Abstract
Pompe disease, which results from mutations in the gene encoding the glycogen-degrading lysosomal enzyme acid α-glucosidase (GAA) (also called “acid maltase”), causes death in early childhood related to glycogen accumulation in striated muscle and an accompanying infantile-onset cardiomyopathy. The efficacy of enzyme replacement therapy (ERT) with recombinant human GAA was demonstrated during clinical trials that prolonged subjects’ overall survival, prolonged ventilator-free survival, and also improved cardiomyopathy, which led to broad-label approval by the U.S. Food and Drug Administration. Patients who lack any residual GAA expression and are deemed negative for cross-reacting immunologic material (CRIM) have a poor response to ERT. We previously showed that gene therapy with an adeno-associated virus (AAV) vector containing a liver-specific promoter elevated the GAA activity in plasma and prevented anti-GAA antibody formation in immunocompetent GAA-knockout mice for 18 wk, predicting that liver-specific expression of human GAA with the AAV vector would induce immune tolerance and enhance the efficacy of ERT. In this study, a very low number of AAV vector particles was administered before initiation of ERT, to prevent the antibody response in GAA-knockout mice. A robust antibody response was provoked in naive GAA-knockout mice by 6 wk after a challenge with human GAA and Freund’s adjuvant; in contrast, administration of the AAV vector before the GAA challenge prevented the antibody response. Most compellingly, the antibody response was prevented by AAV vector administration during the 12 wk of ERT, and the efficacy of ERT was thereby enhanced. Thus, AAV vector–mediated gene therapy induced a tolerance to introduced GAA, and this strategy could enhance the efficacy of ERT in CRIM-negative patients with Pompe disease and in patients with other lysosomal storage diseases.
Infantile-onset Pompe disease (also known as “glycogen storage disease II” [MIM 232300]) is associated with muscle weakness, hypotonia, and lethal cardiomyopathy during infancy, whereas late-onset Pompe disease features progressive weakness without significant cardiomyopathy.1,2 The histopathology of Pompe disease includes progressive lysosomal accumulation of glycogen in cardiac and skeletal muscle. The in vivo efficacy of enzyme-replacement therapy (ERT) for Pompe disease was demonstrated first in acid α-glucosidase (GAA)–deficient Japanese quail by both clinical and metabolic correction3 and then later in the GAA-knockout (GAA-KO) mouse model, by reducing the glycogen accumulation and restoring the GAA activity in the heart and skeletal muscle.4,5 The preclinical data justified an initial phase I/II clinical trial.3,6 Further development of recombinant human GAA (rhGAA) involved two pivotal clinical trials that differed primarily in age at study entry. Study 1 enrolled subjects aged <6 mo and demonstrated prolonged survival in response to rhGAA therapy; furthermore, all 18 patients were alive at age 18 mo, and 15 (83%) showed invasive ventilator–free survival at age 18 mo.7 Study 2 enrolled subjects aged 6–36 mo and demonstrated improved survival in response to ERT, although no difference in ventilator dependence was realized. Both protocols improved cardiomyopathy, growth, and motor development; however, the more-robust outcomes in study 1 emphasized the value of early treatment in infantile-onset Pompe disease.
The main limitation of ERT in Pompe disease is a well-recognized variability of response by skeletal muscle. Potential factors involved in this variability include the extent of muscle damage at the start of ERT, the lower number of mannose-6-phosphate receptors in skeletal muscle in comparison with in the heart, the resistance to correction by type II myofibers, and the formation of high-titer antibodies in cross-reacting immunologic material (CRIM)–negative patients.7–9
Animal and human studies have suggested that formation of antibodies to rhGAA reduced the efficacy of ERT. For instance, GAA-KO mice produced anti-GAA antibodies in response to intravenously administered rhGAA and died after subsequent injections.5 In the first pilot study of ERT that used Chinese hamster ovary (CHO) cell–derived rhGAA, both CRIM-negative subjects with Pompe disease had markedly reduced efficacy of ERT in association with high-titer antibodies against human GAA (hGAA).6 Phase II and III studies revealed that patients with the highest sustained titers of antibody had the least favorable outcome.7,9 The similarity with regard to the antibody response in GAA-KO mice and in CRIM-negative patients with Pompe disease could be linked to the lack of residual GAA protein expression.
Intravenous administration of adenovirus vectors encoding GAA transiently corrected the glycogen storage in the striated muscle of GAA-KO mice,10,11 although glycogen gradually reaccumulated coincident with the formation of anti-GAA antibodies.12 Even when GAA-KO mice were rendered immunotolerant to hGAA by neonatal administration of the recombinant enzyme, only a subset of those mice failed to produce anti-GAA antibodies in response to administration of an adeno-associated virus (AAV) vector encoding hGAA.13 In marked contrast, administration of an AAV vector containing a liver-specific promoter showed evasion of immune responses to introduced hGAA in response to only 1010 vector particles and achieved near-total clearance of accumulated glycogen from skeletal muscle with a 10-fold higher vector quantity.14,15
Liver-specific expression has accomplished immune tolerance to therapeutic proteins in several models of genetic diseases that result from a null mutation, including mice with Pompe disease. Immune tolerance was established through high-level liver-specific expression, as demonstrated through dose-reduction experiments in mice with hemophilia B (MIM 306900).16 Furthermore, the use of a muscle-specific promoter failed to prevent antibody responses to the therapeutic protein in mice with either hemophilia B or Pompe disease.17,18 A unique liver-specific promoter derived from the thyroid hormone–binding globulin promoter sequence (denoted “LSP”) prevented the antibody response against factor IX and against GAA in immunocompetent mice,14,19 and its activity was highly restricted to the liver as compared with muscle.14 The relevance of liver-specific expression in response to therapy in lysosomal storage disorders was further emphasized by the ability of a different liver-specific promoter to prevent the formation of antibodies against α-galactosidase in mice with Fabry disease (MIM 301500).20,21 The mechanism for achieving immune tolerance, although incompletely understood, clearly depends on the induction of regulatory T cells that repress the formation of cytotoxic T cells.16,21,22 The LSP reduced the γ-interferon response to introduced GAA expression in comparison with a universally active promoter, which is consistent with abrogation of the cytotoxic T-cell responses.14 Thus, GAA expression with a liver-specific promoter could potentially prevent both humoral and cellular immune responses to introduced GAA, thereby enhancing the response to therapy in Pompe disease.
