Abstract
Objective
Language skills continue to develop rapidly in children during the school-age years and the “snapshot” view of the neural substrates of language provided by current neuroimaging studies cannot capture the dynamic changes associated with brain development. The aim of this study was to conduct a five-year longitudinal investigation of language development using fMRI in healthy children.
Methods
Thirty subjects enrolled at ages 5, 6, or 7 were examined annually for 5 years using a 3T MRI scanner and a verb generation task. Data analysis was conducted based on a general linear model that was modified to investigate developmental changes while minimizing the potential for missing data.
Results
With increasing age, there is progressive participation in language processing by the inferior/middle frontal, middle temporal, and angular gyri of the left hemisphere and the lingual and inferior temporal gyri of the right hemisphere and regression of participation of the left posterior insula/extrastriate cortex, left superior frontal and right anterior cingulate gyri, and left thalamus.
Conclusion
The age-related changes observed in this study provide evidence of increased neuroplasticity of language in this age group and may have implications for further investigations of normal and aberrant language development.
Keywords: longitudinal language development, language, fMRI, child, verb generation, brain, development, functional MRI
Introduction
The human brain is a dynamic organ, undergoing remarkable changes throughout the lifespan. These changes are coincident with and support the acquisition of cognitive abilities. Among the most uniquely human of these cognitive abilities may be the capacity to communicate through a complex linguistic system. The scientific study of the brain basis of human language is now centuries old.1,2 However, study of the adult brain has predominated this work initially because investigators relied on “accidents of nature” such as stroke or injury to infer the function of specific brain regions. The laws of probability and aging favor these events occurring at a much higher rate among adults. Consequently, classical models of brain-language relations have reflected static systems with language fully developed prior to insult. These models initially emphasized regions supporting language that were localized within the left hemisphere (i.e., Broca’s area, Wernicke’s area). Later, the conceptualization was broadened to include the idea of left hemisphere networks that supported language.3–7 More recent neuroimaging work suggests that language is lateralized to rather than localized within the left hemisphere with bilaterally distributed networks contributing to language function in a task-specific manner that may differ in degree of lateralization.8 As with earlier studies, the vast majority of language studies have been done with adult subjects whose language systems are mature.
For centuries the question of how language becomes represented by the brain has stimulated neuroscientists. The initial description of a gross anatomical asymmetry of the planum temporale (included within Wernicke’s area on the left) seemed to suggest that there was an anatomical bias towards language lateralization to the left hemisphere.9 Subsequent work described asymmetries in frontal language areas as well.10,11 Support for the notion that these asymmetries supported the eventual lateralization of language came from imaging studies that tied structural asymmetries in both the inferior frontal gyrus and planum temporale to language dominance as measured by WADA testing.12,13
Brain asymmetries linked to language functions first arise during the course of prenatal brain development.14,15 Brain development, including changes in cortical thickness, white matter volumes and the volume of subcortical structures occur throughout childhood.16 Maturational changes in white matter myelination can be detected across ages during childhood as well.17–19 It is likely that brain maturation and the development of behavioral skills are an interactive and synergistic process. However, the relation of behavioral skills and brain structure may be more pronounced at earlier than later ages in childhood.20 This suggests that the early bias conferred by brain structure may be diminished as children become more proficient at language-based skills.
Changes in cerebral volumes throughout child development and improved functional connectivity are thought to be linked to development of the white matter tracts and may be realized by way of selective regression, although others put forward two opposite theories.21,22 Today, it is known that both regression and progression contribute to brain development during childhood and beyond. As children mature, there is progressive loss of synaptic connections and decreased connectivity in certain brain areas as measured by microscopic and neuroimaging studies.18,23,24 Functional MRI studies also provide evidence for the second theory: as children mature, there is evidence of either increase or change in BOLD signal in frontal and other areas of the brain.25–29 Positive changes in brain connectivity are also observed.18,30
The anatomical data suggest that early brain development may lead to an underlying architecture that preferentially supports language within the left hemisphere. However, later aspects of development may shape the system so that distributed networks are used with increasing efficiency. The protracted period of development of the frontal lobe17,18,31,32 may make the functions supported by this region particularly dynamic through the course of childhood. White matter changes within the temporal and parietal lobes may likewise affect contributions of posterior regions during development.18 Finally, physiologic changes may reflect either increases or decreases in the contribution of specific regions if the strength of connections between components of the language network increase or decrease with age through progressive or regressive events.
