Abstract
Secretory proteins are exported from the endoplasmic reticulum (ER) by bulk flow and/or receptor-mediated transport. Our understanding of this process is limited because of the low number of identified transport receptors and cognate cargo proteins. In mammalian cells, the lectin ER Golgi intermediate compartment 53-kD protein (ERGIC-53) represents the best characterized cargo receptor. It assists ER export of a subset of glycoproteins including coagulation factors V and VIII and cathepsin C and Z. Here, we report a novel screening strategy to identify protein interactions in the lumen of the secretory pathway using a yellow fluorescent protein–based protein fragment complementation assay. By screening a human liver complementary DNA library, we identify α1-antitrypsin (α1-AT) as previously unrecognized cargo of ERGIC-53 and show that cargo capture is carbohydrate- and conformation-dependent. ERGIC-53 knockdown and knockout cells display a specific secretion defect of α1-AT that is corrected by reintroducing ERGIC-53. The results reveal ERGIC-53 to be an intracellular transport receptor of α1-AT and provide direct evidence for active receptor-mediated ER export of a soluble secretory protein in higher eukaryotes.
Introduction
The lumen of the ER provides a unique oxidizing environment for protein folding and modification. One third of all newly synthesized proteins are translocated into the ER and processed by multiple ER resident enzymes. An elaborate quality-control system monitors the conformational state of the nascent proteins and retains them in the ER during the folding process (Ellgaard and Helenius, 2003). Upon correct folding, proteins are exported from the ER in coat protein II (COPII) vesicles (Lee et al., 2004). Although transmembrane proteins can directly interact with the cytosolic COPII coat (Barlowe, 2003), some soluble luminal proteins require cargo receptors for their selective recruitment into COPII vesicles (Belden and Barlowe, 2001; Baines and Zhang, 2007). The best characterized mammalian cargo receptor is the oligomeric type I membrane lectin ERGIC-53 (Schweizer et al., 1988). ERGIC-53 cycles between the ER and ER Golgi intermediate compartment (ERGIC), captures soluble glycoproteins in the lumen of the ER, and binds COPII by means of a dihydrophobic ER export motif in its cytosolic tail (Hauri et al., 2000; Appenzeller-Herzog and Hauri, 2006). As a cargo receptor, ERGIC-53 mediates ER export of coagulation factors V and VIII and cathepsin C and Z (Nichols et al., 1998; Vollenweider et al., 1998; Appenzeller et al., 1999). Furthermore, ERGIC-53 was recently shown to assist the assembly of IgM polymers in the ER (Anelli et al., 2007). With only few ERGIC-53 cargo proteins identified, however, it is not known whether receptor-mediated ER export is the predominant mechanism for the transport of soluble proteins. A major problem in identifying cargo–receptor complexes is their transient nature in a unique ionic and oxidizing environment. Further, existing proteomic techniques for identifying protein–protein interactions (e.g., affinity purification/mass spectrometry or the yeast two-hybrid system) are not easily applicable or are not appropriate to the study of membrane proteins. However, protein fragment complementation assays (PCAs) do allow for detection of both transient and dynamic protein–protein interactions in intact living cells, including those for membrane proteins (Remy et al., 1999; Michnick et al., 2007). A PCA based on a YFP variant (citrine) has been demonstrated to be applicable to protein–protein interactions inside the lumen of the secretory pathway (Nyfeler et al., 2005). In the YFP PCA, nonfluorescent N- and C-terminal fragments of YFP (YFP1 and YFP2) are individually fused to the coding sequences of two separate proteins and expressed in mammalian cells. If the two fusion proteins interact, the fragments of YFP are brought into proximity, permitting folding and reconstitution of fluorescent YFP in vivo. Using this specific and sensitive assay, we have demonstrated that the oligomerization of ERGIC-53 and its interactions with multiple coagulation factor deficiency protein 2 (MCFD2) and cathepsin C and Z were readily visible in living cells. Here, we describe a YFP PCA–based cDNA library screening strategy for the identification of novel ERGIC-53 cargo proteins. The screening of a human adult liver cDNA library identified α1-antitrypsin (α1-AT) as novel interaction partner of ERGIC-53 and our validation studies establish ERGIC-53 as a transport receptor of α1-AT.
Results and discussion
Based on a general PCA cDNA library screening strategy (Remy and Michnick, 2004a,b), we developed a screening approach designed to identify proteins that bind to cargo receptors in the ER lumen. ERGIC-53 was used as bait and was N-terminally tagged with YFP2, which localizes YFP2 to the luminal side of the membrane and allows screening for protein–protein interactions inside the lumen of the ER. Prey proteins were C-terminally tagged with YFP1 and expressed from a cDNA-YFP1 fusion library (Fig. 1). This fusion orientation assures that library-encoded secretory and membrane proteins reach the lumen of the ER because N-terminal signal sequences are not perturbed. However, we do not expect to identify known ERGIC-53 interaction partners with this cDNA-YFP1 library because of specific characteristics of these proteins or because of the specific orientation of the prey YFP1 toward cytosol. For example, C-terminal tagging cathepsin Z and C impedes the interaction with ERGIC-53 (Nyfeler et al., 2005), whereas MCFD2-YFP1 is unlikely to be identified because its interaction with ERGIC-53 strictly depends on the full-length MCFD2 protein (Nyfeler et al., 2008). Further, the interaction of ERGIC-53 with itself cannot be detected because the oligomerization of ERGIC-53 requires its transmembrane domain (Nufer et al., 2003), which localizes the YFP1 of all oligomerization-competent YFP1–ERGIC-53 prey proteins to the cytosol. This compartment-specific topological requirement is a unique feature of the PCA approach, providing information about the orientation of membrane-associated prey proteins. Our C-terminal tagging strategy requires that library inserts lack their stop codon to avoid termination of translation in front of the YFP1 sequence. To this end, we used cDNAs generated by randomly primed reverse transcription of mRNA, which enriches the 5′ ends. cDNA inserts were subcloned from a human adult liver cDNA library into mammalian pcDNA3 expression vectors containing YFP1 in all three reading frames. The resulting cDNA-YFP1 library contained ∼106 clones and inserts ranged from ∼1 to ∼2.5 kb in size. As expected for a library generated from a secretory tissue like liver, cDNAs encoding secretory proteins were well represented (Fig. S1, available at http://www.jcb.org/cgi/content/full/jcb.200709100/DC1).