The potential role for gene therapy as an immunomodulatory agent in CRIM-negative infantile Pompe disease has become evident. Therefore, we evaluated the hypothesis that immune tolerance to hGAA could be achieved by administration of a subtherapeutic dose of the AAV vector containing the LSP in GAA-KO mice. End points included motor performance, avoidance of antibody responses, and biochemical correction. Immune tolerance to hGAA was evaluated through antibody levels in response to an immune challenge in vector or mock-treated GAA-KO mice. The concept of AAV vector–mediated immunomodulatory gene therapy can thus be advanced in CRIM-negative infantile Pompe disease.
Methods
Preparation of AAV Vector
In brief, 293 cells were transfected with the pAAV-LSPhGAApA vector plasmid,14 the AAV packaging plasmid p5E18-VD 2/8 (courtesy of Dr. James M. Wilson, University of Pennsylvania, Philadelphia),23 and pAdHelper (Stratagene). Cell lysate was harvested 48 h after infection, was freeze-thawed three times, and was isolated by sucrose-cushion pelleting followed by two cesium chloride–gradient centrifugation steps. AAV stocks were dialyzed against three changes of Hanks buffer, and aliquots were stored at −80°C. The number of vector DNA containing–particles was determined by DNase I digestion, DNA extraction, and Southern-blot analysis. All viral vector stocks were handled in accordance with Biohazard Safety Level 2 guidelines published by the National Institutes of Health (NIH).
The LSP in pAAV-LSPhGAApA was subcloned from pAAV-LSP-cFIX (courtesy of Dr. Inder Verma, Salk Institute, La Jolla, CA; sequence is available on request). This LSP24 contains a thyroid hormone–binding globulin (thyroxine-binding globulin of serum [MIM 188600]) promoter sequence (−475 through +4)25 downstream from two copies of an α1-microglobulin/bikunin precursor (MIM 176870) enhancer sequence (−2804 through −2704).26
In Vivo Analysis of AAV Vector
The AAV type 8 pseudotyped (AAV2/8) vector stocks were administered intravenously (via the retro-orbital sinus) in 3-mo-old GAA-KO mice.27 At the indicated postinjection time points, plasma or tissue samples were obtained and were processed as described in the following two paragraphs. All animal procedures were done in accordance with guidelines approved by the Duke University Institutional Animal Care and Use Committee.
Rotarod testing was performed as described elsewhere.28 GAA activity and glycogen content were analyzed as described elsewhere.10 A P value <.05 indicated a statistically significant difference between the observed values for each group of GAA-KO mice after AAV vector administration and for the control group of PBS-injected GAA-KO mice.
Western blotting of hGAA was performed as described elsewhere,28 by use of the hGAA monoclonal antibody (courtesy of Genzyme), lysosomal-associated membrane protein 2 (LAMP-2) rabbit polyclonal antibody (Abcam), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) rabbit polyclonal antibody (Abcam). ELISA was performed as described elsewhere.29 All samples yielded absorbance values that were within the linear range of the assay at this dilution.
ERT in GAA-KO Mice
ERT was modeled in GAA-KO mice by retro-orbital injection of rhGAA (5 mg/ml [supplied by Genzyme]) over ∼15 s at either 20 mg/kg or 100 mg/kg. When high-dose rhGAA (100 mg/kg) was administered, pretreatment with diphenhydramine (5 mg/kg) by intraperitoneal injection preceded rhGAA administration by 10 min.
