recombinant Ag85A from M. ulcerans was very effective in increasing the protection (Figure 6D) (p<0.001 compared to M. ulcerans DNA alone, p<0.01 compared to DNA encoding Ag85A from M. tuberculosis boosted with the protein of M. tuberculosis). Median survival time in the M. ulcerans DNA primed- M. ulcerans protein boosted mice was 17 weeks. This protection was comparable to that conferred by BCG (p>0.05).
Figure 6. Evaluation of footpad size (A and C) and Survival curves (B and D) of vaccinated B6 mice after M. ulcerans infection with 105 AFB in the right footpad.
Experiment 2 using MPL-A as adjuvant and 6 weeks as rest between vaccination and infection. Mice were vaccinated with empty vector (* n = 9), with 0.2 mg of BCG (▵ n = 10), with Ag85-DNA from M. ulcerans (□ n = 9), with DNA encoding 85A from M. tuberculosis (○ n = 10), with Ag85A DNA-M. ulcerans boosted with the Ag85A protein from M. ulcerans (▪ n = 9), or with DNA encoding Ag85A boosted with Ag85 protein from M. tuberculosis (• n = 10).
Discussion
Buruli ulcer belongs to the family of neglected tropical diseases [48]. In 1998 the first International Conference on Buruli Ulcer was organized in Côte d'Ivoire, expressing the poor knowledge about this disease and calling on the international scientific community to support control and research efforts. Currently, no specific vaccine exists against this disease. In 1957, Fenner demonstrated that a high degree of protection was conferred, in an experimental mouse model, against challenge infection with small doses of M. ulcerans by prior inoculation with M. ulcerans, M. balnei and M. bovis BCG (BCG). Footpad and intravenous BCG administration gave considerable protection against a small dose and a slight protection against a large dose of M. ulcerans given in the other footpad [49]. More recently we have shown in a similar experimental mouse model that BCG vaccine protects to some extent against infection with M. ulcerans but that a booster vaccination with the same BCG vaccine does not increase the protective effect [31],[47]. In 1969, a clinical study performed in Uganda reported on a protection rate of 47% of the BCG vaccine. However, protection turned out to be short-lived and was only detected in the first 6 months following BCG vaccination [24]. In 1976, Smith et al reported another BCG vaccination trial against Buruli ulcer in Uganda giving similar short lived (one year) protection rates of about 50% [25]. Although not very effective at preventing the classical skin lesions of Buruli ulcer, the BCG vaccine seems to exert a significant protective effect against its severe, disseminated osteomyelitis form both in children and in adults [26],[27].
A more effective M. ulcerans vaccine would certainly help to control this debilitating disease that affects particularly children. Unfortunately, the nature of the protective immune response and the precise antigens involved are not fully defined at the moment. Based on biopsy specimens, M. ulcerans was originally thought to reside exclusively as free extracellular bacilli, implying that humoral responses might be protective. However, Coutanceau et al recently demonstrated that the initial phase of M. ulcerans infection proceeds by internalization of bacilli by phagocytic cells and that the extracellular stage results from mycolactone inducing host cell death [6],[50]. Therefore, recognition of the early intracellular stage by an effective Th1 type immune response may contribute to the control of the infection, that is in so far as it can help to reduce the mycolactone production. Hence, magnitude of mycobacteria-specific Th1 type immune response is a plausible correlate of protection that can be used to analyze the potential of new, experimental vaccines.