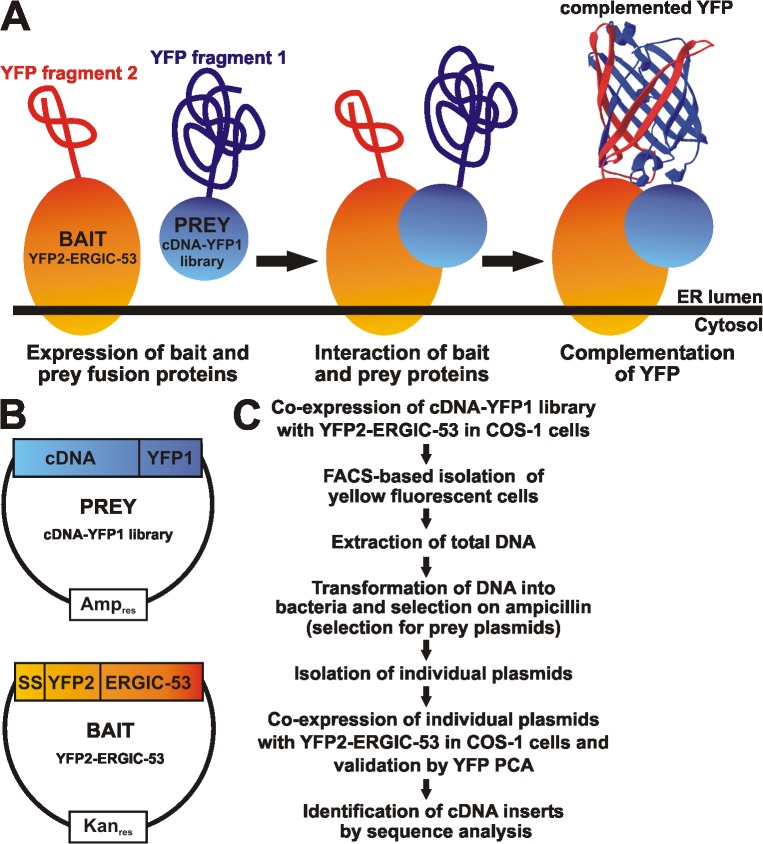
Figure 1.
Strategy of the ERGIC-53 cargo hunt based on YFP PCA. (A) Schematic representation of the YFP PCA approach to screen for ERGIC-53 cargo proteins in the lumen of the secretory pathway. YFP PCA is based on the reconstitution of functional YFP from two nonfluorescent fragments that are brought into close proximity by two interacting proteins. YFP2 was fused to the ERGIC-53 bait protein, whereas YFP1 was fused to a human liver cDNA library to express YFP1-tagged prey proteins. (B) The YFP2–ERGIC-53 bait protein was expressed from the kanamycin-resistant pCMV vector, whereas the cDNA-YFP1 library was constructed in the ampicillin-resistant pcDNA3 vector. (C) Flow chart of the screening strategy.
The YFP PCA–based cDNA library screen was performed in COS-1 cells, which express the large T antigen and replicate plasmids containing the SV40 eukaryotic origin of replication. Because of this feature, cDNA-YFP1 library plasmids will be replicated upon transfection, which ensures sufficient expression of individual prey proteins and simplifies recovery and analysis of positive clones. Coexpression of YFP2–ERGIC-53 and the cDNA-YFP1 library resulted in the detection of 1.63% yellow fluorescent COS-1 cells, and YFP PCA can fully account for the detected signal because few positive cells were obtained upon expression of YFP2–ERGIC-53 or the cDNA-YFP1 library alone (Fig. 2 A). In the screen, YFP2–ERGIC-53 and the cDNA-YFP1 library were transfected at a ratio of 10:1 to reduce the number of library plasmids transfected per cell. This lowered the percentage of positive cells to 0.12% (unpublished data). Several hundred YFP-positive cells were collected by FACS and prey clones were recovered, 48 of which were individually reanalyzed by YFP PCA in COS-1 cells. Coexpression with YFP2–ERGIC-53 resulted in positive YFP signals for prey plasmids numbered 17, 32, 33, and 44 (Fig. 2 B). The recovery of only 4 positives out of 48 reanalyzed prey plasmids is not because of the unspecificity of our assay but rather the uptake of several prey plasmids per transfected cell. A YFP-positive cell contains the positive prey plasmid as well as many cotransfected negative plasmids, which will be subsequently also recovered (Fig. S2, available at http://www.jcb.org/cgi/content/full/jcb.200709100/DC1).
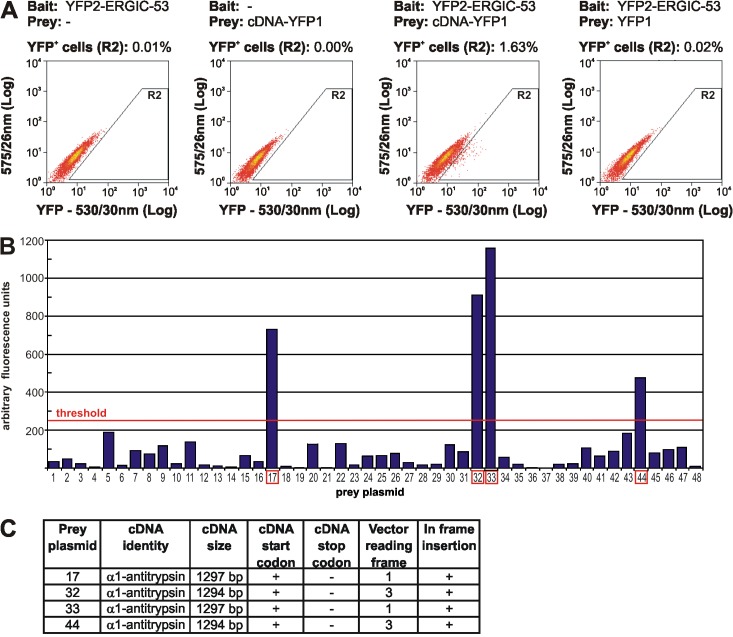
Figure 2.
