Abstract
Objective:
Implantation of synthetic temporomandibular joint (TMJ) disc replacements aimed to alleviate pain and restore functional losses caused by TMJ disorders. Unfortunately, these synthetic replacements have been largely unsuccessful and in some instances have incited severe immune responses. Tissue engineering, however, may provide viable TMJ disc replacements. Towards this end, we have studied TMJ disc gene expression as a measure of protein production potential. With passage, collagen type I and aggrecan gene expression decrease in TMJ disc cell cultures. We hypothesize that surfaces coated with TMJ disc proteins may rapidly recover the lost gene expression in passaged TMJ disc cells.
Design:
To study these effects, passage 0, 1, and 2 TMJ disc cells were plated in wells coated with aggrecan, collagen type I, collagen type II, or decorin. Safranin O staining was conducted to visualize cell aggregation.
Results:
At passage 0, cultures appeared similar on each surface; however, by passage 1 and 2, aggrecan-coated and decorin-coated surfaces appeared to have more cell aggregates. Gene expression data did not correspond to these visual changes. No treated surface offered a significant change in aggrecan, collagen type I, or decorin expression relative to untreated controls. Furthermore, aggrecan and collagen type I gene expression dropped relative to samples taken prior to plating.
Conclusions:
These results indicate that, despite visual changes described by cell aggregates, protein coatings have limited effects for recovering TMJ disc gene expression in monolayer cultures.
Introduction
The temporomandibular joint (TMJ) is composed of three primary structures: the mandibular condyle, glenoid fossa-articular eminence of the temporal bone, and an interarticular disc. Malformation, misalignment, or remodeling of the primary TMJ components can result in severe pain, headaches, and joint crepitation.(1;2) In addition, persons suffering from these disorders can experience a loss of functional motions, thus making common jaw movements difficult. The origin or onset of TMJ disorders is incompletely understood, but they are believed to derive from a variety of factors including displacement or dislocations of the interarticular disc, changes to the musculature and joint loading, trauma, and diseases such as osteo- and rheumatoid-arthritis.(1-4) Surgical treatments aim to restore essential functions and reduce pain;(3;5;6) however, TMJ tissues are likely severly remodeled, permanently injured, or scarred when surgical intervention is indicated. Towards this end, we have previously proposed tissue engineering as an avenue by which viable replacement tissues may be generated for the TMJ.(7)
The interarticular disc, or TMJ disc, is the joint's central element, located between the mandibular condyle and fossa-eminence of the temporal bone. This fibrocartilaginous tissue divides the joint space into inferior and superior compartments and assists in joint motions, distribution of load, and the decrease of incongruencies between the joint's bony structures. When displaced, injured, or malformed, the TMJ disc can be removed from its functional position in the joint, which may lead to tissue remodeling, folding, and/or thinning.(2) Restoring the TMJ disc's function in the impaired joint via the implantation of a TMJ disc equivalent may improve on current treatment modalities.(7) Synthetic implants have been largely unsuccessful,(8-10) and for this reason, the TMJ disc has been a prime target for tissue engineers.(7;11)
Thus far, the majority of TMJ disc tissue engineering studies have used cells that were passaged and expanded in monolayer prior to the tissue engineering attempt.(12-18) Recent work demonstrated that monolayer expansion of TMJ disc cells resulted in rapid changes to TMJ disc gene expression.(19) Most alarming in this work were gene expression losses indicating TMJ disc cells were losing their ability to produce key extracellular matrix (ECM) components, namely collagen type I and aggrecan.(19) However, previous work on the chondrocytic potential of fibroblasts demonstrated that chondrocytic gene expression could be induced in a fibroblastic population by plating the cells over aggrecan-coated surfaces.(20) In these experiments, two cues were described: 1) cell nodule/aggregate formation by the plated cells, and 2) changes in aggrecan and collagen type II gene expression.(20) In addition, this same plating technique reduced the de-differentiation effects of monolayer expansion for articular chondrocytes.(21) Losses in chondrocytic markers, primarily collagen type II and aggrecan gene expression, were lessened when articular chondrocytes were plated on aggrecan-coated surfaces.(21)
In the present study, we investigated the effects of protein-coated surfaces on TMJ disc cells. Cells were isolated from porcine TMJ discs, then either plated for monolayer expansion or plated on a aggrecan-coated, collagen type I-coated, collagen type II-coated, decorin-coated or a no protein coating control surface. To investigate nodule formations, safranin O/fast green staining was conducted. Cellular responses were investigated using real-time RT-PCR for aggrecan, collagen type I, collagen type II, and decorin gene expression. Thus, the aim of this study was to recover essential TMJ disc gene expression lost during the expansion of TMJ disc cells.
