Abstract
Patients with defective ectodysplasin A (EDA) are affected by X-linked hypohidrotic ectodermal dysplasia (XLHED), a condition characterized by sparse hair, inability to sweat, decreased lacrimation, frequent pulmonary infections, and missing and malformed teeth. The canine model of XLHED was used to study the developmental impact of EDA on secondary dentition, since dogs have an entirely brachyodont, diphyodont dentition similar to that in humans, as opposed to mice, which have only permanent teeth (monophyodont dentition), some of which are very different (aradicular hypsodont) than brachyodont human teeth. Also, clinical signs in humans and dogs with XLHED are virtually identical, whereas several are missing in the murine equivalent. In our model, the genetically missing EDA was compensated for by postnatal intravenous administration of soluble recombinant EDA. Untreated XLHED dogs have an incomplete set of conically shaped teeth similar to those seen in human patients with XLHED. After treatment with EDA, significant normalization of adult teeth was achieved in four of five XLHED dogs. Moreover, treatment restored normal lacrimation and resistance to eye and airway infections and improved sweating ability. These results not only provide proof of concept for a potential treatment of this orphan disease but also demonstrate an essential role of EDA in the development of secondary dentition.
In X-linked hypohidrotic ectodermal dysplasia (XLHED [MIM #305100]) in humans (caused by a defect in ED1), affected individuals have a developmental disorder characterized by sparse or absent hair, missing and/or malformed teeth, and hypoplastic eccrine glands.1–5 There is significant morbidity and mortality in affected children due to hyperthermia, caused by their inability to sweat. Lack of other glands leads to an increased risk of respiratory-tract infections and dry eye resulting in ocular disease.1,3,5,6 Tooth abnormalities include delayed primary and secondary dentition, abnormal occlusion, conical tooth crowns (peg-shaped teeth), and oligodontia, which lead to difficulties with mastication, growth retardation, poor appearance, and speech impairment.3,7,8
The proteins encoded by the two longest transcripts of ED1 (EDA-A1 and EDA-A2) are type II transmembrane proteins with a short intracellular domain, a transmembrane domain, a collagen motif, and a tumor necrosis factor (TNF)–ligand motif that associate into a homotrimer.9 An extracellular furin site allows for cleavage of the protein, making it a soluble ligand, which is required for binding to its receptor (EDAR) and for proper signaling. About half the mutations causing XLHED are missense mutations, most of which are located in either (1) the putative transmembrane/extracellular junction domain, (2) the furin cleavage site, (3) the collagenous domain, which is thought to be necessary for ligand oligomerization, or (4) the TNF domain, which mediates receptor binding.10 These mutations either alter the overall structure and folding of ectodysplasin A (EDA) or specifically impair one of the functional domains.
In recent experiments, recombinant EDA (Fc:EDA1) was administered pre- and postnatally to Tabby mice, the murine homologue of humans and canines with XLHED.11 The protein was designed such that, when injected intravenously (IV) into pregnant dams, the Fc portion (of human immunoglobulin G1) would allow for transfer across the placenta into the affected fetus.11 Because there is virtually no intrauterine transfer of immunoglobulins in dogs, we chose to treat the XLHED dogs postnatally. This also more closely reflects the clinical situation, in which the diagnosis is often not made until after birth unless there is a family history of ectodermal dysplasia. Postnatal injections in neonatal Tabby mice resulted in normalization of the eyelid opening and the appearance of sweat glands and tail hair. However, correction of the lack of ear hair, guard and zigzag hair, and abnormal molar shape was achieved only when fetal Tabby mice, but not neonatal mice, were exposed to the recombinant protein.