Screening of the cDNA-YFP1 library for ERGIC-53 interaction partners. (A) COS-1 cells were transfected with the indicated bait and prey constructs and analyzed by FACS as described in Materials and methods. Coexpression of YFP2–ERGIC-53 and the cDNA-YFP1 library resulted in the specific detection of 1.63% YFP positive (YFP+) cells. In a nonsaturating screen, ∼500 fluorescent cells were collected by FACS and total DNA was extracted and transformed into bacteria, which resulted in the recovery of several hundred prey clones. (B) 48 prey clones were randomly selected and plasmids were isolated and individually assayed by YFP PCA with YFP2–ERGIC-53 in COS-1 cells. Fluorometric analysis revealed that prey plasmids 17, 32, 33, and 44 (indicted by red boxes) were positive and reconstitute fluorescent YFP when expressed with YFP2–ERGIC-53. Bars represent fluorometric values of a single screening experiment. The threshold for a positive hit was set to 250 arbitrary fluorescence units, which corresponds to a ∼1.5-fold induction in YFP fluorescence in comparison to untransfected cells. (C) Sequence analyses identified α1-AT as a cDNA insert in all four positive prey plasmids.
DNA sequence analysis identified α1-AT as the corresponding cDNA insert in prey plasmids 17, 32, 33, and 44. Two types of α1-AT inserts were found, a 1294-bp and a 1297-bp variant. Both inserts were in frame and covered the complete coding sequence of α1-AT with the exception of the last two C-terminal amino acids (Fig. 2 C). The ERGIC-53–α1-AT interaction was validated by YFP PCA in HeLa cells using full-length α1-AT N-terminally tagged with YFP2 (Fig. 3 A).
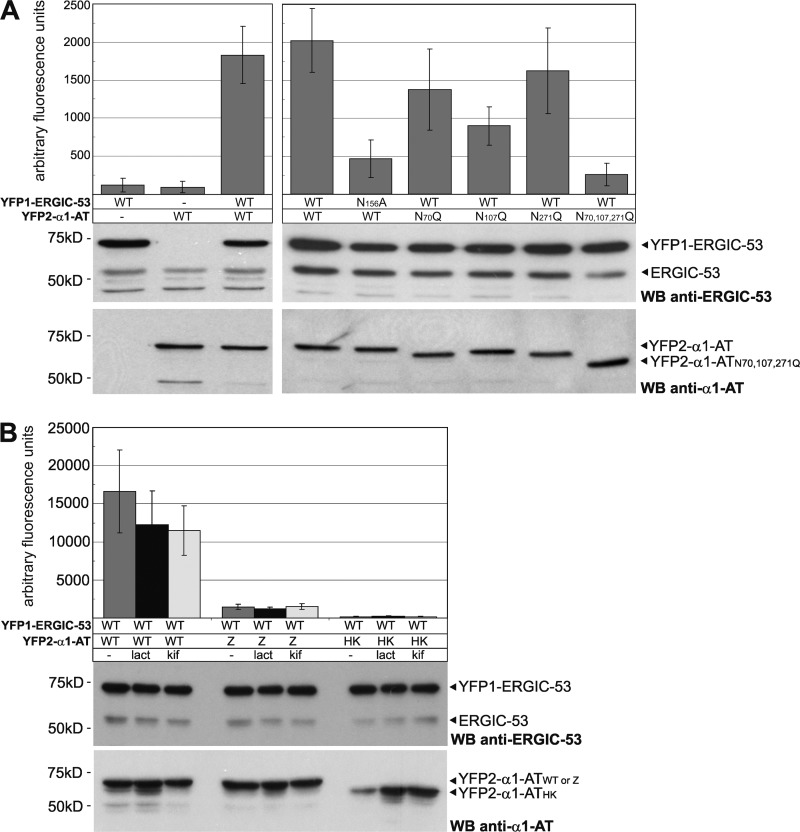
Figure 3.
ERGIC-53 captures α1-AT in a carbohydrate- and conformation-dependent manner. The indicated YFP PCA constructs were expressed in HeLa cells for 24 (A) and 48 h (B). 20 μM Lactacystin (lact) and 200 μM kifunensine (kif) were applied where indicated for 24 h. YFP complementation was analyzed by fluorometric analysis of cell suspensions in microtiter plates and expression levels of the different constructs were probed by Western blotting (WB) using polyclonal antibodies against ERGIC-53 and α1-AT. Endogenous ERGIC-53 functions as an input control. Bars represent mean ± SD (n = 3).
