Abstract
Although brain development abnormalities and brain cancer predisposition have been reported in some Fanconi patients, the possible role of Fanconi DNA repair pathway during neurogenesis is unclear. We thus addressed the role of fanca and fancg, which are involved in the activation of Fanconi pathway, in neural stem and progenitor cells during brain development and adult neurogenesis. Fanca−/− and fancg−/− mice presented with microcephalies and a decreased neuronal production in developing cortex and adult brain. Apoptosis of embryonic neural progenitors, but not that of postmitotic neurons, was increased in the neocortex of fanca−/− and fancg−/− mice and was correlated with chromosomal instability. In adult Fanconi mice, we showed a reduced proliferation of neural progenitor cells related to apoptosis and accentuated neural stem cells exhaustion with ageing. In addition, embryonic and adult Fanconi neural stem cells showed a reduced capacity to self-renew in vitro. Our study demonstrates a critical role for Fanconi pathway in neural stem and progenitor cells during developmental and adult neurogenesis.
Keywords: ageing, apotosis, DNA repair, Fanconi, neural progenitor
Introduction
Neural stem and progenitor cells (NSPCs) self-renew, proliferate and give rise to the three major neural cell types (neurons, astrocytes, and oligodendrocytes) during central nervous system development (Temple, 2001). NSPC proliferation remains in restricted regions of the adult brain: the subgranular layer of the dentate gyrus in the hippocampus and the subventricular zone (SVZ) of the lateral ventricles (Alvarez-Buylla and Lim, 2004). The adult neurogenic niches maintain neurogenesis during life for brain homeostasis and repair after trauma or stresses. Due to their intense self-renewal and proliferation, NSPCs are exposed to a lot of environmental and replicative stresses, and thus require appropriate DNA repair system to maintain their genetic integrity and ensure correct brain development and homeostasis.
DNA double-stand breaks (DSBs) trigger a signalling cascade that leads to repair and the resolution of the breaks by two main pathways, (i) non-homologous end joining (NHEJ), and (ii) homologous recombination (HR), or lead to apoptosis (for review, see Downs et al, 2007). The HR pathway copies matched base pairs either from complementary template found on the homologous chromosome, or from sister chromatid requiring that the cell are in late S or G2 phase of the cell cycle. By contrast, NHEJ has a predominant role in DSB repair in the G0/G1 phase.
Disruption of genes involved in NHEJ, such as DNA ligase IV and Xrcc4, results in DNA damage-induced apoptosis of differentiated neural cells during brain development (Gao et al, 1998; Orii et al, 2006). Loss of Xrcc2, a DNA repair gene involved in HR, provokes neural progenitor apoptosis, and highlights a critical role of HR DNA repair during brain development (Orii et al, 2006). DNA repair is therefore required for normal brain development and specificity of DNA repair pathway may depend on the nature of cells, that is, NHEJ for postmitotic neurons and HR for proliferating neural stem and progenitor cells (Orii et al, 2006). Since HR DNA repair deficiency results in embryonic lethality, no data are available concerning the importance of HR DNA repair pathways for NSPCs in the adult forebrain. BRCA2/FANCD1, which is involved in HR, is required for neurogenesis within the cerebellum (Frappart et al, 2007).
The genomic instability syndrome Fanconi anaemia (FA) is a recessive disorder characterized by genetic cancer-susceptibility syndrome, congenital abnormalities, bone marrow failure, and cellular sensitivity to DNA cross-linking agents (D'Andrea and Grompe, 2003). Although the precise functions of the 13 FA genes are not fully understood, the FA/BRCA pathway has been implicated in HR DNA-repair pathway. Eight FA proteins (FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, and FANCM) form the core complex in the nucleus, which has a presume ubiquitin–E3 ligase activity (Grompe and van de Vrugt, 2007). In response to DNA damages induced by mitomycin C, ionizing radiation, ultraviolet, or during the S phase of the cell cycle, the FA core complex mediates monoubiquitination of FANCD2 (Wang and D'Andrea, 2004), which is translocated to DNA-repair sub-nuclear foci where it colocalizes with DNA-repair proteins involved in HR such as BRCA1 (Garcia-Higuera et al, 2001), RAD51 (Taniguchi et al, 2002), and BRCA2/FANCD1 (Wang et al, 2004). In support of a role of the FA/BRCA pathway in HR, cell lines deficient for the FA/BRCA pathway have been reported to have defective HR (Kennedy and D'Andrea, 2005).
Congenital abnormalities such as microcephaly and microphtalmia are frequently observed in FA patients (Faivre et al, 2000; Tischkowitz and Hodgson, 2003) and fanca and fancd2-knockout mice show only partial FA phenotype, including microphtalmia (Houghtaling et al, 2003; Wong et al, 2003). However, the role of FA/BRCA pathway has never been examined in adult forebrain neurogenesis. FANCA and FANCG are both involved in the FA core complex, and the disruption of one of these proteins destabilizes the FA core complex, resulting in the abolition of FANCD2 activation and the absence of FA/BRCA pathway functionality (Garcia-Higuera et al, 2000). We thus investigated the role of FA/BRCA in NSPCs during embryogenesis and in adult brain using fanca- and fancg-deficient mice. We demonstrated that the loss of fanca or fancg was associated with increased apoptosis of proliferating NSPCs, but not of postmitotic neurons, which correlated to chromosomal instability. Remarkably, adult FA mice showed progressive exhaustion of the NSPC pool, which accentuated with ageing. All together, our results demonstrated a critical requirement of the FA/BRCA pathway in NSPC renewal during brain development and in adult brain homeostasis.
Results
Inactivation of FA pathway leads to abnormal cortex development
We examined the requirement of FA pathway during neurogenesis by using fanca- or fancg-deficient transgenic mice. Although adult fanca−/− and fancg−/− mice did not show obvious behavioural abnormalities, they had a smaller forebrain size in comparison with wild-type (WT) littermate controls (Supplementary Figure 1). These microcephalies related to loss of fanca or fancg suggested an important role of the FA pathway during development of the central nervous system.
