Abstract
Multiple myeloma is a clonal plasma cell malignancy that accounts for slightly more than 10% of all hematologic cancers. In this paper, we present a historically focused review of the disease, from the description of the first case in 1844 to the present. The evolution of drug therapy and stem-cell transplantation for the treatment of myeloma, as well as the development of new agents, is discussed. We also provide an update on current concepts of diagnosis and therapy, with an emphasis on how treatments have emerged from a historical perspective after certain important discoveries and the results of experimental studies.
Introduction
Multiple myeloma (MM) is a malignant plasma cell disorder that accounts for approximately 10% of all hematologic cancers.1,2 It usually evolves from an asymptomatic premalignant stage of clonal plasma cell proliferation termed “monoclonal gammopathy of undetermined significance” (MGUS). MGUS is present in more than 3% of the population above the age of 50 and progresses to myeloma or related malignancy at a rate of 1% per year.3,4 In some patients, an intermediate asymptomatic but more advanced premalignant stage, referred to as “smoldering multiple myeloma” (SMM), is clinically recognized.5 The annual incidence of myeloma, age-adjusted to the 2000 US population, is 4.3 per 100 000.6 Myeloma and MGUS are twice as common in blacks compared with whites and slightly more common in males than females.
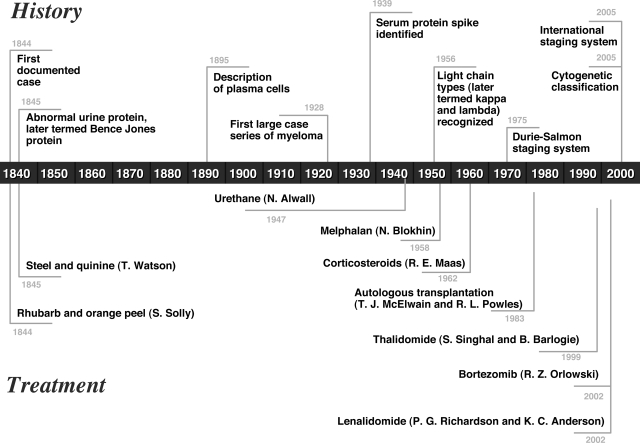
We provide a historically based review of myeloma from its first description to the present, with an emphasis on the evolution of diagnosis and therapy for the disease over the past 160 years (Figure 1).
Figure 1.
Timeline depicting the history and treatment of multiple myeloma from 1844 to the present.
Historical overview
Identification and description of the disease
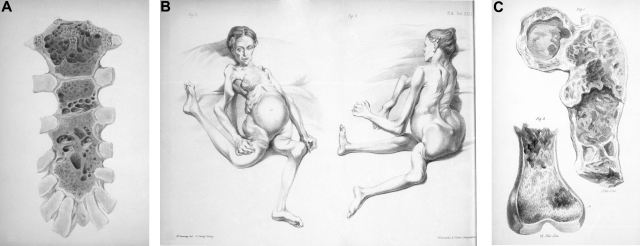
Although multiple myeloma has most likely been present for thousands of years, the first well-documented case was the second patient described by Solly in 1844.7 This 39-year-old woman, Sarah Newbury, developed fatigue and bone pain from multiple fractures (Figure 2). At autopsy, 4 years after the onset of symptoms, the bone marrow was found to be replaced by a red substance whose cells were very similar to those found at the autopsy of Thomas Alexander McBean. Solly thought that the disease was an inflammatory process and that it began with a “morbid action” of the blood vessels in which the “earthy matter of the bone is absorbed and thrown out by the kidneys in the urine.” Was he contemplating the role of angiogenesis in the pathophysiology of the disease?
Figure 2.
Sarah Newbury, the first reported patient with multiple myeloma. (A) Bone destruction in the sternum. (B) The patient with fractured femurs and right humerus. (C) Bone destruction involving the femur. Adapted from Solly7 with permission.
The best known case of multiple myeloma is that of Thomas Alexander McBean, “a highly-respectable tradesman,” 45 years of age. He had developed fatigue and had noted that his “body linen was stiffened by his urine.” While on holiday in September 1844, he vaulted out of an underground cavern and he suddenly “felt as if something had snapped or given way within the chest” and for some minutes he lay unable to stir because of severe pain. The pain was temporarily relieved by a “strengthening plaster to the chest” but recurred 3 to 4 weeks later. A pound of blood was removed and leeches were applied for “maintenance therapy.” This was followed by considerable weakness for 2 to 3 months, but his pain resolved. The pain recurred in the spring of 1845; cupping and therapeutic phlebotomy were not helpful and only made him weaker. Dr Thomas Watson, his physician, prescribed steel and quinine, which resulted in rapid improvement. That summer, he traveled to Scotland where he bounded over hills as “nimbly as any of his companions.”8 That fall, his pain recurred, and despite a variety of therapies, he died on January 1, 1846. On autopsy, soft, brittle, readily fractured ribs and a “gelatiniform substance of a blood-red colour and unctuous feel” was found in the bones. Histologic examination of the bone marrow revealed round or oval cells that were one-half to twice as large as an average blood cell and contained one or 2 nuclei and a bright-colored nucleolus.9
McBean had been seen in consultation on October 30, 1845, by Dr William Macintyre, a Harley Street consultant. His urine was examined and the following note and a sample of urine were sent to Henry Bence Jones (Figure 3) at St. George's Hospital.
Figure 3.
(A) Henry Bence Jones. (B) Otto Kahler (courtesy of Dr Heinz Ludwig, Vienna). (C) Jan Waldenström (courtesy of Giampaolo Merlini, Pavia, Italy).
Saturday, November 1, 1845:
Dear Dr Jones,
The tube contains urine of very high specific gravity. When boiled it becomes slightly opaque. On the addition of nitric acid, it effervesces, assumes a reddish hue, and becomes quite clear; but as it cools, assumes the consistence and appearance which you see. Heat liquefies it. What is it?10
Jones had already established a reputation as a chemical pathologist.9 Henry Bence Jones studied the urine from Mr McBean in great detail and confirmed the physical properties described by Macintyre. Jones concluded that the protein was the “hydrated deutoxide of albumen.”11 However, Jones emphasized its role in the diagnosis of myeloma for he said, “I need hardly remark on the importance of seeking for this oxide of albumen in other cases of mollities osseum” (softening of the bone).10 Jones' obituaries described his work on renal stones, diabetes mellitus, and malignant and tuberculous involvement of the kidney with an emphasis on the value of microscopic examination of the urine. There was no mention of his papers on the unique urinary protein that bears his name. (Incidentally, a hyphen does not occur in Jones' name in his more than 40 publications for which he used H. Bence Jones.)
The term “Kahler's disease” was once used to describe myeloma and resulted from a case report of a physician named Dr Loos by Prof Otto Kahler of Prague and subsequently Vienna (Figure 3). The patient had progressive bone pain, proteinuria with the typical heat characteristics of Bence Jones protein, and at autopsy, the presence of large, round cells consistent with multiple myeloma.