Materials and Methods
The porcine model was selected based upon previous studies on animal models for human TMJ disc research.(22;23) Female pigs weighing more than 60 kg (135 lbs) post dressing were obtained from a local slaughter house; pigs in this weight range are skeletally mature (approx. 8 – 12 months old). A TMJ from three porcine heads was isolated using an en bloc dissection technique. TMJs were placed in 70% ethanol and transferred to a sterile hood. Joints were then opened by rupture of the joint capsule, and the TMJ discs were removed, minced, and placed in a Petri dish. All discs were digested overnight in 40 mL of 2 mg/mL collagenase type II (Worthington). Cells were then isolated via centrifugation, counted, and suspended in Dulbecco's modified eagle's medium with Glutamax (Gibco) containing 10% fetal bovine serum (Gemini), 25 μg/mL ascorbic acid (Sigma), 1% non-essential amino acids (Gibco), and 1% penicillin-streptomycin-fungizone (Biowhittaker). Cells were then either plated on tissue culture plastic (TCP) 6-well plates at approximately 33% confluence, lysed for passage 0 controls, or reserved for seeding on a protein-coated surface. Full media changes were conducted daily in monolayer expansion cultures. Upon reaching confluence, cells were passaged using trypsin/EDTA (Gibco), isolated via centrifugation (1500 rpm for 5 min), and then counted with a hemocytometer. Passaged cells were either lysed for a passage controls or reserved for seeding on a protein-coated surface.
Protein-coated surfaces included treatments with either aggrecan, collagen type I, collagen type II, decorin, or no protein for control. These surfaces were created in TCP treated 24-well plates. To coat, 250 μL of protein in sterile water (0.02 μg/μL) was added to each target well and allowed to evaporate overnight in a sterile hood. Cells were plated on the protein-coated surface at a concentration of 200,000 cells per well for 24 hrs.(20) At which time, media were removed, and samples were either lysed in TriZol reagent (Invitrogen) for gene expression characterization or processed for histological investigation.
RNA was isolated from the TriZol reagent using a protocol provided by the manufacturer. Briefly, 1 mL of TriZol was added to each well, agitated, and transferred to a 1.5 mL Eppendorf tube. Chloroform (0.2 mL) was mixed in each sample, centrifuged at 12,000 g for 15 min, and the topmost supernatant was transferred to a fresh 1.5 mL Eppendorf tube. Isopropyl alcohol (0.5 mL) was added to the supernatant, and RNA was precipitated by centrifugation. Ethanol (75%, 1 mL) was then added to wash the RNA pellet. To remove the RNA pellet from the ethanol, samples were briefly centrifuged (9,000 g for 5 min), ethanol was removed, and residual ethanol droplets were allowed to evaporate (∼5 to 10 min). RNA was then dissolved in 40 μL of RNase free water, and RNA concentration and purity was assessed on a spectrophotometer (Nanodrop).
Stratascript™ First Strand Synthesis System (Stratagene) was used to reverse transcribe RNA to cDNA, according to the manufacturer's protocol. Briefly, 350 ng RNA per sample was transferred to a 0.2 mL PCR tube and suspended in RNAse-free water, such that the total volume was 17.7 μL. This process reduced variation in RNA concentration between samples, such that we assume a comparable RT reaction efficiency for different samples. Random primers were annealed to the sample RNA by incubating with primers, buffer, and dNTPs at 65°C for 5 min. Samples were then cooled to room temperature (≈ 10 min) before reverse transcriptase and RNase block were added to the sample. This was followed by an incubation at 42°C for 60 min followed by a reaction termination at 70°C for 15 min.
