Abstract
Altered expression of Bcl-2 family proteins plays central roles in apoptosis dysregulation in cancer and leukemia, promoting malignant cell expansion and contributing to chemoresistance. In this study, we compared the toxicity and efficacy in mice of natural product gossypol and its semisynthetic derivative apo-gossypol, compounds that bind and inhibit antiapoptotic Bcl-2 family proteins. Daily oral dosing studies showed that mice tolerate doses of apogossypol 2- to 4-times higher than gossypol. Hepatotoxicity and gastrointestinal toxicity represented the major adverse activities of gossypol, with apogossypol far less toxic. Efficacy was tested in transgenic mice in which Bcl-2 is overexpressed in B cells, resembling low-grade follicular lymphoma in humans. In vitro, Bcl-2–expressing B cells from transgenic mice were more sensitive to cytotoxicity induced by apogossypol than gossypol, with LD50 values of 3 to 5 μM and 7.5 to 10 μM, respectively. In vivo, using the maximum tolerated dose of gossypol for sequential daily dosing, apogossypol displayed superior activity to gossypol in terms of reducing splenomegaly and reducing B-cell counts in spleens of Bcl-2–transgenic mice. Taken together, these studies indicate that apogossypol is superior to parent compound gossypol with respect to toxicology and efficacy, suggesting that further development of this compound for cancer therapy is warranted.
Introduction
Overexpression of Bcl-2 and other antiapoptotic members of the Bcl-2 family occurs in many human cancers and leukemias.1–3 Bcl-2 and related antiapoptotic proteins suppress tumor cell death induced by chemotherapy, radiation, hormonal therapies (including glucocorticoids), and other therapeutics used in the treatment of malignancy.4–6 Thus, agents that inhibit antiapoptotic Bcl-2 family proteins are desired as potential new therapeutics for restoring apoptosis sensitivity and improving clinical outcomes for patients with cancer or leukemia.
Bcl-2 has been validated as a target for improving treatment of B-cell malignancies using Bcl-2 antisense oligodeoxynucleotides to reduce Bcl-2 protein expression.7 The Bcl-2 antisense drug candidate, oblimersen sodium (Genasense; Genta, Berkeley Heights, NJ), for example, improved complete response rates and prolonged response duration in a randomized phase 3 clinical trial involving patients with relapsed or refractory chronic lymphocytic leukemia (CLL).8 Moreover, the BCL-2 gene becomes activated by chromosomal translocations or gene amplification in the majority of non-Hodgkin B-cell lymphomas (B-NHLs), while its overexpression is found in most chronic lymphocytic leukemias (CLLs) in association with chromosomal deletions of microRNA (miR)–encoding genes that normally suppress Bcl-2 expression.9–11
In this study, we compared the toxicity and efficacy in mice of gossypol (NSC19048) and apogossypol (NSC736630), a semisynthetic analog of natural product gossypol, in which 2 reactive aldehydes were eliminated from the compound.12 Gossypol and apogossypol are orally active compounds that mimic endogenous BH3 peptide–containing antagonists of antiapoptotic Bcl-2 family proteins, competing with the BH3 peptide-binding sites on Bcl-2, Bcl-XL, Mcl-1, Bcl-W, and Bcl-B, but not Bfl-1, with IC50s of 0.5 to 2 μM.13 These compounds thus represent broad-spectrum antagonists of antiapoptotic Bcl-2 family proteins, and consequently are expected to be useful for malignancies in which expression of 2 or more antiapoptotic Bcl-2 family proteins are simultaneously elevated. The (−) enantiomer of gossypol (AT-101; Ascenta Pharmaceuticals, San Diego, CA) is currently under clinical evaluation in phase 1/2 clinical trials involving patients with solid tumors, lymphoma, and leukemia. Although AT-101 shows clinical activity, it was associated with hepatotoxicity (elevation of serum levels of AST and ALT) and gastrointestinal (GI) toxicity (partial paralytic ileus). Indeed, GI toxicity was found to be a dose-limiting toxicity in CLL patients (T. Kipps, University of California San Diego [UCSD], oral communication, April 2006).
Because the affinity of gossypol for Bcl-2 and related antiapoptotic proteins is only modest,13 it is likely that relatively high doses will be required to effectively neutralize Bcl-2 family proteins. The aldehydes in gossypol make this compound reactive, thus effectively reducing the available concentrations of active drug and causing toxicity. For this reason, we analyzed analogs of gossypol in which the aldehydes were removed, and showed previously that apogossypol (1,1′,6,6′,7,7′-hexahydroxy-3,3′-dimethyl-5,5′-bis (1-methylethyl)-[2.2′-binaphthalene]) retains full activity against antiapoptotic Bcl-2 family proteins but without the problematic aldehydes.12 Recently we evaluated the single-dose pharmacokinetic characteristics of apogossypol in mice, revealing superior blood concentrations over time (area under curve)14 compared with gossypol, due to slower clearance of the compound.15
The present study was undertaken to compare the toxicity and efficacy in mice of gossypol and apogossypol. These compounds have received development support from the National Cancer Institute as NSC19048 and NSC736630, respectively. The preclinical data presented here show superior efficacy and markedly reduced toxicity of apogossypol compared with gossypol, and thus indicate that further development of apogossypol for B-cell malignancies is warranted.
Methods
Bcl-2–transgenic mice
Transgenic mice expressing Bcl-2 have been described as the B6 line.16 The BCL-2 transgene represents a minigene version of a t(14;18) translocation in which the human BCL-2 gene is fused with the immunoglobulin heavy-chain (IgH) locus and associated IgH enhancer. The transgene was propagated on the Balb/c background. These mice develop polyclonal B-cell hyperplasia with asynchronous transformation to monoclonal aggressive lymphomas beginning at approximately 6 months of age, with approximately 90% of mice undergoing transformation by the age of 12 to 24 months. All animals used here had not yet developed aggressive lymphoma.
Patient specimens
Peripheral blood mononuclear cells (PBMCs) freshly acquired from patients with CLL were obtained from the UCSD branch of the CLL Research Consortium (San Diego, CA). The blood samples were collected after obtaining informed consent in accordance with the Declaration of Helsinki. PBMCs were isolated by density gradient centrifugation using Histopaque 1077 (Sigma-Aldrich, St Louis, MO). All patients met the NCI International Workshop (IW) CLL criteria for diagnosis of CLL.17,18 The samples used contained 95% or more CD19+ and CD5+ cells, as assessed by flow cytometry. CLL samples were cultured in RPMI media containing 10% fetal bovine serum (FBS; HyClone, Logan, UT or Mediatech, Herndon, VA) at 37°C in 5% CO2/95% air.