α1-AT is a liver glycoprotein of 52 kD carrying three N-linked glycans. After its synthesis in hepatocytes, α1-AT is secreted into the blood, where it acts as a serine protease inhibitor, mainly against neutrophil elastase. As one of the major glycoproteins synthesized in the liver, α1-AT is likely to be highly abundant in our nonnormalized library. When YFP2–ERGIC-53 and the cDNA-YFP1 library were expressed in COS-1 cells after siRNA-mediated silencing of α1-AT, a 30–50% reduction in YFP-positive cells was observed (unpublished data). Hence, α1-AT seems responsible for up to half of all observed YFP-positive cells in our screen. N-linked glycosylation of α1-AT is strictly required for its secretion (Gross et al., 1982) and it was suggested that the N-glycans may serve as recognition sites for an ER-to-Golgi transport receptor (Lodish and Kong, 1984). Is ERGIC-53 an intracellular transport receptor of α1-AT? We first analyzed the carbohydrate specificity of the ERGIC-53–α1-AT interaction by performing a YFP PCA with the N156A mutant of ERGIC-53 and a triple N70,107,271Q mutant of α1-AT. ERGIC-53N156A is unable to bind to N-linked oligosaccharides on glycoproteins (Appenzeller et al., 1999) and α1-ATN70,107,271Q remains unglycosylated because of site-directed mutagenesis of all three N-glycosylation consensus sites. In comparison to their wild-type counterparts, ERGIC-53N156A and α1-ATN70,107,271Q showed a markedly reduced YFP PCA signal (Fig. 3 A). These findings suggest that ERGIC-53 binds α1-AT in a carbohydrate-dependent manner. We also analyzed N70Q, N107Q, and N271Q single mutants of α1-AT and found that mutagenesis of the second N-glycosylation site (N107Q) has the strongest effect on the ERGIC-53–α1-AT interaction (Fig. 3 A). This result is in full agreement with a previous study showing that the N107Q mutant of α1-AT has a significantly longer intracellular retention time than the other mutations (Samandari and Brown, 1993). Interestingly, all three N-glycosylation single site mutants have similar degradation rates and solubility (Samandari and Brown, 1993). Hence, the carbohydrate attached at N107 is particularly important for ER export.
Next, we addressed the conformation specificity of the ERGIC-53–α1-AT interaction by analyzing two misfolded versions of α1-AT known as Z (α1-ATZ) and Hong Kong (α1-ATHK) mutants (Stoller and Aboussouan, 2005). Although α1-ATHK is soluble and degraded by ER-associated degradation involving the proteasome, α1-ATZ aggregates in the ER and is subjected to proteasomal as well as lysosomal degradation (Cabral et al., 2000; Teckman et al., 2001). Strikingly, in our YFP PCA, neither α1-ATHK nor α1-ATZ bound to ERGIC-53 (Fig. 3 B). YFP2–α1-ATZ was expressed at a similar intracellular level as YFP2–α1-ATWT and was not affected by the two inhibitors of proteasomal degradation lactacystin and kifunensine. We cannot rule out the possibility that aggregation decreases the pool of soluble, ERGIC-53 binding-competent α1-ATZ, thereby lowering the YFP PCA signal. In the case of α1-ATHK, inhibition of proteasomal degradation by lactacystin and kifunensine clearly increased intracellular levels of YFP2–α1-ATHK. Although the amount of YFP2–α1-ATHK was thereby restored to that of the wild-type construct, the YFP PCA signal was not enhanced (Fig. 3 B). These data suggest that the ERGIC-53–α1-AT interaction is conformation-dependent and supports a function of ERGIC-53 in secondary quality control by capturing only native cargo proteins for ER export (Ellgaard and Helenius, 2003; Appenzeller-Herzog et al., 2005).
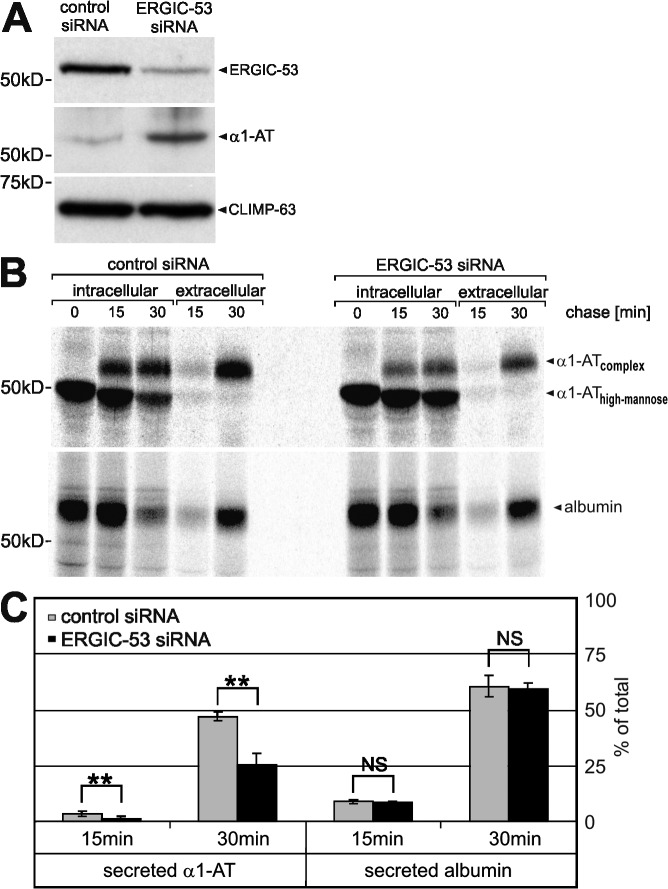
To confirm that ERGIC-53 captures α1-AT for ER export, we analyzed the transport of endogenous α1-AT in HepG2 cells in which ERGIC-53 was knocked down by siRNA. Silencing of ERGIC-53 for 96 h reduced total ERGIC-53 protein levels to below 20% (Fig. 4 A). Intriguingly, the knock down of ERGIC-53 led to substantial steady-state accumulation of α1-AT inside the cell (Fig. 4 A). This effect was further analyzed by studying transport and secretion of α1-AT in pulse chase experiments using [35S]methionine. α1-AT is synthesized as high-mannose glycoprotein, undergoes complex glycosylation in the Golgi, and is subsequently secreted into the culture medium. In HepG2 cells transfected with control siRNA, high-mannose α1-AT was rapidly converted to its complex glycosylated form and about half of the protein was already secreted after a 30-min chase. In contrast, in ERGIC-53–silenced cells, α1-AT remained considerably longer in its high-mannose form and only ∼15% of the protein was secreted after 30 min (Fig. 4, B and C). The inefficient secretion of α1-AT is not caused by a general secretion defect because the secretion of endogenous albumin is unchanged (Fig. 4, B and C). Furthermore, previous studies in HeLa cells have already shown that neither depletion nor mislocalization of ERGIC-53 change the morphology of the secretory pathway or affect overall protein secretion (Vollenweider et al., 1998; Nyfeler et al., 2006). Hence, reduced levels of ERGIC-53 significantly delay the secretion of α1-AT in a specific manner.