Therefore, we studied the consequences of fanca or fancg loss on embryonic brain development, and focused our analysis on the early phases of neuron production in the cerebral cortex, that is, E13.5 and E14.5. Although we did not observe any size difference in the neocortex in coronal sections at E13.5 (data not shown), a reduction of neocortex thickness was observed at E14.5 in FA embryos compared with WT embryos and it correlated with a decrease in the number of cell layers from the ventricular to the pial surfaces (Figure 1). This thinning was observed at different rostro-caudal levels, and was more pronounced in the dorsal pallium than in the lateral pallium of fanca−/− and fancg−/− neocortices (Figure 1, and data not shown). To determine the cell type affected by FA gene loss in neocortices, we performed immunostaining for nestin and βIII-tubulin to label NSPCs and neurons, respectively. Thickness of the VZ, containing nestin-positive NSPCs, was unaffected in FA forebrains, whereas the βIII-tubulin-positive zone significantly thinned and this correlated with a decreased number of neuron layers in the dorsal pallium of fanca−/− and fancg−/− (Figure 1D and E). These results demonstrate that FANCA and FANCG are required for neuron production during brain development.
Figure 1.
FA embryos have a reduced neocortex size. Embryonic brains (E14.5) were cut into coronal sections at different rostro-caudal levels (I, II, III) as shown in the sagittal representation. (A) A typical DAPI staining of caudal sections (III) is shown for fancg+/+ and fancg−/− brains. (A′) βIII-Tubulin (red) and nestin (green) immunostainings corresponding to white boxes on the left panel. (B, C) Neocortices of fanca−/− and fancg−/− showed a size reduction in the dorsal pallium (DP boxes in A′) compared with WT controls at each rostro-caudal level. (D, E) The thickness of the βIII-tubulin zone, containing neurons, was decreased in dorsal pallium of the fanca−/− and fancg−/− neocortex compared with WT controls at each rostro-caudal level. The number of mice is indicated within the bars. V, ventricle. Scale bars, 100 μm. *P<0.05; **P<0.01; ***P<0.005.
Increased apoptosis in the SVZ of FA neocortices
Brain size depends on a strictly controlled balance between apoptosis and cell proliferation during embryonic development.
Proliferating NSPCs could be labelled in the VZ by bromodeoxyuridine (BrdU) incorporation during replication of their DNA (Takahashi et al, 1995). We did not observe any difference in BrdU incorporation for fanca−/− and fancg−/− neocortex in comparison with WT controls (Supplementary Figure 2A and B). The number of BrdU-positive NSPCs and their localization in the VZ was similar between fanca−/−, fancg−/−, and WT controls, suggesting that S phase was not altered in FA mice during neocortex development.
Analysis of H3 histone phosphorylation (γ-H3 histone), a marker of mitosis (Hans and Dimitrov, 2001), showed that the number of mitotic cells in the VZ was similar in FA and in the WT (Supplementary Figure 2C).
To determine if reduced neocortex size may account for an elevated cell death in FA embryos, the extent of apoptosis was examined by detection of pycnotic nuclei (4',6-diamidino-2-phenylindole (DAPI) staining), and was confirmed by cleaved-caspase-3 immunochemistry (Figure 2A). Neocortices of fanca−/− and fancg−/− embryos showed an increase in the number of pycnotic nuclei (Figure 2B), revealing a higher level of apoptosis that was confirmed by cleaved-caspase-3 immunostaining (Figure 2C). Increased apoptosis was observed with similar magnitude at E13.5 and E14.5 (Figure 2C), and apoptotic cells were almost exclusively localized in the proliferative VZ and they expressed nestin (Figure 2D). Apoptotic cells were sometimes observed as doublets of apoptotic cells in the VZ, suggesting postmitotic death (Figure 2A′). The βIII-tubulin-positive zone was more developed at E14.5; however, no apoptosis was observed in this zone of FA mice. These results suggest that abnormal cortical structures in fanca−/− and fancg−/− embryos did not result from death of postmitotic neurons, but from NSPC apoptosis in the VZ, suggesting that the FA pathway may be required during NSPC proliferation.
Figure 2.
Apoptosis is increased in FA VZ. (A) DAPI and cleaved-caspase-3 immunostaining are shown in a fanca−/− E14.5 neocortex section. Arrows pointed out the apoptotic cells in the VZ. Nonspecific staining was observed on blood vessels (stars) and on meninges (dotted lines). Colocalization of pycnotic nuclei and cleaved-caspase-3 staining is shown in the enlargement of the white box (A′). White arrows pointed out the isolated apoptotic cells and red arrows pointed out a doublet of apoptotic cells. (B) Quantifications of pycnotic nuclei and (C) cleaved-caspase-3-positive cells in the VZ of E13.5 and E14.5 embryonic brains show a significant increase of apoptosis in fanca−/− and fancg−/− compared with fanca+/+ and fancg+/+ controls. (D) Apoptotic cells in the fancg−/− neocortex express nestin, an NSPC marker. The number of mice is indicated within the bars. V, ventricle. Scale bars, 10 μm. *P<0.05; ***P<0.005.
All together, our data did not reveal any difference in the cell-cycle progression of fanca−/− and fancg−/− NSPCs compared with WT NSPCs during brain development, but showed that FANCA and FANCG are required for their survival.
Proliferating NSPCs display chromosomal instabilities in the FA neocortex
The hallmarks of FA are genomic instability and hypersensitivity to DNA cross-linking agents, however the FA/BRCA pathway is also required during replication to prevent accumulation of DSBs (Sobeck et al, 2006). Phosphorylation of histone H2AX (referred to as γ-H2AX) occurs rapidly after DNA DSBs (Rogakou et al, 1998). Analysis of H2AX phosphorylation was performed by immunochemistry in developing brain of FA mice. As we have previously reported, two patterns of γ-H2AX staining could be observed in the neocortex after DNA damage: (i) nuclear γ-H2AX foci signalling DSBs and (ii) a bright and diffuse γ-H2AX staining related to DNA fragmentation during apoptosis (Nowak et al, 2006). As a result of increased apoptosis, bright γ-H2AX staining was observed in the VZ of FA mice (data not shown). Of note, cells localized in the VZ of fancg−/− showed numerous γ-H2AX foci, which were rarely observed in WT embryos, suggesting that numerous DSBs occurred during unperturbed neurogenesis in FA NSPCs (Figure 3A).
Figure 3.
FA NSPCs display chromosomic aberrations. (A) γ-H2AX foci were numerous in the VZ of fancg−/− embryos, but infrequent in fancg+/+ controls. Cytogenetic analyses were performed on embryonic NSPCs in culture. Figures of abnormal metaphases are shown to illustrate the most frequent aberrations that were observed in fanca−/− NSPC cultures; (B) the arrow points a chromatid break; (C) a complex rearrangement is surrounded (C′); and an endoreplication is shown (arrowheads pointed out a pair of chromosomes) (D). (E) Quantifications were performed on 300 metaphases obtained from four embryos at the first and second passage in culture, and they showed increased levels of chromatid breaks and rearrangements (radial formation, endoreplication) in fanca−/− compared with fanca+/+ controls. **P<0.01; ***P<0.005.