Gossypol and apogossypol preparation and formulation
Apogossypol (NSC736630) was cocrystalized with ascorbic acid at 1:1 molar ratio. Gossypol (NSC19048) was lyophilized in acetic acid form. Both compounds were provided by NCI–Developmental Therapeutics Program (DTP; Rapid Access to Intervention Development [RAID]-program). Compounds were dissolved in 100% sesame oil just before oral administration. Vehicle control consisted of corresponding concentration of ascorbic acid suspended in 100% sesame oil.
Mouse experiments
Gossypol and apogossypol were administered orally to mice daily at doses of 60 μmol/kg or 120 μmol/kg, using a straight-type oral gavage needle (18-G, 3-inch, straight 2.25-mm ball; Braintree Scientific, Braintree, MA). The volume of administration was 10 mL/kg19—typically 0.2 mL per 20-g mouse. Normal Balb/c mice of 7 to 8 weeks of age at the initiation of the study were used for toxicity studies, while Bcl-2–transgenic mice on Balb/c background of more than 6 months of age were used for efficacy studies. To mimic the oncology drug delivery schedule used for some intravenous drugs now in clinical use, age-matched, sex-matched mice were dosed 5 times weekly, using a regimen of daily dosing for 5 consecutive days (Monday through Friday), followed by a 2-day rest (Saturday, Sunday), before resuming dosing. At conclusion of treatments, mice were killed within 24 hours via intraperitoneal injection of 0.7 mL tribromoethanol and whole blood was collected into Yellow-Top Serum Separator tubes (Becton Dickinson Vacutainer Systems; Becton Dickinson, Franklin Lakes, NJ). Spleens were removed and weighed.
Hematology studies
Whole blood (250 μL) was collected in EDTA-coated glass tubes (purple top; Microtainer Brand Tube with EDTA; Becton Dickinson) via either cardiac puncture or severing the brachial artery of anesthetized mice. After thorough mixing, specimens were analyzed using a VetScan HM2 (Abaxis, Union City, CA) hematology analyzer, measuring white blood cell count (WBC), red blood cell count (RBC), platelet (PLT) count, leukocyte differential (including percentage lymphocyte, percentage monocyte, and percentage granulocyte), hematocrit (Ht), and hemoglobin (Hb).
Serum chemistries
Approximately 500 μL whole blood was collected in glass tubes (yellow top; MICROTAINER Brand, Serum Separator Tube, Catalog no. 365956; Becton Dickinson, Franklin Lakes, NJ) and kept on ice for 30 minutes, then centrifuged at 15 000g for 2 minutes to separate serum from cells and fibrin clot. The resulting serum specimens were analyzed using an automated blood chemistry analyzer (“COBAS MIRA Classic”; Roche, Indianapolis, IN) to measure alanine aminotransferase (ALT) and aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine.
Cardiac toxicity
Immediately after ultrasound imaging, electrocardiogram (ECG) analysis of anesthetized mice was performed using an MP150 (Biopac Systems, München, Germany).
Histology
Vital organs, including liver, kidneys, spleen, heart, stomach, small intestines, large intestines, and lungs, were fixed in Z-fix solution (Anatech, Battle Creek, MI) for 3 days, rinsed 3 times with phosphate-buffered saline (PBS, pH 7.4), and then embedded in paraffin blocks. Thin sections were cut (0.5 μM) stained with hematoxylin-eosin (H&E). An automated image analysis system (Aperio Technologies, Vista, CA) was employed to digitalize the slides for histologic evaluation. Images were captured using ScanScope software, version 8 (Aperio Technologies). For the images presented, 10× and 40× digital zoom lenses were used. In addition, unstained sections were analyzed by the terminal deoxynucleotidyl transferase end-labeling (TUNEL) method to stain cells with DNA fragmentation indicative of apoptosis, as described.20 The percentage of TUNEL-positive cells was evaluated by a morphometric method using an automated image analysis system (Aperio Technology, Vista, CA) and applying a nuclear scoring algorithm.
Splenocyte isolation
Spleens were excised from killed mice and cell suspensions treated with a mouse erythrocyte lysing kit (R & D Systems, Minneapolis, MN). Total splenocyte count was determined by trypan blue dye exclusion assays using hemocytometers. The percentage of B lymphocytes was determined by fluorescence-activated cell sorter (FACS) analysis (FACS-CANTO; Becton Dickinson, Mountain View, CA) following staining cells with phycoerythrin (PE)–conjugated anti-CD19 or -B220 antibodies (Becton Dickinson, San Jose, CA).
Cell culture and cytotoxicity studies
Splenocytes were suspended at 1 × 106 cells/mL in RPMI 1640 medium (Mediatech) containing 10% fetal bovine serum (Mediatech) and penicillin/streptomycin (Mediatech). Human B-CLL cells and 3 B-NHL cell lines, including RS11846, DOHH2, and 380 cells, were cultured in RPMI 1640 medium (Mediatech) containing 10% fetal bovine serum (Mediatech) and penicillin/streptomycin (Mediatech). Cells were cultured with various concentrations of gossypol, apogossypol, or ascorbic acid for 1 to 2 days. The percentage of viable cells was determined by annexin V and propidium iodide (PI) labeling, using an Apoptosis Detection kit (BioVision, Mountain View, CA), analyzing stained cells by flow cytometry (FACSort; Becton Dickinson, Mountain View, CA). Cells that were annexin V negative and PI negative were considered viable.
Testing of gossypol and apogossypol against the NCI 60 human cancer cell line panel
The anticancer drug screening test was conducted as previously described using the NCI's panel of 60 human tumor cell lines.21 Cells were grown in RPMI 1640 culture medium supplemented with 5% FBS and 2 mM l-glutamine for 24 hours at 37°C to allow stabilization prior to addition of gossypol and apogossypol. Stock solutions of gossypol and apogossypol in DMSO were serially diluted with the RPMI 1640 medium and added immediately to the microtiter plates to produce 5 concentrations of the compounds: 10−4, 10−5, 10−6, 10−7, and 10−8 M. The compounds were incubated with cells for 48 hours before fixing cells in situ using 10% trichloroacetic acid. Plates were washed 5 times with water and dried. Sulforhodamine B (0.4% in 1% acetic acid), a protein stain binding to basic amino acids of cellular macromolecules, was added to each well, and incubated for 10 minutes at room temperature. Unbound sulforhodamine B was removed by washing 5 times with acetic acid. Plates were then air-dried. Bound stain was solubilized with Tris buffer, and the optical densities were read at 515 nm. The optical densities generated from sulforhodamine B staining are a function of cell mass and growth rate. The dose-response curves were created by plotting the percentage growth against the log10 of concentrations of the corresponding compound for each cell line. Molar concentrations of gossypol or apogossypol that caused 50% growth inhibition (GI50) and 50% cell killing (LC50) of the cell lines were determined as described.21 GI50 data for apogossypol and gossypol were compared by Spearman correlation analysis. LD50 data were compared by chi-square test, empirically dichotomizing into resistant (LD50 < 100 μM) and sensitive (LD50 > 100 μM) categories.