In this study, we focused on a plasmid DNA vaccine encoding Ag85A from M. ulcerans. Protective efficacy was evaluated using two approaches, in one experiment by enumerating the number of AFB in the footpad at nine weeks after M. ulcerans challenge and in the other experiment by monitoring footpad swelling and long term survival of the mice. We have previously reported that footpad swelling is correlated with bacterial replication and can be used as an alternative read-out for protection against infection [47]. DNA prime–protein boost strategy using specific M. ulcerans antigen 85A was clearly the most effective, reducing about one hundred fold the bacterial number and offering a protection of comparable magnitude as the one induced by the BCG vaccine. Nevertheless, and as for the BCG vaccine, immune protection was not sterilizing and eventually all mice developed footpad swelling. We hypothesize that the vaccines reduced or delayed temporarily mycolactone production by the virulent type 1 strain 04-855 but that immunity was not strong enough to completely block the ML synthesis. Targeting ML production by specific antibodies or by interfering with its synthesis might help to overcome this problem. A study made by Fenner, in 1956 showed that the apparition of footpad swelling depends of the number of viable AFB in the inoculum, small doses of bacilli showing delayed appearance of footpad lesion [51]. As we used a high inoculum size of 105 AFB in our studies, it is possible that more sustained protections could have been observed if we had administered a lower number of bacteria.
The Gerbu adjuvant is less well known as immunomodulator than other adjuvants such as alumn or monosphoshoryl-lipd-A (MPL-A) [52]. Here we have shown that this adjuvant has a strong Th1 inducing capacity, as indicated by the elevated levels of antigen-specific IL-2 and IFN-γ that could be detected in spleen cell cultures from mice vaccinated with protein in this adjuvant. Antibodies of both IgG1 but also of IgG2b isotype were induced, which was another indication of its Th1 favouring properties. Vaccination with recombinant M. ulcerans Ag85A protein in Gerbu adjuvant induced comparable Th1 cytokine and antibody levels as the prime-boost DNA vaccination. This protein vaccine also induced considerable protection (albeit somewhat lower that the DNA based vaccine) as indicated by significantly reduced number of AFB in the footpad at nine weeks after M. ulcerans challenge. We have previously shown that DNA vaccination induces a broader T cell repertoire (more protein epitopes recognized) than infection with tuberculosis [53],[54], vaccination with BCG [44] or with protein [46] and this may explain the better protection conferred by the DNA prime-protein boost vaccination. It is also possible that immune memory induced with this combined immunization protocol was stronger and longer lasting than immune memory induced with protein only vaccination.
Analysis of the H-2b restricted Th1 T cell epitopes of antigen 85A from M. ulcerans and from M. tuberculosis revealed some extent of species specificity, both in the NH2-terminal and in the COOH-terminal half of the protein. In contrast to the response induced with DNA encoding M. tuberculosis Ag85A, which was preferentially directed against Ag85A peptide spanning aa 261–280, T cell response induced with DNA encoding the M. ulcerans protein was directed preferentially against peptide spanning aa 240–259. Remarkably, mice vaccinated with the M. tuberculosis DNA reacted more strongly to this peptide region of M. ulcerans (25,000 cpm of IL-2/5,000 pg of IFN-γ) than to the same region in M. tuberculosis (10,000 cpm of IL-2/1,000 pg of IFN-γ). We have previously reported that B6 mice vaccinated with DNA encoding Ag85B from M. tuberculosis also react more strongly to 85B peptide spanning aa 244–260 than to peptide spanning aa 262–279 [46]. Sequence analysis of the 241–260 region of Ag85 revealed that the Ag85A sequence from M. ulcerans is more similar to the Ag85B sequence of M. tuberculosis (only 1 aa (A–D) change in position 242) than to the Ag85A sequence of M. tuberculosis (4 aa changes). Interestingly, it was demonstrated by Yanagisawa et al that vaccination of B6 mice with killed M. tuberculosis triggered preferentially a vβ11+ CD4+ T cell response against the peptide spanning amino acids 240 to 254 of Ag85B [55]. All these data taken together seem to indicate that the M. ulcerans Ag85A241–260 region is more immunogenic than the corresponding M. tuberculosis Ag85A region and this may explain the better protective efficacy that we have observed with the species specific M. ulcerans vaccine.