Anatomical and pathological studies of the brain in health and disease inform us about the morphological changes that the brain undergoes during development and about the pathological processes that impede or affect such development. These studies, though, allow us only to evaluate the correlation between anatomy or pathology and function by inference. Direct evidence for involvement of certain brain areas in cognitive functions is missing from these studies. Until recently, there have been limited means of examining the dynamics of the normally developing brain as it acquires language skills. Functional MRI has opened new avenues for examination of language development even in infants.33 Studies utilizing fMRI have explored the changes in cortical substrates supporting language during the normal course of child development.26–28,34–37 Although language development begins much earlier than many children can cooperate with the demands of fMRI, we have successfully performed fMRI studies of language in children as young as 5 years of age.34,37,38
Despite the promise of fMRI for studying language development, the existing literature suffers from a number of significant limitations. First, the number of validated studies in children is low relative to the number of and breadth of studies available in adults. With few exceptions,26,28,34,37,39–41 the majority of pediatric studies have included relatively small sample sizes over relatively restricted age ranges within childhood, and have very few children represented at any one age within the range studied. All of the available studies including our own work35,37 have inferred developmental trends from cross-sectional data. Cross sectional data, relative to longitudinal data, is not as sensitive to small magnitude changes and can misrepresent processes of organization that occur within the individual.42
In this study, we sought to overcome these limitations by studying longitudinally a cohort of normally developing children throughout the course of language emergence during the early school years. We employed a verb-generation task for study of language development beginning at the age of 5 when many children begin kindergarten in the USA. The current report follows language development with the verb generation task in these children over the course of 5 years. This task was selected for multiple reasons. First, it taps semantic and lexical skills, which have a protracted period of development relative to other aspects of language. Verb generation, and other verbal fluency tasks, have been widely used in studies of both children and adults who have undergone either PET or fMRI studies and results have been replicable across studies.27,43–51 These studies consistently describe a left lateralized network that includes both Broca’s and Wernicke’s areas as well as additional regions of activation including dorsolateral prefrontal regions, middle temporal cortex, parietal and cingulate cortices. Previous studies of children and adults have shown that the overall lateralization of this task shifts across age with age accounting for 7.5 to 34% of the variability in language lateralization.35,37 Neuroimaging studies using language tasks other than the verb generation used here have found similar trends with age accounting for 5 to 24% of variability in language lateralization.52,53 The task has demonstrated effectiveness for examining changes in brain language organization over time. However, changes in the neural networks supporting the verb generation task in individual children as they mature have not been investigated. Finally, the pattern of activation for this task shows minimal variation related to sex differences,40 which reduces factors as possible confounds to interpretation.
The purpose of this study is to examine maturational changes in brain systems that support a semantic/lexical language task. By using a within-subject methodology with children between the ages of 5 and 11 years, we sought to characterize changes that have been suggested by anatomical studies of brain maturation. In addition, we will present an analysis approach to longitudinal fMRI data that accommodates the challenges of repeatedly scanning young children who are performing a cognitive task.
Materials and Methods
Subjects
Functional MRI and behavioral data was successfully collected from 30 children (16 F, 14 M). The children were either 5, 6, or 7 years of age when they began the study. Twenty-six of them were right-handed, one was ambidextrous, and three were left-handed as determined by the Edinburgh Handedness Inventory (EHI).54 The children were followed longitudinally over a period of five years, being scanned once per year. Many of the children began the study in the summer of 2000 and were scanned five times; a subset of children who began the study later was scanned between 2 and 4 times (Table 1).