We chose to use the canine model12 for further therapeutic trials with Fc:EDA1, because the disease in dogs more closely mirrors that seen in human patients. In our model, XLHED is caused by a point mutation in the splice-acceptor site of intron 8, which results in a truncated, nonfunctional protein.13 The XLHED dogs have symmetrical hairlessness, nasal crusting, and dry eye from decreased lacrimation and are unable to sweat. As in most human patients with XLHED, we have found an increased rate of pulmonary infectious diseases, attributable to the lack of bronchial glands, which are necessary for normal ciliary function.14
The tooth abnormalities are also quite similar, in that the number of teeth is greatly reduced, and those teeth that are present are generally all peg shaped in affected dogs. Tooth development in dogs and humans is very similar: deciduous teeth are formed before birth, erupt after birth, and are followed by permanent teeth, which develop as a bud arising from the dental lamina of the deciduous tooth.15 Adult humans and dogs have brachyodont dentition consisting of 32 and 42 teeth, respectively, including incisors, canines, premolars, and molars. Mice differ in that they have only 16 permanent teeth, with incisors that grow continuously (aradicular hypsodont), and they lack canines and premolars.16,17 In the Tabby mouse, the third molar is missing in ∼50% of the mice, the teeth are generally smaller, and molars have less prominent cusps,18,19 but the overall appearance is not dramatically different from that of normal mice. In this study, we demonstrate that postnatal administration of Fc:EDA1 in dogs has a prominent effect on permanent dentition and significantly improves long-term resistance to eye and airway infections, probably through restoration of glandular functions.
Material and Methods
Treatment and Analysis of XLHED Dogs
All dogs were cared for in accordance with the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and in the International Guiding Principles for Biomedical Research Involving Animals. Affected females were produced by breeding heterozygous females to XLHED males, and their phenotype does not differ from that seen in male dogs. Three affected pups (two females and one male) received a single injection of 1 mg (3–4 mg/kg) of Fc:EDA111 IV via the jugular vein at age 2 d (T1x1 protocol) (table 1). One female XLHED pup was injected with 1 mg Fc:EDA1 at ages 2, 5, 8, and 11 d (T4x1 protocol), and five male XLHED dogs received a higher dose of 2 mg (6–7 mg/kg) of Fc:EDA1 at ages 2, 5, 8, 11, and 14 d (T5x2 protocol). The clinical appearances of hair, teeth, eyes, and sweat glands were evaluated in the treated and sex-matched control (nontreated) XLHED dogs, as were other clinical signs of disease. Dental examinations were performed at ages 4 wk (when eruption of deciduous teeth begins), 8–12 wk (when all deciduous teeth are present), 16 wk (when eruption of adult teeth begins), and 36 wk (when all adult teeth are present), with attention paid to the presence or absence of teeth, crown shape, and tooth location. Intraoral radiographs were obtained from all dogs in the study with the use of dental film (D-speed, size 4 [Kodak]) and a dental radiograph machine (Schick CDR Discovery DC 60/70). Radiographs of the maxilla and rostral mandible were taken using the bisecting-angle technique, whereas radiographs of the caudal mandible were taken using the parallel technique.20
Table 1. .
Injection Protocol[Note]
| Fc:EDA1 Injection per Protocol(mg) |
|||
| Age (d) |
T1x1a | T4x1b | T5x2c |
| 2 | 1 | 1 | 2 |
| 5 | … | 1 | 2 |
| 8 | … | 1 | 2 |
| 11 | … | 1 | 2 |
| 14 | … | … | 2 |
Note.— XLHED dogs received Fc:EDA1 IV at doses and ages specified in the table.
The T1x1 protocol used three dogs: one male and two female.
The T4x1 protocol used one female dog.
The T5x2 protocol used five male dogs.
Lacrimal Function
The Shirmer tear testing was performed 30 min after oral treatment with 4 mg pilocarpine HCl 1% (a cholinergic agent with muscarinic action that increases secretion from exocrine glands [Alcon Laboratories]), to standardize tear production. Shirmer tear test strips (Schering Plough) were placed into the medial canthus of each eye, and the amount of fluid produced was determined by measuring the length of the resulting blue dye after 1 min. XLHED dogs produce ∼25% less lacrimal fluid than do wild-type dogs.
Sweat Testing
As opposed to humans, dogs and mice have eccrine sweat glands only in their footpads. To assess sweat-gland function in the dog, we developed a modified starch-iodine sweating assay. Wild-type and XLHED-affected dogs were given 4 mg pilocarpine orally. Thirty minutes later, the footpads of one paw were painted with 3% (wt/vol) iodine in ethanol. After 1 min, the paw was placed on a Whatman No. 3 filter paper coated with cornstarch. The paw was held in place for 1 min, to allow the iodine in the presence of sweat droplets to mix with the cornstarch, resulting in dark brown spots on the filter paper. The procedure was then repeated for the other paws. Semiquantitative measurements were obtained by scanning the filter papers, calculating the area of the footpad, and calculating the area of brown staining caused by sweating, with the use of Image-Pro (Media Cybernetics), a computer imaging analysis software. The ratio of the brown staining was divided by the area of the footpad and was multiplied by 100 to give the percentage of sweat-droplet coverage of the footpad. Testing was performed at least three times at 1-mo intervals for each dog after age 16 wk, at which time sweat glands are fully functional in wild-type dogs.