Results
Apogossypol is less toxic than gossypol
We compared the toxicities of gossypol and apogossypol in normal female Balb/c mice. Preliminary maximum tolerated dose (MTD) studies suggested that apogossypol was less toxic than gossypol whether delivered orally or by intraperitoneal injection. Previous NCI-sponsored studies determined that racemic gossypol and (−)gossypol are nonlethal and show antitumor activity when dosed orally at 60 μmol/kg daily for up to 21 days (L.J., unpublished data, November 2003). We therefore compared orally administered gossypol and apogossypol, dosing animals empirically with twice the standard gossypol dose (120 μmol/kg). Ascorbic acid was used as a control, because apogossypol is formulated at 1:1 molar ratio with this weak acid, which renders the compound stable upon storage.22 Compounds or vehicle control were dosed 15 times over 3 weeks; compounds were given daily for 5 consecutive days (Monday-Friday), resting on weekends.
In terms of gross appearance, gossypol was toxic in 23 of 23 mice, as evidenced by lethargic behavior as well as scruffy and rough hair. No such toxicity was seen in 38 of 40 mice treated with apogossypol or in 20 of 20 mice treated with vehicle control.
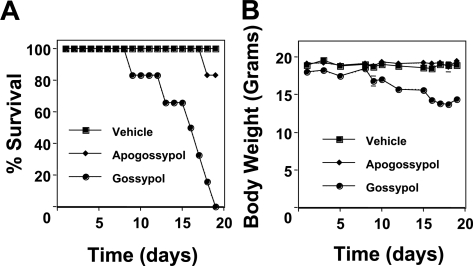
Figure 1 shows a representative experiment in which cohorts of 6 mice each were dosed orally with vehicle control, gossypol, or apogossypol. By the second week of treatment, gossypol-treated mice began to die, and all were dead within 19 days (Figure 1A). In contrast, no deaths occurred in the apogossypol-treated mice until day 18, and more than 80% of mice remained alive after the treatment regimen (5 of 6 apogossypol mice surviving compared with 0 of 6 gossypol mice; P < .001). Weights of mice treated with gossypol also declined, while apogossypol did not cause weight loss (Figure 1B). In the first week of dosing, gossypol-induced weight loss appeared to be reversible, with mice regaining most of their pretreatment weight during the 2-day rest periods over weekends. Thereafter, weight loss was persistent.
Figure 1.
Toxicity profiles of gossypol versus apogossypol in normal mice. Female Balb/c mice (7 weeks old) (n = 6 per group) were orally administered vehicle control (■), 120 μmol/kg gossypol (•), or 120 μmol/kg apogossypol (♦) 5 times weekly for 3 weeks. (A) Percentage survival of mice is shown over time. (B) Body weight is shown over time, in grams (mean ± standard deviation [SD]). Data are representative of several experiments involving a total of 20 gossypol-treated, 40 apogossypol-treated, and 20 vehicle control–treated mice.
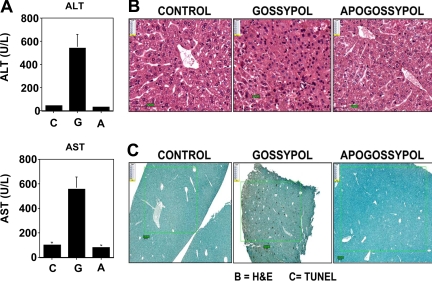
The effects of gossypol and apogossypol on hepatotoxicity were assessed by monitoring serum levels of liver enzymes, ALT and AST. For these experiments, cohorts of 6 mice per group were orally administered compounds daily at 120 μmol/kg daily for 5 days for 3 weeks (15 total doses). Gossypol treatment induced an average increase in ALT of approximately 10-fold and in AST of approximately 5-fold, while apogossypol-treated mice showed no increase in ALT or AST (Figure 2A). Because gossypol is lethal upon repeated daily dosing at 120 μmol/kg, mice were carefully monitored and serum specimens were collected for ALT and AST measurements when animals were close to death. For apogossypol and vehicle control, serum was obtained within 24 hours after the last dose. In contrast to ALT and AST, levels of γ-glutaminyl transferase (GGT) were not increased by these compounds (not shown), excluding biliary obstruction and favoring direct hepatotoxicity. Serum levels of direct and indirect bilirubin were also not elevated (data not shown).
Figure 2.
Gossypol is more hepatotoxic than apogossypol. Balb/c mice were orally administered apogossypol, gossypol, or vehicle control at a daily dose of 120 μmol/kg 5 times weekly for 3 weeks (6 mice per group). (A) Serum levels of ALT and AST were measured at conclusion of treatment (mean ± SD). (B) H&E histology of liver sections is presented. Note area of necrosis in gossypol-treated animal shown. Representative of 3 of 6 mice evaluated for each treatment group. (C) TUNEL-stained sections of liver are shown (representative of 3 of 6 mice for each group). Size bars represent 100 μM.
Liver tissue was also analyzed histologically (Figure 2B). No pathologic alterations were observed in liver tissue from mice receiving vehicle control or apogossypol. However, regions of hepatocellular necrosis were evident in gossypol-treated animals (Figure 2B). TUNEL staining demonstrated increased apoptosis in gossypol-treated mice (5.5% ± 0.9%) compared with vehicle control (0.2% ± 0.04%) and apogossypol-treated animals (0.1% ± 0.02%), with P less than .001 for gossypol versus vehicle control or apogossypol (Figure 2C).
Unlike the markers of liver toxicity, neither gossypol nor apogossypol significantly affected indicators of renal function: BUN or creatinine (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Both compounds also had no significant effects on WBC, RBC, Hb, Ht, or PLT count (Figure S1). Gossypol, however, caused lymphopenia, with an average 44% plus or minus 4% reduction, whereas apogossypol did not (Figure S1). Neither gossypol nor apogossypol showed evidence of cardiotoxicity by ECG analysis, revealing no arrhythmias, fibrillation, PQ-interval prolongation, T-inversion, or other abnormalities.