Real-time PCR was performed for collagen type I, collagen type II, aggrecan, decorin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene expression using a Rotor-gene 3000 real-time PCR machine (Corbett Research). HotStarTaq (Qiagen) combined with buffer (Qiagen, 1x), MgCl (Qiagen, 3.5 mM), dNTPs (Promega, 0.2 mM), RNase-free water, and the gene-specific primer-probe sets were combined with 1 μL of sample (conducted in triplicate). The PCR reaction commenced with a 15 min denaturing step at 95°C followed by 50 cycles of 15 s at 95°C and 30 s at 60°C; fluorescence measurements (on FAM, CalRed, and Quasar 670) at 60°C were taken for each cycle. The GAPDH primer-probe set was included in every PCR reaction to serve as a verification gene and to insure consistent reaction loading. GAPDH is abundant in TMJ disc cells and is easily detectable in the PCR reactions. This practice allows for the separation of samples with expression below the limit of detection from samples with a failed PCR reaction. Primer-probe sequences for GAPDH are available in Darling and Athanasiou(24); all other primer-probe sequences are available in Upton et al.(25)
The PCR reaction efficiency (EFF) was determined by dilutions of a standard porcine sample (1x, 10x, 100x, and 1000x). Sample and standard take-off cycles (Ct), where Ct is equal to the cycle at which 30% of the maximum slope for a PCR reaction occurs, were calculated with the comparative quantification package available within the Rotogene software package. Abundance of the gene of interest (AGOI) can be calculated for each sample via the following equation:
| (1) |
These values were then utilized for the quantitative comparison of TMJ disc gene expression.
In order to visualize nodule formation, Safranin O/fast green staining was performed.(20) After each passage, media was removed from the wells, and samples were washed with phosphate buffer solution. Samples were first fixed in formalin (10 min), rinsed with water, and then stained with Fast Green (10 min). Fast Green was removed, with excess stain removed via additional water washes. A brief incubation in acetic acid was preformed (< 2 min), followed by staining with Safranin O for 2 min. Samples were rinsed once with water and photographed using a Nixon CoolPix 990 digital camera mounted on a Nikon Eclipse TS-100 inverted microscope at 4x and 10x magnification.
The gene expression of TMJ disc cells seeded over aggrecan, collagen type I, collagen type II, or decorin was compared using an analysis of variance approach (ANOVA). A two-factor ANOVA was used in which passage and treatment were considered as factors. The passage levels included passage 0, 1, and 2; the treatment levels included plating on aggrecan, collagen type I, collagen type II, decorin, no protein treatment control, and passage controls. No treatment controls were cells place on a non-protein-treated surface; passage controls represent the sample gene expression prior to plating.
Results
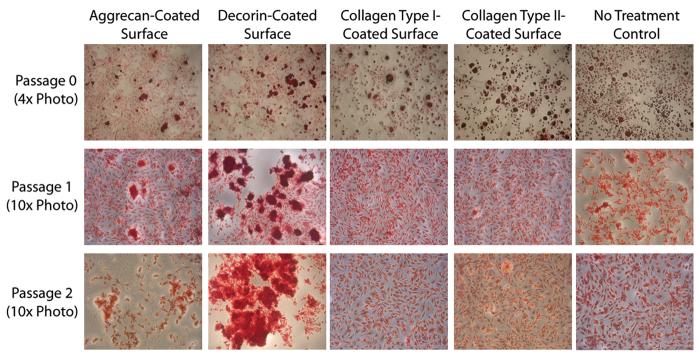
Seeding on a proteoglycan-coated surface caused visual changes to the cell culture at higher passages. At both passage 1 and passage 2, TMJ disc cells formed cell aggregates when plated on aggrecan-coated or decorin-coated surfaces; Figure 1 - columns 1 & 2 describe the cell aggregates as stained with Safranin O. On the decorin-coated surface, the cell aggregates were generally concentrated near the center of the well and became larger with passage. Aggregates in the aggrecan-coated wells were randomly dispersed throughout the well and of consistent size between passages 1 & 2. Cells plated over collagen-coated surfaces, either type I or type II, generally had a spindle-like shape; the gross appearance of these cultures did not differ visually from that of no treatment controls. At passage 0, cell cultures appeared similar across plate treatments. Each well, regardless of surface coating, had small cell nodule formations with spindle shaped cells around an aggregate's periphery (Fig. 1, top row). These small aggregates could be found on each surface treatment at each passage; however, aggregates were far less frequent on collagen-coated surfaces and no treatment controls relative to that of proteoglycan-coated surfaces at higher passages.