The term “plasma cell” was introduced by Waldeyer in 1875.12 It is probable that he was describing tissue mast cells rather than plasma cells. Ramon y Cajal, the neuroanatomist, was the first to accurately describe the plasma cell. Marschalko, in 1895, published the best description of plasma cells, which included blocked chromatin, eccentric position of the nucleus, a perinuclear pale area (hof), and a spherical or irregular cytoplasm. Wright13 thought that the tumor cells of myeloma consisted of plasma cells or immediate descendants of these cells. In 1929, Arinkin's14 introduction of bone marrow aspiration increased the recognition of multiple myeloma. For example, Rosenthal and Vogel15 reported that only 3 cases of multiple myeloma had been recognized in Mount Sinai Hospital in New York from 1916 to 1935 but that 13 cases were found in the succeeding 2.5 years. In 1928, Geschickter and Copeland16 reported on 412 cases of multiple myeloma found in the literature from 1848 to 1928. They emphasized the presence of pathologic fractures, Bence Jones proteinuria, anemia, and chronic renal disease. However, they did not recognize the abnormalities of the sedimentation rate or blood proteins.
Bence Jones protein and light chain isotypes
Although Heller17 in 1846 described a protein in urine that precipitated when warmed above 50°C and then disappeared on further heating, he did not recognize its precipitation when it cooled. The term “Bence Jones protein” was first used by Fleischer in 1880.18 Bayne-Jones and Wilson19 described 2 groups of Bence Jones protein in 1922. Using the Ouchterlony test in 1956, Korngold and Lipari, his technician,20 identified different classes of Bence Jones proteins. They also demonstrated that antisera to Bence Jones protein also reacted with the myeloma protein in the blood. As a tribute to Korngold and Lipari, the 2 classes of Bence Jones proteins have been designated kappa and lambda. In 1962, Edelman and Gally21showed that light chains prepared from an IgG monoclonal protein in the serum and the Bence Jones protein from the same patient's urine had an identical amino acid composition as well as multiple other properties. The light chains had the same heat properties as Bence Jones protein, thus solving the mystery of the origin of this unique protein 115 years after the work of Henry Bence Jones.
Identification of the serum monoclonal protein
Hyperproteinemia was first demonstrated in multiple myeloma in 1928 by Perlzweig et al.22 Tiselius, using the moving-boundary method of electrophoresis in his doctoral dissertation in 1930, demonstrated the homogeneity of certain serum globulins. Seven years later, Tiselius23 separated serum globulins into 3 components, which he designated as alpha, beta, and gamma. It is interesting to note that his first manuscript was rejected by Biochemical Journal because it was “too physical.” In 1939, Tiselius and Kabat24 demonstrated antibody activity in the gamma globulin fraction.
The moving-boundary electrophoresis apparatus, using a U-tube, became commercially available, but it was very cumbersome. A single electrophoresis run required a full day of effort and interpretation was difficult. The tall, narrow-based, “church spire” peak characteristic of multiple myeloma was recognized in 1939.25 In 1951, filter paper as a support permitted the separation of protein into discrete zones, which could be stained with a variety of dyes.26 Cellulose acetate supplanted filter paper and currently electrophoresis on agarose gel or capillary electrophoresis is used in most laboratories. Immunoelectrophoresis was described by Grabar and Williams in 1953.27 Eleven years later, immunofixation was introduced by Wilson.28
A critical milestone was the concept of monoclonal vs polyclonal gammopathies presented in the Harvey Lecture Series by Jan Waldenström (Figure 3) in 1961.29 He described patients with a narrow band of hypergammaglobulinemia on electrophoresis as having a monoclonal protein. Many of these patients had multiple myeloma or macroglobulinemia, but others had no evidence of malignancy and he considered them to have “essential hypergammaglobulinemia” or a “benign monoclonal protein.” Today, the preferred term is MGUS, because multiple myeloma, macroglobulinemia, light-chain (AL) amyloidosis, or a related disorder may subsequently develop.30 Waldenström regarded the broad band in hypergammaglobulinemia as a polyclonal increase in proteins. A distinction between monoclonal versus polyclonal gammopathies is important because patients with a monoclonal gammopathy either have a neoplastic process or may develop a malignancy, whereas patients with a polyclonal gammopathy have an inflammatory or reactive process.30
Origins of alkylator and corticosteroid-based therapy
The current treatment of multiple myeloma has improved markedly compared with the rhubarb pill and infusion of orange peel that was given to Sarah Newbury in 1844.7 Mr McBean obtained benefit from phlebotomy and the application of leeches, which was used as “maintenance therapy.” Later, his response to steel and quinine was impressive.7–9
In 1947, Alwall31 reported that urethane produced a reduction in serum globulin, an increase in hemoglobin, disappearance of proteinuria, and a decrease in bone marrow plasma cells in a patient with multiple myeloma. This was the standard of therapy for more than 15 years. In 1966, Holland et al32 randomized 83 patients with treated or untreated multiple myeloma to receive urethane or a placebo consisting of cherry and cola–flavored syrup. No difference was seen in the objective improvement or in survival between the 2 treatment groups.
Blokhin et al33 in 1958 reported the benefit of sarcolysin (melphalan) in 3 of 6 patients with multiple myeloma. In 1962, Bergsagel et al34 reported significant improvement in 8 of 24 pa-tients with multiple myeloma who were treated with melphalan. Subsequently, Hoogstraten et al found that melphalan given as a loading dose for 1 week followed by maintenance therapy produced some responses in 78% of 64 patients with newly diagnosed or previously treated multiple myeloma.35
Corticosteroids were first tested by Maas, who determined in a placebo-controlled double-blind trial that prednisone as a single agent produced significant decreases in serum globulin and an increase in hematocrit but no difference in survival compared with a placebo.36 In another study, prednisone, in a single dose of 200 mg every other morning, was reported to produce benefit in 8 of 10 patients with poor-risk myeloma, and toxic reactions were minimal.37 In a reanalysis of 2 Cancer and Leukemia Group B myeloma treatment protocols, prednisone as a single agent produced a 44% objective response rate.38 The classic regimen of melphalan plus prednisone (MP) was established in a randomized trial of 183 myeloma patients led by Alexanian et al, in which survival was 6 months longer with MP compared with melphalan alone.39
Harley et al introduced the combination of carmustine, melphalan, cyclophosphamide, and prednisone in the treatment of multiple myeloma. In 1974, Lee et al treated 36 myeloma patients with carmustine, cyclophosphamide, melphalan, vincristine, and prednisone (M-2 protocol) and reported that 60% had excellent subjective and objective responses.40 Subsequently, Case et al reported an 87% response rate in 73 patients with myeloma given the M-2 protocol.41
In a large meta-analysis of individual data of 4930 persons from 20 randomized trials comparing MP with various combinations of therapeutic agents, response rates were significantly higher with combination chemotherapy (60%; MP, 53%; P < .001). However, there was no significant difference in response duration or overall survival.42 MP thus remained the mainstay of myeloma therapy for decades.