Mucociliary Clearance
To examine the effect of absent bronchial glands, mucociliary clearance studies were performed in 6-mo-old dogs. One radioactive marker was taped to the lateral chest wall at the level of the carina (fifth intercostal space), and a second marker was taped 15–20 cm cranial at the level of the trachea, depending on the size of the dog. With the use of general anesthesia and a sleeved polystyrene catheter, a droplet (25–50 μl) of 99mTechnetium-macroaggregated albumin containing 200–300 μCi of radioactivity was placed through the intubation tube onto the tracheal mucosa at the level of the distal (carina) marker. Static images were acquired with the Technicare Omega 500 camera interfaced with a NuQuest Veterinary nuclear medicine computer every 5 min for 20–30 min, and the tracheal mucociliary velocity (in mm/min) was calculated using these images.21 The dogs were clinically healthy, and there was no indication of respiratory disease at the time of the study.
Statistical Analysis
Statistical analyses were performed using the Mann-Whitney test (nonparametric) for small sample sizes that were not distributed normally (InStat version 2.03 for Macintosh [GraphPad Software]).
Results
Normally, untreated XLHED dogs show a decrease in weight gain at age ∼2 d when compared with their normal and heterozygote littermates. With proper supplementation and care, the XLHED pups usually become equal in size and weight to their littermates by ages 2–3 wk. In this study, all dogs that received Fc:EDA1 gained weight without supplementation at a rate comparable to their wild-type littermates, but no change in hair growth development was observed (table 2). None of the treated dogs showed any side effects during or after the injections.
Table 2. .
Outcome of Treatment of XLHED-Affected Dogs with Fc:EDA1
| Clinical Observation | Untreated (n=30) |
T1x1a (n=3) |
T4x1b (n=1) |
T5x2c (n=5) |
| Improved weight gain | 0 | 3 | 1 | 5 |
| Normal lacrimation | 0 | 3 | 1 | 5 |
| Ability to sweat | 0 | 2 | 1 | 4 |
| Full set of adult teethd | 0 | 0 | 0 | 4 |
| Normalization of shape of some teeth | 0 | 0 | 0 | 4 |
| Mucociliary clearance | 0 | 2 | 1 | 4 |
| Bronchopneumonia | 16 | 0 | 1 | 0 |
| Change in hair distribution | 0 | 0 | 0 | 0 |
One milligram given IV at age 2 d.
One milligram given IV at ages 2, 5, 8, and 11 d.
Two milligrams given IV at ages 2, 5, 8, 11, and 14 d.
Dogs in which there was no significant difference in number of teeth when compared with normal dogs.
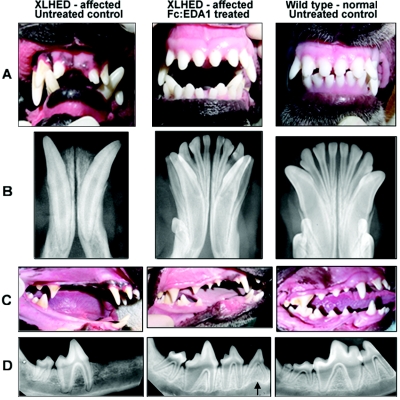
Whereas wild-type adult dogs have a total of 12 incisor, 4 canine, 16 premolar, and 10 molar teeth, with the occasional absence of 1 or 2 premolars or molars, XLHED dogs have a significant reduction in incisors (P=.008) and premolars (P=.008) (table 3). Although no overt rescue of deciduous tooth abnormalities was observed, four of five XLHED dogs that received the extended T5x2 protocol developed an almost full set of adult teeth, with no significant differences from wild type when incisors (P=.31) and premolars (P=.10) were compared (table 3). Many of the incisor teeth and some of the canines, premolars, and molars of the dogs that responded to treatment were clinically and radiographically normal (fig. 1). The most common abnormality noted in the dentition of the XLHED dogs in the T5x2 group was slightly narrowed canine teeth compared with those of normal age- and size-matched control dogs (fig. 1A and 1B). In affected dogs, the maxillary canine teeth were tipped forward (mesioversion), and the mandibular canines often exhibited both mesioversion and labioversion (outward tipping) (fig. 1C). The positioning of the canines was markedly improved in the T5x2-protocol treated XLHED dogs, so that no corrective orthodontic procedures were necessary. At age 2 years, the dentition remains corrected, with no changes in tooth position or loss of structure in the oldest of the T5x2-protocol dogs.