Cytogenetic analyses were performed to determine whether these DSBs could induce chromosomal instability in proliferating NSPCs. NSPCs from telencephalons were cultured as aggregates, named neurospheres (Reynolds and Weiss, 1992), and metaphase spreads were performed on primary cultures at the first and second passages. Quantitative analyses were performed on 300 metaphases (from four embryos) and showed two times more abnormal metaphases in fanca−/− compared with that in fanca+/+ controls (Figure 3E). We mainly observed chromatid breaks (Figure 3B) and the percentage of metaphases containing two or more chromatid breaks was significantly increased in fanca−/− NSPCs compared with WT controls (25.2±4.5 versus 5.0±2.6%). Less frequently, more complex rearrangements (Figure 3C and C′) and endoreplications (Figure 3D), were also observed in fanca−/− metaphases; however, they were totally absent from control metaphases (Figure 3E).
Cytogenetic analyses were also performed under low oxygen tension (3% O2) to rule out that chromosomal instability was due to susceptibility of FA cells to ambient oxygen (Joenje and Oostra, 1983). We observed that chromatid breaks were significantly increased in fanca−/− NSPCs in 3% O2 cultures compared with WT controls (Supplementary Figure 3). Levels of chromatid breaks in fanca−/− NSPCs were similar in 3 and 21% O2 cultures, showing that chromosomal instability is not due to culture under ambient oxygen.
All together, our results demonstrate that FA NSPCs exhibit spontaneous DSBs and chromosomal instability, which could account for increased NSPC apoptosis in the developing neocortex.
Loss of the FA pathway reduces proliferation and induces apoptosis in adult brain
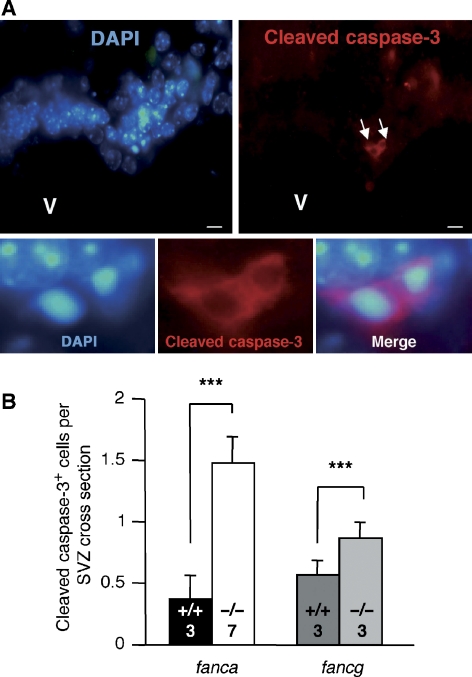
We further determine the involvement of fanca and fancg genes in the adult SVZ, where proliferation of NSPCs persists. Both cleaved-caspase-3 immunostaining and pycnotic nucleus analyses showed an increased level of apoptosis in the SVZ of fanca−/− and fancg−/− mice compared with their littermate controls (Figure 4).
Figure 4.
Apoptosis is increased in the adult FA SVZ. (A) Pycnotic nuclei and cleaved-caspase-3 staining (white arrows) are shown in the SVZ of adult fancg−/− mice. Colocalization of pycnotic nuclei and cleaved-caspase-3 staining is showed in an enlargement (lower panel). (B) Quantifications of cleaved-caspase-3-positive cells per SVZ cross section showed significant elevated apoptosis in the SVZ of fanca−/− and fancg−/− compared with fanca+/+ and fancg+/+ controls. The number of mice is indicated within the bars. V, ventricle. ***P<0.005.
To determine if loss of fanca or fancg induced proliferation defects in adult brain, BrdU was administered to label proliferating NSPCs in the SVZ. Loss of fanca or fancg resulted in a significant decrease in the percentage of BrdU-positive cells in the SVZ in comparison with their littermate controls (Figure 5A and B, and data not shown). In addition, amounts of cells expressing the proliferation marker Ki67 were also decreased in fanca−/− mice compared with the WT (Figure 5C). Furthermore, the number of mitotic cells, identified by γ-H3 histone immunostaining, was reduced in FA mice compared with WT mice (Figure 5D).
Figure 5.
Proliferation is decreased in the SVZ of adult FA mice. (A) Proliferation of NSPCs was examined after BrdU incorporation for 3 h in fanca−/− and fanca+/+ control mice. (B, C) Quantifications of BrdU-positive and Ki67-positive cells in the SVZ showed a decreased percentage of proliferating cells in the fanca−/− SVZ compared with fanca+/+ controls. (D) γ-H3-histone immunostaining showed a decreased number of mitotic cells in the fanca−/− and fancg−/− SVZ in comparison with fanca+/+ and fancg+/+ controls. Data were obtained with 3–5 mice, as indicated within the bars, representing from 3024 to 5671 nuclei. *P<0.05; ***P<0.005.
NSPCs in the SVZ generate a progeny of neuroblasts, which migrate into the olfactory bulbs where they differentiate into neurons (Lledo et al, 2006). To examine the production of new neurons in the olfactory bulbs, mice received BrdU for 14 days followed by a 7-days-chase. We observed a twofold decrease in the number of BrdU-positive cells in olfactory bulbs of fanca−/− mice compared with WT mice (Supplementary Figure 4), illustrating that reduced NSPC proliferation in the SVZ of FA mice was followed by a decline in neuron production.
Proliferating progenitors, identified as NG2-positive cells, are widespread throughout the brain, outside neurogenic niches (Dawson et al, 2003). We have thus analysed whether those progenitors were altered in the corpus callosum of adult FA mice. The number of NG2/Ki67-double-positive cells was lower in fanca−/− mice compared with WT mice (Supplementary Figure 5), suggesting that all neural progenitors spread throughout the brain were decreased in adult FA mice.
Overall, our data demonstrate that FANCA and FANCG are required during adult neurogenesis for NSPC proliferation and survival.