Figure 1. Safranin O staining of TMJ disc cells plated on protein-coated surfaces.
Safranin O/Fast Green staining was used to detect nodule formation on the protein-coated surfaces. At passage 0, all surfaces had both nodules and spindle-shaped cells, and cell shape and GAG production appeared similar on all surfaces. At passage 1, aggrecan-coated and decorin-coated surfaces caused large nodule formations, while collagen type I-coated, collagen type II-coated, and no treatment controls had relatively more spindle-shaped cells. At passage 2, aggrecan-coated and decorin-coated surfaces again caused large nodule formations. Nodules on the aggrecan-coated surface were spread across the well, while nodules on the decorin-coated surface were large and concentrated near the center of the well. Nodule formation was observed on each surface at each passage; however, the size and prevalence of nodules was lower on collagen-coated surfaces and no treatment controls.
TMJ disc gene expression changes were compared relative to two different controls. First, passage controls represent a cell population's gene expression levels prior to plating over any surface; this control is represented by solid black horizontal lines in Figures 2-5. Second, no treatment controls represent a cell population's gene expression when plated on a non-protein-treated surface; this control is represented by solid black vertical bars in Figures 2-5. In passage controls, collagen type I and aggrecan gene expression decreases with each subsequent passage. Conversely, decorin gene expression increases slightly with each passage. Collagen type II gene expression was near the lower limits of detection at passage 0; by passage 1, samples were below the lower limits of detection for this gene.
Figure 2. Collagen type I gene expression of TMJ disc cells plated on protein-coated surfaces.
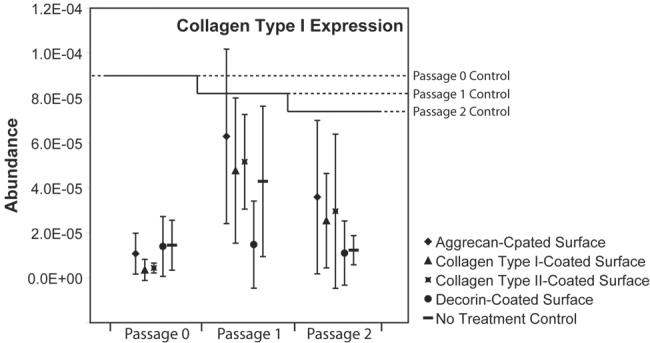
Collagen type I gene expression dropped in 2-dimensional cultures relative to passage controls (p < 0.05). At passage 0, these drops were larger than drops at passage 1 and passage 2. No surface coating demonstrated a significant advantage in up-regulating collagen type I gene expression relative to no treatment controls.
Relative to passage controls, collagen type I gene expression dropped regardless of the surface treatment or passage (Figure 2). These drops at passage 0 were approximately 8.5 fold; by passage 1 and passage 2, decreases were lower in magnitude – approximately 0.86 and 2.2 fold, respectively. Thus, samples taken immediately following passaging had higher levels of collagen type I gene expression than samples taken from any tested monolayer surface (p < 0.05). No differences between surfaces were observed.
Relative to passage controls, aggrecan gene expression also dropped regardless of the surface or passage (Figure 3). These drops were approximately 18 fold at passage 0, 3.3 fold at passage 1, and over 50 fold at passage 2. Thus, samples taken immediately following passaging had higher levels of aggrecan gene expression than samples taken from any tested monolayer surface (p < 0.05). No differences between surfaces were observed.
Figure 3. Aggrecan gene expression of TMJ disc cells plated on protein-coated surfaces.
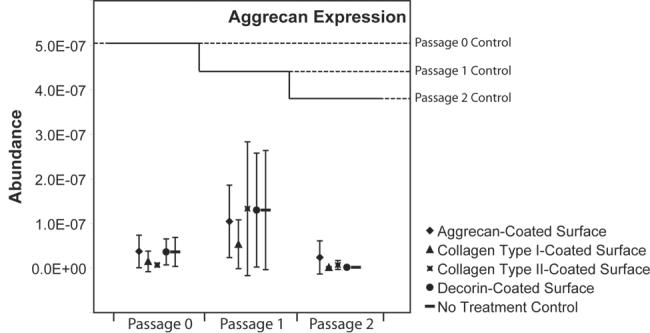
At each passage, aggrecan dropped significantly on each surface relative to passage controls. (p < 0.05). No differences between protein-coated surfaces and no treatment controls were observed in aggrecan gene expression.