In conclusion, our results show that specific Ag85A-DNA priming followed by protein boosting is an effective way to induce robust Th1 type immune responses and strong protection against experimental footpad infection with M. ulcerans in mice. This is a promising vaccination approach that warrants further analysis. Combination with vaccines targeting mycolactone or with vaccines targeting enzymes involved in mycolactone synthesis may be a way to strengthen its protective efficacy.
Acknowledgments
We are grateful to Prof. F. Portaels (Institute of Tropical Medicine in Antwerp, Belgium) for providing us the virulent African strain 04-855 of M. ulcerans. We also thank P.Y. Adnet, F. Jurion, and R. Laali for excellent technical assistance, V. Rosseels for help with figure files, and E. Jongert for help in statistical analysis.
Footnotes
The authors have declared that no competing interests exist.
This work was partially supported by grants from the Damiaanaktie-Belgium, from FWO-Vlaanderen (Krediet aan Navorsers 1.5.144.04), and from Research Transversal Programme PTR 212. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Marsollier L, Robert R, Aubry J, Saint Andre JP, Kouakou H, et al. Aquatic insects as a vector for Mycobacterium ulcerans. Appl Environ Microbiol. 2002;68:4623–4628. doi: 10.1128/AEM.68.9.4623-4628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsollier L, Severin T, Aubry J, Merritt RW, Saint Andre JP, et al. Aquatic snails, passive hosts of Mycobacterium ulcerans. Appl Environ Microbiol. 2004;70:6296–6298. doi: 10.1128/AEM.70.10.6296-6298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott SnyderD, Small PLC. Uptake and cellular actions of mycolactone, a virulence determinant for Mycobacterium ulcerans. Microbial Pathogenesis. 2003;34:91–101. doi: 10.1016/s0882-4010(02)00210-3. [DOI] [PubMed] [Google Scholar]
- 4.Egidio Torrado, Sarojini Adusumilli, Fraga Alexandra G, Small Pamela LC, Castro Antonio G, et al. Mycolactone-Mediated Inhibition of Tumor Necrosis Factor Production by Macrophages Infected with Mycobacterium ulcerans Has implications for the Control of Infection. Infect Immun. 2007;75:3979–3988. doi: 10.1128/IAI.00290-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emmanuelle Coutanceau, Jeremie Decalf, Angelo Martino, Aurélie Babon, Nathalie Winter, et al. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. JEM. 2007;204:1395–1403. doi: 10.1084/jem.20070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutanceau E, Marsollier L, Brosch R, Perret E, Goossens P, et al. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell Microbiol. 2005;7:1187–1196. doi: 10.1111/j.1462-5822.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 7.Daniel Alexa K, Lee Richard E, Françoise Portaels, Small PLC. Analysis of Mycobacterium Species for the Presence of a Macrolide Toxin, Mycolactone. Infect Immun. 2004;72:123–132. doi: 10.1128/IAI.72.1.123-132.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mve-Obiang A, Lee RE, Umstot ES, Trott KA, Grammer TC, et al. A newly discovered mycobacterial pathogen isolated from laboratory colonies of Xenopus species with lethal infections produces a novel form of mycolactone, the Mycobacterium ulcerans macrolide toxin. Infect Immun. 2005;73:3307–3312. doi: 10.1128/IAI.73.6.3307-3312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranger BS, Mahrous EA, Mosi L, Adusumilli S, Lee RE, et al. Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F. Infect Immun. 2006;74:6037–6045. doi: 10.1128/IAI.00970-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann SH, Flesch IE. Cytokines in antibacterial resistance: possible applications for immunomodulation. Lung. 1990;168:1025–1032. doi: 10.1007/BF02718240. [DOI] [PubMed] [Google Scholar]