In vitro self-renewal of FA NSPCs is reduced
Long-term neurosphere cultures were performed with NSPCs of FA mice obtained from embryonic telencephalons (E14.5) and adult SVZ. Neurosphere cultures of embryonic telencephalons and adult SVZ from WT mice could be expanded for numerous passages, revealing efficient self-renewal of neural stem cells (Figure 6A and D). Proliferation of heterozygous fanca+/− embryonic NSPCs did not show any difference with WT (Figure 6A). In contrast, population doublings of fanca−/− embryonic NSPCs were significantly reduced in comparison with controls, although FA embryonic neurosphere cultures could be expanded for long term (Figure 6A). This reduction was not related to an alteration in DNA replication phase, as BrdU incorporation experiments did not evidence any S-phase alteration (Figure 6B). In contrast, reduced population doublings of embryonic FA NSPCs correlated with a higher apoptotic level illustrated by cleaved-caspase-3 immunostaining (Figure 6C).
Figure 6.
NSPC self-renewal in vitro is altered in FA mice. Growth, proliferation, and apoptosis were examined in neurosphere cultures from embryonic (A–C) and adult (D–F) NSPCs. (A) Population doublings of fanca−/− embryonic NSPCs were reduced in comparison with fanca+/+ controls. (B, E) Proliferating NSPCs were determined by flow cytometry after BrdU incorporation (30 min or 1 h for embryonic or adult NSPCs, respectively). (C, F) Analysis of cleaved-caspase-3 by flow cytometry revealed increased level of apoptosis in fanca−/− and fancg−/− embryonic and adult NSPCs in comparison with WT controls. (D) Population doublings of adult NSPCs were drastically reduced compared with WT controls. The number of mice is indicated within the bars. **P<0.01.
Decrease in population doublings of fanca−/− NSPCs was also obtained under low oxygen tension (3% O2) (Supplementary Figure 6), showing that reduction in cell proliferation was not due to ambient oxygen level, but rather due to intrinsic defects in the FA pathway within NSPCs.
NSPCs from adult WT SVZ could be expanded for more than 10 passages, whereas those from FA SVZ did not significantly expand and died at the seventh and ninth passages for fanca−/− and fancg−/−, respectively (Figure 6D). These proliferation defects of adult FA NSPCs were related to a reduced BrdU incorporation and a higher apoptotic level observed in fanca−/− and fancg−/− neurosphere cultures (Figure 6E and F, and data not shown).
In conclusion, our results suggest that alterations of NSPC survival and proliferation could be responsible for reduced self-renewal capacity in FA mice.
Exhaustion of the NSPC pool in the SVZ of FA mice is accentuated with ageing
With regards to the reduced in vitro self-renewal capacity of adult FA NSPCs, we hypothesized that the loss of fanca or fancg may provoke NSPC defect during ageing, accentuating progenitor decline observed in older mice (Tropepe et al, 1997).
Fast-proliferating progenitors and slowly dividing neural stem cells were analysed by immunochemistry in the SVZ from young adults (2–3 months) and old adults (10–12 months). Fast-proliferating NG2/Ki67-double-positive cells in the SVZ have been identified as multipotent progenitors, or type C-like cells (Aguirre et al, 2004). The number of NG2/Ki67 progenitors was reduced by 40% in young fanca−/− mice and by 72% in old adults in comparison with the age-matched WT (Figure 7A). In addition, we have quantified slowly dividing neural stem cells in the SVZ by their capacity to retain BrdU labelling for long term (Morshead et al, 1998; Doetsch et al, 1999). BrdU-label-retaining cells were decreased by 20% in young adult fanca−/− mice in comparison with WT mice (Figure 7B). The number of BrdU-label-retaining cells showed a reduction with ageing in both fanca+/+ and fanca−/− mice. However, the decrease in BrdU-label-retaining cells was considerably more pronounced in old fanca−/− mice, reaching 70% reduction, in comparison with age-matched fanca+/+ (Figure 7B).
Figure 7.
Exhaustion of the NSPC pool in the SVZ of FA mice is accentuated with ageing. (A) Quantification of NG2/Ki67-double-positive cells in the adult SVZ shows decreased level of proliferating progenitors in the SVZ of young (2–3 months) and old (10–12 months) fanca−/− adults compared with fanca+/+ age-matched controls. (B) BrdU-label-retaining cells in the adult SVZ, recognized as slowly cycling neural stem cells, have a reduced level in the SVZ of young (2–3 months) and old (10–12 months) fanca−/− adults compared with fanca+/+ age-matched controls. (C) The NSPC pool was determined by culturing freshly harvested SVZ in a semi-solid medium that allows discrimination between neural stem cell-derived colonies and neural progenitor-derived colonies. The number of neural progenitor-derived colonies was decreased in the SVZ of young (2–3 months) fanca−/− and fancg−/− adults compared with fanca+/+ and fancg+/+ age-matched controls. Both neural stem cell- and progenitor-derived colonies were profoundly reduced in old fancg−/− mice. Data were obtained with 2–6 mice, as indicated within or below (n=) the bars, representing in panels A and B from 1908 to 2894 nuclei (except the number of nuclei for NG2/Ki67 in old fanca−/− mice, which was 798). *P<0.05; **P<0.01; ***P<0.005.
A functional approach was further used to confirm the alterations of neural stem and progenitor pools in the SVZ of adult FA mice. NSPCs form neurosphere-related colonies in semisolid medium in the presence of epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF-2), and after 3 weeks, neural stem cell-derived colonies can be distinguished from neural progenitor-derived colonies by their larger size due to their higher proliferative capacities (Mouthon et al, 2006). Moreover, neural stem cell-derived colonies can be thereafter maintained in liquid medium for at least three passages, and had the ability to differentiate in both neuronal and glial lineages when transferred in differentiation medium. According to immunochemistry analyses, the number of neural progenitor-derived colonies was reduced by 50% in young fanca−/− and fancg−/− mice and further by 90% in older fancg−/− mice, in comparison with WT age-matched controls (Figure 7C). In addition, neural stem cell-derived colonies appeared to be unaltered in young FA mice, whereas they were no more detected in older fancg−/− mice (Figure 7C).
Together, the data from examination of NSPC pools demonstrated that the loss of fanca and fancg results largely in progenitor decline and a progressive exhaustion of neural stem cells during ageing, suggesting that the FA pathway is required to maintain neural stem cell and progenitor pools in the adult SVZ.