History of stem-cell transplantation
It is surprising to note that more than one-half of the scientific session at the first organizational meeting of the American Society of Hematology held in the Aescalapian Room of the Harvard Club in Boston, Massachusetts, on April 7, 1957, was devoted to the preservation and transplantation of human bone marrow. That same year, Thomas et al43 treated 6 human patients (1 had myeloma) with total body irradiation or chemotherapy followed by an intravenous infusion of bone marrow cells. The first successful syngeneic bone marrow transplantation for myeloma was reported in 2 physician brothers.44 Fefer et al45 described 5 myeloma patients who received a syngeneic bone marrow transplant. Gahrton et al46 reported that 10 of 14 patients with multiple myeloma who received an allogeneic bone marrow transplant from an HLA-compatible sibling donor survived for a median of 12 months.
McElwain and Powles47 first reported autologous bone marrow transplantation in a patient with plasma cell leukemia. The patient was given 140 mg/m2 of melphalan and required platelet support and antibiotics; after relapse 16 months later, he was again given 140 mg/m2 of melphalan followed by an intravenous autograft obtained from his remission marrow. Two of 4 previously untreated myeloma patients obtained a complete response, whereas 1 of 4 previously treated patients had a complete response.47 Selby et al48 reported that 11 (27%) of 41 patients with previously untreated multiple myeloma obtained a complete remission after a single intravenous dose of melphalan (140 mg/m2). Unfortunately, most of the patients relapsed with a median duration of remission of 19 months.
Barlogie et al49 used melphalan 140 mg/m2 and total body irradiation (850 cGy) followed by autologous or allogeneic bone marrow transplantation in 6 patients with multiple myeloma refractory to chemotherapy. Subsequently, Barlogie developed intense treatment programs using autologous transplantation (“total therapy”) that eventually played a major role in establishing high-dose therapy and stem cell rescue as standard therapy for myeloma.
New drugs
In the past decade, there have been major advances in the treatment of myeloma. Thalidomide,50 bortezomib,51,52 and lenalidomide53,54 have emerged as highly active agents in the treatment of myeloma. Each has a unique and interesting historical perspective.
Thalidomide
Historical perspective.
Chemie Grünenthal, a German pharmaceutical company, introduced thalidomide (α-N-[phthalimido] glutarimide) into the market as a sedative on October 1, 1957.55 By 1960, it was sold in more than 40 countries and became popular both as a sedative and as treatment for morning sickness of pregnancy. It was marketed under various commercial names, such as Contergan, Distaral, Softenon, Neurosedyn, Isomin, Kedavon, Telargan, and Sedalis.56
On November 18, 1961, Widukind Lenz, a German physician and geneticist, determined that thalidomide was associated with severe teratogenic malformations.56 In December 1961, independent confirmation came from William McBride, an Australian obstetrician.57 Fetal malformations occurred inevitably when the drug was taken in the first trimester between days 35 and 49 after the last menstrual period. By the end of 1961, thalidomide was taken off the market in most countries, but almost 10 000 infants had already been affected. The United States was spared because the drug had been denied approval by Dr Frances Kelsey at the US Food and Drug Administration (FDA), who was concerned about the lack of safety data.
Shortly after its teratogenic properties were discovered, it is important to note that thalidomide was considered as a possible treatment against cancer.58 Two clinical trials were undertaken in patients with advanced cancer,59,60 but no significant activity was seen. These 2 trials did enroll a few myeloma patients, but the antimyeloma activity of thalidomide was not evident in either trial, illustrating that clinically important activity can be missed if trials do not target specific malignancies.
Thalidomide persisted as a therapeutic agent due to promising activity later seen in leprosy (1964), Behçet disease (1979), graft-versus-host disease (1988), and human immunodeficiency virus (HIV)–associated oral ulcers and wasting (1989).55 Under pressure from HIV activists and to discourage illegal drug distribution networks, the FDA approved thalidomide for the treatment of erythema nodosum leprosum in July 1998 with a built-in safety system, termed the System for Thalidomide Education and Prescribing Safety program.
In 1994, D'Amato et al61 described for the first time the significant antiangiogenic properties of thalidomide in the rabbit cornea micropocket assay. In late 1997, based on the increasing awareness of angiogenesis in the pathogenesis of cancer and the evidence of increased angiogenesis in myeloma, the spouse of an affected myeloma patient convinced Barlogie and colleagues at the University of Arkansas to initiate a compassionate-use trial of “antiangiogenic therapy.” The idea to use thalidomide in this setting came from Folkman, in a direct telephone conversation with Barlogie. Barlogie should be fully credited for pursuing this strategy, in a landmark trial that enrolled 84 patients.50 Remarkably, 32% of patients responded to thalidomide, making it the first new drug with single-agent activity for myeloma in more than 3 decades.50
Activity in myeloma.
Initial results with thalidomide observed in the Arkansas study were then confirmed by many other centers, in all phases of the disease.62 Response rates in relapsed disease are approximately 50% with the combination of thalidomide and steroids, and 65% with a 3-drug combination of thalidomide, steroids, and cyclophosphamide. Several other combination chemotherapy regimens containing thalidomide have since been developed.
Bortezomib
Historical perspective.
The multicatalytic ubiquitin-proteasome pathway is responsible for the orderly degradation of eukaryotic cellular proteins.63 In 2004, Aaron Ciechanover (Israel), Avram Hershko (Israel), and Irwin Rose (United States) shared the Nobel Prize in Chemistry for their discovery of the role of ubiquitination in protein degradation.
The 26S proteasome consists of a core 20S catalytic complex and a 19S regulatory complex. Ubiquitin-tagged proteins are recognized by the 19S regulatory complex where the ubiquitin tags are removed. Proteins then move into the 20S proteasome cylinder for hydrolysis into small polypeptides. Inhibition of the proteasome leads to cellular apoptosis, with malignant, transformed, and proliferating cells being more susceptible.64,65
Numerous proteasome inhibitors were developed, but the initial compounds lacked specificity and were not suitable for clinical use. Subsequently, Adams et al64 designed and developed several boronic acid derived compounds that inhibit the proteasome pathway in a highly specific manner. Bortezomib, a boronic acid dipeptide, was then selected for preclinical and clinical testing.51 Preclinical studies demonstrated that bortezomib had potent cytotoxic and growth inhibitory effects. The initial clinical study with bortezomib in advanced hematologic malignancies was led by Robert Orlowski at the University of North Carolina.66 Leading up to the trial, Orlowski's laboratory was actively investigating the proteasome pathway, an area of research his father, Marian Orlowski, had pioneered years earlier. Marian Orlowski (working in collaboration with Sherwin Wilk) was the first to discover the multicatalytic intracellular proteinase complex (later known as the 20S proteasome) and developed the first proteasome inhibitor, benzyloxycarbonyl-prolyl-prolinal (a peptide aldehyde inhibitor).67,68
Bortezomib demonstrated striking antimyeloma activity in the initial phase 1 study.66 It also showed activity against myeloma cells in several preclinical models in a series of experiments conducted in the laboratories of Kenneth Anderson at the Dana-Farber Cancer Institute.69
Activity in myeloma.