Results
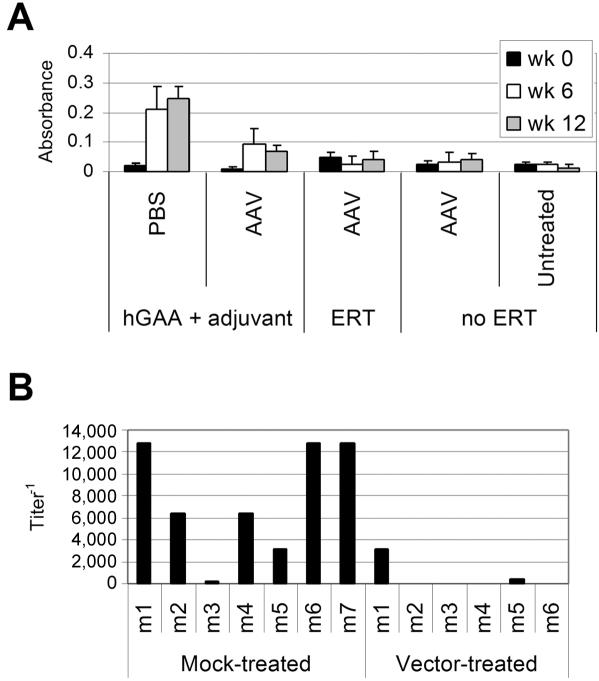
The potential role of immune tolerance in mediating the response to ERT among CRIM-negative subjects with Pompe disease was evaluated in GAA-KO mice. A low, subtherapeutic number of AAV2/8 vector14 particles was administered to 3-mo-old GAA-KO mice, at 6 wk before an immune challenge with rhGAA. Naive, PBS-treated mice served as mock-treated controls. An immune challenge consisting of rhGAA (20 mg/kg, the standard dose for humans7,9) was administered with modified Freund’s adjuvant by intraperitoneal injection at age 4.5 mo to vector-treated and mock-treated GAA-KO mice. Anti-hGAA antibodies were detected only in the mock-treated GAA-KO mice at ages 6 mo and 7.5 mo (absorbance >0.2) (fig. 1A). The titer for mock-treated GAA-KO mice was significantly elevated at age 6 mo, in comparison with AAV vector–treated GAA-KO mice (P=.007) (fig. 1B). The absence of significant anti-hGAA antibodies suggested immune tolerance to hGAA after AAV2/8 vector administration, in support of the hypothesis that gene therapy could fulfill an immunomodulatory role in CRIM-negative infantile Pompe disease.
Figure 1. .
ELISA for anti-hGAA IgG in GAA-KO mice after AAV2/8 vector administration. Mice were administered the AAV vector (AAV), followed by ERT (n=6) or no ERT (n=6 except at age 7.5 mo, when n=4), PBS without ERT (PBS, n=5), or immune challenge with rhGAA + Freund’s adjuvant at age 4.5 mo after AAV vector administration (AAV, n=6) or mock treatment (PBS, n=7). A, Absorbance for 1:200 dilution of plasma from the indicated groups of mice after hGAA + Freund’s adjuvant challenge or ERT. The mean and SD are shown. B, Titer of anti-GAA IgG 12 wk after hGAA + Freund’s adjuvant challenge. Each bar (mn) represents a single mouse. Absorbance values were deemed positive if >0.2. The P value was calculated with a two-tailed homoscedastic Student’s t test.
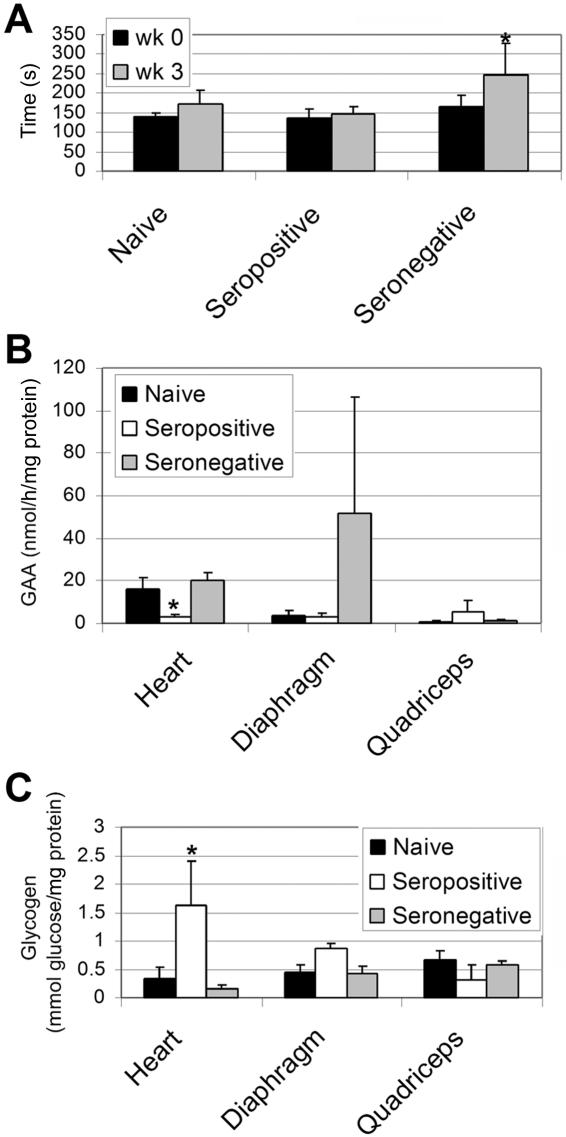
The effect of pre-existing immunity was evaluated in GAA-KO mice after immune challenge. High-dose rhGAA (100 mg/kg) was administered once at age 9 mo, after immune challenge with rhGAA and Freund’s adjuvant at age 4.5 mo, to seropositive mock-treated GAA-KO mice, to seronegative vector-treated GAA-KO mice, and to naive GAA-KO mice that had not received rhGAA previously. This enzyme-replacement regimen has reduced glycogen accumulation in the heart but not skeletal muscle of GAA-KO mice within 3 wk after treatment (authors' unpublished data). Endurance was significantly improved only for vector-treated mice, indicating that seropositivity prevented efficacy of ERT (fig. 2A). Biochemical correction was demonstrated only for seronegative vector-treated GAA-KO mice. GAA activity was significantly elevated in the heart for seronegative GAA-KO mice, in comparison with seropositive GAA-KO mice (fig. 2B). Glycogen content was similarly reduced in the heart for seronegative GAA-KO mice (fig. 2C). Partial biochemical correction was observed in the diaphragm of vector-treated or naive GAA-KO mice (fig. 2B and 2C).