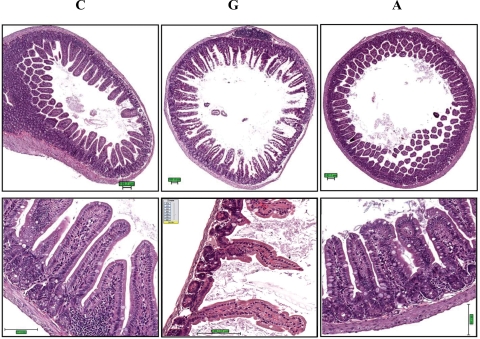
GI toxicity was evident for mice given 0.12 mmol/kg gossypol, but not apogossypol at the same dose. Specifically, gross pathology revealed distension of intestines, suggestive of partial paralytic ileus and color changes (darkening of tissue) in gossypol-treated mice. Ultrasound imaging of live mice confirmed this finding, as evidenced by distension of intestines (ie, distended up to 7 mm in diameter [not shown]). In contrast, the intestines of apogossypol-treated mice appeared normal on gross pathology examination, and evidence of intestinal dilatation was not found by ultrasound (not shown). Histologically, intestinal tissue from gossypol-treated mice showed diffuse mucosal damage, including loss of surface epithelium, marked thinning and atrophy of the intestinal wall, epithelial vacuolation, ulceration, and necrosis (Figure 3). In contrast, intestinal tissue from apogossypol-treated mice was indistinguishable from vehicle control mice, showing that apogossypol has no appreciable GI toxicity at these doses and for the duration of treatment used here (3 weeks).
Figure 3.
Gossypol has greater gastrointestinal toxicity than apogossypol. Balb/c mice were orally administered apogossypol (A), gossypol (G), or vehicle control (C) at a daily dose of 120 μmol/kg 5 times weekly for 3 weeks (6 mice per group). Representative H&E histology is presented of intestinal tissue (duodenum) of treated mice in cross-section (top) and longitudinal section (bottom). Note denuding of mucosal epithelium in gossypol-treated mice, with shortening and loss of villi. Size bars represent 100 μM.
Gossypol and apogossypol display cytotoxic activity against cultured human B-cell lymphoma and leukemia cells
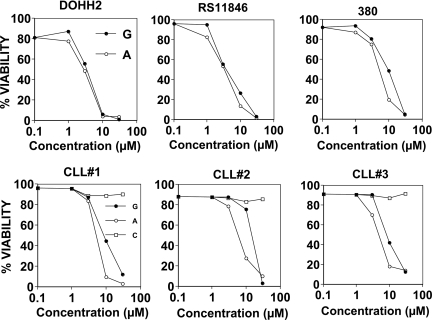
The cytotoxic activities of gossypol and apogossypol were compared in vitro, using cultured B-cell lymphoma and CLL leukemia cells and assessing cell viability by staining with annexin V and PI. Treatment of the t(14;18)-containing B-NHL cell lines DoHH2, RS11846, and 380 showed similar cytotoxic activity of gossypol and apogossypol in culture, with average lethal dose 50% (LD50) for the 3 B-NHL cell lines of 5.32 μM (95% confidence interval: 3.29-8.61) for gossypol compared with 4.16 μM (95% confidence interval: 3.27-5.29) for apogossypol (Figure 4 top panels). Killing of NHL B-cell lines by apogossypol was rapid, with reductions in cell viability detected as early as 1 hour after treatment and maximum killing generally obtained by 6 hours (Figure S2).
Figure 4.
Comparison of cytotoxic activity of apoossypol and gossypol against human B-cell malignancies. NHL B-cell lines bearing t(14;18) translocations (DoHH2, RS11846, and 380) (top) and primary CLL specimens (bottom) were cultured at 106 cells/mL for 48 hours in the absence (vehicle control [white squares]) or presence of various concentrations (log-scale) of gossypol (black circles) or apogossypol (white circles). Percentage viability was determined by staining with FITC–annexin V and PI, scoring viable cells as annexin V negative/PI negative. Data for NHL are representative of several experiments. For CLLs, primary data are shown for 3 patient specimens that had low spontaneous rates of cell death in culture (Rai stage II [n = 1]; Rai stage I [n = 2]), derived from previously untreated patients. Differences in percentage viability between CLLs treated with apogossypol versus gossypol were statistically significant (P < .025 by 2-way ANOVA analysis). An additional 3 CLL specimens were also tested and showed similar sensitivity to gossypol and apogossypol, but higher levels of background cell death precluded determination of LD50 (not shown).
We also treated primary CLL B cells derived from peripheral blood of patients, because these cells have high Bcl-2 and defective programmed cell death. CLL B cells were cultured for 2 days with various concentrations of gossypol or apogossypol, revealing superior cytotoxic activity of apogossypol (Figure 4 bottom panels), which was approximately 2-fold more potent (LD50 = 5.24 μM [95% confidence interval: 4.58-5.98] for apo-gossypol vs LD50 = 11.0 μM [95% confidence interval: 8.34-14.51] for gossypol) (P < .025 by 2-way ANOVA analysis). Although 3 of 3 specimens tested here showed sensitivity to apogossypol and gossypol, future studies of larger numbers of CLL patient specimens are needed to define the overall frequency of responders. Apogossypol-induced killing of CLL cells in culture was rapid, with cell death evident within 4 hours (not shown).
Gossypol and apogossypol display cytotoxic activity against cultured murine B cells derived from Bcl-2–transgenic mice
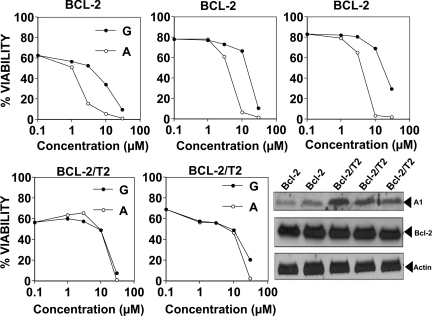
In preparation for in vivo analysis of gossypol and apogossypol in Bcl-2–transgenic mice, we first studied the cytotoxic activity of these compounds on cultured B cells derived from Bcl-2–transgenic mice in which the human BCL-2 gene is fused with the immunoglobulin heavy-chain (IgH) locus and associated IgH enhancer. With age, B cells accumulate in these mice, producing a histologic picture highly similar to low-grade follicular lymphomas within 3 months.16 Treating cultured splenocytes from Bcl-2–transgenic mice with gossypol or apogossypol resulted in cell death (Figure 5 top panels), with apogossypol more potent than gossypol (average LD50 = 3.9 μM [95% confidence interval: 3.01-5.07 μM] for apogossypol vs LD50 = 16.7 μM [95% confidence interval: 11.7-23.8 μM] for gossypol; P < .005 by 2-way ANOVA analysis). Note that B cells typically constitute more than 80% of the splenocytes of these Bcl-2–transgenic animals. Similar conclusions were reached when using multicolor FACS analysis to assess viability of the B220+ B cells (Figure S3) or CD19 cells (not shown) within the splenocyte cultures. Seeding Bcl-2–transgenics splenocytes at higher density (10 million cells/mL instead of 1 million cells/mL) resulted in higher LD50s, but the superiority of apogossypol compared with gossypol was still evident (Figure S4).