The first phase 2 trial with bortezomib was conducted in 202 patients with relapsed refractory myeloma. Approximately one-third responded to bortezomib therapy, with an average response duration of 1 year.51 These results led to the approval of bortezomib by the FDA in May 2003. In a subsequent randomized trial, time to disease progression was found to be superior with bortezomib compared with dexamethasone alone in patients with relapsed, refractory myeloma.52 Bortezomib has recently been effectively combined with intravenous liposomal doxorubicin in a trial that demonstrated, for the first time in a randomized manner, the antimyeloma activity of anthracyclines.70
Lenalidomide
Historical perspective.
Shortly after thalidomide was determined to be a teratogen, several analogs of thalidomide were synthesized to determine the mechanism of its teratogenicity. Similarly, in the 1990s, several thalidomide analogs were synthesized in an attempt to increase efficacy and minimize toxicity. Lenalidomide, a 4-amino substituted analog of thalidomide formerly called CC-5013, belongs to a class of thalidomide analogs termed immunomodulatory drugs. Thus, in contrast to the clinical development of thalidomide, the development of lenalidomide was inevitable.
Based on the preclinical studies conducted by the manufacturer and at the Dana-Farber Cancer Institute, lenalidomide was tested in phase 1 trials in relapsed refractory myeloma.71
Activity in myeloma.
Richardson et al54 conducted a multicenter randomized phase 2 trial that enrolled 102 patients with relapsed/refractory myeloma. Overall response rate with single-agent lenalidomide was 17%. In a phase 2 trial conducted at Mayo Clinic, 31 of 34 patients (91%) with newly diagnosed myeloma achieved an objective response with lenalidomide plus dexamethasone.53 Two large phase 3 trials have since shown significantly superior time to progression with lenalidomide plus dexamethasone compared with placebo plus dexamethasone in relapsed myeloma.72,73 Lenalidomide plus dexamethasone was approved by the FDA in June 2006 for the treatment of myeloma in patients who have failed one prior therapy.
Current therapy
Since 1975, the Durie-Salmon staging system has been used to stratify patients with multiple myeloma.74 However, this staging system had limitations, especially in the categorization of bone lesions. Recently, Greipp et al,75 developed an International Staging System based on data from 11 171 patients. Besides stage, important prognostic factors that stratify patients into high risk and standard risk are deletion 13 or hypodiploidy on conventional karyotyping, deletion 17p− or immunoglobulin heavy chain translocations t(4;14) or t(14;16) on molecular genetic studies, and plasma cell labeling index of 3% or higher.76 The presence of any one or more of these high-risk factors classifies a patient as having high-risk myeloma. The median survival of patients with high-risk features is only 2 to 3 years, even with tandem stem-cell transplantation, compared with 5 or more years in patients with standard-risk disease.
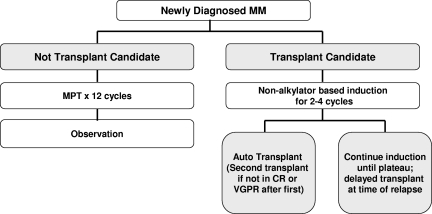
There is no evidence that early treatment of patients with asymptomatic (smoldering) multiple myeloma prolongs survival. However, clinical trials are ongoing to determine whether newer agents can delay progression. The treatment of symptomatic myeloma depends on eligibility for stem-cell transplantation and risk assessment (Figure 4).76 Table 1 lists selected landmark phase 3 trials that have altered the treatment of myeloma over the years.52,72,73,77–85
Figure 4.
Algorithm outlining the current approach to the treatment of newly diagnosed myeloma. CR indicates complete response; MPT, melphalan, prednisone, thalidomide; and VGPR, very good partial response.
Table 1.
Results of selected randomized trials that have had a major impact on myeloma therapy
| Trial, treatment comparison | Total no. of patients studied | CR/VGPR, % | Median PFS, mo | Median OS, mo | Comments |
|---|---|---|---|---|---|
| Stem-cell transplantation | |||||
| IFM 90*77 | |||||
| Conventional chemotherapy | 100 | 13 | 18 | 44 | Established role of ASCT |
| ASCT | 100 | 38 | 28 | 57 | — |
| MRC VII*78 | |||||
| Conventional chemotherapy | 201 | 8 | 20 | 42 | Confirmed role of ASCT |
| ASCT | 200 | 44 | 32 | 54 | — |
| MAG 90*79 | |||||
| Delayed ASCT | 94 | 21 | 13 | 65 | Demonstrated delayed ASCT as an alternative |
| Early ASCT | 91 | 32 | 39 | 64 | — |
| United States Intergroup S9321*80 | |||||
| Delayed ASCT | 255 | 15§ | 21 | 64 | Demonstrated delayed ASCT as an alternative; dampened enthusiasm for allogeneic SCT |
| Early ASCT | 261 | 17§ | 25 | 58 | — |
| Allogeneic SCT | 36 | 17§ | NR | 6 | — |
| IFM 94*81 | |||||
| Single ASCT | 199 | 42 | 25 | 48 | Established role of tandem ASCT if CR/VGPR not achieved with first ASCT |
| Double ASCT | 200 | 50 | 30 | 58 | — |
| PETHEMA†82 | |||||
| Conventional chemotherapy | 83 | 11§ | 33 | 66 | Demonstrated that ASCT may have limited value in patients responding well to induction |
| ASCT | 81 | 30§ | 42 | 61 | — |
| Italian*83 | |||||
| Tandem ASCT | 82 | NA | 29 | 54 | Demonstrated efficacy of single ASCT followed by nonmyeloablative SCT in selected patients |
| Single ASCT followed by nonmyeloablative SCT | 80 | NA | 35 | 80 | — |
| New therapies | |||||
| Italian GIMEMA group*84 | |||||
| MP | 126 | 12 | 27 | 64% at 3 y | Demonstrated potential value of MPT over the classic MP regimen |
| MPT | 129 | 36 | 54 | 80% at 3 y | — |
| IFM 99-06*85 | |||||
| MP | 196 | 7 | 18 | 33 | Changed standard of care in elderly to MPT (after 3 decades of MP) |
| MPT | 125 | 47 | 28 | 52 | — |
| Tandem intermediate-dose ASCT | 126 | 43 | 19 | 38 | — |
| APEX‡52 | |||||
| Bortezomib | 333 | 13¶ | 6‖ | NR | Led to full approval of bortezomib in the United States |
| Dex | 336 | 2¶ | 3‖ | NR | — |
| MM-010‡72 | |||||
| Len/Dex | 176 | 24¶ | 11‖ | Not reached | Pivotal trial establishing role of Len |
| Placebo/Dex | 175 | 5¶ | 5‖ | 21 | — |
| MM-009‡73 | |||||
| Len/Dex | 177 | 24¶ | 11§ | 30 | Led to approval of Len in the United States |
| Placebo/Dex | 176 | 2¶ | 5§ | 20 | — |
CR indicates complete response; VGPR, very good partial response; PFS, progression-free survival; OS, overall survival; IFM, InterGroupe Francophone du Myélome; MRC, Medical Research Council; MAG, Myélome Autogreffe; PETHEMA, Programa para el Estudio y Tratamiento de las Hemopatias Malignas; GIMEMA, Gruppo Italiano Malattie Ematologiche dell'Adulto; ASCT, autologous hematopoietic stem-cell transplantation; NA, not applicable; SCT, stem-cell transplantation; MP, melphalan plus prednisone; MPT, melphalan, prednisone, thalidomide; NR, not reported; Dex, dexamethasone; MM, multiple myeloma; and Len, lenalidomide.
