Abstract
Neuroglobin (Ngb) is a novel vertebrate globin expressed principally in neurons. Ngb expression is induced by hypoxia and ischemia, and Ngb protects neurons against these insults. The mechanism of Ngb's protective action is unknown, but its ability to bind NO suggests that NO scavenging might be involved. To test this hypothesis, we treated wild type and Ngb-transfected HN33 (mouse hippocampal neuron × N18TG2 neuroblastoma) cells with NO donors and compared their sensitivity to NO-induced cell death. Ngb overexpression shifted concentration-toxicity curves to the right, indicating reduced susceptibility to NO or is metabolites. The results suggest that the ability of Ngb to neutralize the neurotoxic effects of reactive nitrogen species may be an important contributor to its neuroprotective properties.
Keywords: Neuroglobin, nitric oxide, hypoxia
Neuroglobin (Ngb) is a monomeric globin that is distantly related to hemoglobin and myoglobin and is expressed predominantly in brain neurons [3]. Ngb expression is induced by neuronal hypoxia and cerebral ischemia [21] and Ngb, in turn, appears to modulate hypoxic-ischemic brain injury. Thus, enhancing Ngb expression reduces—and knocking down Ngb expression increases—hypoxic neuronal injury in vitro [21] and ischemic cerebral injury in vivo [22]. In addition, Ngb-overexpressing transgenic mice are resistant to cerebral infarction from occlusion of the middle cerebral artery [10, 11]. Ngb also reduces the toxicity of H2O2 [5], paraquat [5] and β-amyloid [13] in vitro. However, the mechanisms that underlie neuroprotection by Ngb are unknown.
Ancestral globins served primarily as NO oxygenases, converting NO to nitrate, whereas their O2-transporting function evolved later [20]. This primordial oxygenase activity is still apparent in the capacity of hemoglobin [6] and myoglobin [4] to scavenge NO, and appears to have pathophysiological significance, since overexpression of inducible NO synthase (NOS) causes heart failure in myoglobin-knockout mice [7]. Because Ngb binds NO [23], its neuroprotective action may also relate to NO scavenging [1, 8].
Several lines of evidence point to a connection between Ngb and NO. In addition to the ability of Ngb to bind NO [23], some studies have shown parallelism between the distribution of Ngb and of neuronal NOS (nNOS) [15], and NOS expression is increased in several tissues of Ngb-overexpressing transgenic mice [11]. Autooxidation of Ngb yields NgbO2, which is thought to react rapidly with NO to form a peroxynitrite (ONOO−)-bound intermediate that decays to yield Ngb and (nontoxic) nitrate [1]. An alternative scheme, in which Ngb reacts directly with NO, has also been proposed [9].
To explore the possible role of NO scavenging in neuroprotection by Ngb, we examined the toxicity of NO and OONO− donors in wild type and Ngb-overexpressing HN33 (mouse hippocampal neuron × N18TG2 neuroblastoma) cells. HN33 cells, provided originally by Dr. Bruce Wainer and grown subsequently in our laboratory, were plated at 1 × 105 cells/well on uncoated, 24-well plastic dishes and maintained as described [21]. Full-length mouse Ngb cDNA was cloned into a pcDNA 3.1 plasmid with CMV promoter (Clontech) [21]. The recombinant plasmid (pcDNA-Ngb) or vector alone (pcDNA) was transfected into HN33 cells for 48 h using FuGENE 6 (Roche), followed by screening with G418 (Life Technology), and overexpression of Ngb was confirmed by Western blot [21].
S-nitroso-N-acetyl-DL-penicillamine (SNAP), diethylenetriamine NONOate (DETA/NONOate), and sydnonimine (SIN-1) were purchased from Sigma (St. Louis, MO, USA). Cell viability was measured by treating cultures for 24 h with or without these drugs for 24 h, then incubating cultures with 5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) at 37°C for 2 h and measuring absorbance at 570 nm in solubilized cells using a Cytofluor Series 4000 multi-well plate-reader (PerSeptive Biosystems) [21].
If Ngb inhibits hypoxic [21], ischemic [22] and possibly other forms of neuronal death by blocking NO toxicity, it should also protect neurons from the direct toxic effects of NO donors. To test this hypothesis, wild type or Ngb-transfected HN33 cultures were treated with NO donors and cell death was measured 24 hr later. Increased expression of Ngb protein in these Ngb-transfected HN33 cultures has been documented previously [21].
Because NO donors differ with respect to chemical structure, bioactive breakdown products (ferrocyanide, amines), the predominant NO species they release (NO, NO+, NO− or ONOO−), and the rate at which they release it [16, 17, 24], we used three different donors in these experiments: the S-nitrosothiol NO donor, S-nitroso-N-acetyl-DL-penicillamine (SNAP); the NONOate NO donor, diethylenetriamine NONOate (DETA-NONOate); and the 3-morpholinosydnonimine ONOO− donor, sydnonimine (SIN-1). Donors were screened at a range of concentrations found previously to be toxic in cortical neuron cultures [14]. Notwithstanding that the half-lives for liberation of NO or ONOO- by the various donors differed, MTT assays were conducted at 24 hr in all cases, to detect both acute and delayed cell death.