Figure 5.
Comparison of cytotoxic activity of gossypol and apogossypol against cultured murine B cells from transgenic mice: Bcl-2 versus Bcl-2/TRAF2ΔN. Splenocytes from age- and sex-matched Bcl-2–transgenic mice (top; n = 3 pairs) and Bcl-2/TRAF2ΔN double-transgenic mice (bottom; n = 2 pairs) were cultured at 106 cells/mL for 18 hours in the absence (vehicle control) or presence of various concentrations (log-scale) of gossypol (closed symbols) or apogossypol (open symbols). Percentage viability was determined by staining with FITC–annexin V/PI, scoring viable cells as annexin V negative/PI negative. Immunoblot analysis (bottom right) was performed using whole-cell lysates from splenocytes of Bcl-2–transgenic (n = 2) and Bcl-2/TRAF2ΔN–transgenic (n = 3) mice, normalized for protein content (50 μg/lane). Blots were probed with antibodies recognizing murine Bfl-1 ortholog A1 (∼ 15-kDa band, probably corresponding to A1c isoform), human Bcl-2, and beta-actin.
We also tested the effects of gossypol and apogossypol on B cells derived from double-transgenic mice, in which the same Bcl-2–transgenic mice were bred with mice expressing an N-terminal truncated (ΔN) version of TRAF2 that mimics TRAF1 in B cells.23 These double-transgenic mice develop a CLL-like phenotype with aging, and their B cells display enhanced resistance to apoptosis.23 Note that aggressive NHLs and CLLs often have elevated TRAF1 expression.24 Gossypol and apogossypol induced concentration-dependent killing of splenocytes from Bcl-2/TRAF2ΔN mice, with average LD50s of 17.1 μM (95% confidence interval: 13.9-21.0 μM) for gossypol compared with LD50 = 14.6 μM (95% confidence interval: 14.1-15.1 μM) for apogossypol (Figure 5 bottom panels). Thus, Bcl-2/TRAF2DN double-transgenic splenocytes are more resistant to apogossypol and gossypol than Bcl-2 single-transgenic splenocytes. The immunophenotypes of the B-cell populations that accumulate in the Bcl-2 and Bcl-2/TRAF2ΔN mice have been previously reported.16,23,24
Immunoblot analysis revealed the levels of Bcl-2 are similar in the splenocytes of Bcl-2 single-transgenic and Bcl-2/TRAF2ΔN double-transgenic mice, but levels of A1 (Bfl-1) protein are markedly elevated in double transgenics (Figure 5 bottom, right panel). Given that gossypol and apogossypol have little in vitro activity against the Bfl-1 protein,13 it is possible that TRAF2ΔN-induced increases in A1 (Bfl-1) protein may account for reduced sensitivity of double-transgenic splenocytes to these compounds.
Comparison of cytotoxicity of apogossypol and gossypol against NCI 60 tumor cell line panel
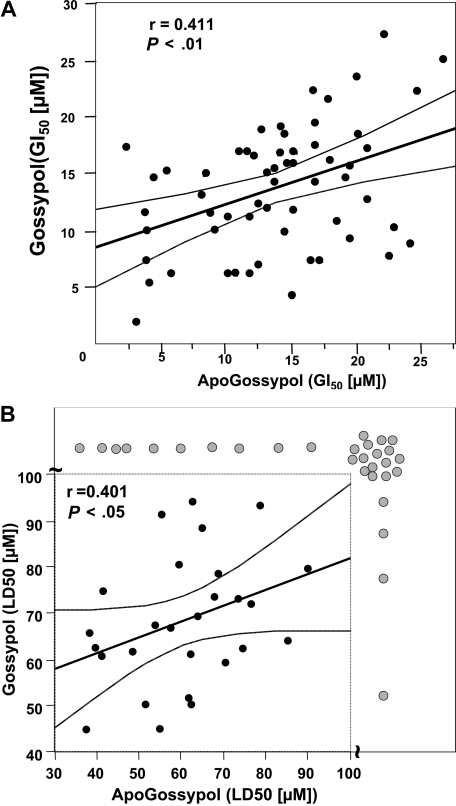
Because levels of several antiapoptotic Bcl-2 family proteins or their encoding mRNAs have previously been determined for the NCI 60 tumor cell line panel (n = 59 tumor lines actual), we compared the growth inhibitory (GI50) and cytotoxic (LD50) activities of gossypol and apogossypol against these cancer and leukemia cell lines, and then performed correlations. Comparison of the GI50 results for gossypol and apogossypol by Spearman correlation analysis showed a significant correlation (r = 0.411; P < .01), suggesting that apogossypol and gossypol suppress in vitro growth of these tumor cell lines at least in part through a common mechanism (Figure 6A). Clear exceptions, however, were noted among some cell lines (such as SNB-19, SF-295, SK-OV-3, NCI-H322M, and UACC-62), where concentrations of compound required to suppress growth by 50% (GI50) were 3- to 5-fold different, suggesting overlapping but nonidentical mechanisms.
Figure 6.
Comparison of growth inhibitory activities of apogossypol and gossypol using NCI 60 tumor cell line panel. Tumor cell lines were cultured for 48 hours with various concentrations of apogossypol or gossypol, and relative cell number was determined by a protein staining method, calculating the concentration required to inhibit growth by 50% (GI50) (A) and to reduce cells by 50% (LD50) (B). The thick line represents the best-fit line, using Spearmen correlation analysis, while thin lines represent 95% confidence intervals. The axis breaks in panel B indicate cell lines (gray circles) where LD50 is more than 100 μM. Spearman correlation analysis for panel B excluded the LD50 data.
Correlations of GI50 data with Bcl-2 and Bcl-XL protein expression and with Bfl-1 mRNA expression (protein data unavailable) as either continuous variables or as dichotomous variables (segregating protein expression values at the median into high versus low) failed to reveal an association with sensitivity to apogossypol or gossypol (data not shown). Mcl-1 protein expression data showed a weak correlation with reduced sensitivity to gossypol (P = .04) but not apogossypol (P = .16) when dichotomizing data at the median (unpaired t test), but not when analyzed as continuous variables.