Newly diagnosed disease stage.
Postinduction (responding patients only) disease stage.
Relapsed, refractory (1-3 prior therapies) disease stage.
CR only; VGPR not reported.
CR or near CR.
PFS data not available; numbers reflect median time to progression.
Initial therapy in patients eligible for transplantation
Vincristine, doxorubicin, dexamethasone (VAD) was used for many years as pretransplantation induction therapy.86 However, VAD is no longer recommended or used as initial therapy after the introduction of several newer induction regimens. The most common induction regimens used today are thalidomide–dexamethasone (Thal/Dex), bortezomib-based regimens, and lenalidomide–dexamethasone (Rev/Dex).
Thal/Dex.
In the last few years, Thal/Dex has emerged as the most commonly used induction regimen for the treatment of newly diagnosed myeloma in the United States. In an Eastern Cooperative Oncology Group (ECOG) randomized trial of 202 patients, the best response within 4 cycles was significantly higher with Thal/Dex compared with dexamethasone alone (63% vs 41%, respectively, P = .002).87 Based on this trial, in May 2006 the FDA granted accelerated approval for Thal/Dex for the treatment of newly diagnosed myeloma. Preliminary results from a separate randomized, double-blind, placebo-controlled study comparing Thal/Dex vs dexamethasone alone as primary therapy in 470 patients with newly diagnosed myeloma confirm these findings.88
Rev/Dex.
In a phase 2 trial conducted at Mayo Clinic, 91% of patients with newly diagnosed myeloma achieved an objective response, including 56% who achieved very good partial response or better.53,89 ECOG recently reported preliminary findings of a randomized trial testing lenalidomide/high-dose dexamethasone (dexamethasone 40 mg days 1-4, 9-12, 17-20) versus lenalidomide/low-dose dexamethasone (dexamethasone 40 mg once weekly).90 Preliminary results show significantly better overall survival and safety with lenalidomide/low-dose dexamethasone.
Bortezomib-based regimens.
In newly diagnosed myeloma, high response rates (approximately 70%-90%) have been observed with bortezomib plus dexamethasone, bortezomib, thalidomide, dexamethasone (VTD), and other bortezomib-based combinations.91,92 Harousseau et al93 recently reported preliminary results of a randomized trial comparing VAD versus bortezomib/dexamethasone as pretransplant induction therapy. With more than 400 patients enrolled, preliminary results show superior response rates with bortezomib/dexamethasone.
Initial therapy in patients not eligible for transplantation
Patients who are not transplant candidates are treated with standard alkylating agent therapy. The 3 most commonly used regimens are discussed below.
Melphalan, prednisone, thalidomide (MPT).
Three recent randomized trials have compared MP with MPT.84,85,94 Palumbo et al84 randomized patients either to standard-dose MP for 6 months or to MPT for 6 months followed by maintenance thalidomide. There was a trend toward an improved 3-year overall survival with MPT. Facon et al85 randomized 447 patients (age, 65-75 years) to MP versus MPT versus tandem autologous stem-cell transplantation (ASCT) with reduced-dose melphalan (100 mg/m2). Significantly higher response and progression-free survival rates were observed with MPT compared with either MP or tandem ASCT groups. More importantly, the trial demonstrated a significant survival advantage with MPT (median overall survival not reached at 52 months, 33 months, and 38 months, respectively; P < .001). Hulin et al94 confirmed a survival advantage with MPT compared with MP in a randomized trial in patients over the age of 75 years. After numerous attempts to improve on MP over the years with a variety of combination chemotherapy regimens, the results of these 3 randomized trials finally changed the standard of care for elderly patients.
Melphalan, prednisone, bortezomib (MPV).
Mateos et al95 studied the novel combination of MPV in newly diagnosed myeloma in patients 65 years of age or older. Therapy was associated with a response rate of 89%, including 32% complete response rate. A phase 3 trial comparing MPV with MP has recently been closed by a data monitoring committee in view of superior response rates and survival associated with MPV.
Melphalan, prednisone, lenalidomide (MPR).
Palumbo et al96 tested MPR in 54 newly diagnosed patients older than 65 years. At the maximum tolerated dose, the overall response rate was 81%, with 48% of patients achieving at least very good partial response or better, and 24% of patients achieving complete response. An ECOG randomized trial is comparing MPR with MPT.
Hematopoietic stem-cell transplantation
ASCT.
Although not curative, ASCT improves complete response rates and prolongs median overall survival in myeloma by approximately 12 months.77,78 The mortality rate is 1% to 2%. Melphalan, 200 mg/m2, is the most widely used conditioning regimen.
Randomized trials show that survival is similar whether ASCT is done early (immediately after 4 cycles of induction therapy) or delayed (at the time of relapse as salvage therapy).79,80 Thus, the decision on timing of ASCT is based on patient and physician preference and the ability to cryopreserve stem cells. In a Spanish randomized trial,82 patients responding to induction therapy had similar overall and progression-free survival with either ASCT or 8 additional courses of chemotherapy, raising a question concerning the benefit of ASCT in patients responding to induction chemotherapy. The need for early ASCT in an era of new drugs is the most important clinical question in myeloma today.
Tandem transplantation.
With tandem (double) ASCT, patients receive a second planned ASCT after recovery from the first procedure. The InterGroupe Francophone du Myélome (IFM) 94 randomized trial found significantly better event-free and overall survival in recipients of double versus single ASCT.81 A similar trend was also demonstrated in a randomized trial conducted in Italy.97 In both the French and Italian trials, the benefit of a second ASCT was restricted to patients failing to achieve a complete response or very good partial response with the first procedure.
Allogeneic transplantation.
Only a limited number of myeloma patients are candidates for allogeneic transplantation because of age, availability of an HLA-matched sibling donor, and adequate organ function. The high treatment-related mortality, mainly related to graft-versus-host disease, has made conventional allogeneic transplants unacceptable for most patients with myeloma. Studies using a concept of an ASCT followed by a planned reduced intensity (RIC) allogeneic stem-cell transplant have shown promise.98 A French randomized trial tested this approach in high-risk patients.99 Based on biologic randomization, patients were allocated either to tandem ASCT or to ASCT followed by an RIC allogeneic SCT. There was no significant difference between the 2 arms with a median follow-up of 24 months. In another randomized trial, a significant survival advantage was seen with ASCT followed by an RIC allogeneic SCT compared with tandem ASCT. On an intent-to-treat basis, the median overall and event-free survival were longer with autologous SCT followed by an RIC compared with tandem ASCT (80 months vs 54 months, P = .01; and 35 months vs 29 months, P = .02).83 However, the sample size was modest (n = 162), and additional confirmation is needed.