Table 3. .
Effect of Treatment with Fc:EDA1 on Dentition
| No. of Teeth(Mean±SD) |
|||||
| Group | n | Incisor | Canine | Premolar | Molar |
| Wild type | 5 | 12.0±.0 | 4.0±.0 | 15.2±1.2 | 10.0±.0 |
| Untreated XLHED | 5 | 6.8±2.6a | 3.2±1.1 | 4.2±.8a | 9.6±.5 |
| Treated XLHED | 5 | 10.0±3.5b | 3.8±.4 | 10.6±5.4b | 9.0±1.0 |
| Treated XLHEDc | 4 | 11.8±.5b | 3.8±.5 | 12.8±2.8b | 9.5±1.0 |
Significant difference at P<.05 between untreated XLHED-affected dogs and normal dogs.
Significant difference at P<.05 between untreated XLHED-affected dogs and XLHED-affected dogs treated with the T5x2 protocol.
Results from treated group, with the data from the dog that did not respond to treatment removed.
Figure 1. .
Photographs (A and C) and mandibular dental radiographs (B and D) of wild-type (right), untreated XLHED (left), and treated XLHED (middle) dogs. All incisor and canine teeth were present in the XLHED dog treated with Fc:EDA1 (A and B). The maxillary incisors are normal in shape and number, but both mandibular first incisors and canine teeth were narrowed in diameter compared with their normal counterparts (A). The number of adult premolars is increased in the XLHED dog treated with Fc:EDA1 in comparison with the untreated control XLHED dog, in which most premolars were absent (C). The third premolar (arrow) has the radiographic appearance of a deciduous tooth (D). However, it appears to be an adult tooth on the basis of the clinical appearance of its crown.
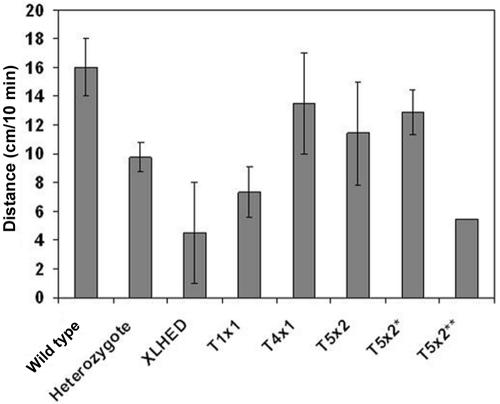
Mucociliary clearance studies were performed to evaluate the effect of Fc:EDA1 on respiratory clearance functions in treated and untreated dogs. As expected, mucociliary clearance was decreased in affected untreated dogs when compared with wild-type dogs (fig. 2). Two of the dogs that received single injections of Fc:EDA1 at age 2 d were able to clear a radioactive tracer at approximately the same rate as the heterozygote dogs, whereas the third dog in this group showed a lower clearance rate (5.5±0.5 cm/min), which was not significantly different from that found in affected dogs (4.5±3.5 cm/min). Dogs that received multiple injections showed the highest clearance rate of all the treated dogs, exceeding even heterozygote levels (fig. 2). However, in one of the XLHED dogs that underwent the T5x2 protocol and also did not sweat, neither clearance (5.45±0 cm/min) nor dentition improved (table 2).
Figure 2. .
Mucociliary clearance in XLHED dogs after a single-dose (T1x1, n=3) or multiple-dose (T4x1, n=1; T5x2, n=5; and T5x2*, n=4) treatment with Fc:EDA1. Multiple measurements were obtained for each dog on separate days, to obtain a mean and SD. For illustrative purposes, the data from the dog that did not respond to treatment were removed from the group (T5x2*) and are shown separately (T5x2**).