Similar results were obtained using LD50 results for the NCI cell line panel, instead of GI50 (Figure 6B). Statistical analysis, however, was more cumbersome because the LD50 was not reached for several of the tumor lines treated with gossypol or apogossypol, at concentrations up to 100 μM. By chi-square analysis, where tumor cell lines were arbitrarily dichotomized into sensitive (LD50 < 100 μM) or resistant (LD50 > 100 μM) categories based on data where 100 μM was the maximal concentration tested, a strong correlation between the cytotoxicity of gossypol and apogossypol was observed (P < .001), with congruous results for 45 of 59 tumor cell lines. However, 14 cell lines showed incongruous results in terms of LD50, with 10 gossypol-sensitive lines showing resistance to apogossypol and 4 apogossypol-sensitive lines showing resistance to gossypol (Figure 6B). Thus, gossypol and apogossypol presumably have overlapping but nonidentical cytotoxic mechanisms. With respect to the cytotoxic mechanism, apogossypol did not kill Bax/Bak double knockout cells at concentrations of 10 μM or less (Figure S5), as expected for an agent that kills by neutralizing antiapoptotic Bcl-2 family proteins.
Comparisons of in vivo activity of gossypol and apogossypol in Bcl-2–transgenic mice
The in vivo activities of orally delivered gossypol and apogossypol were compared in Bcl-2–transgenic mice, using spleen size as an end point for assessing activity. Spleen weight was measured in cross-sectional studies where pairs of age-matched, sex-matched littermates were treated with either vehicle control or active compound, then mice were killed and spleens weighed. In preliminary studies, we noted that spleen weight is highly consistent in age- and sex-matched untreated littermates of the Bcl-2–transgenic line (< 5% variation).
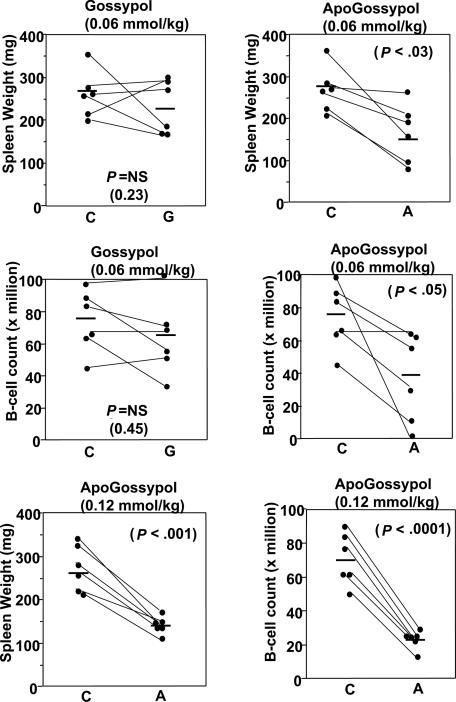
At the standard dose and regimen used for previous gossypol studies conducted by NCI-DTB (60 μmol/kg oral daily dosing), both gossypol and apogossypol were well tolerated and reduced splenomegaly. For mice given 3 weeks of therapy at 60 μmol/kg, and then killed and compared with age- and sex-matched littermates, spleen weights were 35% plus or minus 11% lower in the apogossypol-treated compared with vehicle control-treated mice (P < .03 by unpaired t test; Figure 7). B-cell counts in excised spleens of the apogossypol-treated mice were also significantly lower, as assessed by flow cytometry 73.9 plus or minus 8.1 million versus 40.6 plus or minus 11.9 million (P < .05 by unpaired t test; Figure 7). Although oral gossypol at 60 μmol/kg also reduced spleen weight and B-cell counts in spleen, the results did not reach statistical significance (Figure 7).
Figure 7.
Comparison of in vivo activity of apogossypol and gossypol in Bcl-2–transgenic mice. Pairs of age-matched (starting ages ranged from 39 to 73 weeks), sex-matched Bcl-2–transgenic mice were orally administered vehicle control (C) and either apogossypol (A) or gossypol (G), at either 60 μmol/kg or 120 μmol/kg (as indicated), 5 times weekly for 3 weeks. At conclusion of treatment, mice were killed and spleens removed and weighed (top), then splenocytes were isolated and analyzed by FACS to determine the number of B cells, typically using either B220 or CD19 as B-cell markers (bottom).
Next, we tested apogossypol at 120 μmol/kg, a well-tolerated dose that is 2 times the dose at which gossypol can be delivered due to its toxicity. A cross-sectional design was used, where age- and sex-matched littermates were treated for 3 weeks with either 1:1 apogossypol–ascorbic acid formulation or with ascorbic acid control, then killed and spleens analyzed. Apogossypol reduced spleen weight by an average of 40% plus or minus 4% (P < .001 by unpaired t test) and reduced B-cell counts in spleen by an average of 66% plus or minus 4% (P < .001; Figure 7). We conclude therefore that oral apogossypol has in vivo single-agent cytoablative activity in this mouse Bcl-2–transgenic model.
Discussion
In this study, we have documented the preclinical cytoablative activity and safety of a semisynthetic analog of the natural product gossypol in Bcl-2–transgenic mice. This compound, which we have termed apogossypol, neutralizes 5 of the 6 antiapoptotic Bcl-2 family proteins13,25 but lacks the reactive aldehydes found in the parent compound. Bcl-2 and related antiapoptotic proteins are commonly overexpressed in cancers, contributing to tumor cell survival (reviewed in Cory et al26). Consequently, compounds that neutralize these antideath proteins are desired for improved treatment of cancer and leukemia. Bcl-2 is the founding member of the family, which in humans includes Bcl-2, Bcl-XL, Mcl-1, Bcl-W, Bfl-1, and Bcl-B. Many examples of pathological overexpression of Bcl-2 have been identified in B-cell malignancies due to chromosomal translocations directly involving the BCL-2 gene, amplification of the BCL-2 gene, and loss of miRs that target Bcl-2 mRNAs.10,11 Thus, Bcl-2 plays a central incontrovertible role in B-cell malignancies, particularly non-Hodgkin lymphoma (NHL) and CLL. However, other antiapoptotic members of the Bcl-2 family also make important contributions to survival of hematopoietic malignancies and solid tumors (reviewed in Reed et al2). Thus, broad-spectrum inhibitors of antiapoptotic Bcl-2 family proteins might be required to effectively induce apoptosis of malignant cells, if the side effects of those inhibitors are tolerable.