Figure 4.
α1-AT secretion is impaired in ERGIC-53 knockdown cells. HepG2 cells were transiently transfected for 96 h with control and ERGIC-53–specific siRNA duplexes. (A) Western blotting using monoclonal anti–ERGIC-53 and anti–CLIMP-63 and polyclonal anti–α1-AT antibodies. ERGIC-53 is efficiently silenced, which results in intracellular accumulation of α1-AT. CLIMP-63, an ER-resident membrane protein, functions as an input control. (B) [35S]methionine pulse chase of endogenous α1-AT and albumin. HepG2 cells were labeled for 15 min with [35S]methionine and chased for the indicated times, and α1-AT and albumin were recovered by immunoprecipitation from cell lysates (intracellular) and conditioned medium (extracellular) using anti–α1-AT and anti-albumin antibodies, respectively. (C) The amount of intracellular and extracellular α1-AT and albumin was quantified by densitometric scanning of the band intensities and the secreted fraction of total protein [extracellular/(intracellular + extracellular)] was determined for each time point. Bars represent mean ± SD (n = 3). Results analyzed by paired t test: NS, P > 0.05; **, P < 0.01.
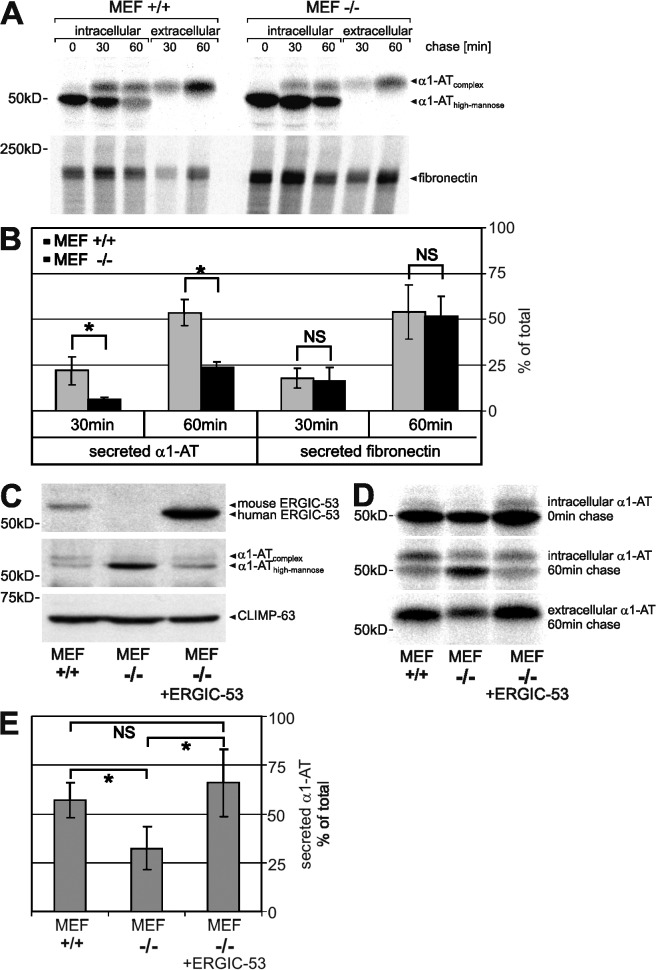
To determine if the remaining secretion of α1-AT is caused by residual ERGIC-53, we studied the transport of α1-AT transfected into mouse embryonic fibroblasts (MEFs) derived from ERGIC-53 knockout (−/−) and wild-type (+/+) mice. Pulse chase experiments revealed a half-time of secretion of ∼60 min in +/+ MEFs. ERGIC-53 knockout MEFs secreted in the same time only ∼25% of the newly synthesized α1-AT and showed significantly slower conversion of high-mannose to complex glycosylated α1-AT (Fig. 5, A and B). A general secretion defect can again be excluded because ERGIC-53 knockout MEFs secreted endogenous fibronectin as efficiently as wild-type MEFs (Fig. 5, A and B). These results demonstrate that α1-AT secretion is significantly delayed without ERGIC-53 but that alternative, less efficient ER export pathways exist.
Figure 5.
ERGIC-53 is an intracellular transport receptor of α1-AT. α1-AT was transfected into wild-type (+/+) and ERGIC-53 knockout (−/−) MEFs and expressed for 24 h. (A) [35S]methionine pulse chase of α1-AT and endogenous fibronectin. MEFs were labeled with [35S]methionine for 15 min and chased for the indicated times, and α1-AT and fibronectin were recovered by immunoprecipitation from cell lysates (intracellular) and conditioned medium (extracellular) using anti–α1-AT and anti-fibronectin antibodies, respectively. (B) The secreted fraction of α1-AT and fibronectin was quantified as described in Fig. 4 C. (C) α1-AT was coexpressed with human ERGIC-53 in ERGIC-53 knockout MEFs (MEF −/− plus ERGIC-53). Western blotting was performed using polyclonal antibodies against ERGIC-53, α1-AT, and CLIMP-63. (D) [35S]methionine pulse chase of α1-AT. (E) The secreted fraction of α1-AT after a 60-min chase was quantified by densitometric scanning and calculated as in Fig. 4 [extracellular/(intracellular + extracellular)]. Bars represent mean ± SD (n = 3). Results analyzed by paired t test: NS, P > 0.05; *, P < 0.05.