Although bronchopneumonia occurred at a rate of >50% in untreated XLHED dogs,14 sometimes in a recurrent manner, none of the treated XLHED dogs showed any clinical signs of disease, with the exception of the T4x1 protocol dog (table 2). This dog had a body temperature of 40.5°C (normal 38.3°C–38.9°C) and increased respiratory sounds over the left medial lung field, consistent with a mild pneumonia, which were resolved after a short course of antibiotics.
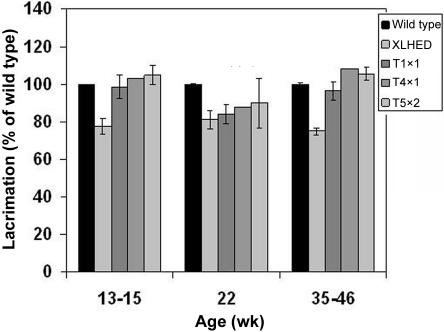
XLHED dogs produce ∼25% less lacrimal fluid than do wild-type dogs, which may contribute to frequent neonatal eye infections (in >90% of XLHED dogs) and keratoconjunctivitis sicca in older affected dogs.14 For dogs given single injections and dogs given multiple injections of either 1 or 2 mg Fc:EDA1, tear production was greatly improved and often approached or exceeded wild-type levels (fig. 3). Neonatal eye infections or keratoconjunctivitis sicca were not observed in any of the treated dogs at any age (table 2).
Figure 3. .
Lacrimal secretions in XLHED dogs after a single-dose (T1x1) or multiple-dose (T4x1 and T5x2) treatment with Fc:EDA1, in percentage of wild type (±SD). The SD is too small to be seen in the normal dogs.
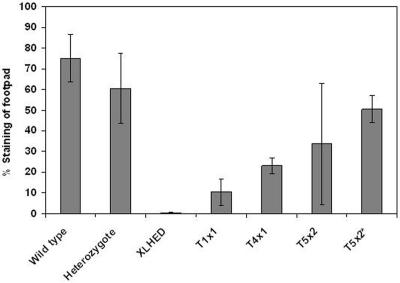
We have found that, in wild-type dogs, eccrine sweat glands are present microscopically at birth but are not fully developed and do not respond to pilocarpine, a cholinergic agent, until age 16 wk. Hence, we assessed the ability to sweat at later time points, using the modified starch-iodine sweat test,11 in wild-type dogs, heterozygote dogs, untreated XLHED dogs, and Fc:EDA1-treated XLHED dogs. By age 28 wk, a few sweat droplets were observed in the foot pads of two of the three XLHED dogs of the T1x1 protocol, and multiple sweat droplets were observed in all footpads (one large palmar and four smaller toe footpads) of the T4x1 protocol dog. Sweat testing at age 13 mo revealed sweat droplets that were often too numerous to count in four of the five T5x2 dogs (fig. 4 and table 2).
Figure 4. .
Semiquantitative sweat testing in XLHED dogs after a single-dose (T1x1, n=3) or multiple-dose (T4x1, n=1; T5x2, n=5; and T5x2*, n=4) treatment with Fc:EDA1. The ability to sweat was measured on all four feet after oral administration of pilocarpine to induce sweating. For illustrative purposes, the data from the dog that did not respond to treatment were removed from the group (T5x2*).
Discussion
The current experiment showed that postnatal administration of a recombinant protein in dogs with XLHED, a developmental genetic disease, was able to induce a more complete and anatomically correct adult dentition and to partially or totally rescue a number of other morphological and pathological features of the disease. In mice, tooth correction by postnatal administration of Fc:EDA1 was never observed.11 However, with use of the highest-dosing protocol, four of five XLHED dogs developed a nearly full set of permanent teeth with near-normal appearance of the crowns. The murine and canine data suggest that the treatment must be administered before the development of the permanent teeth, which occurs earlier in mice because they lack a deciduous dentition. In the one dog that did not appear to respond to the T5x2 treatment (hereafter, called the “poorly responsive” dog), only 4 of 12 incisors, 4 of 4 canines, 2 of 16 premolar teeth, and 8 of 10 molar teeth were present. Mucociliary clearance and sweating ability did not improve in this dog either, but lacrimation was normalized. Failure of Fc:EDA1 to rescue dentition in this dog was not due to the development of neutralizing antibodies (data not shown), which raises the question of whether the threshold for Fc:EDA1 action varies between individual, noninbred dogs. Of note, the T1x1 (n=3) and T4x1 (n=1) protocols did not improve tooth phenotype, despite improvement of other clinical parameters, such as lacrimation. This suggests that a positive response of the secondary dentition to treatment requires either higher concentrations of Fc:EDA1 or the presence of Fc:EDA1 at later time points ∼14 d after birth.