Discussion
In this study, we showed that fanca- and fancg-deficient embryos developed microcephalies due to apoptosis of proliferating NSPCs and the resulting decline in neuron production. The normal FA/BRCA pathway was required for maintaining NSPC genomic integrity and survival during replicative stress. We also demonstrated for the first time that the loss of fanca and fancg largely resulted in decline of fast-proliferating progenitors in the adult SVZ, leading to a progressive exhaustion of slowly dividing neural stem cells during ageing.
Mutations or deletions in fanca and fancg genes account for 75% of FA patients. Our results are in accordance with genomic instability and microcephalies reported in some FA patients (Gozdasoglu et al, 1980; Balta et al, 2000; Gennery et al, 2004), indicating that FA loss in the mouse recapitulates neural aspects of this disease. In addition, loss of fanca and fancg gave similar developmental abnormalities, DNA repair defects, apoptosis, and NSPC pool exhaustion. In fact, FANCA and FANCG are both involved in the FA core complex and disruption of one of these proteins destabilizes the FA core complex, resulting in the absence of FA/BRCA pathway activation (Garcia-Higuera et al, 2000; Gurtan et al, 2006; Sobeck et al, 2006). Therefore, alterations of embryonic and adult neurogenesis, which we observed in fanca−/− and fancg−/− mice, are most likely related to the inability of the FA/BRCA pathway to be activated. Recently, Frappart et al (2007) have reported that inactivation of BRCA2, another member of the FA/BRCA pathway, leads to microcephaly and cerebellar defects. Together, these data illustrate the importance of the FA/BRCA pathway in nervous system development.
The FA pathway is critical for survival of NSPCs
We showed an increase in apoptosis in nestin-positive NSPCs contained within the VZ of FA embryonic neocortices and in the SVZ of adult FA mice, but not in the zone containing postmitotic neurons, and further in vitro analyses demonstrated an increased apoptosis in proliferating FA NSPCs. Neural progenitors are very sensitive to DNA damages, such as DSBs induced by ionizing radiation (Nowak et al, 2006) and telomere damages (Cheng et al, 2007), which provoke apoptosis. However, telomere damage is probably not involved in neural progenitors apoptosis in fanca- and fancg-deficient NSPCs, as fancg-deficient mouse cells (both haematopoietic and non-haematopoietic) have been shown to display normal telomere length, normal telomerase activity, and normal chromosome end capping (Franco et al, 2004). The FA pathway is thought to be involved in the repair of irregular DNA structures, including those encountered by the moving replication fork, and to recruit HR to repair DSBs. FA-deficient cells are defective in HR-mediated repair of extremely cytotoxic DSBs, particularly for proliferating cells (Niedernhofer et al, 2005). We showed that FA neural progenitors have increased DSBs as indicated by γ-H2AX foci in vivo and chromosomal breaks in vitro. Hence, apoptosis of proliferating neural progenitors in the embryonic VZ and in adult SVZ of FA mice is almost certainly due to DNA repair defects in FA.
Interestingly, loss of Xrcc2, which is involved in HR, also specifically induces apoptosis of neural progenitors in the VZ of embryonic neocortices (Orii et al, 2006), and inactivation of BRCA2/FANCD1 has been also involved in NSPC apoptosis in the postnatal cerebellum (Frappart et al, 2007). Although the amount of cell death in fanca−/− and fancg−/− neocortices was less pronounced than disruption of Xrcc2 and BRCA2/FANCD1 (Orii et al, 2006; Frappart et al, 2007), it exceeded the normal level and was undoubtedly responsible for the decline in neuron production and subsequent microcephalies. Similarly, increase in apoptosis of proliferating NSPCs in the SVZ of FA adult mice is most likely responsible for the reduction in neuron production in the olfactory bulbs.
These data strongly suggest that the FA/BRCA pathway and activation of the HR DNA-repair pathway are required for survival of proliferating NSPCs.
The FA pathway is critical for genetic stability of NSPCs
Our data pointed out the critical importance of the FA pathway in maintaining NSPC genomic stability even in the absence of exogenous stress. Indeed, we observed the presence of DSBs, that is, γ-H2AX foci within proliferating NSPCs during brain development of FA mice and increased levels of abnormal metaphases with chromatid breaks and chromosomal rearrangements in NSPC cultures from FA mice both under ambient and low (3%) oxygen levels. The increase in γ-H2AX foci in FA NSPCs is in accordance with the FA/BRCA pathway connecting with H2AX, preventing chromosomal instability (Bogliolo et al, 2007). Characterization of FANCD2 foci with BRCA1 and RAD51 during S phase suggested that the FA/BRCA pathway is involved in HR repair of DSBs during S phase (D'Andrea and Grompe, 2003). Moreover, using a cell-free assay (Xenopus extracts) it has been shown that association of FA proteins with chromatin occurs specifically when replication forks encounter certain DNA lesions (Sobeck et al, 2006). Due to their intense proliferation during nervous system development and in adulthood, NSPCs are exposed to replicative stresses that could lead to alterations in DNA, including DSBs. All together, these data strongly suggest that the FA pathway is specifically required for HR induction to prevent accumulation of DSBs and consequent apoptosis during unperturbed NSPC proliferation.
FA disease is a cancer-susceptibility syndrome and FA patients may develop brain tumours, mainly medulloblastoma. By targeting inactivation of fancd1 (or BRCA2) in NSPCs under the nestin promoter, Frappart et al (2007) have shown that fancd1 is a potent tumour suppressor in the cerebellum. However, there is a low chance that FANCA and FANCG, which are contained in the FA core complex, may also have a tumour-suppressor function, as FANCA and FANCG patients show only a low risk of brain tumours in comparison with patients belonging to the complementation FANCD1 and FANCN/PALB2 groups, which form a complex directly involved in HR (Offit et al, 2003; Reid et al, 2007).
FA is required for long-term maintenance of the NSPC pool
We showed that loss of fanca and fancg dramatically reduced fast-proliferating progenitors in the adult SVZ both in vivo and in vitro, whereas proliferation rates were not altered in embryonic NSPCs in vivo and few in vitro. Our data also illustrate that neural progenitor spread throughout the adult brain may be affected by loss of FA pathway. Furthermore, we demonstrated that loss of FA genes provoked only a slight decline in slowly dividing stem cells in young adult SVZ, whereas it was dramatic in older mice. Therefore, loss of the FA pathway primarily affects neural progenitors and provokes progressive exhaustion of neural stem cell pool in FA mice during ageing, which is almost certainly due to DNA damage-induced apoptosis. Due to their high self-renewal capacities, embryonic neural stem cells may allow neural progenitor deficit to be replenished, but continual death of fast proliferating progenitors in adult probably leads to premature exhaustion of adult neural stem cells. It is also possible that neural stem cells have low thresholds for damage checkpoint activation, exploiting apoptosis to limit the potential harmful impact of genetic damage, which may contribute to their exhaustion during ageing.