Figure 2. .
End points and biochemical correction depending on antibody status after administration of rhGAA (100 mg/kg). Mean ± SD P values were calculated with Kruskal-Wallis and Dunn’s multiple comparison tests and were considered significant if P<.05 (indicated by an asterisk [*]). A, Rotarod testing. The Rotarod time was evaluated 2 wk after administration of rhGAA. Prior to rhGAA administration (100 mg/kg) at age 10 mo, 3-mo-old GAA-KO mice were administered the AAV vector, followed by immune challenge with rhGAA + Freund’s adjuvant (“Seronegative”: n=6; n=4 at 2 wk), or PBS followed by rhGAA + Freund’s adjuvant (“Seropositive”: n=7; n=6 at 2 wk). Naive GAA-KO mice received no prior treatment, before rhGAA administration (100 mg/kg) at age 10 mo (“naive”: n=5; n=4 at 2 wk). Mice unavailable for repeat testing at 2 wk after rhGAA administration died >24 h after rhGAA injection, and these deaths were attributed to Pompe disease. B, Tissue GAA assayed for GAA-KO mice 3 wk after administration of rhGAA. C, Glycogen content in tissues for mice in panel B.
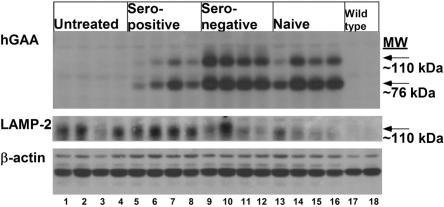
Western blotting detected higher levels of hGAA in the heart of seronegative and naive GAA-KO mice, in comparison with seropositive mice; therefore, the presence of anti-GAA antibodies in seropositive mice interfered with the receptor-mediated uptake of rhGAA by the heart (fig. 3). LAMP-2, a marker for lysosomal accumulation, was reduced in the heart for the majority of seronegative and naive GAA-KO mice at 3 wk after rhGAA (100 mg/kg); however, seropositive GAA-KO mice had persistently elevated LAMP-2 consistent with a lack of efficacy of a single high dose of rhGAA treatment (fig. 3). These data indicate that the presence of anti-hGAA antibodies severely impacted the efficacy of ERT, whereas immune tolerance through gene therapy greatly improved the efficacy of ERT.
Figure 3. .
Western blot detection of hGAA in cardiac muscle after administration of rhGAA (100 mg/kg). Cardiac muscle was analyzed 3 wk after administration of rhGAA. Three different proteins were detected, hGAA, LAMP-2, and β-actin. β-actin served as a control to indicate equal loading of each lane. Samples were from GAA-KO mice; either untreated, or rhGAA-treated seropositive, seronegative, and naive mice, n=4 for each group; and from C57BL/6 wild-type controls (n=2). Each lane represents an individual mouse.
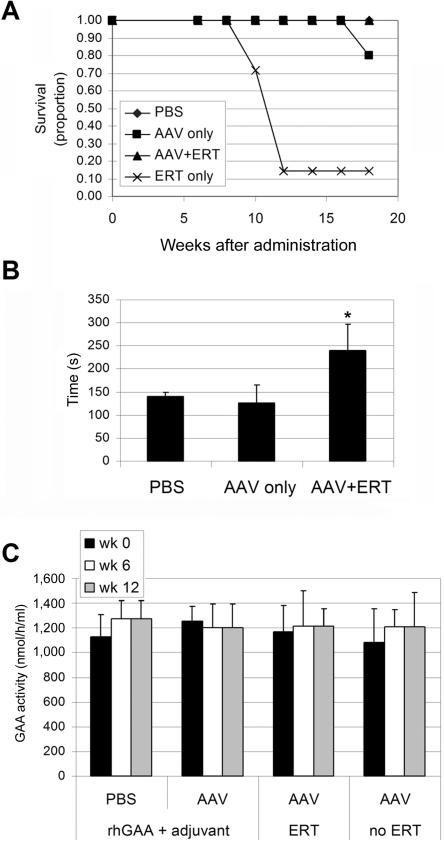
The impact of immune tolerance on long-term ERT was evaluated by comparison of the efficacy in vector-treated and mock-treated GAA-KO mice. ERT was administered every other week for 12 wk, starting at age 4.5 mo, at doses of 20 mg/kg, consistent with recommended clinical dosages.7 Mock-treated GAA-KO mice died within hours after the second or third dose of rhGAA, which is consistent with anaphylaxis (fig. 4A), as was reported for nontolerant GAA-KO mice.5 Endurance was significantly improved for the 10.5-mo-old vector-treated GAA-KO mice after 12 wk of sustained ERT, in comparison with vector-treated GAA-KO mice that received no ERT (fig. 4B). Taken together, these data indicate efficacy of ERT only in GAA-KO mice rendered immunotolerant to rhGAA through gene therapy. The possibility that low-dose vector–treated mice produce secreted hGAA was evaluated by enzyme analysis of plasma, which revealed no elevation of plasma activity either after ERT or with no ERT (fig. 4C); consistent with this hypothesis, efficacy was associated with ERT, not with gene therapy alone.
Figure 4. .
