Abstract
Low serum 25 hydroxyvitamin D3 (vitamin D3) is known to perturb cellular function in many tissues, including the endocrine pancreas, which are involved in obesity and type II diabetes mellitus (TIIDM). Vitamin D3 insufficiency has been linked to obesity, whether obesity is assessed by body mass index (BMI) or waist circumference (waist). Central obesity, using waist as the surrogate, is associated with the metabolic syndrome (MetSyn), insulin resistance, TIIDM and atherosclerotic cardiovascular disease (CVD). We tested how vitamin D3 was related to measures of fat mass, MetSyn markers, haemoglobin A1c (HbA1c) and MetSyn in a cross-sectional sample of 250 overweight and obese adults of different ethnicities. There were modest inverse associations of vitamin D3 with body weight (weight) (r = -0.21, p = 0.0009), BMI (r = -0.18, p = 0.005), waist (r = -0.14, p = 0.03), [but not body fat % (r = -0.08, p = 0.24)], and HbA1c (r = -0.16, p = 0.01). Multivariable regression carried out separately for BMI and waist showed a decrease of 0.74 nmol/L (p = 0.002) in vitamin D3 per 1 kg/m2 increase in BMI and a decrease of 0.29 nmol/L (p = 0.01) per 1 cm increase in waist, with each explaining approximately 3% of the variation in vitamin D3 over and above gender, age, ethnicity and season.
The similar relationships of BMI and waist with vitamin D3 may have been due to associations between BMI and waist, or coincidental, where different mechanisms relating hypovitaminosis D3 to obesity occur concurrently. Previously reviewed mechanisms include that 1) low vitamin D3, may impair insulin action, glucose metabolism and various other metabolic processes in adipose and lean tissue 2) fat soluble-vitamin D3 is sequestered in the large adipose compartment, and low in serum, 3) obese people may be sensitive about their body shape, minimising their skin exposure to view and sunlight (not tested). We showed evidence for the first theory but no evidence to support the second.
In the current study, serum vitamin D3 was inversely related to weight, BMI and markers of TIIDM (large waist, raised HbA1c) but not to adipose mass nor to MetSyn per se.
Background
It is now known that insufficient serum 25 hydroxyvitamin D3 (calcifediol, vitamin D3) alters metabolite function causing perturbation of many cellular functions, including that of the endocrine pancreas [1]. Recently, there has been a resurgence of hypovitaminosis D3 in many populations [2], including young, pale-skinned adults [3] in addition to those with pigmented skin. Suggested recent recommendations of ideal vitamin D3 serum levels for metabolic health are >70–100 nmol/L (previously >50 nmol/L) [4,5]. In parallel, there has been a world-wide increase in the prevalence of obesity [6]. Links between hypovitaminosis D3 and obesity have been reported when obesity is defined using body mass index (BMI) [7,8] and waist circumference (waist) [9]. Large waist, a surrogate for abdominal obesity, is the key marker required for the metabolic syndrome (MetSyn) as defined by the International Diabetes Federation (IDF) [10]. Whilst there is overlap in total body and abdominal obesity, the diverse metabolic processes of different adipose depots and lean tissue may underpin dissimilar hypotheses for the mechanisms proposed for the inverse relationship of vitamin D3 and obesity.
This study was designed to 1) assess the relationships between vitamin D3 and anthropometric, metabolic syndrome and TIIDM markers, 2) determine whether whole body [BMI] or central [waist] adiposity was significantly related to vitamin D3, and if so whether one was related independently of the other when corrected for well-known influences in mixed-ethnicity adults.
Methods
Population and anthropometry
250 ambulant adults in Auckland, New Zealand were recruited into a body weight (weight) loss trial with primary criteria including BMI 28–50 kg/m2, age >18 y, not currently using weight loss agents nor participating in commercial weight loss programmes, and a desire to lose weight. Baseline data from this study were analysed for relationships with vitamin D3. Ethnicity was the surrogate for skin pigment. The lightly pigmented skin sub-group consisted of Caucasians and the variably pigmented skin sub-group included all other ethnicities which were New Zealand Maori, Pacific Peoples (Tongan, Samoan,) and Asian (East, South or Indian). For anthropometry, participants were lightly clad and measurements were taken in duplicate. Weight, height, waist and blood pressure were measured using standard methods as detailed in our previous publication [11]. Body fat percentage (fat%) was assessed indirectly by multi-frequency bioelectrical impedance analysis (SFB3 MFBIA, Impedimed, Australia). All participants provided written informed consent. Ethics approval for this study was obtained from the Auckland Ethics Committee, Auckland, New Zealand.