Patients with FA display multi-organ defects and most develop bone marrow failure in childhood. Progressive bone marrow failure observed in FANCC deficiency has been considered as a proliferative defect of haematopoietic stem cells (Haneline et al, 1999), which corroborates our data on NSPCs with FANCA and FANCG. We could thus hypothesize that FA mutants may have a general stem cell defect, the FA/BRCA pathway being required for their efficient replication.
The ataxia–telangiectasia regulated (ATR) (Seckel syndrome) DNA-repair protein plays a role in the activation of the FA/BRCA pathway in response to replication stress (Wang and D'Andrea, 2004). Interestingly, it has been recently reported that deletion of ATR provokes premature ageing phenotype and defects in tissue homeostasis, which correlated with stem cell and progenitor exhaustion in tissues (Ruzankina et al, 2007). In addition, recent studies with DNA-repair mutants of NHEJ evidence that accumulation of DNA damage in adult haematopoietic stem cells leads to their exhaustion and contributes to the ageing phenotype (Nijnik et al, 2007; Rossi et al, 2007). Our data are also consistent with DNA-damage accrual being a physiological mechanism of neural stem cell ageing, which may contribute to the diminished capacity of aged brain to maintain homeostasis.
In conclusion, our study demonstrates a critical role for the FA/BRCA pathway in neural stem and progenitor cells during developmental and adult neurogenesis.
Materials and methods
Mice
All animal procedures were carried out in accordance with French government regulations (Services vétérinaires de la santé et de la production animale, Ministère de l'Agriculture). fanca+/− mice (C57Bl/6xSV129) and fancg+/− mice, previously established (Cheng et al, 2000; Koomen et al, 2002), were backcrossed into C57BL/6J strain and bred to generate fanca−/− or fancg−/− and WT mice in our animal facilities. Mice were used at 2–3 months as young adults and at 10–12 months as old adults. Embryonic preparations were obtained on day 13.5 or 14.5 of gestation (E13.5 or E14.5). The genotype of the offsprings was determined by polymerase chain reaction analysis using DNA extracts.
Experiments were performed with fanca−/− or fancg−/−, and with the corresponding controls of the same genetic background, that is, fanca+/+ or fancg+/+, and within the same offspring when possible.
BrdU administration
Single administration of BrdU (1 mg; Sigma) was given intraperitoneally 3 and 1 h before euthanasia for adult and embryos (pregnant mice).
Alternatively, for long-term treatment of adult mice, BrdU was given in drinking water (1 mg/ml) for 14 days, and then followed by a 7-days chase (Doetsch et al, 1999).
Antibodies
Primary antibodies purchased were as follows: anti-nestin (mouse, 1:200; Becton Dickinson), anti-βIII-tubulin (rabbit, 1:200; Covance), anti-cleaved-caspase-3 (rabbit, 1:200; Cell Signaling), anti-γ-H3-histone (rabbit, 1:200; Cell Signaling), anti-γ-H2AX (mouse, 1:500; Cell Signaling), anti-Ki67 (mouse, 1:100; Vector), anti-NG2 (rabbit, 1:200; Chemicon), and mouse anti-BrdU (mouse, 1:100; Amersham). Secondary antibodies (all used at 1:400) purchased from Invitrogen were as follows: goat anti-rabbit AlexaFluor®488, goat anti-rabbit Alexafluor®594, chicken anti-mouse AlexaFluor®647, goat anti-mouse Alexafluor®488, goat anti-mouse Alexafluor®546, and goat anti-mouse IgG1 Alexafluor®350.
Immunohistochemistry
Adult mouse brains were fixed overnight by immersion in 4% paraformaldehyde (PFA), washed in phosphate-buffered saline (PBS), and then transferred to 30% sucrose for 24 h. Coronal sections (8 μm) of frozen adult mouse brains were cut on a cryostat (Leica) and fixed for 10 min in 4% PFA.
Embryonic brains were fixed overnight by immersion in 4% PFA, or 1 h in cold methanol, dehydrated, and embedded in paraffin (VIP; Leica). Coronal sections (5 μm) were cut with a microtome for histologic analysis. Tissue sections were deparaffinized and unmasked in citrate solution of pH 6.
Adult and embryonic brain sections were incubated for 10 min in 0.5% Triton X-100. For BrdU immunostaining brain sections were digested with DNAse I (1 mg/ml; Sigma) for 1 h at 37°C. Sections were blocked for 1 h with the Vector® MOM immunodetection kit (Vector Laboratories) and incubated overnight at 4°C with the required primary antibodies. After washing in PBS, slides were then incubated for 1 h with the appropriate secondary antibodies; nuclei were stained with DAPI (13 ng/ml) and the slides were mounted in Fluoromount (Southern Biotechnologies).
Isolation and neurosphere cultures of embryonic and adult NSPCs
Brains were isolated from embryos on day 14.5 of gestation, meninges were removed from the forebrain in PBS 0.6 g/l glucose, and telencephalons were dissected under a binocular microscope and incubated in collagenase I (100 U/ml; Invitrogen)/DNAse I (100 U/ml; Sigma) in PBS for 10–20 min at 37°C, and then mechanically dissociated. Cells were plated at a density of 105 cells per 25-cm2 flask in 5 ml of Euromed® (Euroclone) supplemented with hormone mix (6 g/l glucose, 60 mmol/l putrescine, 20 nmol/l progesterone, 30 nmol/l sodium selenite, 25 μg/ml insulin, and 100 μg/ml apo-transferrin) and human recombinant EGF and FGF-2 (Sigma), both at the final concentration of 20 ng/ml.
NSPCs were also obtained from the SVZ of adult mice aged of 2–3 months. Coronal brain slices (2-mm thick) were cut between +0.5 and −1.5 mm relative to bregma. The SVZ region was microdissected under a binocular microscope, incubated in 1 mg/ml papain (Worthington) for 30–40 min at 37°C, and then mechanically dissociated. Cells were plated at a density of 3.105 SVZ cells per 25 cm in NeuroCult (Stem Cell Technologies) supplemented with heparin, EGF (20 ng/ml), and FGF2 (10 ng/ml). Neurosphere cultures of embryonic and adult SVZ NSPCs were mechanically dissociated, numbered, and plated in fresh medium every week.