End points after ERT. Mean ± SD P values were calculated with Kruskal-Wallis and Dunn’s multiple comparison tests and were considered significant if P<.05 (indicated by an asterisk [*]). A, Proportion of mice surviving in each group at the indicated age. B, Rotarod testing. Rotarod time was evaluated at age 7.5 mo. Mice were administered the AAV vector, followed by ERT (n=5), no ERT (n=4), or PBS without ERT (n=5). P values were calculated with a two-tailed homoscedastic Student’s t test. C, Plasma GAA activity after hGAA + Freund’s adjuvant challenge or ERT for mice in panel B.
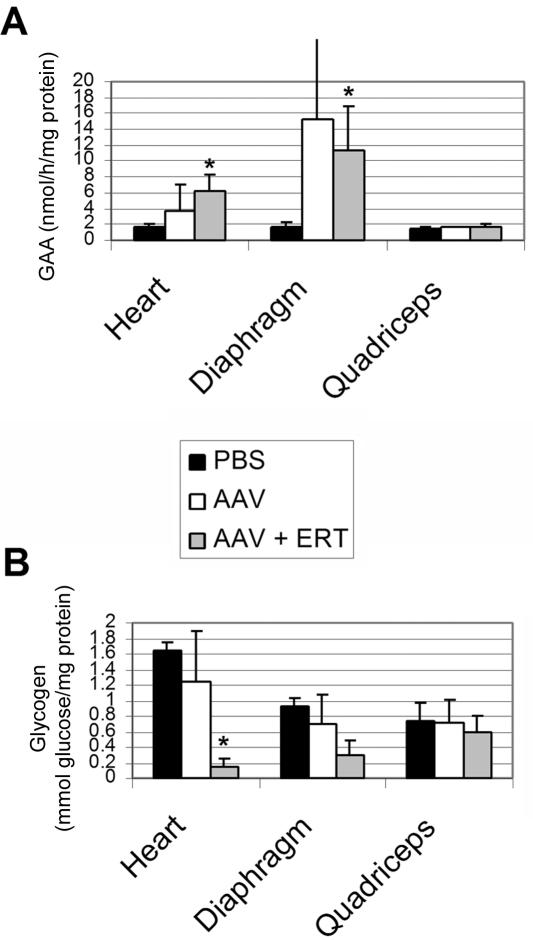
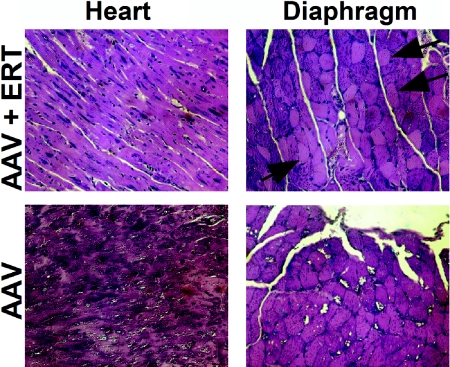
The efficacy of 12-wk sustained ERT was further evaluated in vector-treated GAA-KO mice through biochemical correction of GAA deficiency and glycogen accumulations (fig. 5), which should be corrected to prevent the cardiorespiratory failure associated with this disorder.2 In contrast to a single infusion of high-dose rhGAA, sustained ERT increased the GAA activity significantly in vector-treated GAA-KO mice at 2 wk after the last injection of rhGAA, in comparison with mock-treated GAA-KO mice (fig. 5A). The correction of glycogen content in the heart previously predicted efficacy in GAA-KO mice.15,28 Glycogen content was reduced significantly only in the heart of vector-treated mice after ERT, in comparison with that of mock-treated GAA-KO mice, although the difference between the glycogen contents of the diaphragm in these groups also approached signficance (P=.055) (fig. 5B). Glycogen vacuolation was markedly reduced in the heart and diaphragm after ERT in vector-treated mice (fig. 6). The quadriceps were not biochemically corrected (fig. 5), which is consistent with the need for higher doses of rhGAA to accomplish clearance of glycogen from the skeletal muscle of GAA-KO mice compared with patients with Pompe disease.7,30 Thus, AAV2/8 vector pretreatment mediated the clearance of stored glycogen from the heart and diaphragm after ERT.
Figure 5. .
Biochemical correction after ERT. Mean ± SD is shown. P values were calculated with Kruskal-Wallis and Dunn’s multiple comparison tests and were considered significant if P<.05 (indicated by an asterisk [*]). Mice were administered the AAV vector, followed by ERT (n=5), no ERT (n=4), or PBS without ERT (n=5). A, Tissue GAA assayed for GAA-KO mice 18 wk after administration of the AAV vector or PBS. B, Glycogen content in tissues for mice in panel A.
Figure 6. .
Glycogen staining. Periodic acid-Schiff staining to visualize glycogen in striated muscle of GAA-KO mice. Arrows indicate corrected myofibers (blackened arrowheads) or foci of glycogen vacuolation (unblackened heads). Original magnification = 200×.