Table 1.
Breakdown of total number of visits, age at the first visit, number of visits where the verb generation paradigm was successfully administered, number of frames with acceptable amounts of subject motion for each paradigm run, and number of acceptable frames available per subject.
| Total Visits | Age at the first visit | Visits with Verb Paradigm | Visits with Verb Paradigm | Number of Acceptable Frames | |||||
|---|---|---|---|---|---|---|---|---|---|
| 05F003* | 5 | 5 | 3 | 17 | 3 | 4 | 24 | ||
| 05F004 | 5 | 5 | 4 | 94 | 99 | 19 | 33 | 245 | |
| 05F008 | 2 | 5 | 2 | 81 | 51 | 132 | |||
| 05M002 | 5 | 5 | 5 | 4 | 5 | 21 | 13 | 23 | 66 |
| 05M005 | 4 | 5 | 2 | 80 | 60 | 140 | |||
| 05M008 | 3 | 5 | 3 | 91 | 60 | 99 | 250 | ||
| 05M019 | 3 | 5 | 3 | 39 | 27 | 21 | 87 | ||
| 05M024 | 3 | 5 | 3 | 31 | 10 | 18 | 59 | ||
| 06F001 | 5 | 6 | 5 | 52 | 100 | 38 | 42 | 21 | 253 |
| 06F011 | 3 | 6 | 3 | 48 | 18 | 37 | 103 | ||
| 06F014 | 3 | 6 | 3 | 86 | 9 | 89 | 184 | ||
| 06F018 | 3 | 6 | 3 | 21 | 21 | 31 | 73 | ||
| 06M001 | 5 | 6 | 4 | 74 | 83 | 38 | 38 | 233 | |
| 06M005 | 5 | 6 | 5 | 73 | 99 | 52 | 14 | 58 | 296 |
| 06M012 | 3 | 6 | 3 | 31 | 72 | 36 | 139 | ||
| 07F001 | 4 | 7 | 3 | 100 | 26 | 41 | 167 | ||
| 07F002 | 4 | 7 | 3 | 67 | 9 | 70 | 146 | ||
| 07F005 | 5 | 7 | 5 | 48 | 71 | 99 | 26 | 99 | 343 |
| 07F007 | 5 | 7 | 4 | 66 | 78 | 61 | 59 | 264 | |
| 07F009 | 5 | 7 | 5 | 14 | 24 | 13 | 18 | 12 | 81 |
| 07F010 | 5 | 7 | 5 | 38 | 26 | 100 | 40 | 100 | 304 |
| 07F015 | 5 | 7 | 5 | 66 | 64 | 96 | 35 | 54 | 315 |
| 07F021 | 3 | 7 | 3 | 97 | 18 | 91 | 206 | ||
| 07F024 | 3 | 7 | 3 | 24 | 15 | 24 | 63 | ||
| 07M001 | 5 | 7 | 5 | 100 | 99 | 45 | 59 | 19 | 322 |
| 07M004 | 5 | 7 | 5 | 87 | 99 | 29 | 30 | 48 | 293 |
| 07M005 | 5 | 7 | 4 | 22 | 24 | 23 | 3 | 72 | |
| 07M006 | 5 | 7 | 5 | 46 | 29 | 13 | 12 | 27 | 127 |
| 07M009 | 5 | 7 | 3 | 15 | 10 | 38 | 63 | ||
| 07M012 | 4 | 7 | 4 | 58 | 64 | 41 | 100 | 263 | |
data on this subject not included in the analysis
To participate in this study, children had to be healthy, native English speakers, and have normal neurological examinations. Subjects with significant medical (e.g., diabetes) or any neurological co-morbidities (e.g., migraine or head trauma) were excluded. Subjects were recruited from a larger cohort of children who were enrolled in a cross-sectional study of normal language development (RO1 HD38578). All subjects signed informed consent approved by the Institutional Review Board of the Cincinnati Children’s Hospital Medical Center; assent was obtained from children and consent from their parents.