Laboratory samples
Participants attended our community clinic for collection of fasting blood. 200 women and 43 men provided evaluable samples. Vitamin D3 was analysed using Vit D25 pre-extraction with acetonitrile, double antibody radioimmunoassay (DiaSorin Inc Stillwater, MN, USA). Fasting plasma glucose (FPG), serum lipids and haemoglobin A1c (HbA1c) were measured using standard methods [11].
Statistical Analysis
Linear regression analysis was used to investigate the relationships of the demographic data, anthropometry and laboratory tests with vitamin D3. Multivariable regression was performed with vitamin D3 as the outcome variable. Explanatory variables were gender, age, ethnicity, season, and either BMI or waist as these were likely to be highly correlated. An analysis including both BMI and waist was also carried out to investigate if one variable contributed over and above the other. SAS 8.0 statistical software (Cary, NC, 2003) was employed for analyses.
Results
Baseline data shows an obese population with prevalences of MetSyn and TIIDM ≈ 40% and 5% in 234 participants with available FPG. Table 1.
Table 1.
Baseline data from 250 female and male overweight and obese participants
| Parameter | Evaluable N1 | Mean(SD) |
| Age (y) | 243 | 47.6(11.6) |
| Weight (kg) | 243 | 97.3(18.2) |
| Height (cm) | 243 | 166(8) |
| Body mass index (kg/m2) | 243 | 35.4(5.2) |
| Body fat (%) | 243 | 38.2(6.6) |
| 2Waist (cm) | 243 | 100.4(12.8) |
| 2Systolic blood pressure (mmHg) | 243 | 123(18) |
| 2Diastolic blood pressure (mmHg) | 243 | 70(10) |
| 2Triglyceride (mmol/L) | 243 | 1.56(0.82) |
| 2High density lipoprotein-cholesterol (mmol/L) | 243 | 1.33(0.34) |
| 2Fasting plasma glucose (mmol/L) | 234 | 5.32(1.38) |
| Haemoglobin A1c (%) | 217 | 5.25(0.82) |
| 2Metabolic syndrome marker count | 234 | 2.4(1.1) |
| Vitamin D3nmol/L* | ||
| Total | 243 | 62.2(22.7) |
| Women | 200 | 62.4(21.9) |
| Men | 43 | 61.7(26.3) |
| Skin pigment, light | 206 | 64.8(22.0) |
| Skin pigment, variable | 37 | 47.5(20.0) |
| Summer | 141 | 68.7(21.9) |
| Winter | 102 | 53.3(20.6) |
| 2Metabolic syndrome, no | 135 | 61.4(22.8) |
| 2Metabolic syndrome, yes | 99 | 63.8(22.4) |
| Non-type II diabetes mellitus | 223 | 62.7(22.7) |
| Type II diabetes mellitus | 11 | 55.5(20.1) |
* Raw data in pair sets. See text for multivariable regression results. 1Although anthropometry was collected on all 250 participants, data is only shown for 243, as evaluable serum samples for vitamin D3 analysis were available for this number (N) only. 6 samples were not obtained due to unsuccessful venepuncture and 1 sample was lost in transit to the laboratory. For the sub-groups some samples were not evaluable due to sample quality or loss. Sample number (N) is shown for each category. 2 Metabolic syndrome (N=234) is defined by the International Diabetes Federation as 1) Waist circumference: Europids and Undefined groups [such as Maori and Pacific Peoples] ≥ 94 cm (men), ≥ 80 cm (women), Asian (based on a Chinese, Malay and Indian Asian population) ≥ 90 cm (men) and ≥ 80 cm (women). Waist must be included, plus two or more of 2) Systolic and diastolic blood pressure ≥ 130/85 mmHg, 3) High density lipoprotein – cholesterol <1.03 mmol/L (<40 mg/dL) men, <1.29 mmol/L (<50 mg/dL) women 4) Triglyceride ≥ 1.69 mmol/L (150 mg/dL) 5) Fasting plasma glucose ≥ 5.6 mmol/L (≥ 100 mg/dL) and/or on treatment medication for the latter 4 conditions.