Analysis of apoptosis and BrdU incorporation by flow cytometry
To analyse NSPCs in the S phase, on day 5 of neurosphere cultures, BrdU was added at a final concentration of 10 μM to the culture medium for 30 min or 1 h. Neurosphere cultures were dissociated, fixed with 1% PFA for 10 min, and then permeabilized with 0.1% Triton X-100 for 5 min. After washing with 0.15% PBS–BSA, detection of BrdU incorporation was performed after DNAse digestion using the APC BrdU Flow kit® (BD Biosciences) as recommended by the manufacturer. Cells were incubated for 1 h with anti-cleaved-caspase-3 phycoerythrin coupled (Cell Signalling) or with a matched isotopic control to set the gate of positivity. Cytometry analyses were performed on a FACScalibur (BD Biosciences).
Neural colony-forming cell assay
Freshly isolated SVZ cells of young (2–3 months) and old (10–12 months) adult mice were obtained as described above for neurosphere cultures, and plated with EGF and FGF2 using the mouse NeuroCult® neural colony-forming cell assay (NCFC; StemCell Technologies) according to the manufacturer's instructions. Cultures were incubated in a humidified atmosphere with 5% CO2. Medium and growth factors were refreshed every 7 days. After 21 days, colonies were scored according to their size. Colony diameters were measured using an eyepiece reticule on an inverted light microscope under phase-contrast optic. Large clones (diameter ⩾2 mm) were considered to have been derived from neural stem cells, whereas smaller clones (1 mm⩽diameter<2 mm) were considered to have been derived from neural progenitor cells.
Analysis of chromosomal aberrations
Embryonic neurosphere cultures under 21% O2 were treated with the antiapoptotic compound Z-VAD (Z-Val-DL-Asp-fluoromethylketone; Bachem) at a final concentration of 10 μM during 24 h to minimize cell loss. For 3% O2 cultures, Z-VAD was not added in the medium. Cells were then incubated for 1.5 h with colchicine (0.1 μg/ml final concentration). Cells were treated with a hypotonic solution containing KCl 0.75 mM and human serum diluted to 1/6 (1:1) for 20 min at 37°C, and then fixed in ethanol:acetic acid (3:1). Air-dried preparations were stained with 3% aqueous Giemsa solution for 10 min to analyse chromosomal aberrations.
Data analyses
Data in the graphs are represented as mean±s.d. of replicate experiments, with the number of mice as indicated in the figures. Immunohistochemistry estimates were made on 3–6 cross sections per mouse, and the number of mice analysed is indicated in each figure legend. Data obtained from FA mice were compared with those from WT littermate controls using Student's t-test. For all analyses, data from fanca−/− or fancg−/− mice were compared with those of the corresponding control of the same genetic background, that is, fanca+/+ or fancg+/+. P-value is indicated in the graphs (*P<0.05; **P<0.01; ***P<0.005). The level of statistical significance was set at P<0.05.
Supplementary Material
Supplementary Figures 1
Supplementary Figures 2
Supplementary Figures 3
Supplementary Figures 4
Supplementary Figures 5
Supplementary Figures 6
Supplementary Figures Legends
Acknowledgments
We thank V Neuville, C Chauveau, and S Leblay for their technical assistance in the animal facilities, and M Vernet and F Rosselli for their helpful discussions and access to facilities of low oxygen cultures. This work was supported by grants from Electricité de France and Association pour la Recherche sur le Cancer (ARC3900 to F Boussin).
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V (2004) NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol 165: 575–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA (2004) For the long run: maintaining germinal niches in the adult brain. Neuron 41: 683–686 [DOI] [PubMed] [Google Scholar]
- Balta G, de Winter JP, Kayserili H, Pronk JC, Joenje H (2000) Fanconi anemia A due to a novel frameshift mutation in hotspot motifs: lack of FANCA protein. Hum Mutat 15: 578. [DOI] [PubMed] [Google Scholar]
- Bogliolo M, Lyakhovich A, Callen E, Castella M, Cappelli E, Ramirez MJ, Creus A, Marcos R, Kalb R, Neveling K, Schindler D, Surralles J (2007) Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. EMBO J 26: 1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Shin-ya K, Wan R, Tang SC, Miura T, Tang H, Khatri R, Gleichman M, Ouyang X, Liu D, Park HR, Chiang JY, Mattson MP (2007) Telomere protection mechanisms change during neurogenesis and neuronal maturation: newly generated neurons are hypersensitive to telomere and DNA damage. J Neurosci 27: 3722–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NC, van de Vrugt HJ, van der Valk MA, Oostra AB, Krimpenfort P, de Vries Y, Joenje H, Berns A, Arwert F (2000) Mice with a targeted disruption of the Fanconi anemia homolog Fanca. Hum Mol Genet 9: 1805–1811 [DOI] [PubMed] [Google Scholar]
- D'Andrea AD, Grompe M (2003) The Fanconi anaemia/BRCA pathway. Nat Rev Cancer 3: 23–34 [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R (2003) NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24: 476–488 [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703–716 [DOI] [PubMed] [Google Scholar]
- Downs JA, Nussenzweig MC, Nussenzweig A (2007) Chromatin dynamics and the preservation of genetic information. Nature 447: 951–958 [DOI] [PubMed] [Google Scholar]
- Faivre L, Guardiola P, Lewis C, Dokal I, Ebell W, Zatterale A, Altay C, Poole J, Stones D, Kwee ML, van Weel-Sipman M, Havenga C, Morgan N, de Winter J, Digweed M, Savoia A, Pronk J, de Ravel T, Jansen S, Joenje H et al. (2000) Association of complementation group and mutation type with clinical outcome in Fanconi anemia. European Fanconi Anemia Research Group. Blood 96: 4064–4070 [PubMed] [Google Scholar]
- Franco S, van de Vrugt HJ, Fernandez P, Aracil M, Arwert F, Blasco MA (2004) Telomere dynamics in Fancg-deficient mouse and human cells. Blood 104: 3927–3935 [DOI] [PubMed] [Google Scholar]
- Frappart PO, Lee Y, Lamont J, McKinnon PJ (2007) BRCA2 is required for neurogenesis and suppression of medulloblastoma. EMBO J 26: 2732–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Sun Y, Frank KM, Dikkes P, Fujiwara Y, Seidl KJ, Sekiguchi JM, Rathbun GA, Swat W, Wang J, Bronson RT, Malynn BA, Bryans M, Zhu C, Chaudhuri J, Davidson L, Ferrini R, Stamato T, Orkin SH, Greenberg ME et al. (1998) A critical role for DNA end-joining proteins in both lymphogenesis and neurogenesis. Cell 95: 891–902 [DOI] [PubMed] [Google Scholar]