Behavioral Testing
Children were administered a battery of norm-referenced tests in order to determine their general level of language and cognitive functioning. The Oral and Written Language Scales (OWLS) was individually administered at the start of the study and again at years 3 and 5. This test measures semantic, morphosyntactic and pragmatic features of language in the receptive and expressive domains.55 Administration of the OWLS requires on average 30–60 minutes and is done within one session. Construct validity of all scales in children 5–11 years old is moderate (0.3 to 0.73) and may depend on the level of instruction received during their educational experiences. Internal reliability of the language composite is above 0.9 for each 5–11 years old age group with test-retest reliability in these groups at 0.9. OWLS/language composite results correlate well with other measures of language and cognitive abilities including Peabody Picture Vocabulary Test-Revised (PPVT-R; r = 0.72), Clinical Evaluation of Language Fundamentals-Revised (CELF-R; r ≥ 0.54), Wechsler Intelligence Scale for Children-Third Edition (WISC-III; r ≥ 0.7), and Kaufman Brief Intelligence Test (K-BIT; r ≥ 0.59).55 The mean language composite score on the OWLS in this study was 110 SD ± 13, indicating normal language functioning. A non-significant increase in composite OWLS scores was noted with age (p = 0.18). The average Full-Scale IQ, as determined by the Wechsler Intelligence Scale for Children – III (WISC-III), was 118 ± 14. There was a slight difference in mean Full-Scale IQ between the female (121) and male subjects (115), but it was not statistically significant (p > 0.20, Student’s T-test). Although the IQ of children included in this study appears to be higher than the average IQ of the population, we believe this is related to the Flynn effect and the fact that the children were tested using WISC – III which includes old norms. After re-adjusting to the current norms (WISC – IV) using the conversion factor determined by Flynn, the average IQ in this study would be approximately 115, which is within 1SD of the mean for the population.56,57
FMRI Task Paradigm
The fMRI task was that of silent verb generation, described in detail in our previous publications.35,37 Briefly, a 30 seconds on-off block-design fMRI paradigm was used. During the “active” epochs, the children covertly generated verbs, such as “throw” or “kick”, to binaurally presented nouns such as “ball”. One noun was presented every 5 seconds. The same set of nouns was used during each scanning session. Children were trained to complete this task prior to entering the scanner, with an alternative set of verbs used for this purpose. Active epochs were alternated with “control” epochs. Several challenges were considered in the design of a control epoch for use with young children. First, the control task had to serve its primary purpose as a contrast to the active epochs. Therefore, children needed to stop thinking of words during this time so that BOLD signal associated with verb generation could be measured. However, this type of task compliance (not engaging in an activity for 30 seconds) is difficult for young children. Therefore, it was advantageous to provide an alternative task (in this case, serial finger tapping), which is relatively incompatible with continued engagement in the verbal processes of the active epoch. We know, for example, that finger tapping decreases the ability to generate fluent speech in adult subjects58,59 and that performance on such dual tasks decreases with age.60 Therefore, use of a task that makes continued generation of verbal items difficult is likely to encourage our child subjects to stop this activity when told to do so during the control epochs.
During the control epochs, the subjects tapped their fingers (sequentially on both hands) in response to a target tone presented binaurally every 5 seconds (FM tones centered on 400Hz with 25% modulation). These tones also provided a control for cortical activation associated with sublexical auditory processing.
The use of a finger tapping control task also provided a validity check because the robust pattern of activation associated with finger-tapping has been well described. Therefore, if our verb generation paradigm had produced a pattern divergent from that previously described for adults, a typical finger tapping pattern might assure us that results were due to the age of our participants rather than spurious factors. This seemed an appropriate caution at the time the study was designed, which predated most fMRI studies of children. Subsequently, we have shown that neither the distribution nor the BOLD signal levels change significantly in sensory/motor areas associated with the finger tapping task over the age range of 5–18 years.29 This provides additional confidence that age-related changes in language-related activation can indeed be attributed to the neurocognitive process supporting language rather than global cerebro-vascular changes that may influence the BOLD effect as a function of age.