If ERGIC-53 is a transport receptor of α1-AT, reintroduction of ERGIC-53 should correct the secretion defect of α1-AT in ERGIC-53 knockout cells. Indeed, when human ERGIC-53 was coexpressed with α1-AT, intracellular α1-AT was considerably reduced in −/− MEFs at a steady state (Fig. 5 C). Moreover, ERGIC-53 increased the secretion of α1-AT in −/− MEFs to the level of +/+ MEFs after a 1-h chase (Fig. 5, D and E). Hence, overexpression of ERGIC-53 in ERGIC-53 knockout MEFs can completely restore α1-AT secretion. These rescue experiments clearly establish ERGIC-53 as an intracellular transport receptor of α1-AT. Previous studies on ERGIC-53 function were mainly based on genetic analysis (Nichols et al., 1998), transport studies with a dominant-negative ER-retained mutant of ERGIC-53 (Vollenweider et al., 1998; Appenzeller et al., 1999), or the characterization of ERGIC-53–cargo interactions (Appenzeller et al., 1999; Appenzeller-Herzog et al., 2005; Nyfeler et al., 2005; Zhang et al., 2005). The current study provides now direct evidence for active receptor-mediated ER export of a soluble secretory protein in mammalian cells. Furthermore, our data indicate that transport receptors render cargo transport faster and more efficient, but cargo transport is not entirely blocked in their absence. α1-AT may possess a second transport receptor or may exit the ER by bulk flow to a certain extent. The screening approach we described here could be equally applied using α1-AT as bait to identify novel cargo receptors. With α1-AT, we now identified an attractive model secretory protein to study the mechanism of receptor-mediated protein export from the ER in detail.
In conclusion, this study not only identifies an intracellular transport receptor of α1-AT but also opens an unprecedented avenue to genome-wide screening for protein–protein interactions in the secretory pathway. The successful identification of a novel ERGIC-53 cargo protein by YFP PCA–based screening of a complex cDNA-YFP1 fusion library provides a general strategy to identify novel luminal protein complexes. With normalized libraries and optimized transfection conditions, saturating and genome-wide screens will be feasible in the near future. Further, YFP PCA has a promising potential for high-throughput screening of chemical and molecular chaperones (Burrows et al., 2000) that can rescue conformational defects of α1-AT mutants and render them secretion-competent by promoting their interaction with ERGIC-53.
Materials and methods
Antibodies and inhibitors
The following antibodies were used: mouse mAbs against human ERGIC-53 (G1/93; Qbiogene), human CLIMP-63 (G1/296; Qbiogene) and GFP (Roche), rabbit polyclonal antibodies (pAbs) against ERGIC-53 (Schweizer et al., 1988), human CLIMP-63, and horse fibronectin (provided by M. Chiquet, Friedrich Miescher Institute, Basel, Switzerland), goat pAb against human α1-AT (MP Biomedicals) and sheep pAb against human albumin (The Binding Site). Lactacystin and kifunensine were obtained from EMD.
cDNA-YFP1 library construction
The cDNA-YFP1 library was constructed in the pcDNA3 vector (Invitrogen) into which the coding sequences for the (GGGGS)2 linker followed by YFP1 (amino acids 1–158 of YFP) was inserted. Note that all three reading frames of YFP1 were covered and that YFP1 contains the citrine mutation (Q69M; Nyfeler et al., 2005). Two SfiI restriction sites were introduced in front of the linker-YFP1 sequences by inserting a pair of annealed oligonucleotides. The pcDNA3(SfiI-linker-YFP1) vectors used for library construction are illustrated in Fig. S1. cDNA inserts were excised from a human adult liver cDNA-NubG library (Dualsystems Biotech) by SfiI restriction digestion and separated by agarose gel electrophoresis. The major cDNA insert fraction, ranging from ∼1 to ∼2.5 kb in size, was purified from the agarose gel and ligated via the SfiI restriction sites into the pcDNA3(SfiI-linker-YFP1) vectors. Ligation products were ethanol precipitated and electroporated into competent MC1061 bacteria. Transformed bacteria were grown overnight at 30°C on ampicillin-containing plates and harvested by scraping into liquid broth medium, and plasmids were isolated using the Plasmid Maxi kit (QIAGEN).
YFP PCA plasmids
The YFP2–ERGIC-53 bait was generated by subcloning SS–YFP2–ERGIC-53 (Nyfeler et al., 2005) into the pCMV-Script vector (Stratagene). YFP fragment 2 contains amino acids 159–239 of YFP. YFP1-MCFD2 was constructed by inserting MCFD2 without its signal sequence into pcDNA3(SS-YFP1) (Nyfeler et al., 2005). YFP2–α1-ATWT and YFP2–α1-ATZ were generated by inserting the corresponding cDNAs, PCR-amplified without the endogenous signal sequences from pECEM(A1Pi) and pECEM(A1PiZ) (provided by M. Spiess, Biozentrum, University of Basel, Basel, Switzerland) into pcDNA3(SS-YFP2) (Nyfeler et al., 2005). YFP2–α1-ATHK, YFP2–α1-ATN70Q, YFP2–α1-ATN107Q, YFP2–α1-ATN271Q, and YFP2–α1-ATN70,107,271Q were generated by QuickChange site-directed mutagenesis (Stratagene). Cloning of HA-MCFD2, YFP1-ERGIC-53WT, and YFP1-ERGIC-53N156A has been described previously (Nyfeler et al., 2005, 2006).