- 11.Gooding travis M, Johnson Paul DR, Campbell Dianne E, Hayman John A, Hartland Elizabeth L, et al. Immune Response to Infection with Mycobacterium ulcerans. Infect Immun. 2001;69:1704–1707. doi: 10.1128/IAI.69.3.1704-1707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gooding travis M, Johnson Paul DR, May Smith, Kemp Andrew S, Robins-Browne Roy M. Cytokine Profiles of Patients Infected with Mycobacterium ulcerans and Unaffected Household Contacts. Infect Immun. 2002;70:5562–5567. doi: 10.1128/IAI.70.10.5562-5567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prévot G, Bourreau E, Pascalis H, Pradinaud R, Tanghe A, et al. Differential production of systemic and intralesional gamma interferon and interleukin-10 in nodular and ulcerative forms of Buruli disease. Infect Immun. 2004;72:958–965. doi: 10.1128/IAI.72.2.958-965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorothy Yeboah-Manu, Elisabetta Pedruzzi, Ernestina Mensah-Quainoo, Adwoa Asante-Poku, David ofori-Adjei, et al. Systemic Suppression of Interferon-y responses in Buruli ulcer patients resolves after surgical excision of the lesions caused by the extracellular pathogen Mycobacterium ulcerans. Journal of Leukocyte Biology. 2006;79:1150–1156. doi: 10.1189/jlb.1005581. [DOI] [PubMed] [Google Scholar]
- 15.Phillips R, Horsfield C, Laing Mangan K, Awuah Etuaful P, Nyarko K, et al. Cytokine mRNA Expression in Mycobacterium ulcerans-Infected Human Skin and Correlation with Local Inflammatory Response. Infect Immun. 2006;74:2917–2924. doi: 10.1128/IAI.74.5.2917-2924.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiszewski AE, Becerril E, Aguilar LD, I.T.A K, Myers W, et al. The local immune response in ulcerative lesions of Buruli disease. Clinical and Experimental Immunology. 2006;143:445–451. doi: 10.1111/j.1365-2249.2006.03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobos Karen M, Spotts Ellen A, Marston Barbara J, HorsburghRobert C, KingHarold C. Serologic Response to Culture Filtrate Antigens of Mycobacterium ulcerans during Buruli Ulcer Disease. Emerging Infectious Diseases. 2000;6:158–164. doi: 10.3201/eid0602.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okenu DM, Ofielu LO, Easley KA, Guarner J, Spotts Whitney EA, et al. Immunoglobulin M Antibody Responses to Mycobacterium ulcerans Allow Discrimination between Cases of Active Buruli Ulcer Disease and Matched Family Controls in Areas Where the Disease Is Endemic. Clinical and Diagnostic Laboratory Immunology. 2004;11:387–391. doi: 10.1128/CDLI.11.2.387-391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz D, Dobeli H, Yeboah-Manu D, Mensah-Quainoo E, Friedlein A, et al. Use of the immunodominant 18-kiloDalton small heat shock protein as a serological marker for exposure to Mycobacterium ulcerans. Clin Vaccine Immunol. 2006;13:1314–1321. doi: 10.1128/CVI.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duker AA, Portaels F, Hale M. Pathways of Mycobacterium ulcerans infection: a review. Environ Int. 2006;32:567–573. doi: 10.1016/j.envint.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Chauty A, Ardant MF, Adeye A, Euverte H, Guedenon A, et al. Promising clinical efficacy of the combination streptomycin-rifampicin for the treatment of Buruli ulcer (Mycobacterium ulcerans disease). Antimicrob Agents Chemother. 2007;25 doi: 10.1128/AAC.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etuaful S, Carbonnelle B, Grosset J, Lucas S, Horsfield C, et al. Efficacy of the combination rifampicin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother. 2005;49:3182–3186. doi: 10.1128/AAC.49.8.3182-3186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huygen K. Prospects for vaccine development against Buruli disease. Expert Rev Vaccines. 2003;2:561–569. doi: 10.1586/14760584.2.4.561. [DOI] [PubMed] [Google Scholar]