The finger tapping control task also provided us with a gross assessment of task performance by the subjects. Subjects were monitored via closed circuit television to insure that they engaged in the finger tapping exercise during appropriate intervals of the paradigm. This performance assessment is critical to insure the participation of young children in an otherwise passive language paradigm.
In typical fMRI verb-generation paradigms, participants are asked to signal when they have thought of a verb via button press. This is of questionable validity in very young children, who may press a button because they know this is expected, and not because they actually generated a verb. The finger-tapping control task effectively prevented use of a button response as well. To assess task compliance, we have previously administered a post-scan recall quiz to selected pediatric subjects entering the study to test their attention to the fMRI verb generation task (N = 51). This quiz consists of testing the recall of nouns given during the active part of the task. The correct recall ranged on average from 82–88%, which is well above chance. There were no significant differences between younger and older children in noun recall (p = 0.18); the recall accuracy was 82% for children 7–8 years old and of 88% for children 10–18 years old.61
FMRI Data Acquisition
EPI-fMRI scan parameters were: TR = 3000 ms, TE = 38 ms, BW = 125 kHz, matrix = 64 × 64, FOV = 25.6 × 25.6 cm, slice thickness = 5 mm, 24 slices acquired covering the whole brain. 110 time frames were acquired, for a total scan time of 5’30”; the first 10 frames were discarded to allow the spins to reach relaxation equilibrium. In addition to the fMRI data, a multi-echo reference scan was collected for geometric distortion correction as well as a whole-brain T1-weighted scan for anatomical co-registration.62
A high-resolution T1-weighted 3-D anatomical scan was obtained using a modified driven equilibrium Fourier transform (MDEFT) protocol: TR = 15 ms, TI = 550ms, TE = 4.3 ms, FOV = 25.6×19.2×16.2, flip angle = 20° to provide images for anatomical localization of the activation maps.63,64 This acquisition took approximately 9 minutes and yielded spatial resolution of 1×1.5×1.5 mm with sufficient signal to noise ratio and contrast between grey and white matter for manual and semi-automated segmentation of regional brain volumes.24 Twenty-four fMRI scan planes were extracted from this 3D anatomical data set by interpolation for use as an anatomical underlay for activation maps.
FMRI Data Analysis
The EPI images were corrected for Nyquist ghosts and geometric distortion using the multi-echo reference method.62 The fMRI data was retrospectively corrected for motion using a pyramid co-registration algorithm,65 and then spatially normalized into Talairach space using landmarks found from the whole-brain anatomical images.66
The data post-processing procedure was developed specifically to handle the challenges of longitudinal data from pediatric subjects. The general approach was based on the General Linear Model,67 but was modified for longitudinal analyses and specifically designed to handle the case of missing data. In the pediatric population, despite the good success rates obtained by our laboratory in pediatric neuroimaging, failure rates in children are still larger than in adults due to subject motion and failure to endure an hour of lying awake and motionless in the MRI scanner.38 A partial failure occurs if the subject moves midway through or part of the time during the fMRI scan run; however we still wish to use the frames that are unadulterated by subject motion. A complete failure occurs if the subject either refuses to enter the MRI scanner altogether or fails to tolerate the scan session for a sufficient length of time to acquire the necessary data (data from 3 other fMRI paradigms, in randomized order, were also acquired during the same scanning session, leading to a higher failure rate). In addition, some families may move away from the vicinity partway through the longitudinal experiment. Thus, we developed specialized statistical image analysis methods in order to fully utilize all available data points even when the full complement of longitudinal data is not available.