Discussion
These studies show that immune tolerance to rhGAA was achieved for >18 wk in adult, immunocompetent GAA-KO mice, through a single administration of a subtherapeutic number of AAV vector particles encoding liver-specific hGAA. The immune tolerance in vector-treated GAA-KO mice was in marked contrast to mock-treated Pompe disease mice that mounted an antibody response against rhGAA, which reduced the efficacy of subsequent ERT. Immune tolerance was demonstrated through an immune challenge with rhGAA, during which only vector-treated Pompe disease mice failed to produce interfering antibodies and demonstrated efficacious responses to ERT. The relevance of pre-existing antibodies against hGAA was confirmed by the lack of efficacy of high-dose rhGAA in seropositive Pompe disease mice. These results support the findings that ERT had little or no efficacy in CRIM-negative patients with Pompe disease after introduction of rhGAA and subsequent antibody formation, as reported during clinical trials of ERT.6,7,9
The complete lack of efficacy of ERT for Pompe disease mice, which have complete deletion of exon 6 in the murine Gaa gene, might have a more severe outcome than that for CRIM-negative patients with Pompe disease. Indeed, most GAA-KO mice died after the second or third dose of rhGAA here and in previous studies,5 although pretreatment with clemastinum prolonged the survival of another strain of GAA-KO mice over a 6-mo duration of ERT.4 The generation of transgenic, liver-expressing, immunotolerant GAA-KO mice also facilitated long-term ERT in a Pompe mouse.5 During the initial phase I/II clinical trial and subsequent trials, CRIM-negative subjects with Pompe disease produced very high levels of anti-hGAA antibodies and demonstrated markedly reduced efficacy of ERT.6 The interfering antibody response in GAA-KO mice and in CRIM-negative patients with Pompe disease indicated that GAA deficiency stemming from an underlying null mutation(s) will not respond efficaciously in the long-term to ERT.
The need for immunomodulation in CRIM-negative patients with Pompe disease follows from the lack of efficacy of ERT. Similar immunological complications have been encountered in hemophilia B mutations and animal models, which have been addressed in preclinical studies by administration of immunosuppression with cyclophosphamide.31 Attempts to induce tolerance by this method after high-titer anti-GAA antibodies developed in CRIM-negative patients with Pompe disease were not successful.6 An alternative strategy, which avoids the risks of immunosuppression, is provided by AAV vector–mediated induction of immune tolerance. AAV vectors have been promoted for gene therapy in genetic disease because of a lack of toxicity and demonstrated long-term transgene expression.32 However, antibody production has prevented long-term efficacy with AAV vectors in early experiments that used GAA-KO mice.13,28,29 We previously found that administration of an AAV2/8 vector containing a liver-specific promoter evaded the humoral response to hGAA and achieved near-total clearance of accumulated glycogen from skeletal muscle, through the induction of immune tolerance to hGAA.14 A dose-response experiment revealed elevated GAA activity in plasma for 12 wk after administration of as few as 3×1010 particles of the vector containing a liver-specific promoter,15 which was validated by demonstrating the efficacy of subsequent ERT here. The persistent correction of hGAA deficiency and lack of anti-hGAA antibodies over 18 wk were consistent with immune tolerance to hGAA after administration of a low dose of the AAV vector, which was within the range of doses safely administered in a clinical trial of an AAV vector in patients with hemophilia B.33,34 The low risk of genotoxic effects from chromosomal integration, which are very infrequent with AAV vectors, would be reduced further by administration of a low number of vector particles.32,35 Finally, it is possible that the AAV vector need not persist for the lifetime of the subject with Pompe disease, once immune tolerance and efficacy of ERT are established. If so, the obstacles to readministration may be overcome with the current strategy.36–38
The current AAV2/8 vector appeared to induce tolerance to hGAA when it was administered in higher particle numbers in GAA-KO mice, on the basis of lack of ELISA and ELISpot responses against hGAA. By contrast, use of a ubiquitously active cytomegalovirus enhancer–chicken β-actin promoter provoked both humoral and cellular immune responses by use of the same assays.14 AAV vectors containing this liver-specific promoter also prevented an antibody response against coagulation factor IX (FIX) in hemophilia B mice and dogs.19,39 Several factors determine the ability to avoid antibody responses against foreign protein by liver-specific expression. Higher levels of FIX were associated with the induction of tolerance to liver-specific FIX expression.16 Acquisition of tolerance to FIX required the induction of regulatory CD4+ T cells, most likely CD25+/CD4+ T-regulatory cells, which suppressed neutralizing antibody formation.16 Similarly, a liver-specific promoter induced tolerance to α-galactosidase in Fabry disease mice, and the transfer of splenocytes from vector-treated mice prevented the antibody response against an α-galactosidase challenge in recipient Fabry mice.21 Taken together, these data strongly support the ability of an AAV vector containing a liver-specific promoter to induce immune tolerance to an introduced foreign protein. Here, we show that this phenomenon can be exploited to achieve efficacy of ERT in the otherwise immunocompetent GAA-KO mouse.
The proposed strategy for immunomodulatory gene therapy would be attractive because a low dose of a nontoxic viral vector might induce immunotolerance to ERT in patients who are likely to have a poor response to ERT because of immune responses against hGAA. The alternative strategy, immune suppression to prevent antibody formation, has potentially severe side effects, such as bone marrow suppression, secondary infections, and malignancy. Immunomodulatory gene therapy with the approach described here may be relevant to therapy for other disorders that are complicated by immune responses, including treatment of other lysosomal storage disorders and the hemophilias.
Further preclinical studies of immunomodulatory gene therapy are clearly indicated in immunocompetent Pompe disease mice and Japanese quail.3,27 The immunomodulatory approach to gene therapy in Pompe disease has special relevance, because ∼40% of patients with infantile Pompe disease are CRIM negative, including the majority of African American patients.6,7,9 These CRIM-negative subjects would not be expected to respond to ERT once antibodies develop. Therefore, infants with Pompe disease should be screened for CRIM status before initiation of ERT, and CRIM-negative patients should be eligible for a clinical trial of immunomodulatory, AAV vector–mediated gene therapy. Indeed, this subpopulation of subjects with Pompe disease represents an important unmet need for therapy, despite the recent approval of rhGAA.