cDNA library screening
COS-1 cells were grown in 100-mm dishes and transfected with 2.5 μg DNA and 7.5 μl FuGENE6 (Roche). pCMV(SS–YFP2–ERGIC-53) and the cDNA-YFP1 library were cotransfected at a ratio of 10:1. 48 h after transfection, cells were harvested in PBS containing 0.1% bovine serum albumin and 5 mM EDTA, and YFP-positive cells were sorted using a FACS Vantage SE (Becton Dickinson). Fluorescence was excited at a wavelength of 488 nm and recorded at 530/30 nm (YFP signal) and 575/26 nm. In a forward versus side scatter linear dot plot, the cell population of interest was defined as region R1 and gated into a 530/30 nm versus 575/26 nm logarithmic dot plot. Plotting of 530/30 nm versus 575/26 nm fluorescence resulted in autofluorescent cells lying in the diagonal of the plot, whereas cells expressing YFP were exclusively found in region R2 (Fig. 2 A). Cells in R2 were sorted into PBS and total DNA was isolated using the DNeasy Tissue kit (QIAGEN), ethanol precipitated, and transformed into XL-10 gold ultracompetent bacteria (Stratagene), which were then selected on ampicillin-containing plates. Note that transformants that take up genomic DNA or pCMV(SS–YFP2–ERGIC-53) cannot grow on ampicillin. Plasmids were isolated from liquid overnight cultures of single bacterial transformants using the Plasmid Mini kit (Sigma-Aldrich) and were individually cotransfected with pCMV(SS–YFP2–ERGIC-53) into COS-1 in 6-well plates. Fluorometric analysis of COS-1 cells was performed 48 h after transfection as described previously (Nyfeler et al., 2005) and plasmids resulting in a positive YFP signal were subjected to nucleotide sequence analyses.
Cell culture and transfection
HeLa cells (CCL-2; American Type Culture Collection) and MEFs were grown in DME supplemented with 10% fetal bovine serum, 1× nonessential amino acids, and antibiotics. COS-1 cells (CRL-1650; American Type Culture Collection) were grown in DME supplemented with 10% fetal bovine serum and antibiotics. HepG2 cells (HB-8065; American Type Culture Collection) were grown in MEM supplemented with 10% fetal bovine serum and antibiotics. HeLa and COS-1 cells were transfected using FuGENE6 (Roche). HepG2 cells were transfected with control and ERGIC-53 siRNA using HiPerfect (QIAGEN) as described previously (Nyfeler et al., 2006). MEFs were transfected by electroporation using the Amaxa Nucleofector, program T-20, and MEF solution 2 (Amaxa Biosystems). α1-AT and ERGIC-53 were expressed from pECEM(A1Pi) and pECE(ERGIC-53) (Schindler et al., 1993), respectively.
Immunoblotting
Protein samples were prepared by boiling cell suspensions in protein sample buffer and were separated by SDS-PAGE, transferred to nitrocellulose membranes, immunoblotted with the indicated antibodies, and visualized by enhanced chemiluminescence (GE Healthcare).
PCR screening
DNA from heat-killed bacterial transformants was amplified with two primers that anneal onto the pcDNA3 vector backbone and amplify ∼1,150 and ∼650 bp fragments for pcDNA3(SS-YFP1-MCFD2) and pcDNA3(SS-HA-MCFD2), respectively.
Preparation of wild-type and ERGIC-53 knockout MEFs
MEFs were generated from embryos at 13.5 d postcoitus as described previously (A. Nagy, 2003) by mating heterozygous mice with an ERGIC-53 knockout allele and transformed with the SV40 large T antigen, and immortalized cell lines were established.
[35S]methionine metabolic labeling
Cells were deprived of l-methionine for 30 min, pulsed for 15 min with 100 μCi [35S]methionine (Perkin Elmer) and chased for the indicated times in culture medium containing 10 mM l-methionine. Cells were lysed in 1% Triton X-100, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM CaCl2, and PMSF, and lysates were cleared by centrifugation at 100,000 g for 1 h. The chase medium was cleared from cell debris by centrifugation at 20,000 g for 5 min. Cleared samples were immunoprecipitated with the indicated antibodies, immunoprecipitates were separated by SDS-PAGE, and radiolabeled bands were imaged and quantified using a phosphorimager (Molecular Dynamics).
Statistical analysis
Mean, standard deviation, and t test were calculated by using Excel 2002 (Microsoft). A two-tailed, paired t test was used to calculate the statistical significance between two indicated samples. P-values >0.05 were considered not significant and p-values <0.05 and <0.01 were considered significant.
Online supplemental material
Fig. S1 shows the assessment of the human adult liver cDNA-YFP1 library. Fig. S2 shows the assessment of plasmid transfection and recovery. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200709100/DC1.
Supplementary Material
Acknowledgments
We thank Käthy Bucher for expert technical assistance and Verena Jäggin for professional assistance in FACS analysis and sorting.
This work was supported by the University of Basel, the Swiss National Science Foundation, and the Roche Research Foundation.
Abbreviations used in this paper: α1-AT, α1-antitrypsin; COPII, coat protein II; ERGIC, ER Golgi intermediate compartment; MCFD2, multiple coagulation factor deficiency protein 2; MEF, mouse embryonic fibroblast; PCA, protein fragment complementation assay.