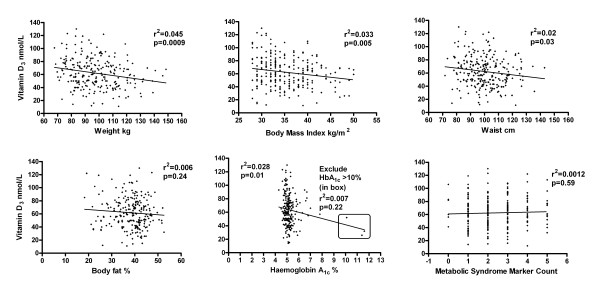
There were modest but significant inverse relationships of vitamin D3 with weight (p = 0.0009), BMI (p = 0.005) and waist (p = 0.03) but no relationship could be shown with fat %. Figure 1. Vitamin D3 could not be shown to be related to any of the non-waist MetSyn markers (MetSynM) or MetSynMcount (number of markers added together) or the presence of MetSyn (p > 0.05, all). Vitamin D3 and HbA1c, alone of the individual metabolic and MetSynM were weakly inversely related (p = 0.01) but this relationship was not significant after exclusion of the three HbA1c values >10% (p = 0.22). Abnormal FPG was related to hypovitaminosis D3, again only when the three values >10 mmol/L were included (r2 = 0.17, p = 0.005). Figure 1.
Figure 1.
The relationship between vitamin D3 and anthropometric and metabolic markers in 250 overweight and obese men and women.
Multivariable regression showed an estimated decrease of 0.74 nmol/L (p = 0.002) in vitamin D3 per 1 kg/m2 increase in BMI with the total model explaining 22% of the variation in vitamin D3 levels and BMI explaining 3% of the variation. On replacing BMI with waist, there was a decrease of 0.29 nmol/L (p = 0.01) vitamin D3 per 1 cm increase in waist, with the total model explaining 21% of the variation in vitamin D3 and waist explaining 3%. When both BMI and waist were included neither could be demonstrated to contribute over and above the other (p = 0.25 and 0.67 respectively), nor could an association of vitamin D3 with gender (p = 0.52) or age (p = 0.52) be shown. However there was strong evidence of its association with ethnicity (p < 0.0001) and season (p < 0.0001).
Discussion
In the current study we showed that low levels of circulating vitamin D3 were inversely related to markers of TIIDM (large waist and raised HbA1c), rather than total adipose mass, non-waist MetSynM or MetSyn per se.
Of the three anthropometric variables that were significantly inversely correlated with vitamin D3 only BMI and waist were further investigated as weight is considered too crude a measure of obesity. Vitamin D3 showed inverse relationships separately, but of the same magnitude, with both BMI and waist when corrected for confounders. Neither could be shown to be related to vitamin D3 given the level of the other. This may indicate either similar mechanisms, or that different metabolic processes are occurring, coincidentally producing similar outcomes.
Hypotheses from the literature are discussed in light of our findings
Whole body obesity, as defined by BMI, has been associated with or contributes to low vitamin D3 status [8,12]. Wortsman et al., found lower vitamin D3 in the serum of obese participants after experimental UV irradiation, deducing that "obesity-associated vitamin D insufficiency is likely due to the decreased bioavailability of vitamin D3 from cutaneous and dietary sources because of its deposition in body fat compartments" [12]. It was unclear from that trial which fat compartments were involved. In the current study MFBIA fat% did not correlate with vitamin D3, in contrast to that of Arunabh et al., [13] where DEXA was performed in women, BMI<24 kg/m2. Total body fat includes both peripheral adipose at the hip and thigh, with beneficial metabolic effects in both women and men [14], as well as less healthy upper body and central fat depots [15]. The opposing effects of these adipose depots could possibly weaken any correlation with vitamin D3. Furthermore, the lack of relationship of fat% with vitamin D3 may reflect influences of fat-free compartments of bone, muscle [16] and abdominal organs (liver, kidney, gut [17]).
The links between the metabolic syndrome and vitamin D3 are not clear. In the present study, apart from waist, none of MetSynM alone, MetSynMcount, nor the presence of MetSyn (defined by three of five positive markers [9]), was correlated with vitamin D3. This lack of relationship of vitamin D3 and MetSyn has been reported previously [18], and two studies of vitamin D3 in the morbidly obese report conflicting relationships [19,20]. However, in the large USA NHANES dataset Ford et al., found that abdominal obesity as measured by waist alone, in addition to MetSyn, was related to low vitamin D3, notably affecting mixed-ethnicity participants equally [21].