Basic development of deciduous and adult dentition in the dog follows the same pattern as that described in humans. Formation of the dental lamina has been shown to begin at 25 d of the 63-d gestation in the dog and, in humans, between the 5th and 6th wk of gestation. The dental lamina gives rise to tooth buds of the deciduous teeth. The first tooth buds for permanent teeth form in humans around the 17th wk of gestation until age 5 years. Studies performed in canine fetuses demonstrate the presence of tooth buds for some permanent premolars and molars at 42 d gestation and the emergence of tooth buds for the first premolars (a permanent tooth in the dog without deciduous predecessor). However, little is known regarding timing of the formation of the other tooth buds.22 In the XLHED dog, molars are present yet misshapen. Treatment with the recombinant protein does not appear to affect these teeth, yet all the teeth that are preceded by deciduous teeth show responses to treatment with Fc:EDA1. Perhaps incompletely developed deciduous teeth of affected dogs are capable of inducing postnatal development of permanent tooth buds after administration of the protein that would have otherwise not developed in affected dogs. Experiments are currently under way, examining tooth development to understand why recombinant protein so profoundly affects tooth development in affected dogs.
After treatment with Fc:EDA1, no change in hair growth development was observed. In the normal dog, the first tactile hair follicles are recognized at 30–32 d gestation (canine pregnancy is 63 d), and they develop into whiskers by 38 d.23 By 43 d gestation, hair follicles are present, and, at 45 d, the body hair begins to form. The hair cover is complete by 53 d, ∼10 d before birth. Histological studies that use conventional12 and electron microscopy (M. L. Casal and E. A. Mauldin, unpublished data) have shown that affected dogs have a complete absence of hair follicles in affected areas at birth. Therefore, it is likely that the treatment was administered too late during development to change hair growth. Interestingly, postnatal treatment of Tabby mice with the recombinant protein resulted in restoration of some hair.11 However, normal mice are born hairless, unlike dogs, suggesting that the specific triggering events have already taken place at birth, resulting in an unresponsiveness of the canine ectodermal structures to the effects of the protein. These results suggest that postnatal treatment may be effective in human patients with XLHED, since there are two waves of scalp hair growth in the neonatal child, which is complete by ages 8–12 wk.24,25
In human patients, lacrimation is reduced, and Meibomian glands may be hypoplastic or missing. Dry eye is a significant problem requiring daily lubrication and leads to corneal lesions and scarring if left untreated.3 In our XLHED dogs, lacrimation is reduced by ∼25%, but Meibomian glands are present and appear to function normally. The 25% reduction must therefore be significant enough to cause dry eye similar to that found in human patients. The results presented here demonstrate long-term stable correction of lacrimation with even the lowest dose of the recombinant protein, suggesting that development of the lacrimal glands is not complete at the time of treatment. Neonatal administration of the recombinant protein led to a significant improvement of the quality of life, by preventing the need to apply eye medications twice daily to avoid corneal lesions.
The higher doses and longer administration of Fc:EDA1 appeared to increase the degree of sweating in the affected dogs. Sweat testing was performed on a monthly basis, and the results for each dog remained virtually the same once they were old enough to sweat, demonstrating the durability of the treatment. Whereas all protocols used resulted in complete correction of lacrimation, the lower dose appears to be insufficient to induce sweating to any degree that would be therapeutic. It is not clear why sweating was not induced in the poorly responsive dog of the T5x2 protocol, despite normalization of lacrimal function. A higher dose of EDA might have been required in this particular dog if some variation exists in disease expression of XLHED. To our knowledge, the onset of eccrine gland development in the dog is not known, but we have shown that normal dogs are born with incompletely functional but formed glands, suggesting that, for a more complete reversion, treatment would have to be initiated earlier in life. The inability of humans with XLHED to sweat causes most of the morbidity and mortality associated with this disease. These results and those from the Tabby mouse studies suggest that the recombinant protein can permanently induce and maintain the ability to sweat.