As shown in Figure 1, all three NO donors killed >80% of wild type HN33 cells at maximally effective concentrations (∼3 mM). However, in Ngb-transfected cells, the concentration-response curves describing NO donor toxicity were shifted to the right, without change in their maximal effects, indicating that Ngb reduced the potency with which NO donors caused cell death.
Fig 1.
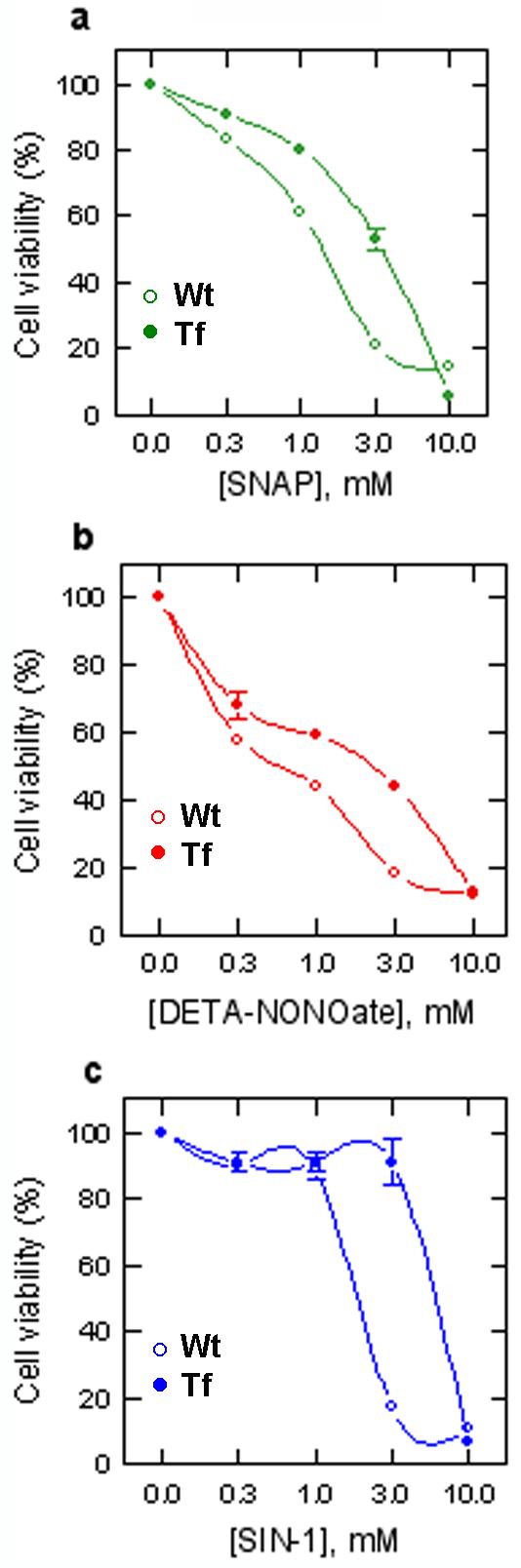
Effect of Ngb overexpression on NO toxicity in HN33 cells. Cells were not (Wt) or were (Tf) transfected with Ngb and exposed to NO donors for 24 hr at the indicated concentrations (mM). Percent viability was measured using MTT. Data shown are from a triplicate experiment repeated 3 times with similar results.
Although Ngb binds O2, enhanced O2 delivery is considered unlikely to explain its protective effect against hypoxic or ischemic injury, because its intraneuronal concentration (∼1 μM) is too low to account for appreciable changes in intracellular O2 levels [2]. In addition, Ngb appears to reduce neuronal death from other insults besides hypoxia or ischemia [5, 13]. Consequently, alternative protective mechanisms have been proposed, including scavenging of reactive oxygen or nitrogen species [1] and promoting the dissociation of G-protein βγ subunits [26] that activate antiapoptotic pathways [19]. In addition, studies of protein-protein interactions have revealed Ngb binding partners, providing further clues to its mode of action. For example, Ngb co-immunoprecipitates with the raft-associated membrane protein flotillin-1 [25], suggesting a possible interaction with raft-associated membrane complexes that regulate cell death.
In our original in vitro study of Ngb neuroprotection, we did not find an effect of Ngb overexpression on toxicity of the NO donor, sodium nitroprusside (SNP) [21]. However, only a single concentration of SNP was used, and SNP releases not only NO, but also ferrocyanide, which may have toxic effects of its own [12]. In the present study, we studied several chemically distinct NO donors at several concentrations, which revealed a protective action of Ngb against NO toxicity.
Interactions between Ngb and NO may result not only in NO scavenging, but also in nitrosative modification of proteins, including Ngb itself [18], which could alter the activity of these target proteins and trigger additional, downstream neuroprotective pathways. H2O2, against which Ngb is also protective [5], can likewise induce modifications in Ngb and other proteins, notably involving cysteine oxidation. Such reactions might ultimately alter the sensitivity of neurons to hypoxia, ischemia or related insults.