Maintenance therapy
Observation is still the standard following initial therapy as described (Figure 4). Interferon does not appear to provide significant clinical benefit.80 A recent trial (IFM 99-02) randomized 597 patients (age < 65 years) after tandem ASCT to no maintenance, pamidronate, or pamidronate plus thalidomide.100 The 4-year overall survival rate was superior with thalidomide (77%, 74%, and 87%, respectively, P < .04), but these results need further confirmation. Clinical trials are currently evaluating thalidomide, lenalidomide, bortezomib, and other novel approaches as maintenance therapy.
Treatment of high-risk myeloma
Patients with high-risk myeloma tend to do poorly with median overall survival of approximately 2 years even with tandem ASCT.101 Incorporation of new agents early in the disease course is a major option for treatment.76 ASCT followed by RIC-allogeneic SCT may also be an option in selected patients. A third option is to incorporate routine maintenance therapy after treatment as outlined earlier for patients with standard-risk disease.
Advances in supportive care
Numerous improvements in supportive care have greatly improved the outcome of myeloma patients, but a detailed discussion is beyond the scope of this paper. Some of the most important advances are the advent of bisphosphonates to treat hypercalcemia and to prevent myeloma bone disease, the use of vertebroplasty and kyphoplasty to treat vertebral fractures, and judicious use of prophylactic antibiotics in selected patients.
Acknowledgment
This work was supported in part the by the National Cancer Institute, Bethesda, MD (grants CA62242, CA85818, CA93842, and CA100080).
Biographies
Robert A. Kyle We were required to spend six months in a laboratory during our fellowship (residency) in Internal Medicine at the Mayo Clinic. The options were Physiology, Pathology, and Hematology. The major emphasis in Physiology was cardiovascular, and the resident was required to personally have a cardiac catheterization. Pathology did not sound very stimulating, but hematology, which was glossed over in my medical school and internship, was foreign to me. Furthermore, I thought that learning to read peripheral blood smears and bone marrows would enhance my future internal medicine practice in California. My laboratory project consisted of a study of acquired hemolytic anemia in chronic lymphocytic leukemia and the lymphomas. I emphasized that these patients often responded to corticosteroids and that the Coombs' test was positive in a number of the patients. After completing the study, I found in the literature that Pappenheim, 50 years before my report, had emphasized the role of hemolytic anemia in lymphocytic leukemia. The only contribution that I made was that the Coombs' test was often positive and the patients responded to corticosteroids—both of which had been discovered recently. I enjoyed laboratory hematology very much but realized that I had little experience in the clinical aspects. Consequently, I asked to be assigned to the clinical hematology hospital service. I noted an electrophoretic pattern and asked the attending physician what it meant. He stated that a spike was often seen in patients with multiple myeloma and Waldenström's macroglobulinemia and suggested that I look into the matter. I reviewed the 6500+ electrophoretic patterns that had been performed at Mayo Clinic from 1956 to 1959 and developed a formula for their recognition. Patients with multiple myeloma or Waldenstrom's macroglobulinemia usually had a spike with a height:width ratio > 4:1, while patients with chronic liver disease or other inflammatory processes often had a broad-based peak and a ratio of < 4:1. This was before Waldenström's seminal paper on the concept of monoclonal versus polyclonal gammopathies and before the clinical use of immunoelectrophoresis or immunofixation. On that same hospital service, a woman was admitted with a diagnosis of scleroderma. Someone requested a dermatology consultation and, surprisingly, she was found to have primary systemic amyloidosis. I knew nothing of this entity, so I then reviewed the 81 cases that had been identified at Mayo Clinic from 1935 to 1959. We emphasized that patients with primary amyloidosis had abnormal plasma cells in the bone marrow in all instances; in fact, almost one-half had classic multiple myeloma.
I began collecting sera from patients with plasma cell disorders in 1961 when I joined the Mayo Clinic staff, and this has developed into a bank containing more than 177 000 specimens. In 1968, I started the Special Protein Laboratory and, 2 years later, began the Dysproteinemia Clinic where patients with plasma cell disorders were seen. This resulted in the opportunity to see patients with gammopathies both in the laboratory and clinically. This led to a program project grant from the National Cancer Institute in the early 1970s and continues to the present.
The Special Protein Laboratory, serum bank, and program project eventually led to the description and long-term follow-up of monoclonal gammopathy of undetermined significance (MGUS), smoldering multiple myeloma (SMM), idiopathic Bence Jones proteinuria and a lifetime working in the field of plasma cell disorders. It has been a fascinating experience. I would recommend to those entering the field to evaluate and observe their patients carefully. Every patient has something to tell us.
I became interested in hematology as a third-year medical student at the Christian Medical College, Vellore, India, inspired by an excellent hematologist, Dr. Mammen Chandy. The opportunity to pursue hematology as a research career arose during my fellowship training at the Mayo Clinic. During this period, and subsequently as a faculty member at Mayo, I was privileged to work with a number of noted hematologists including Morie Gertz, Philip Greipp, Ayalew Tefferi, and my mentor, Bob Kyle. With their steadfast support and encouragement, I pursued clinical and laboratory investigations exploring the role of novel therapy and angiogenesis in myeloma and related disorders. Also critical to my career has been leadership roles in randomized clinical trials, and the myeloma committee of the Eastern Cooperative Oncology Group (ECOG). Hematology is moving at a rapid pace, and advances in molecular medicine and targeted therapy are making a mark in our field more than any other. I consider myself privileged to be part of this community.