Collagen type II gene expression was very low in all samples. At passage 0, collagen type II gene expression was detected in only about half of the samples. By passage 1 and 2, collagen type II gene expression was below the limits of detection. Thus, statistical analysis of the effects of protein-coated surfaces on collagen type II gene expression could not be conducted due to lack of measures. Decorin gene expression was not altered by the protein-coated surfaces (Figure 4); furthermore, at each passage, decorin gene expression in monolayer cultures fell within the range of passage controls.
Figure 4. Decorin gene expression of TMJ disc cells plated on protein-coated surfaces.
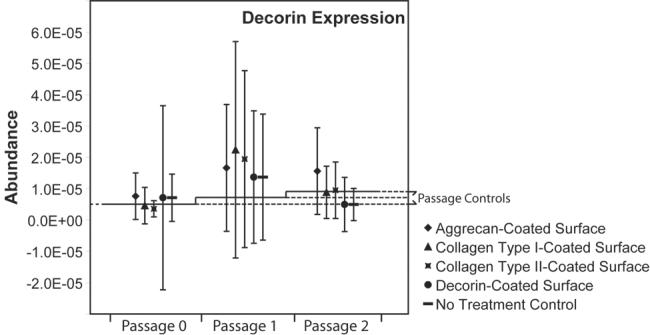
Decorin gene expression on the protein-coated surfaces was not significantly different from passage or no treatment controls at any of the examined passages.
Discussion
Aggrecan-coated and decorin-coated surfaces appeared to promote the formation of cell aggregates in TMJ disc cells that had been passaged at least once. Cells in aggregates have a rounded appearance. This shape may be morphologically approaching the shape of articular chondrocytes.(26) However, the presence of cell aggregates in the TMJ disc cell cultures did not correlate to chondrocyte-like gene expression at 24 hours. Instead, the act of plating TMJ disc cells over any surface, either treated or untreated, resulted in the down-regulation of gene expression indicative of TMJ disc ECM production. Most notable were the down-regulation of collagen type I and aggrecan gene expression. These proteins are the TMJ disc's primary collagen and proteoglycan constituents respectively.(27)
Proteoglycan-coated surfaces have previously been noted for their induction of spindle-shaped fibroblastic cells into a more spherical shape.(20;26;28) This shape change is believed to result from the negatively charged protein-coated surface interacting with a negatively charged cell membrane, thus reducing the ability of fibroblastic cells to spread across a surface. Furthermore, this change in cell morphology has been linked to transcriptional level collagen type II changes in RAB-9 dermal fibroblasts relative to a control monolayer surface.(20) It is unclear as to why the protein coated-surface had minimal transcriptional level effects on TMJ disc cells relative to our prior experiments with skin derived fibroblasts. While we did observe changes in cell morphology, we did not observe such a chondrocytic shift with TMJ disc cells. RAB-9 cells were pretreated with insulin-like growth factor I prior to plating over a proteoglycan-coated surface; the author however comments that preliminary trials with freshly isolated dermal cells did not require a growth factor pretreatment.(20) Within our data, similar morphological differences between surfaces were observed, but a transcriptional level change was not. It may be possible that collagen type II gene expression was altered relative to control surfaces, but expression levels were below the limits of detection. Regardless, these low levels of expression are unlikely to correlate to a sufficient level of protein production in tissue engineering studies.