Conversion of vitamin D3 to its derivative 1,25 vitamin D3 is complex and involves other hormones. 1,25 vitamin D3, via its receptor which is present in insulin-producing beta-islet cells, is known to be a potent regulator of cell proliferation and differentiation [22,23]. However, there is evidence that low vitamin D3 itself is associated with TIIDM irrespective of 1,25 vitamin D3 [8]. The inverse relationship of vitamin D3 with high to extreme HbA1c [24,25] and/or FPG [7,8] may indicate that it is the long-term, severely abnormal (carbohydrate) metabolism of TIIDM [7,26,27] and muscle insulin resistance [28], that is associated with hypovitaminosis D3. HbA1c, a glycated protein, is a predictor of 2-hour glucose in oral glucose tolerance testing, [29] an indicator of chronic hyperglycaemia, protein glycation damage [30] and oxidative stress [31]. Many new, profound and interacting mechanisms link hypovitaminosis D with other correlates of the metabolic syndrome, including renin regulation [1]. Vitamin D-upregulated protein-1 reportedly modulates endothelial oxidative stress, macrophage and smooth muscle function, depending on the stage of atherosclerosis [32,33].
Limitations of the present study include the cross sectional design where cause cannot be attributed. Lifestyle, body shape sensitivity [34,35] or cultural reasons [36] for precluding skin exposure to view, and ultraviolet light for efficient vitamin D3 synthesis, may selectively affect obese people but were not examined.
In the current study low serum vitamin D3 was inversely related to weight and BMI, but not fat mass, and to markers indicative of TIIDM (large waist and raised HbA1c), rather than MetSyn per se. The link between hypovitaminosis D3 and metabolic disorders, including obesity, MetSyn, TIIDM and CVD requires further investigation, particularly for those most at risk of these combined conditions.
Abbreviations
vitamin D3: serum 25 hydroxyvitamin D3; TIIDM: type II Diabetes Mellitus; BMI: body mass index; waist: waist circumference; weight: body weight; fat%: body fat percentage; MFBIA: multi-frequency bioelectrical impedance analysis; DEXA: dual energy x-ray absorptiometry; MetSyn: metabolic syndrome; MetSynM: metabolic syndrome marker; MetSynMcount: metabolic syndrome marker count (the number of metabolic syndrome markers added together, ranging from 0–5. A count of 3, obligatorily including waist, indicates the metabolic syndrome); CVD: atherosclerotic cardiovascular disease; FPG: fasting plasma glucose; S/DBP: systolic/diastolic blood pressure; TAG: triglyceride; HDL-C: high density lipoprotein-cholesterol; HbA1c: haemoglobin A1c; IDF: International Diabetes Federation.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
ATM conceived the study and was the senior author during manuscript preparation. ATM, FEL, SDP and CMS contributed to the planning, conduct, and reporting of this study. JMS, ATM and CMS did the data entry and statistical analysis. ATM, FEL, SDP and CMS contributed to manuscript preparation. All authors read and approved the final manuscript. Funds were raised by ATM and SDP as part of a wider programme grant.
Acknowledgments
Acknowledgements
We thank the Health Research Council of New Zealand for funding the main ECHO trial and Healtheries Ltd, New Zealand for funding the vitamin D3 assays. We also thank the Clinical Trials Research Unit, University of Auckland, Jane Easton (Study Manager), Santuri Rungan, Chao-Yuan Chen, David Anderson, Laura Gerulitis, Pia Nielson, Jeannette Eis, Cathelijne Reincke, Shannon McCarthy, Jenneke van Drunen (Research Assistants) and the 250 participants.
Contributor Information
Anne-Thea McGill, Email: at.mcgill@auckland.ac.nz.
Joanna M Stewart, Email: j.stewart@auckland.ac.nz.
Fiona E Lithander, Email: fiona.lithander@tcd.ie.
Caroline M Strik, Email: c.strik@auckland.ac.nz.
Sally D Poppitt, Email: s.poppitt@auckland.ac.nz.