Acknowledgments
This work was supported by NIH grant R01 HL081122-01A1 from the National Heart, Lung, and Blood Institute. D.D.K. was supported by the Muscular Dystrophy Association. GAA-KO mice were provided courtesy of Dr. Nina Raben at the NIH (Bethesda). The AAV8 packaging plasmid, p5E18-VD 2/8, was provided courtesy of Dr. James M. Wilson at the University of Pennsylvania (Philadelphia).
D.D.K. has received research/grant support from Genzyme Corporation. P.K. has received research/grant support from Genzyme Corporation and is a member of the Pompe Disease Advisory Board for Genzyme Corporation. The clinical trials with rhGAA have been supported by a grant from Genzyme Corporation at the various sites where patients received treatment. rhGAA, in the form of Genzyme’s product Myozyme, has now been approved by the U.S. Food and Drug Administration and the European Union as therapy for Pompe disease. Duke University and the inventors of the method of treatment and predecessors of the cell lines used to generate the enzyme (rhGAA) used in this clinical trial may benefit financially pursuant to the University’s Policy on Inventions, Patents and Technology Transfer, even if those cell lines are not used in the commercialized therapy.
Web Resource
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Pompe disease, hemophilia B, Fabry disease, thyroxine-binding globulin of serum, and α1-microglobulin/bikunin precursor)
References
- 1.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D (2006) A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr 148:671–676 10.1016/j.jpeds.2005.11.033 [DOI] [PubMed] [Google Scholar]
- 2.Hirschhorn R, Reuser AJJ (2001) Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular basis for inherited disease. McGraw-Hill, New York, pp 3389–3419 [Google Scholar]
- 3.Kikuchi T, Yang HW, Pennybacker M, Ichihara N, Mizutani M, Van Hove JL, Chen YT (1998) Clinical and metabolic correction of Pompe disease by enzyme therapy in acid maltase-deficient quail. J Clin Invest 101:827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijvoet AG, Van Hirtum H, Kroos MA, Van de Kamp EH, Schoneveld O, Visser P, Brakenhoff JP, Weggeman M, van Corven EJ, Van Der Ploeg AT, et al (1999) Human acid α-glucosidase from rabbit milk has therapeutic effect in mice with glycogen storage disease type II. Hum Mol Genet 8:2145–2153 10.1093/hmg/8.12.2145 [DOI] [PubMed] [Google Scholar]
- 5.Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL, Mattaliano RJ, Nagaraju K, Plotz PH (2003) Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab 80:159–169 10.1016/j.ymgme.2003.08.022 [DOI] [PubMed] [Google Scholar]
- 6.Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, Mackey J, Kishnani P, Smith W, McVie-Wylie A, et al (2001) Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med 3:132–138 [PubMed] [Google Scholar]
- 7.Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, McDonald M, et al (2007) Recombinant human acid α-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 68:99–109 10.1212/01.wnl.0000251268.41188.04 [DOI] [PubMed] [Google Scholar]
- 8.Raben N, Fukuda T, Gilbert AL, de Jong D, Thurberg BL, Mattaliano RJ, Meikle P, Hopwood JJ, Nagashima K, Nagaraju K, et al (2005) Replacing acid α-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol Ther 11:48–56 10.1016/j.ymthe.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 9.Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, Herman GE, Amalfitano A, Thurberg BL, Richards S, et al (2006) Chinese hamster ovary cell-derived recombinant human acid α-glucosidase in infantile-onset Pompe disease. J Pediatr 149:89–97 10.1016/j.jpeds.2006.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amalfitano A, McVie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P, Chen YT (1999) Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-α-glucosidase. Proc Natl Acad Sci USA 96:8861–8866 10.1073/pnas.96.16.8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauly DF, Fraites TJ, Toma C, Bayes HS, Huie ML, Hirschhorn R, Plotz PH, Raben N, Kessler PD, Byrne BJ (2001) Intercellular transfer of the virally derived precursor form of acid α-glucosidase corrects the enzyme deficiency in inherited cardioskeletal myopathy Pompe disease. Hum Gene Ther 12:527–538 10.1089/104303401300042447 [DOI] [PubMed] [Google Scholar]
- 12.Ding EY, Hodges BL, Hu H, McVie-Wylie AJ, Serra D, Migone FK, Pressley D, Chen YT, Amalfitano A (2001) Long-term efficacy after [E1−, polymerase−] adenovirus-mediated transfer of human acid-α-glucosidase gene into glycogen storage disease type II knockout mice. Hum Gene Ther 12:955–965 10.1089/104303401750195917 [DOI] [PubMed] [Google Scholar]
- 13.Cresawn KO, Fraites TJ, Wasserfall C, Atkinson M, Lewis M, Porvasnik S, Liu C, Mah C, Byrne BJ (2005) Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid α-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther 16:68–80 10.1089/hum.2005.16.68 [DOI] [PubMed] [Google Scholar]
- 14.Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, Brown T, Young SP, Clay TM, Amalfitano A, et al (2005) Evasion of immune responses to introduced human acid α-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 12:876–884 10.1016/j.ymthe.2005.04.024 [DOI] [PubMed] [Google Scholar]