The pretreatment of TMJ disc cells with chondro-inductive agents may spark these aggregates to behave more chondrocyte-like on the protein coated surface. However, the selection of these agents for TMJ disc cells has very mixed results. Most notably, in a nearly analogous experimental design, transforming growth factor beta 1, transforming growth factor beta 3, and insulin-like growth factor I had little effect on the gene expression of passaged TMJ disc cells.(29) In the tissue engineering environment, these growth factors have had more success increasing protein production, but these results are only observed after 4-6 weeks of continuous growth factor delivery.(13;16;29-31) Furthermore, the effectiveness of insulin-like growth factor I on TMJ disc cells is very mixed and results may depend on a plethora of confounding factors such as scaffolding, substrate, cell isolation techniques, and seeding density.(13;31)
As with dermis cells, TMJ disc cells represent a heterogeneous population with a primarily fibroblastic component. The TMJ disc cell population is approximately 70% fibroblast-like and 30% chondrocyte-like with sparse contributions from other cells types (32). Though cells from both tissue sources experienced shifts in morphology when placed on proteoglycan-coated surfaces, it may be possible that transcriptional level changes occur on different time scales in TMJ disc and skin cell populations. Additionally, TMJ disc cells may be more easily damaged by digestive enzymes during cell isolation and passaging and may, thus, be less sensitive to extracellular matrix components in monolayer culture. Finally, only a subpopulation of dermis-derived cells is aggrecan sensitive (28); this subpopulation may be too small of a component in the TMJ disc to cause transcriptional level changes measured at the gross population level.
As of yet, we have been unable to isolate TMJ disc subpopulations for culture and are unable to comment directly on changes in gene expression in specific TMJ disc cell subpopulations. Morphologically, the average shape of TMJ disc cells in culture becomes more spindle-shaped as cells are passaged. However, it is unclear whether chondrocyte-like cells are shifting toward a fibroblastic phenotype or spreading and complying into a spindle shape. Furthermore, fibroblast-like cells may be proliferating and, thus, drowning out the effects of cells with a chondrogenic potential. Our laboratory has recently isolated a specific subpopulation of aggrecan-sensitive dermis derived cells from skin and demonstrated chondrogenic potential in these cell isolates.(28) We continue to explore similar techniques to isolate specific cell subpopulations in the TMJ disc and identify cellular components with high matrix production potential for TMJ disc tissue engineering. In the future, these techniques may lead to a more complete understanding of the TMJ disc's cells and their mechanisms and identify a cell source with a high potential for TMJ disc tissue engineering.
TMJ disc cells alter their gene expression as a result of passage.(19) Previously, we hypothesized these changes were linked to the passaging event, possibly induced by exposing the cells to a digestive enzyme. The results of this study indicate that drops attributed to passaging may also be attributed to plating the cells. Collagen type I, aggrecan, and collagen type II gene expression was lower in samples taken from a monolayer surface relative to samples taken prior to plating. As cells are plated, they are likely adapting their membrane characteristics to the new surface/substrate. This may result in down regulation of ECM components and up regulation of cell attachment components. As cells expand, gene expression may shift in other directions encouraging rapid division. However, if ECM production is gradually restored during expansion, previous work indicates that expression levels of collagen type I and aggrecan at confluence will remain below that of the previous passage.(19) Also, it should also be noted that cells kept in pellet cultures experience dramatic reduction in ECM gene expression levels.(19) Thus, the monolayer environment may not be an ideal for cell expansion, but the alternative culture may also have severe limitations.
The TMJ disc has a heterogeneous population of cells. These cells can be visually classified as spindle-shaped (fibroblasts), rounded sans visible pericellular matrix (fibrochondrocytes), and rounded with visible pericellular matrix (chondrocytes). In the adult porcine TMJ disc, fibroblasts and fibrochondrocytes dominate, with approximately 70% of the cells resembling spindle-shaped fibroblasts.(32) The vast remainder of cells possess rounded shapes and have only scant traces of pericellular matrix.(32) This distribution of cells fits well with the cell aggregation observed at passage 0. Rounded cells are observed in the aggregates, while spindle-shaped cells cover the remaining surface. Thus, passage 0 cells may be retaining a native morphology; furthermore, this morphology is relatively unaffected by the initial plating surface.
As cells are passaged, the effect of the protein-coated surfaces on cell morphology changes. This may be due to changes to the cell membrane resulting from exposure to trypsin or growth on tissue culture plastic. Decorin nodules appear to get larger with passage; decorin gene expression has also been observed to increase with passage. It is unclear why either event occurs, but it is possible that the presence of decorin allows cells to pack tighter together. The up regulation of decorin as a result of passage may be in response to tighter packing of the cells as they approach confluence.