- 24.Uganda, Buruli, Group. BCG vaccination against Mycobacterium ulcerans infection (Buruli ulcer). Lancet. 1969;i:111–115. [PubMed] [Google Scholar]
- 25.Smith PG, Revill WD, Lukwago E, Rykushin YP. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans R Soc Trop Med Hyg. 1977;70:449–457. doi: 10.1016/0035-9203(76)90128-0. [DOI] [PubMed] [Google Scholar]
- 26.Nackers F, Dramaix M, Johnson RC, Zinsou C, Robert A, et al. BCG vaccine effectiveness against Buruli ulcer: a case-control study in Benin. Am J Trop Med Hyg. 2006;75:768–774. [PubMed] [Google Scholar]
- 27.Portaels F, Aguiar J, Debacker M, Guedenon A, Steunou C, et al. Mycobacterium bovis BCG vaccination as prophylaxis against Mycobacterium ulcerans osteomyelitis in Buruli ulcer disease. Infect Immun. 2004;72:62–65. doi: 10.1128/IAI.72.1.62-65.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portaels F, Aguiar J, Debacker M, Steunou C, Zinsou C, et al. Prophylactic effect of Mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli ulcer). Clin Diagn Lab Immunol. 2002;9:1389–1391. doi: 10.1128/CDLI.9.6.1389-1391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stinear TP, Seemann T, Pidot S, Frigui W, Reysset G, et al. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007;17:192–200. doi: 10.1101/gr.5942807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coutanceau E, Legras P, Marsollier L, Reysset G, Cole ST, et al. Immunogenicity of Mycobacterium ulcerans Hsp65 and protective efficacy of a Mycobacterium leprae Hsp65-based DNA vaccine against Buruli ulcer. Microbes infect. 2006;8:2075–2081. doi: 10.1016/j.micinf.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Tanghe A, Content J, Van Vooren JP, Portaels F, Huygen K. Protective Efficacy of a DNA Vaccine Encoding Antigen 85A from Mycobacterium bovis BCG against Buruli Ulcer. Infect Immun. 2001;69:5403–5411. doi: 10.1128/IAI.69.9.5403-5411.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosseels V, Marché S, Roupie V, Govaerts M, Godfroid J, et al. Members of the 30-32-kilodalton Mycolyl Transferase Family (Ag85) from Culture Filtrate of Mycobacterium avium subsp paratuberculosis Are Immunodominant Th1-type Antigens Recognized Early upon Infection in Mice and Cattle. Infect Immun. 2006;74:202–212. doi: 10.1128/IAI.74.1.202-212.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belisle JT, Vissa VDST, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 34.Ronning DR, Klabunde T, Besra GS, Vissa VD, Belisle J, et al. Crystal structure of the secreted form of the antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000;7:141–146. doi: 10.1038/72413. [DOI] [PubMed] [Google Scholar]
- 35.McShane H, Pathan AA, Sander CR, Keatng SM, Gilbert SC, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 36.Langermans JA, Doherty TM, Vervenne RA, Van der Laan T, Lyashchenko K, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23:2740–2750. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 37.Tanghe A, D'Souza S, Rosseels V, Denis O, Ottenhoff THM, et al. Improved Immunogenicity and Protective Efficacy of a Tuberculosis DNA Vaccine Encoding Ag85 by Protein Boosting. Infect Immun. 2001;69:3041–3047. doi: 10.1128/IAI.69.5.3041-3047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore QC, Bosarge JR, Quin LR, McDaniel LS. Enhanced protective immunity against pneumococcal infection with PspA DNA and protein. Vaccine. 2006;24:5755–5761. doi: 10.1016/j.vaccine.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Miao J, Li X, Liu Z, Xue C, Bujard H, et al. Immune responses in mice induced by prime-boost schemes of the Plasmodium falciparum apical membrane antigen1 (PfAMA1)-based DNA, protein and recombinant Modified Vaccinia Ankara vaccines. Vaccine. 2006;24:6187–6198. doi: 10.1016/j.vaccine.2006.05.099. [DOI] [PubMed] [Google Scholar]