Authorship
Contribution: R.A.K. and S.V.R. did the required background research for this manuscript and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert A. Kyle, Division of Hematology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; e-mail: kyle.robert@mayo.edu.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Kyle RA. Plasma cell disorders. In: Goldman L, Ausiello D, editors. Cecil Textbook of Medicine. 23rd ed. Philadelphia, PA: Saunders; 2007. pp. 1426–1437. [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582–2590. doi: 10.1056/NEJMoa070389. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., 3rd Incidence of multiple myeloma in Olmsted County, Minnesota: trend over 6 decades. Cancer. 2004;101:2667–2674. doi: 10.1002/cncr.20652. [DOI] [PubMed] [Google Scholar]
- 7.Solly S. Remarks on the pathology of mollities ossium with cases. Med Chir Trans Lond. 1844;27:435–461. doi: 10.1177/095952874402700129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macintyre W. Case of mollities and fragilitas ossium, accompanied with urine strongly charged with animal matter. Med Chir Trans Lond. 1850;33:211–232. doi: 10.1177/095952875003300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle RA. Multiple myeloma: an odyssey of discovery. Br J Haematol. 2000;111:1035–1044. doi: 10.1046/j.1365-2141.2000.02318.x. [DOI] [PubMed] [Google Scholar]
- 10.Bence Jones H. Chemical pathology. Lancet. 1847;2:88–92. [Google Scholar]
- 11.Bence Jones H. On the new substance occurring in the urine of a patient with mollities ossium. Philos Trans R Soc Lond. 1848;138:55–62. [Google Scholar]
- 12.Waldeyer W. Ueber bindegewebszellen. Arch Microbiol Anat. 1875;11:176–194. [Google Scholar]
- 13.Wright JH. A case of multiple myeloma. Trans Assoc Am Phys. 1900;15:137–147. [Google Scholar]
- 14.Arinkin MI. Die intravitale Untersuchungsmethodik des Knochenmarks. Folia Haematol. 1929;38:233–240. [Google Scholar]
- 15.Rosenthal N, Vogel P. Value of the sternal puncture in the diagnosis of multiple myeloma. J Mt Sinai Hosp. 1938;4:1001–1019. [Google Scholar]
- 16.Geschickter CF, Copeland MM. Multiple myeloma. Arch Surg. 1928;16:807–863. [Google Scholar]
- 17.Heller JD. Die mikroscopisch-chemisch-pathologische untersuchung. Vienna: Braumuller and Seidel; 1846. [Google Scholar]
- 18.Fleischer R., XXIV Ueber das Vorkommen des sogenannten Bence Jones' schen Eiweisskorpers im normalen Knochenmark. Arch Pathol Anatom Physiol Klin Med. 1880;80:842–849. [Google Scholar]
- 19.Bayne-Jones S, Wilson DW. Immunological reactions of Bence-Jones proteins: II. Differences between Bence-Jones proteins from various sources. Bull Johns Hopkins Hosp. 1922;33:119–125. [Google Scholar]
- 20.Korngold L, Lipari R. Multiple-myeloma proteins“ III. The antigenic relationship of Bence Jones proteins to normal gamma-globulin and multiple-myeloma serum proteins. Cancer. 1956;9:262–272. doi: 10.1002/1097-0142(195603/04)9:2<262::aid-cncr2820090210>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Edelman GM, Gally JA. The nature of Bence-Jones proteins: chemical similarities to polypeptide chains of myeloma globulins and normal gamma-globulins. J Exp Med. 1962;116:207–227. doi: 10.1084/jem.116.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlzweig WA, Delrue G, Geschicter C. Hyperproteinemia associated with multiple myelomas: report of an unusual case. JAMA. 1928;90:755–757. [Google Scholar]
- 23.Tiselius A. A new apparatus for electrophoretic analysis of colloidal mixtures. Trans Faraday Soc. 1937;33:524. [Google Scholar]
- 24.Tiselius A, Kabat EA. Electrophoretic study of immune sera and purified antibody preparation. J Exp Med. 1939;69:119–131. doi: 10.1084/jem.69.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longsworth LG, Shedlovsky T, MacInnes DA. Electrophoretic patterns of normal and pathological human blood, serum, and plasma. J Exp Med. 1939;70:399–413. doi: 10.1084/jem.70.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkel HG, Tiselius A. Electrophoresis of proteins on filter paper. J Gen Physiol. 1951;35:89–118. doi: 10.1085/jgp.35.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabar P, Williams CA. Methode permettant l'etude conjuguee des proprietes electrophoretiques et immunochimiques d'un melange de proteines; application au serum sanguin. Biochim Biophys Acta. 1953;10:193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- 28.Wilson AT. Direct immunoelectrophoresis. J Immunol. 1964;92:431–434. [PubMed] [Google Scholar]
- 29.Waldenström J. Studies on conditions associated with disturbed gamma globulin formation (gammopathies). Harvey Lect. 1961;56:211–231. [PubMed] [Google Scholar]
- 30.Kyle RA. Monoclonal gammopathy of undetermined significance: natural history in 241 cases. Am J Med. 1978;64:814–826. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 31.Alwall N. Urethane and stilbamidine in multiple myeloma: report on two cases. Lancet. 1947;2:388–389. doi: 10.1016/s0140-6736(47)90375-9. [DOI] [PubMed] [Google Scholar]
- 32.Holland JR, Hosley H, Scharlau C, et al. A controlled trial of urethane treatment in multiple myeloma. Blood. 1966;27:328–342. [PubMed] [Google Scholar]
- 33.Blokhin N, Larionov L, Perevodchikova N, Chebotareva L, Merkulova N. Clinical experiences with sarcolysin in neoplastic diseases. Ann N Y Acad Sci. 1958;68:1128–1132. doi: 10.1111/j.1749-6632.1958.tb42675.x. [DOI] [PubMed] [Google Scholar]
- 34.Bergsagel DE, Sprague CC, Austin C, Griffith KM. Evaluation of new chemotherapeutic agents in the treatment of multiple myeloma: IV. L-Phenylalanine mustard (NSC-8806). Cancer Chemother Rep. 1962;21:87–99. [PubMed] [Google Scholar]
- 35.Hoogstraten B, Sheehe PR, Cuttner J, et al. Melphalan in multiple myeloma. Blood. 1967;30:74–83. [PubMed] [Google Scholar]
- 36.Maas RE. A comparison of the effect of prednisone and a placebo in the treatment of multiple myeloma. Cancer Chemother Rep. 1962;16:257–259. [PubMed] [Google Scholar]
- 37.Salmon SE, Shadduck RK, Schilling A. Intermittent high-dose prednisone (NSC-10023) therapy for multiple myeloma. Cancer Chemother Rep. 1967;51:179–187. [PubMed] [Google Scholar]
- 38.McIntyre OR, Pajak TF, Kyle RA, Cornwell GG, 3rd, Leone L. Response rate and survival in myeloma patients receiving prednisone alone. Med Pediatr Oncol. 1985;13:239–243. doi: 10.1002/mpo.2950130502. [DOI] [PubMed] [Google Scholar]
- 39.Alexanian R, Haut A, Khan AU, et al. Treatment for multiple myeloma: combination chemotherapy with different melphalan dose regimens. JAMA. 1969;208:1680–1685. doi: 10.1001/jama.208.9.1680. [DOI] [PubMed] [Google Scholar]
- 40.Lee BJ, Sahakian G, Clarkson BD, Krakoff IH. Proceedings: combination chemotherapy of multiple myeloma with alkeran, cytoxan, vincristine, prednisone, and BCNU. Cancer. 1974;33:533–538. doi: 10.1002/1097-0142(197402)33:2<533::aid-cncr2820330231>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Case DC, Jr, Lee DJ, 3rd, Clarkson BD. Improved survival times in multiple myeloma treated with melphalan, prednisone, cyclophosphamide, vincristine and BCNU: M-2 protocol. Am J Med. 1977;63:897–903. doi: 10.1016/0002-9343(77)90543-5. [DOI] [PubMed] [Google Scholar]
- 42.Myeloma Trialists' Collaborative Group. Combination chemotherapy vs melphalan plus prednisone as treatment for multiple myeloma: an overview of 6633 patients from 27 randomized trials. J Clin Oncol. 1998;16:3832–3842. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 43.Thomas ED, Lochte HL, Jr, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 44.Osserman EF, DiRe LB, DiRe J, Sherman WH, Hersman JA, Storb R. Identical twin marrow transplantation in multiple myeloma. Acta Haematol. 1982;68:215–223. doi: 10.1159/000206984. [DOI] [PubMed] [Google Scholar]