Towards tissue engineering the TMJ disc, no surface caused a marked increase in collagen type I gene expression. Collagen type I is the primary ECM component of the TMJ disc; production of this protein in large amounts is paramount to TMJ disc tissue engineering. This finding coupled with reductions in collagen type I gene expression due to passage is troubling.(19) Currently in TMJ disc tissue engineering, there exists a trade off between the quantity of cells and the cellular potential for matrix production. It may be possible to further alter the culture environment to slow the losses in gene expression associated with TMJ disc cell expansion. Alternatively, the use and differentiation of stem cells for use in TMJ disc tissue engineering must be further explored. From this source, a large cell population with high ECM production potential may be possible.
In conclusion, cell aggregation can be induced in passaged TMJ disc cells via proteoglycan-coated surfaces. These morphological changes, however, do not result in transcriptional level changes of the TMJ disc's primary extracellular matrix components at 24 hrs. Further optimization of the tissue culture conditions, such as pretreatment with a growth factor, could help to prime TMJ disc cells for ECM production. However, recent findings suggest that the positive growth factor effects on TMJ disc cells are only observed after long term, continuous delivery, and not in short-term gene expression recovery experiments. Towards tissue engineering of the TMJ disc, identifying a large population of cells with a strong potential for matrix production is vital. Retention of TMJ disc gene expression over multiple passages should continue to be investigated; alternatively, discovering optimal conditions for stem cell differentiation may prove to be a more viable cell source option for TMJ disc tissue engineering.
Acknowledgements
Research support was provided by NIH #1 R01 DE015038-01A2, and individual support for KDA was provided by NSF IGERT DGE-0114264.
Common Abbreviations
- TMJ
temporomandibular joint
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- TCP
tissue culture plastic
- RT
reverse transcriptase
- PCR
polymerase chain reaction
- ANOVA
analysis of variance
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong ME, Allen KD, Athanasiou KA. The Biomedical Engineering Handbook: Tissue Engineering Section. CRC Press; 2005. Tissue engineering of the temporomandibular joint. In Press. [Google Scholar]
- 2.Wilkes CH. Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg. 1989;115(4):469–477. doi: 10.1001/archotol.1989.01860280067019. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri LG. Considering total temporomandibular joint replacement. Cranio. 1999;17(1):44–48. doi: 10.1080/08869634.1999.11746076. [DOI] [PubMed] [Google Scholar]
- 4.Stegenga B. Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain. 2001;15(3):193–205. [PubMed] [Google Scholar]
- 5.Wolford LM. Temporomandibular joint devices: treatment factors and outcomes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(1):143–149. doi: 10.1016/s1079-2104(97)90105-0. [DOI] [PubMed] [Google Scholar]
- 6.McCarty WL, Farrar WB. Surgery for internal derangements of the temporomandibular joint. J Prosthet Dent. 1979;42(2):191–196. doi: 10.1016/0022-3913(79)90174-4. [DOI] [PubMed] [Google Scholar]
- 7.Detamore MS, Athanasiou KA. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;9(6):1065–1087. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 8.Henry CH, Wolford LM. Treatment outcomes for temporomandibular joint reconstruction after Proplast-Teflon implant failure. J Oral Maxillofac Surg. 1993;51(4):352–358. doi: 10.1016/s0278-2391(10)80343-x. discussion 359-360. [DOI] [PubMed] [Google Scholar]
- 9.TMJ Implants - A Consumer Information Update - 1999. United States Food and Drug Administration; 1999. [Google Scholar]
- 10.FDA Enforcement Report. United States Food and Drug Administration; 1991. [Google Scholar]
- 11.Allen KD, Athanasiou KA. Tissue Engineering of the TMJ disc: a review. Tissue Eng. 2006;12(5):1183–1196. doi: 10.1089/ten.2006.12.1183. [DOI] [PubMed] [Google Scholar]