The lack of eccrine (serous) glands in the respiratory tract of individuals with XLHED prevents the cilia from moving freely, resulting in reduced pulmonary clearance of pathogens. Therefore, respiratory-tract infections are common in humans with XLHED3 and in affected dogs.14 Heterozygote dogs exhibit about 2/3 of wild-type mucociliary clearance, which appears sufficient for function, since the heterozygotes had no clinical signs compatible with pulmonary disease. At all dosing protocols, there was improvement in mucociliary clearance in all but two dogs, one of which received the lowest dose. Clearance also did not improve in the poorly responsive XLHED dog of the T5x2 protocol, in which a higher dose might have been required to correct dentition, sweating, and mucociliary clearance.
In summary, the therapeutic action of Fc:EDA1 was not restricted to its effect on adult teeth but also improved weight gain (nine of nine dogs), restored wild-type levels of lacrimation, preventing eye infections and keratoconjuctivitis sicca (nine of nine dogs), induced the formation of moderate numbers of functional sweat glands (seven of nine dogs), and significantly improved mucociliary clearance in the respiratory tract (seven of nine dogs) that correlated with the marked decrease of pulmonary diseases in the treated dogs (eight of nine dogs; at least 50% of untreated XLHED dogs develop bronchopneumonia). However, patterned hypotrichosis was not corrected (zero of nine dogs), most likely because hair-differentiation fate in the dog is a prenatal event. These findings have implications for the treatment of human patients with XLHED, because the diagnosis is often not made until after birth, and postnatal treatment may still result in improvement of clinical features of the disease.
Acknowledgments
We thank Jürg Tschopp (University of Lausanne) and Mark Haskins (University of Pennsylvania) for their support and critical reading of the manuscript. We also thank, from the University of Pennsylvania, Patricia O'Donnell, Jennifer Scheidt, Vivian Staci (nuclear medicine facility), James Rhodes, and the veterinary students, for the expert care and assistance with the experiments. This work was supported by National Institutes of Health grants AR049817 and RR02512 and by grants from the National Foundation for Ectodermal Dysplasias (to M.L.C.), the Swiss National Research Foundation and the National Center of Competence in Research Molecular Oncology (to P.S.), and Apoxis. The authors declare competing financial interests (M.F., F.P., S.D., and P.S.).
Web Resource
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for XLHED) [PubMed]
References
- 1.Beahrs JO, Lillington GA, Rosan RC, Russin L, Lindgren JA, Rowley PT (1971) Anhidrotic ectodermal dysplasia: predisposition to bronchial disease. Ann Intern Med 74:92–96 [DOI] [PubMed] [Google Scholar]
- 2.Clarke A (1987) Hypohidrotic ectodermal dysplasia. J Med Genet 24:659–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke A, Phillips DI, Brown R, Harper PS (1987) Clinical aspects of X-linked hypohidrotic ectodermal dysplasia. Arch Dis Child 62:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, et al (1996) X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet 13:409–416 10.1038/ng0895-409 [DOI] [PubMed] [Google Scholar]
- 5.Soderholm AL, Kaitila I (1985) Expression of X-linked hypohidrotic ectodermal dysplasia in six males and in their mothers. Clin Genet 28:136–144 [DOI] [PubMed] [Google Scholar]
- 6.Gilgenkrantz S, Blanchet-Bardon C, Nazzaro V, Formiga L, Mujica P, Alembik Y (1989) Hypohidrotic ectodermal dysplasia: clinical study of a family of 30 over three generations. Hum Genet 81:120–122 10.1007/BF00293886 [DOI] [PubMed] [Google Scholar]