Acknowledgments
Supported by NIH grant NS35965 and the Buck Institute for Age Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunori M, Giuffre A, Nienhaus K, Nienhaus GU, Scandurra FM, Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc Natl Acad Sci U S A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunori M, Vallone B. Neuroglobin, seven years after. Cell Mol Life Sci. 2007;64:1259–1268. doi: 10.1007/s00018-007-7090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 4.Flogel U, Merx MW, Godecke A, Decking UK, Schrader J. Myoglobin: A scavenger of bioactive NO. Proc Natl Acad Sci U S A. 2001;98:735–740. doi: 10.1073/pnas.011460298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fordel E, Thijs L, Martinet W, Lenjou M, Laufs T, Van Bockstaele D, Moens L, Dewilde S. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci Lett. 2006;410:146–151. doi: 10.1016/j.neulet.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Gardner PR. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J Inorg Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Godecke A, Molojavyi A, Heger J, Flogel U, Ding Z, Jacoby C, Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem. 2003;278:21761–21766. doi: 10.1074/jbc.M302573200. [DOI] [PubMed] [Google Scholar]
- 8.Herold S, Fago A. Reactions of peroxynitrite with globin proteins and their possible physiological role. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:124–129. doi: 10.1016/j.cbpb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- 10.Khan AA, Sun Y, Jin K, Mao XO, Chen S, Ellerby LM, Greenberg DA. A neuroglobin-overexpressing transgenic mouse. Gene. 2007;398:172–176. doi: 10.1016/j.gene.2007.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiedrowski L, Manev H, Costa E, Wroblewski JT. Inhibition of glutamate-induced cell death by sodium nitroprusside is not mediated by nitric oxide. Neuropharmacology. 1991;30:1241–1243. doi: 10.1016/0028-3908(91)90171-7. [DOI] [PubMed] [Google Scholar]
- 13.Li RC, Pouranfar F, Lee SK, Morris MW, Wang Y, Gozal D. Neuroglobin protects PC12 cells against beta-amyloid-induced cell injury. Neurobiol Aging. 2007 June 7; doi: 10.1016/j.neurobiolaging.2007.05.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustig HS, von Brauchitsch KL, Chan J, Greenberg DA. Ethanol and excitotoxicity in cultured cortical neurons: Differential sensitivity of N-methyl-D-aspartate and sodium nitroprusside toxicity. J. Neurochem. 1992;59:2193–2200. doi: 10.1111/j.1471-4159.1992.tb10111.x. [DOI] [PubMed] [Google Scholar]
- 15.Mammen PP, Shelton JM, Goetsch SC, Williams SC, Richardson JA, Garry MG, Garry DJ. Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J Histochem Cytochem. 2002;50:1591–1598. doi: 10.1177/002215540205001203. [DOI] [PubMed] [Google Scholar]
- 16.Moncada S, Higgs A, Furchgott R. International Union of Pharmacology Nomenclature in Nitric Oxide Research. Pharmacol Rev. 1997;49:137–142. [PubMed] [Google Scholar]
- 17.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- 18.Nicolis S, Monzani E, Ciaccio C, Ascenzi P, Moens L, Casella L. Does human neuroglobin act only as a scavenger? Reactivity and endogenous modification by nitrite and hydrogen peroxide. Biochem J. 2007 Jun 29; doi: 10.1042/BJ20070372. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwindinger WF, Robishaw JD. Heterotrimeric G-protein bg-dimers in growth and differentiation. Oncogene. 2001;20:1653–1660. doi: 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- 20.Sidell BD, O'Brien KM. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J Exp Biol. 2006;209:1791–1802. doi: 10.1242/jeb.02091. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is upregulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Doorslaer S, Dewilde S, Kiger L, Nistor SV, Goovaerts E, Marden MC, Moens L. Nitric oxide binding properties of neuroglobin: a characterization by EPR and flash photolysis. J Biol Chem. 2003;278:4919–4925. doi: 10.1074/jbc.M210617200. [DOI] [PubMed] [Google Scholar]
- 24.Vidwans AS, Kim S, Coffin DO, Wink DA, Hewett SJ. Analysis of the neuroprotective effects of various nitric oxide donor compounds in murine mixed cortical cell culture. J Neurochem. 1999;72:1843–1852. doi: 10.1046/j.1471-4159.1999.0721843.x. [DOI] [PubMed] [Google Scholar]
- 25.Wakasugi K, Nakano T, Kitatsuji C, Morishima I. Human neuroglobin interacts with flotillin-1, a lipid raft microdomain-associated protein. Biochem Biophys Res Commun. 2004;318:453–460. doi: 10.1016/j.bbrc.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 26.Wakasugi K, Nakano T, Morishima I. Oxidized human neuroglobin acts as a heterotrimeric Galpha protein guanine nucleotide dissociation inhibitor. J Biol Chem. 2003;278:36505–36512. doi: 10.1074/jbc.M305519200. [DOI] [PubMed] [Google Scholar]