- 12.Detamore MS, Athanasiou KA. Use of a rotating bioreactor toward tissue engineering the temporomandibular joint disc. Tissue Eng. 2005;11(78):1187–1197. doi: 10.1089/ten.2005.11.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detamore MS, Athanasiou KA. Evaluation of three growth factors for TMJ disc tissue engineering. Ann Biomed Eng. 2005;33(3):383–390. doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bean AC, Almarza AJ, Athanasiou KA. Effects of ascorbic acid concentration on the tissue engineering of the temporomandibular joint disc. Proc Inst Mech Eng [H] 2006;220(3):439–447. doi: 10.1243/09544119JEIM51. [DOI] [PubMed] [Google Scholar]
- 15.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10(1112):1787–1795. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 16.Almarza AJ, Athanasiou KA. Evaluation of three growth factors in combinations of two for TMJ disc tissue engineering. Arch Oral Biol. 2006;51(3):215–221. doi: 10.1016/j.archoralbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Almarza AJ, Athanasiou KA. Effects of hydrostatic pressure on TMJ disc cells. Tissue Eng. 2006;12(5):1285–1294. doi: 10.1089/ten.2006.12.1285. [DOI] [PubMed] [Google Scholar]
- 18.Almarza AJ, Athanasiou KA. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomed Eng. 2005;33(7):943–950. doi: 10.1007/s10439-005-3311-8. [DOI] [PubMed] [Google Scholar]
- 19.Allen KD, Athanasiou KA. Effect of passage and topography on gene expression of temporomandibular joint disc cells. Tissue Eng. 2006;13(1):101–110. doi: 10.1089/ten.2006.0094. [DOI] [PubMed] [Google Scholar]
- 20.French MM, Rose S, Canseco J, Athanasiou KA. Chondrogenic differentiation of adult dermal fibroblasts. Ann Biomed Eng. 2004;32(1):50–56. doi: 10.1023/b:abme.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- 21.Darling EM, Athanasiou KA. Retaining zonal chondrocyte phenotype by means of novel growth environments. Tissue engineering. 2005;11(34):395–403. doi: 10.1089/ten.2005.11.395. [DOI] [PubMed] [Google Scholar]
- 22.Bermejo A, Gonzalez O, Gonzalez JM. The pig as an animal model for experimentation on the temporomandibular articular complex. Oral Surg Oral Med Oral Pathol. 1993;75(1):18–23. doi: 10.1016/0030-4220(93)90399-o. [DOI] [PubMed] [Google Scholar]
- 23.Herring SW. Animal models of temporomandibular disorders: how to choose. In: Sessle BJ, Bryant PS, Dionne RA, editors. Temporomandibular disorders and related pain conditions. IASP Press; Seattle: 1995. pp. 323–328. [Google Scholar]
- 24.Darling EM, Hu JC, Athanasiou KA. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22(6):1182–1187. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21(6):963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 26.Revell CM, Dietrich JA, Scott CC, Luttge A, Baggett LS, Athanasiou KA. Characterization of fibroblast morphology on bioactive surfaces using vertical scanning interferometry. Matrix Biol. 2006;25(8):523–533. doi: 10.1016/j.matbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical Content and Distribution in the Porcine Temporomandibular Joint Disc. Brit J Oral Maxillofac Surg. 2006;44:124–128. doi: 10.1016/j.bjoms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Hu JC, Athanasiou KA. Isolation and chondroinduction of a dermis-isolated, aggrecan-sensitive subpopulation with high chondrogenic potential. Arthritis & Rheumatism. 2007;56(1):168–176. doi: 10.1002/art.22300. [DOI] [PubMed] [Google Scholar]
- 29.Allen KD, Athanasiou KA. Growth factor effects on passaged TMJ disk cells in monolayer and pellet cultures. Orthod Craniofac Res. 2006;9(3):143–152. doi: 10.1111/j.1601-6343.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 30.Detamore MS, Athanasiou KA. Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol. 2004;49(7):577–583. doi: 10.1016/j.archoralbio.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Allen KD, Athanasiou KA. TMJ Disc Engineering: Scaffold and Growth Factor Selection. Journal of Dental Research. 2006 doi: 10.1177/154405910808700205. MS ID 06-0433. [DOI] [PubMed] [Google Scholar]
- 32.Detamore MS, Hegde JN, Wagle RR, Almarza AJ, Montufar-Solis D, Duke PJ, Athanasiou KA. Cell type and distribution in the porcine temporomandibular joint disc. J Oral Maxillofac Surg. 2005;64(2):243–248. doi: 10.1016/j.joms.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]