- Garcia-Higuera I, Kuang Y, Denham J, D'Andrea AD (2000) The Fanconi anemia proteins FANCA and FANCG stabilize each other and promote the nuclear accumulation of the Fanconi anemia complex. Blood 96: 3224–3230 [PubMed] [Google Scholar]
- Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D'Andrea AD (2001) Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell 7: 249–262 [DOI] [PubMed] [Google Scholar]
- Gennery AR, Slatter MA, Bhattacharya A, Barge D, Haigh S, O'Driscoll M, Coleman R, Abinun M, Flood TJ, Cant AJ, Jeggo PA (2004) The clinical and biological overlap between Nijmegen Breakage Syndrome and Fanconi anemia. Clin Immunol 113: 214–219 [DOI] [PubMed] [Google Scholar]
- Gozdasoglu S, Cavdar AO, Arcasoy A, Babacan E, Sanal O (1980) Fanconi's aplastic anemia, analysis of 18 cases. Acta Haematol 64: 131–135 [DOI] [PubMed] [Google Scholar]
- Grompe M, van de Vrugt H (2007) The Fanconi family adds a fraternal twin. Dev Cell 12: 661–662 [DOI] [PubMed] [Google Scholar]
- Gurtan AM, Stuckert P, D'Andrea AD (2006) The WD40 repeats of FANCL are required for Fanconi anemia core complex assembly. J Biol Chem 281: 10896–10905 [DOI] [PubMed] [Google Scholar]
- Haneline LS, Gobbett TA, Ramani R, Carreau M, Buchwald M, Yoder MC, Clapp DW (1999) Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood 94: 1–8 [PubMed] [Google Scholar]
- Hans F, Dimitrov S (2001) Histone H3 phosphorylation and cell division. Oncogene 20: 3021–3027 [DOI] [PubMed] [Google Scholar]
- Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, Grompe M (2003) Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev 17: 2021–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joenje H, Oostra AB (1983) Effect of oxygen tension on chromosomal aberrations in Fanconi anaemia. Hum Genet 65: 99–101 [DOI] [PubMed] [Google Scholar]
- Kennedy RD, D'Andrea AD (2005) The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev 19: 2925–2940 [DOI] [PubMed] [Google Scholar]
- Koomen M, Cheng NC, van de Vrugt HJ, Godthelp BC, van der Valk MA, Oostra AB, Zdzienicka MZ, Joenje H, Arwert F (2002) Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice. Hum Mol Genet 11: 273–281 [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7: 179–193 [DOI] [PubMed] [Google Scholar]
- Morshead CM, Craig CG, van der Kooy D (1998) In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development 125: 2251–2261 [DOI] [PubMed] [Google Scholar]
- Mouthon M-A, Fouchet P, Mathieu C, Sii-Felice K, Etienne O, Silva Lages C, Boussin FD (2006) Neural stem cells from mouse forebrain are contained in a population distinct from the ‘side population'. J Neurochem 99: 807–817 [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Lalai AS, Hoeijmakers JH (2005) Fanconi anemia (cross)linked to DNA repair. Cell 123: 1191–1198 [DOI] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA, Cornall RJ (2007) DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447: 686–690 [DOI] [PubMed] [Google Scholar]
- Nowak E, Etienne O, Millet P, Lages CS, Mathieu C, Mouthon MA, Boussin FD (2006) Radiation-induced H2AX phosphorylation and neural precursor apoptosis in the developing brain of mice. Radiat Res 165: 155–164 [DOI] [PubMed] [Google Scholar]
- Offit K, Levran O, Mullaney B, Mah K, Nafa K, Batish SD, Diotti R, Schneider H, Deffenbaugh A, Scholl T, Proud VK, Robson M, Norton L, Ellis N, Hanenberg H, Auerbach AD (2003) Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst 95: 1548–1551 [DOI] [PubMed] [Google Scholar]
- Orii KE, Lee Y, Kondo N, McKinnon PJ (2006) Selective utilization of nonhomologous end-joining and homologous recombination DNA repair pathways during nervous system development. Proc Natl Acad Sci USA 103: 10017–10022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, Wurm M, Batish SD, Lach FP, Yetgin S, Neitzel H, Ariffin H, Tischkowitz M, Mathew CG, Auerbach AD, Rahman N (2007) Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 39: 162–164 [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710 [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858–5868 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725–729 [DOI] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ (2007) Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1: 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeck A, Stone S, Costanzo V, de Graaf B, Reuter T, de Winter J, Wallisch M, Akkari Y, Olson S, Wang W, Joenje H, Christian JL, Lupardus PJ, Cimprich KA, Gautier J, Hoatlin ME (2006) Fanconi anemia proteins are required to prevent accumulation of replication-associated DNA double-strand breaks. Mol Cell Biol 26: 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS Jr (1995) The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci 15: 6046–6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D'Andrea AD (2002) S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood 100: 2414–2420 [DOI] [PubMed] [Google Scholar]
- Temple S (2001) The development of neural stem cells. Nature 414: 112–117 [DOI] [PubMed] [Google Scholar]
- Tischkowitz MD, Hodgson SV (2003) Fanconi anaemia. J Med Genet 40: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D (1997) Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci 17: 7850–7859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Andreassen PR, D'Andrea AD (2004) Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol Cell Biol 24: 5850–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, D'Andrea AD (2004) The interplay of Fanconi anemia proteins in the DNA damage response. DNA Rep (Amst) 3: 1063–1069 [DOI] [PubMed] [Google Scholar]
- Wong JC, Alon N, McKerlie C, Huang JR, Meyn MS, Buchwald M (2003) Targeted disruption of exons 1 to 6 of the Fanconi anemia group A gene leads to growth retardation, strain-specific microphthalmia, meiotic defects and primordial germ cell hypoplasia. Hum Mol Genet 12: 2063–2076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1
Supplementary Figures 2
Supplementary Figures 3
Supplementary Figures 4
Supplementary Figures 5
Supplementary Figures 6
Supplementary Figures Legends