- 15.Sun B, Zhang H, Benjamin DK Jr, Brown T, Bird A, Young SP, McVie-Wylie A, Chen YT, Koeberl DD (2006) Enhanced efficacy of an AAV vector encoding chimeric, highly secreted acid α-glucosidase in glycogen storage disease type II. Mol Ther 14:822–830 10.1016/j.ymthe.2006.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang YQ, Arruda VR, High KA, Herzog RW (2003) Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest 111:1347–1356 10.1172/JCI200316887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YL, Mingozzi F, Rodriguez-Colon SM, Joseph S, Dobrzynski E, Suzuki T, High KA, Herzog RW (2004) Therapeutic levels of factor IX expression using a muscle-specific promoter and adeno-associated virus serotype 1 vector. Hum Gene Ther 15:783–792 10.1089/1043034041648453 [DOI] [PubMed] [Google Scholar]
- 18.Sun B, Zhang H, Franco LM, Brown T, Bird A, Schneider A, Koeberl DD (2005) Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol Ther 11:889–898 10.1016/j.ymthe.2005.01.012 [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Takabe K, Bidlingmaier SM, Ill CR, Verma IM (1999) Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci U A 96:3906–3910 10.1073/pnas.96.7.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M, Ford C, Barbon CM, Desnick RJ, Gao GP, et al (2004) AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of α-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther 9:231–240 10.1016/j.ymthe.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 21.Ziegler RJ, Cherry M, Barbon CM, Li C, Bercury SD, Armentano D, Desnick RJ, Cheng SH (2007) Correction of the biochemical and functional feficits in Fabry mice following AAV8-mediated hepatic expression of α-galactosidase A. Mol Ther 15:492–500 10.1038/sj.mt.6300066 [DOI] [PubMed] [Google Scholar]
- 22.Hoffman BE, Dobrzynski E, Wang L, Hirao L, Mingozzi F, Cao O, Herzog RW (2007) Muscle as a target for supplementary factor IX gene transfer. Hum Gene Ther 18:603–613 10.1089/hum.2007.042 [DOI] [PubMed] [Google Scholar]
- 23.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM (2002) Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 99:11854–11859 10.1073/pnas.182412299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ill CR, Yang CQ, Bidlingmaier SM, Gonzales JN, Burns DS, Bartholomew RM, Scuderi P (1997) Optimization of the human factor VIII complementary DNA expression plasmid for gene therapy of hemophilia A. Blood Coagul Fibrinolysis 8:S23–S30 [PubMed] [Google Scholar]
- 25.Hayashi Y, Mori Y, Janssen OE, Sunthornthepvarakul T, Weiss RE, Takeda K, Weinberg M, Seo H, Bell GI, Refetoff S (1993) Human thyroxine-binding globulin gene: complete sequence and transcriptional regulation. Mol Endocrinol 7:1049–1060 10.1210/me.7.8.1049 [DOI] [PubMed] [Google Scholar]
- 26.Rouet P, Raguenez G, Tronche F, Yaniv M, N’Guyen C, Salier JP (1992) A potent enhancer made of clustered liver-specific elements in the transcription control sequences of human α1-microglobulin/bikunin gene. J Biol Chem 267:20765–20773 [PubMed] [Google Scholar]
- 27.Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L, LaMarca M, King C, Ward J, Sauer B, et al (1998) Targeted disruption of the acid α-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem 273:19086–19092 10.1074/jbc.273.30.19086 [DOI] [PubMed] [Google Scholar]
- 28.Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A, Amalfitano A, Chen YT, Koeberl DD (2005) Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 11:57–65 10.1016/j.ymthe.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 29.Sun B, Chen YT, Bird A, Xu F, Hou YX, Amalfitano A, Koeberl DD (2003) Packaging of an AAV vector encoding human acid α-glucosidase for gene therapy in glycogen storage disease type II with a modified hybrid adenovirus-AAV vector. Mol Ther 7:467–477 10.1016/S1525-0016(03)00022-4 [DOI] [PubMed] [Google Scholar]
- 30.Raben N, Jatkar T, Lee A, Lu N, Dwivedi S, Nagaraju K, Plotz PH (2002) Glycogen stored in skeletal but not in cardiac muscle in acid alpha-glucosidase mutant (Pompe) mice is highly resistant to transgene-encoded human enzyme. Mol Ther 6:601–608 10.1016/S1525-0016(02)90716-1 [DOI] [PubMed] [Google Scholar]
- 31.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD Jr (2001) Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther 4:192–200 10.1006/mthe.2001.0442 [DOI] [PubMed] [Google Scholar]
- 32.McCarty DM, Young SM Jr, Samulski RJ (2004) Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet 38:819–845 10.1146/annurev.genet.37.110801.143717 [DOI] [PubMed] [Google Scholar]
- 33.High K (2003) Gene transfer for hemophilia: can therapeutic efficacy in large animals be safely translated to patients. J Thromb Haemost 3:1682–1691 10.1111/j.1538-7836.2005.01460.x [DOI] [PubMed] [Google Scholar]
- 34.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, et al (2006) Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 12:342–347 10.1038/nm1358 [DOI] [PubMed] [Google Scholar]
- 35.Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR (2003) Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol 77:3495–3504 10.1128/JVI.77.6.3495-3504.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW, Miller AD (1997) Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol 71:5932–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halbert CL, Standaert TA, Wilson CB, Miller AD (1998) Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 72:9795–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, Figueredo J, Lock M, Wilson JM (2006) Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther 13:77–87 10.1016/j.ymthe.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Nichols TC, Read MS, Bellinger DA, Verma IM (2000) Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther 1:154–158 10.1006/mthe.2000.0031 [DOI] [PubMed] [Google Scholar]