Comparisons of the in vitro activity of gossypol and apogossypol on the NCI panel of 59 tumor cell lines suggested that these compounds have overlapping but nonidentical mechanisms. Instances were found where tumor cell lines were sensitive to either apogossypol or gossypol but not both compounds, as measured by GI50 or LD50 determinations. This finding highlights that inhibition of Bcl-2 family proteins is likely to represent only one of the mechanisms by which these compounds have anticancer activity, a characteristic that is typical of many natural products.
Several natural products and synthetic compounds have been identified that neutralize Bcl-2 and other members of the Bcl-2 family.13 Among those that have gained entry into human clinical trials are GX15-070 (Gemin X Biotechnologies, Montreal, QC), a synthetic broad-spectrum Bcl-2 antagonist; AT-101, which is (−)-gossypol (Ascenta Pharmaceuticals), a purified enantiomer of gossypol; and ABT-263, a synthetic selective inhibitor of Bcl-2, Bcl-XL, and Bcl-W (Abbott Laboratories, Abbott Park, IL). Previously, we compared the potency of these compounds or close analogs with respect to their ability to competitively displace BH3 peptides from the 6 antiapoptotic human Bcl-2 family proteins. While (−)gossypol and GX-07-050 analogs are broad spectrum in their inhibition, their in vitro potency is only modest, with approximately 1 μM IC50 against the purified Bcl-2 family targets.13 Thus, to be effective, these compounds should be given at high doses. In contrast, ABT-263 analog (ABT-737) is potent (< 0.1 μM IC50) but does not inhibit 3 of the 6 antiapoptotic Bcl-2 family proteins, an activity profile that has been associated with resistance to this selective inhibitor in preclinical studies due to, for example, expression of Mcl-1, an antiapoptotic Bcl-2 family member that is not inhibited.27
Although AT-101 was found to be clinically active, its use in humans is associated with hepatotoxicity and GI toxicity. It was previously unclear whether this toxicity was mechanism based or a physicochemical attribute of the particular compound. The preclinical studies reported here, however, strongly argue that hepatotoxicity and GI toxicity are not results of inhibition of Bcl-2 family proteins, but rather due to the reactive aldehyde groups in gossypol, since apogossypol did not exhibit GI or hepatotoxicity, and both compounds showed similar GI absorption rates when given to mice at the same oral dose (60 μmol/kg). Because of the reduced toxicity of apogossypol, we were able to dose animals with twice as much of this compound compared with gossypol, resulting in superior spleen shrinkage in the Bcl-2–transgenic mouse model. Preliminary experiments indicate that apogossypol can be administered orally on a daily scheduled for 3 weeks at even 4 times the tolerated dose of gossypol in nude mice (unpublished observations), suggesting opportunities for still greater antitumor activity using higher doses of this compound.
As a single agent at 120 μmol/kg daily, apogossypol exhibited in vivo cytoablative activity in Bcl-2–transgenic mice as measured by spleen weight, spleen size, and B-cell counts in spleen. In the Bcl-2–transgenic model used here, noncycling, mature B cells accumulate in spleen and lymphoid organs with age due to suppression of programmed cell death.16 These animals develop splenomegaly and lymphadenopathy from the polyclonal expansion of B cells, then eventually transform to aggressive lymphomas beginning at approximately 6 months as additional secondary genetic lesions occur. We analyzed the effects of apogossypol and gossypol in Bcl-2–transgenic mice before transformation, thus ensuring homogeneity of the treated cells and mice. Unlike normal B cells, which are culled in vivo in accordance with physiological cues, the B cells in the transgenic mice survive beyond their intended lifespan in a Bcl-2–dependent manner. Thus, upon neutralizing Bcl-2 with compounds, these Bcl-2–dependent B cells die. Although normal B cells are also dependent on Bcl-2 for their long-term survival, as demonstrated by studies of bcl-2−/− mice,28 we speculate that the excess B cells that accumulate in Bcl-2–transgenic mice may be preferentially sensitive to Bcl-2–neutralizing agents, based in part on the failure of apogossypol to reduce levels of circulating lymphocytes or to cause splenic shrinkage in normal mice.
Comparisons of apogossypol and gossypol suggested that even at low doses (60 μmol/kg), apogossypol has superior in vivo activity compared with gossypol. Since these 2 compounds have similar potency against antiapoptotic Bcl-2 family proteins in vitro, we presume the superior activity seen in vivo is due to the better pharmacokinetic behavior of apogossypol and the nonreactive nature of this compound, which allows for more active drug to be available to neutralize Bcl-2 and related proteins. In this regard, our prior pharmacological studies showed that apogossypol exhibits more sustained plasma concentrations after oral dosing in mice and has better stability in mouse and human liver microsome assays compared with gossypol.15
Compared with Bcl-2 single-transgenic mice, splenocytes from Bcl-2/TRAF2ΔN double-transgenic mice were far more resistant to the cytotoxic activity of gossypol and apogossypol. We found that levels of A1 (Bfl-1), an NF-κB–inducible antiapoptotic member of the Bcl-2 family,29 are elevated in splenocytes of the double-transgenic mice. Because apogossypol and gossypol do not significantly inhibit Bfl-1,13 it is possible that elevated A1 (Bfl-1) renders these Bcl-2/ΔN-TRAF2 double-transgenic cells resistant to these compounds. Future clinical trials involving gossypol or apogossypol therefore might consider incorporating assessment of Bfl1 expression, as patients whose malignancies contain high levels of Bfl-1 proteins may be unlikely to respond.
Overexpression of Bcl-2 and related antiapoptotic proteins contributes to chemoresistance of cancers and leukemias. Thus, combination therapy using Bcl-2 antagonists and cytotoxic anticancer drugs would be expected to produce synergistic results, provided toxicity is not exacerbated. In this regard, apogossypol is nonmyeloablative, thus boding well for its combination with traditional cytotoxic anticancer drugs. Future preclinical studies will be required to assess the toxicity risk of combining apogossypol with cytotoxic anticancer drugs. Taken together, these preclinical animal studies reported here indicate that apogossypol is superior to parent compound gossypol with respect to toxicology and efficacy, suggesting that further development of this compound for cancer therapy is warranted.
Supplementary Material
Acknowledgments
We thank M. Hanaii and T. Siegfried for paper preparation, X. Huang for histology, and S. Krajewski for helpful discussions. Special thanks to Dr Laura Rassenti and the CRC tissue bank for CLL samples.