- 7.Johnson EL, Roberts MW, Guckes AD, Bailey LJ, Phillips CL, Wright JT (2002) Analysis of craniofacial development in children with hypohidrotic ectodermal dysplasia. Am J Med Genet 112:327–334 10.1002/ajmg.10654 [DOI] [PubMed] [Google Scholar]
- 8.Tape MW, Tye E (1995) Ectodermal dysplasia: literature review and a case report. Compend Contin Educ Dent 16:524–528 [PubMed] [Google Scholar]
- 9.Bodmer JL, Schneider P, Tschopp J (2002) The molecular architecture of the TNF superfamily. Trends Biochem Sci 27:19–26 10.1016/S0968-0004(01)01995-8 [DOI] [PubMed] [Google Scholar]
- 10.Schneider P, Street SL, Gaide O, Hertig S, Tardivel A, Tschopp J, Runkel L, Alevizopoulos K, Ferguson BM, Zonana J (2001) Mutations leading to X-linked hypohidrotic ectodermal dysplasia affect three major functional domains in the tumor necrosis factor family member ectodysplasin-A. J Biol Chem 276:18819–18827 10.1074/jbc.M101280200 [DOI] [PubMed] [Google Scholar]
- 11.Gaide O, Schneider P (2003) Permanent correction of an inherited ectodermal dysplasia with recombinant EDA. Nat Med 9:614–618 10.1038/nm861 [DOI] [PubMed] [Google Scholar]
- 12.Casal ML, Jezyk PF, Greek JM, Goldschmidt MH, Patterson DF (1997) X-linked ectodermal dysplasia in the dog. J Hered 88:513–517 [DOI] [PubMed] [Google Scholar]
- 13.Casal ML, Scheidt JL, Rhodes JL, Henthorn PS, Werner P (2005) Mutation identification in a canine model of X-linked ectodermal dysplasia. Mamm Genome 16:524–531 10.1007/s00335-004-2463-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casal ML, Mauldin EA, Ryan S, Scheidt J, Kennedy J, Moore PF, Felsburg PJ (2005) Frequent respiratory tract infections in the canine model of X-linked ectodermal dysplasia are not caused by an immune deficiency. Vet Immunol Immunopathol 107:95–104 10.1016/j.vetimm.2005.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand RW, Isselhard DE (2003) Deciduous dentition. In: Brand RW, Isselhard DE (eds) Anatomy of orofacial structures, 7th ed. Mosby, St. Louis, pp 194–224 [Google Scholar]
- 16.Long PH, Leininger JR (1999) Teeth. In: Maronpot RR (ed) Pathology of the mouse. Cache River Press, Vienna, IL, pp 13–17 [Google Scholar]
- 17.Wiggs RB, Lobprise HB (1997) Veterinary dentistry: principles and practice. Lippincott-Raven, Philadelphia [Google Scholar]
- 18.Gruneberg H (1966) The molars of the tabby mouse, and a test of the “single-active X-chromosome” hypothesis. J Embryol Exp Morphol 15:223–244 [PubMed] [Google Scholar]
- 19.Kristenova-Cermakova P, Peterka M, Lisi S, Lesot H, Peterkova R (2002) Postnatal lower jaw dentition in different phenotypes of tabby mice. Connect Tissue Res 43:283–288 [DOI] [PubMed] [Google Scholar]
- 20.Mulligan TW, Aller MS, Williams CA, Veterinary Learning Systems (1998) Atlas of canine and feline dental radiography. Veterinary Learning Systems, Trenton, NJ [Google Scholar]
- 21.Toal RL, Edwards DF (1996) Mucociliary scintigraphy. In: Berry CR, Daniel GB (eds) Handbook of veterinary nuclear medicine. North Carolina State University, Raleigh, pp 154–157 [Google Scholar]
- 22.Williams RC, Evans HE (1978) Prenatal dental development in the dog, Canis familiaris: chronology of tooth germ formation and calcification of deciduous teeth. Anat Histol Embryol 7:152–163 10.1111/j.1439-0264.1978.tb00665.x [DOI] [PubMed] [Google Scholar]
- 23.Evans HE, Sack WO (1973) Prenatal development of domestic and laboratory mammals: growth curves, external features, and selected references. Zentralbl Veterinarmedizin Reihe C 2:11–45 [DOI] [PubMed] [Google Scholar]
- 24.Fletcher MA (1994) Physical assessment and classification. In: Avery GB, Fletcher MA, MacDonald MG (eds) Neonatology: pathophysiology and management of the newborn. Lippincott, Philadelphia, pp 301–320 [Google Scholar]
- 25.Sams WMJ (1990) Structure and function of the skin. In: Sams WMJ, Lynch PJ (eds) Principles and practice of dermatology. Churchill-Livingstone, New York, pp 3–14 [Google Scholar]