- 40.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–476. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 41.Huygen K, Lene Ljungqvist, Rob Ten Berg, Jean-Paul Van Vooren. Repertoires of Antibodies to Culture Filtrate Antigens in Different Mouse Strains Infected with Mycobacterium bovis BCG. Infect Immun. 1990;58:2192–2197. doi: 10.1128/iai.58.7.2192-2197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huygen K, Content J, Denis O, Montgomery DL, Yawman M, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 43.Gartner T, Baeten M, Otieno S, Revets H, De Baetselier P, et al. Mucosal prime-boost vaccination for tuberculosis based on TLR triggering OprI lipoprotein from Pseudomonas aeruginosa fused to mycolyl-transferase Ag85A. Immunology letters. 2007;111:26–35. doi: 10.1016/j.imlet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Huygen K, Lozes E, Gilles A, Drowart A, Palfliet K, et al. Mapping of Th1 helper T-cell epitopes on major secreted mycobacterial Ag85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huygen K, Abramowicz D, Vandenbussche P, Jacobs F, De Bruyn J, et al. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992;60:2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Souza S, Rosseels V, Romano M, Tanghe A, Denis O, et al. Mapping of Murine Th1 Helper T-Cell Epitopes of Mycolyl Transferases Ag85A, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect Immun. 2003;71:483–493. doi: 10.1128/IAI.71.1.483-493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanghe A, Adnet PY, Gartner T, Huygen K. A Booster Vaccination with Mycobacterium bovis BCG Does Not Increase the Protective Effect of the Vaccine against Experimental Mycobacterium ulcerans Infection in Mice. Infect Immun. 2007;75:2642–2644. doi: 10.1128/IAI.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO. Neglected Tropical Diseases-Hidden Successes, Emerging opportunities. 2006 [Google Scholar]
- 49.Frank Fenner. Homologous and Heterologous Immunity in Infections of Mice with Mycobacterium ulcerans and Mycobacterium balnei. Am Rev Tuberc. 1957;76:76–89. doi: 10.1164/artpd.1957.76.1.76. [DOI] [PubMed] [Google Scholar]
- 50.Torrado E, Fraga AG, Castro AG, Stragier P, Meyers WM, et al. Evidence for an Intramacrophage Growth Phase of Mycobacterium ulcerans. Infect Immun. 2007;75:977–987. doi: 10.1128/IAI.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frank Fenner. The Pathogenic Behavior of Mycobacterium ulcerans and Mycobacterium balnei in the Mouse and the Developing Chick Embryo. Am Rev Tuberc. 1956;73:650–673. doi: 10.1164/artpd.1956.73.5.650. [DOI] [PubMed] [Google Scholar]
- 52.Shu Q, Bir SH, Gill HS, Duan E, Xu Y, et al. Antibody response in sheep following immunization with Streptococcus bovis in different adjuvants. Vet res Commun. 2001;25:43–54. doi: 10.1023/a:1026757917968. [DOI] [PubMed] [Google Scholar]
- 53.Denis O, Tanghe A, Palfliet K, Jurion F, Van den Berg TP, et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romano M, Denis O, D'Souza S, Wang XM, Ottenhoff TH, et al. Induction of in vivo functional Db-restricted cytolytic T cell activity against a putative phosphate transport receptor of Mycobacterium tuberculosis. J Immunol. 2004;172:6913–6921. doi: 10.4049/jimmunol.172.11.6913. [DOI] [PubMed] [Google Scholar]
- 55.Yanagisawa S, Koike M, Kariyone A, Nagai S, Takatsu K. Mapping of V beta 11+ helper T cell epitopes on mycobacterial antigen in mouse primed with Mycobacterium tuberculosis. Int Immunol. 1997;9:227–237. doi: 10.1093/intimm/9.2.227. [DOI] [PubMed] [Google Scholar]