- 45.Fefer A, Cheever MA, Greenberg PD. Identical-twin (syngeneic) marrow transplantation for hematologic cancers. J Natl Cancer Inst. 1986;76:1269–1273. [PubMed] [Google Scholar]
- 46.Gahrton G, Tura S, Flesch M, et al. Bone marrow transplantation in multiple myeloma: report from the European Cooperative Group for Bone Marrow Transplantation. Blood. 1987;69:1262–1264. [PubMed] [Google Scholar]
- 47.McElwain TJ, Powles RL. High-dose intravenous melphalan for plasma-cell leukaemia and myeloma. Lancet. 1983;2:822–824. doi: 10.1016/s0140-6736(83)90739-0. [DOI] [PubMed] [Google Scholar]
- 48.Selby PJ, McElwain TJ, Nandi AC, et al. Multiple myeloma treated with high dose intravenous melphalan. Br J Haematol. 1987;66:55–62. doi: 10.1111/j.1365-2141.1987.tb06890.x. [DOI] [PubMed] [Google Scholar]
- 49.Barlogie B, Alexanian R, Dicke KA, et al. High-dose chemoradiotherapy and autologous bone marrow transplantation for resistant multiple myeloma. Blood. 1987;70:869–872. [PubMed] [Google Scholar]
- 50.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma [see comments]. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 51.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 52.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma [see Comment]. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 53.Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106:4050–4053. doi: 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajkumar SV. Thalidomide: tragic past and promising future. Mayo Clin Proc. 2004;79:899–903. doi: 10.4065/79.7.899. [DOI] [PubMed] [Google Scholar]
- 56.Lenz W. Thalidomide and congenital abnormalities. Lancet. 1962;1:45. doi: 10.1016/s0140-6736(62)91943-8. [DOI] [PubMed] [Google Scholar]
- 57.McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [Google Scholar]
- 58.Woodyatt PB. Thalidomide. Lancet. 1962;1:750. [Google Scholar]
- 59.Grabstad H, Golbey R. Clinical experience with thalidomide in patients with cancer. Clin Pharmacol Ther. 1965;6:298–302. doi: 10.1002/cpt196563298. [DOI] [PubMed] [Google Scholar]
- 60.Olson KB, Hall TC, Horton J, Khung CL, Hosley HF. Thalidomide (N-phthaloylglutamimide) in the treatment of advanced cancer. Clin Pharmacol Ther. 1965;6:292–297. doi: 10.1002/cpt196563292. [DOI] [PubMed] [Google Scholar]
- 61.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimopoulos MA, Anagnostopoulos A, Weber D. Treatment of plasma cell dyscrasias with thalidomide and its derivatives. J Clin Oncol. 2003;21:4444–4454. doi: 10.1200/JCO.2003.07.200. [DOI] [PubMed] [Google Scholar]
- 63.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 64.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 65.Orlowski RZ, Eswara JR, Lafond-Walker A, Grever MR, Orlowski M, Dang CV. Tumor growth inhibition induced in a murine model of human Burkitt's lymphoma by a proteasome inhibitor. Cancer Res. 1998;58:4342–4348. [PubMed] [Google Scholar]
- 66.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 67.Wilk S, Orlowski M. Cation-sensitive neutral endopeptidase: isolation and specificity of the bovine pituitary enzyme. J Neurochem. 1980;35:1172–1182. doi: 10.1111/j.1471-4159.1980.tb07873.x. [DOI] [PubMed] [Google Scholar]
- 68.Wilk S, Orlowski M. Inhibition of rabbit brain prolyl endopeptidase by n-benzyloxycarbonyl-prolyl-prolinal, a transition state aldehyde inhibitor. J Neurochem. 1983;41:69–75. doi: 10.1111/j.1471-4159.1983.tb11815.x. [DOI] [PubMed] [Google Scholar]
- 69.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 70.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized Phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 71.Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- 72.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 73.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 74.Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 75.Greipp PR, San Miguel JF, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 76.Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo stratification of myeloma and risk-adapted therapy (mSMART): Consensus Statement. Mayo Clin Proc. 2007;82:323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- 77.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma: Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 78.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 79.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92:3131–3136. [PubMed] [Google Scholar]
- 80.Barlogie B, Kyle RA, Anderson KC, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 81.Attal M, Harousseau JL, Facon T, et al. Single vs double autologous stem-cell transplantation for multiple myeloma [see comment]. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 82.Blade J, Rosinol L, Sureda A, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–3759. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 83.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 84.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 85.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide vs melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 86.Alexanian R, Barlogie B, Tucker S. VAD-based regimens as primary treatment for multiple myeloma. Am J Hematol. 1990;33:86–89. doi: 10.1002/ajh.2830330203. [DOI] [PubMed] [Google Scholar]
- 87.Rajkumar SV, Blood E, Vesole DH, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 88.Rajkumar SV, Hussein M, Catalano J, et al. A randomized, double-blind, placebo-controlled trial of thalidomide plus dexamethasone vs dexamethasone alone as primary therapy for newly diagnosed multiple myeloma [abstract]. Blood. 2006;108:795. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lacy MQ, Gertz MA, Dispenzieri AA, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82:1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 90.Rajkumar SV, Jacobus S, Callander N, Fonseca R, Vesole D, Greipp P. A randomized phase III trial of lenalidomide plus high-dose dexamethasone vs lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group [abstract]. Blood. 2006;108:799. [Google Scholar]
- 91.Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129:776–783. doi: 10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 92.Harousseau J, Attal M, Leleu X, et al. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- 93.Harousseau J-L, Marit G, Caillot D, et al. VELCADE/dexamethasone (Vel/Dex) vs VAD as induction treatment prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (MM): an interim analysis of the IFM 2005-01 Randomized Multicenter Phase III Trial [abstract]. Blood. 2006;108:56. [Google Scholar]
- 94.Hulin C, Virion J, Leleu X, et al. Comparison of melphalan-prednisone-thalidomide (MP-T) to melphalan-prednisone (MP) in patients 75 years of age or older with untreated multiple myeloma (MM). Preliminary results of the randomized, double-blind, placebo controlled IFM 01-01 trial [abstract]. J Clin Oncol. 2007;25:8001. [Google Scholar]
- 95.Mateos M-V, Hernandez J-M, Hernandez M-T, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–2172. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- 96.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459–4465. doi: 10.1200/JCO.2007.12.3463. [DOI] [PubMed] [Google Scholar]
- 97.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 Clinical Study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 98.Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102:3447–3454. doi: 10.1182/blood-2002-09-2955. [DOI] [PubMed] [Google Scholar]
- 99.Garban F, Attal M, Michallet M, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–3480. doi: 10.1182/blood-2005-09-3869. [DOI] [PubMed] [Google Scholar]
- 100.Attal M, Harousseau J-L, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 101.Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007;21:529–534. doi: 10.1038/sj.leu.2404516. [DOI] [PubMed] [Google Scholar]