References
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Rajakumar K, Greenspan SL, Thomas SB, Holick MF. SOLAR ultraviolet radiation and vitamin D: a historical perspective. Am J Public Health. 2007;97:1746–1754. doi: 10.2105/AJPH.2006.091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–662. doi: 10.1016/S0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86:1657–1662. doi: 10.1093/ajcn/86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM. Obesity, Metabolic Syndrome, and Cardiovascular Disease. J Clin Endocrinol Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract Suppl. 1995;27:181–188. doi: 10.1016/0168-8227(95)01040-K. [DOI] [PubMed] [Google Scholar]
- Need AG, O'Loughlin PD, Horowitz M, Nordin BC. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) 2005;62:738–741. doi: 10.1111/j.1365-2265.2005.02288.x. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program Expert Panel Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- The IDF consensus worldwide definition of the metabolic syndrome http://www.idf.org/webdata/docs/MetS_def_update2006.pdf
- Ni-Mhurchu C, Poppitt SD, McGill A-T, Leahy FE, Bennett DA, Lin RB, Ormrod D, Ward L, Strik C, Rodgers A. The effect of the dietary supplement, Chitosan, on body weight: a randomised controlled trial in 250 overweight and obese adults. Int J Obes Relat Metab Disord. 2004;28:1149–1156. doi: 10.1038/sj.ijo.0802693. [DOI] [PubMed] [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–1197. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Is Visceral Fat Involved in the Pathogenesis of the Metabolic Syndrome? Human Model. Obes Res. 2006;14:20S–24. doi: 10.1038/oby.2006.278. [DOI] [PubMed] [Google Scholar]
- Arabi A, Baddoura R, Awada H, Salamoun M, Ayoub G, El-Hajj Fuleihan G. Hypovitaminosis D osteopathy: Is it mediated through PTH, lean mass, or is it a direct effect? Bone. 2006;39:268–275. doi: 10.1016/j.bone.2006.01.140. [DOI] [PubMed] [Google Scholar]
- Moyad MA. Osteoporosis. Part III–Not just for bone loss: potential benefits of calcium and vitamin D for overall general health. Urol Nurs. 2003;23:69–74. [PubMed] [Google Scholar]
- Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- Botella-Carretero J, Alvarez-Blasco F, Villafruela J, Balsa J, Vazquez C, Escobar-Morreale H. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007 doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Rueda S, Fernández-Fernández C, Romero F, Martínez de Osaba M, Vidal J. Vitamin D, PTH, and the Metabolic Syndrome in Severely Obese Subjects. Obesity Surgery. doi: 10.1007/s11695-007-9352-3. 2008 Jan 4. [DOI] [PubMed] [Google Scholar]
- Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- Mannion CA, Gray-Donald K, Koski KG. Association of low intake of milk and vitamin D during pregnancy with decreased birth weight. Can Med Assoc J. 2006;174:1273–1277. doi: 10.1503/cmaj.1041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerle HJ, Pimenta WP, Meyer C, Gosmanov NR, Szoke E, Szombathy T, Mitrakou A, Gerich JE. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin a1c values. Arch Intern Med. 2004;164:1627–1632. doi: 10.1001/archinte.164.15.1627. [DOI] [PubMed] [Google Scholar]
- Osei K, Rhinesmith S, Gaillard T, Schuster D. Is glycosylated hemoglobin A1c a surrogate for metabolic syndrome in nondiabetic, first-degree relatives of African-American patients with type 2 diabetes? J Clin Endocrinol Metab. 2003;88:4596–4601. doi: 10.1210/jc.2003-030686. [DOI] [PubMed] [Google Scholar]
- Boucher BJ. Inadequate vitamin D status: does it contribute to the disorders comprising syndrome 'X'? Br J Nutr. 1998;79:315–327. doi: 10.1079/BJN19980055. [DOI] [PubMed] [Google Scholar]
- Pittas AG, Dawson-Hughes B, Li T, Dam RMV. Vitamin D and Calcium Intake in Relation to Type 2 Diabetes in Women. Diabetes Care. 2006;29:650–657. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- Lytras A, Tolis G. Assessment of endocrine and nutritional status in age-related catabolic states of muscle and bone. Curr Opin Clin Nutr Metab Care. 2007;10:604–610. doi: 10.1097/MCO.0b013e3282cfa32f. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation.[comment] Diabetic Medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Valeri C, Pozzilli P, Leslie D. Glucose control in diabetes. Diabetes Metab Res Rev. 2004;20:S1–8. doi: 10.1002/dmrr.512. [DOI] [PubMed] [Google Scholar]
- VanderJagt DJ, Harrison JM, Ratliff DM, Hunsaker LA, Vander Jagt DL. Oxidative stress indices in IDDM subjects with and without long-term diabetic complications. Clin Biochem. 2001;34:265–270. doi: 10.1016/S0009-9120(01)00204-1. [DOI] [PubMed] [Google Scholar]
- Billiet L, Furman C, Larigauderie G, Copin C, Page S, Fruchart JC, Brand K, Rouis M. Enhanced VDUP-1 gene expression by PPARgamma agonist induces apoptosis in human macrophage. J Cell Physiol. 2008;214:183–191. doi: 10.1002/jcp.21179. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation. 2008 January 7. [DOI] [PMC free article] [PubMed]
- Compston J, Ledger J, Webb A, Gazet J, Pilkington T, Vedi S. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- Rosen JC, Reitera J. Development of the body dysmorphic disorder examination. Behav Res Ther. 1996;34:755–766. doi: 10.1016/0005-7967(96)00024-1. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]