References
- Anelli, T., S. Ceppi, L. Bergamelli, M. Cortini, S. Masciarelli, C. Valetti, and R. Sitia. 2007. Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. EMBO J. 26:4177–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller, C., H. Andersson, F. Kappeler, and H.P. Hauri. 1999. The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1:330–334. [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog, C., and H.P. Hauri. 2006. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 119:2173–2183. [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog, C., B. Nyfeler, P. Burkhard, I. Santamaria, C. Lopez-Otin, and H.P. Hauri. 2005. Carbohydrate- and conformation-dependent cargo capture for ER-exit. Mol. Biol. Cell. 16:1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines, A.C., and B. Zhang. 2007. Receptor-mediated protein transport in the early secretory pathway. Trends Biochem. Sci. 32:381–388. [DOI] [PubMed] [Google Scholar]
- Barlowe, C. 2003. Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol. 13:295–300. [DOI] [PubMed] [Google Scholar]
- Belden, W.J., and C. Barlowe. 2001. Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science. 294:1528–1531. [DOI] [PubMed] [Google Scholar]
- Burrows, J.A., L.K. Willis, and D.H. Perlmutter. 2000. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc. Natl. Acad. Sci. USA. 97:1796–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral, C.M., P. Choudhury, Y. Liu, and R.N. Sifers. 2000. Processing by endoplasmic reticulum mannosidases partitions a secretion-impaired glycoprotein into distinct disposal pathways. J. Biol. Chem. 275:25015–25022. [DOI] [PubMed] [Google Scholar]
- Ellgaard, L., and A. Helenius. 2003. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4:181–191. [DOI] [PubMed] [Google Scholar]
- Gross, V., T. Geiger, T.A. Tran-Thi, F. Gauthier, and P.C. Heinrich. 1982. Biosynthesis and secretion of alpha 1-antitrypsin in primary cultures of rat hepatocytes. Characterization of differently glycosylated intracellular and extracellular forms. Eur. J. Biochem. 129:317–323. [DOI] [PubMed] [Google Scholar]
- Hauri, H.P., F. Kappeler, H. Andersson, and C. Appenzeller. 2000. ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 113:587–596. [DOI] [PubMed] [Google Scholar]
- Lee, M.C., E.A. Miller, J. Goldberg, L. Orci, and R. Schekman. 2004. Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20:87–123. [DOI] [PubMed] [Google Scholar]
- Lodish, H.F., and N. Kong. 1984. Glucose removal from N-linked oligosaccharides is required for efficient maturation of certain secretory glycoproteins from the rough endoplasmic reticulum to the Golgi complex. J. Cell Biol. 98:1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michnick, S.W., P.H. Ear, E.N. Manderson, I. Remy, and E. Stefan. 2007. Universal strategies in research and drug discovery based on protein-fragment complementation assays. Nat. Rev. Drug Discov. 6:569–582. [DOI] [PubMed] [Google Scholar]
- Nagy, A., M.G. Gertsenstein, K. Vintersten, and R. Behringer, editors. 2003. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 764 pp.
- Nichols, W.C., U. Seligsohn, A. Zivelin, V.H. Terry, C.E. Hertel, M.A. Wheatley, M.J. Moussalli, H.P. Hauri, N. Ciavarella, R.J. Kaufman, and D. Ginsburg. 1998. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 93:61–70. [DOI] [PubMed] [Google Scholar]
- Nufer, O., F. Kappeler, S. Guldbrandsen, and H.P. Hauri. 2003. ER export of ERGIC-53 is controlled by cooperation of targeting determinants in all three of its domains. J. Cell Sci. 116:4429–4440. [DOI] [PubMed] [Google Scholar]
- Nyfeler, B., S.W. Michnick, and H.P. Hauri. 2005. Capturing protein interactions in the secretory pathway of living cells. Proc. Natl. Acad. Sci. USA. 102:6350–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyfeler, B., B. Zhang, D. Ginsburg, R.J. Kaufman, and H.P. Hauri. 2006. Cargo selectivity of the ERGIC-53/MCFD2 transport receptor complex. Traffic. 7:1473–1481. [DOI] [PubMed] [Google Scholar]
- Nyfeler, B., Y. Kamiya, F. Boehlen, K. Yamamoto, K. Kato, P. de Moerloose, H.P. Hauri, and M. Neerman-Arbez. 2008. Deletion of three residues from the C-terminus of MCFD2 affects binding to ERGIC-53 and causes combined factor V and factor VIII deficiency. Blood. 111:1299–1301. [DOI] [PubMed] [Google Scholar]
- Remy, I., and S.W. Michnick. 2004. a. A cDNA library functional screening strategy based on fluorescent protein complementation assays to identify novel components of signaling pathways. Methods. 32:381–388. [DOI] [PubMed] [Google Scholar]
- Remy, I., and S.W. Michnick. 2004. b. Regulation of apoptosis by the Ft1 protein, a new modulator of protein kinase B/Akt. Mol. Cell. Biol. 24:1493–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy, I., I.A. Wilson, and S.W. Michnick. 1999. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 283:990–993. [DOI] [PubMed] [Google Scholar]
- Samandari, T., and J.L. Brown. 1993. A study of the effects of altering the sites for N-glycosylation in alpha-1-proteinase inhibitor variants M and S. Protein Sci. 2:1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler, R., C. Itin, M. Zerial, F. Lottspeich, and H.P. Hauri. 1993. ERGIC-53, a membrane protein of the ER-Golgi intermediate compartment, carries an ER retention motif. Eur. J. Cell Biol. 61:1–9. [PubMed] [Google Scholar]
- Schweizer, A., J.A. Fransen, T. Bachi, L. Ginsel, and H.P. Hauri. 1988. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 107:1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller, J.K., and L.S. Aboussouan. 2005. Alpha1-antitrypsin deficiency. Lancet. 365:2225–2236. [DOI] [PubMed] [Google Scholar]
- Teckman, J.H., J. Burrows, T. Hidvegi, B. Schmidt, P.D. Hale, and D.H. Perlmutter. 2001. The proteasome participates in degradation of mutant alpha 1-antitrypsin Z in the endoplasmic reticulum of hepatoma-derived hepatocytes. J. Biol. Chem. 276:44865–44872. [DOI] [PubMed] [Google Scholar]
- Vollenweider, F., F. Kappeler, C. Itin, and H.P. Hauri. 1998. Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of HeLa cells impairs the secretion of a lysosomal enzyme. J. Cell Biol. 142:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B., R.J. Kaufman, and D. Ginsburg. 2005. LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J. Biol. Chem. 280:25881–25886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.