This work was supported by NCI-RAID and NCI contract funds N01-CM-07110 and N01-CM-52205, by a grant to the CLL Research Consortium (P01-CA081534), and by a NCI Drug Discovery Group award (CA-113318).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.K. designed and performed experiments, analyzed data, and wrote the paper; C.L.K. performed experiments; M.K. performed experiments, analyzed data, and wrote paper; L.J. and M.P. provided key reagents; J.C.R. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: Development rights to apogossypol have been licensed to Coronado Biosciences, under terms where J.C.R. and M.P. would receive milestone and royalty payments if approved for clinical use. All other authors declare no competing financial interests.
Correspondence: John Reed, Burnham Institute for Medical Research, 10901 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: reedoffice@burnham.org.
References
- 1.Campos L, Rouault J-P, Sabido O, et al. High expression of Bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81:3091–3096. [PubMed] [Google Scholar]
- 2.Reed JC, Miyashita T, Krajewski S, et al. Bcl-2 family proteins and the regulation of programmed cell death in leukemia and lymphoma. Cancer Treat Res. 1996;84:31–72. doi: 10.1007/978-1-4613-1261-1_3. [DOI] [PubMed] [Google Scholar]
- 3.Kornblau SM, Thall PF, Estrov Z, et al. The prognostic impact of BCL2 protein expression in acute myelogenous leukemia varies with cytogenetics. Clin Cancer Res. 1999;5:1758–1766. [PubMed] [Google Scholar]
- 4.Reed JC, Kitada S, Takayama S, Miyashita T. Regulation of chemoresistance by the Bcl-2 oncoprotein in non-Hodgkin's lymphoma and lymphocytic leukemia cell lines. Ann Oncol. 1994;5(suppl1):S61–S65. doi: 10.1093/annonc/5.suppl_1.s61. [DOI] [PubMed] [Google Scholar]
- 5.Alnemri ES, Fernandes TF, Haldar S, Croce CM, Litwack G. Involvement of Bcl-2 in glucocorticoid-induced apoptosis of human pre-B-leukemias. Cancer Res. 1992;52:491–495. [PubMed] [Google Scholar]
- 6.Reed JC. Bcl-2 family proteins: regulators of chemoresistance in cancer. Toxicol Lett. 1995;82–83:155–158. doi: 10.1016/0378-4274(95)03551-6. [DOI] [PubMed] [Google Scholar]
- 7.Reed JC, Stein C, Subasinghe C, et al. Antisense-mediated inhibition of BCL2 proto-oncogene expression and leukemic cell growth and survival: comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990;50:6565–6570. [PubMed] [Google Scholar]
- 8.O'Brien S, Moore JO, Boyd TE, et al. Randomized phase 3 trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2007;25:1114–1120. doi: 10.1200/JCO.2006.07.1191. [DOI] [PubMed] [Google Scholar]
- 9.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Nat Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Croce CM. MicroRNA signatures in human cancers. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 11.Calin GA, Croce CM. MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene. 2006;25:6202–6210. doi: 10.1038/sj.onc.1209910. [DOI] [PubMed] [Google Scholar]
- 12.Becattini B, Kitada S, Leone M, et al. Rational design and real time in-cell detection of the pro-apoptotic activity of a novel compound targeting Bcl-Xl. Chem Biol. 2004;11:389–395. doi: 10.1016/j.chembiol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of anti-apoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 14.Bremer T, Man A, Kask K, Diamond C. CACNA1C polymorphisms are associated with the efficacy of calcium channel blockers in the treatment of hypertension. Pharmacogenomics. 2006;7:271–279. doi: 10.2217/14622416.7.3.271. [DOI] [PubMed] [Google Scholar]
- 15.Jia L, Coward LC, Kerstner-Wood C, et al. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemother Pharmacol. 2008;61:63–73. doi: 10.1007/s00280-007-0446-3. [DOI] [PubMed] [Google Scholar]
- 16.Katsumata M, Siegel RM, Louie DC, et al. Differential effects of Bcl-2 on B and T lymphocytes in transgenic mice. Proc Natl Acad Sci U S A. 1992;89:11376–11380. doi: 10.1073/pnas.89.23.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faguet GB, Agee JF. Immunophenotypic diagnosis of clinical and preclinical chronic lymphatic leukemia by using monoclonal antibodies against the cCLLa, a CLL-associated antigen. Blood. 1988;72:679–684. [PubMed] [Google Scholar]
- 18.Matutes E, Owusu-Ankomah K, Morilla R, et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia. 1994;8:1640–1645. [PubMed] [Google Scholar]
- 19.Miller J, Lenz D. Development of an immunoassay for diagnosis of exposure to toxic organophosphorus compounds. J Appl Toxicol. 2001;21:S23–S26. doi: 10.1002/jat.801. [DOI] [PubMed] [Google Scholar]
- 20.Schimmer AD, Welsh K, Pinilla C, et al. Small-cule antagonists of apoptosis-suppressor XIAP exhibit broad anti-tumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 21.Shami PJ, Saavedra JE, Bonifant CL, et al. Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J Med Chem. 2006;49:4356–4366. doi: 10.1021/jm060022h. [DOI] [PubMed] [Google Scholar]
- 22.Coward L, Gorman G, Noker P, et al. Quantitative determination of apogossypol, a pro-apoptotic analog of gossypol, in mouse plasma using LC/MS/MS. J Pharm Biomed Anal. 2006;42:581–586. doi: 10.1016/j.jpba.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Zapata JM, Krajewska M, Morse HC, III, Choi Y, Reed JC. TNF receptor-associated factor (TRAF) domain and Bcl-2 cooperate to induce small B cell lymphoma/chronic lymphocytic leukemia in transgenic mice. Proc Nat Acad Sci U S A. 2004;101:16600–16605. doi: 10.1073/pnas.0407541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zapata JM, Krajewska M, Krajewski S, et al. TRAF-family protein expression in normal tissues and lymphoid malignancies. J Immunol. 2000;165:5084–5096. doi: 10.4049/jimmunol.165.9.5084. [DOI] [PubMed] [Google Scholar]
- 25.Pierce GF, Polmar SH. Lymphocyte dysfunction in cartilage-hair hypoplasia: evidence for an intrinsic defect in cellular proliferation. J Immunol. 1982;129:570–575. [PubMed] [Google Scholar]
- 26.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 27.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Hochman A, Sternin H, Gorodin S, et al. Enhanced oxidative stress and altered antioxidants in brains of Bcl-2-deficient mice. J Neurochem. 1998;71:741–748. doi: 10.1046/j.1471-4159.1998.71020741.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kB-mediated up-regulation of bcl-x and bfl-1/a1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci U S A. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.