A cost function was used as the criterion for the presence of unacceptable motion on a frame-by-frame basis. The specific cost function was the normalized r.m.s. deviation from the template, e.g.: where C(n) is the cost function for the nth frame, Si(n) is the MRI signal intensity for the ith voxel in the nth frame, and Ti is the MRI signal intensity for the ith voxel in the template used as the reference for the co-registration (for this analysis the 11th fMRI frame was used as the template). It was found empirically that the cost function provided a more robust indicator of unacceptable subject motion than the gross amount of subject motion (in mm) estimated via the 3-D retrospective analysis.65 The subject’s head could move midway through the 3 seconds acquisition period, resulting in a corrupted acquisition, detected only via the cost function approach. By visual inspection, a threshold for acceptable subject motion was deemed to be C(n) = 0.0002.
In theory, a three-stage mixed-effects model would be the appropriate analysis procedure as there are three sources of variability: within-session, within-subject, and between-subjects.68 However this would be computationally intensive, involving (assuming no missing data points) 30 subjects, 100 time frames, and 5 scanning sessions, resulting in using a 15000-X-15000 matrix at each voxel. Instead, the “summary statistic” two-stage approach typically used for group fMRI analysis was adapted for the longitudinal analysis.69 This method has been shown to produce similar results to solving the full mixed-effects model. For each subject, the spatially normalized data was concatenated across sessions. The natural logarithm of the data was taken since the parameter of interest is the fractional BOLD signal change from baseline; this does not result in significant deviation from normality for EPI data with SNR on the order of 100:1. A voxelwise GLM analysis was then performed with the design matrix constructed to extract parameters corresponding to BOLD signal change increase per year and BOLD signal at 11 years of age (the oldest subject age at study completion). Assuming that data was acquired during visit j with the subject age in years A(j) at each visit, and that the task reference function (shifted by 3 s to account for the hemodynamic delay) is H(t), t being the frame number, the model for the signal intensity S(V,t) for visit V at frame t is:
where B(j) are the baseline log signal intensities for the jth visit, I is the intercept (BOLD signal intensity at 11 years of age), and m is the slope (BOLD signal change increase per year of age). The GLM analysis will thus yield parameters for the intercept and slope of BOLD activation, as well as standard errors on those parameters. Data analysis for negative BOLD signal associations with age was conducted in the same way except that the intercept was set at age = 5 years, to ensure activation at the beginning, not the end, of the age range. In other words, there should be "real" positive activation at the beginning, and these results don't represent increasing deactivations.
To perform a random-effects group analysis on the GLM parameters, the between-subject variance must be estimated. This problem is more involved than in typical fMRI group analyses, since there are different numbers of data points available for each subject, resulting in heteroscedastic data (i.e. different within-subject variances). Thus, it is not possible to directly solve for the between-subject variance in closed form. However, it may be estimated using an Expectation-Maximization Restricted Maximum Likelihood (EM-ReML) approach. The applicability of EM-ReML to longitudinal data, with separate within- and between-subject analyses has been previously shown.70 The EM algorithm alternates between estimating the second level parameters (e.g. average intercept or slope) and the random-effects variance. For this analysis, the EM-ReML algorithm was simplified by approximating the shape of the GLM parameter T-distributions as Gaussian, as there are a large number of degrees of freedom available for each subject. The EM-ReML algorithm was used on a voxelwise basis to generate T-maps (then converted to Z-score maps) of average slope and average intensity. The Z-score maps were then spatially filtered using a Gaussian kernel of width 6 mm. For the slope of BOLD contrast per year of subject age, analysis was restricted to those voxels with Z > 9 for the intercept, ensuring positive BOLD contrast at 11 years of age (so as not to include regions with decreasing deactivation with age). After computing the Z-score maps for the slope, a previously published Monte Carlo method was used to estimate a corrected p-value using the fit residuals as an estimate of the intrinsic spatial autocorrelation. Subsequently, we used the threshold of Z > 6 and spatial extent threshold of 10 voxels which is significant at a corrected p < 0.05.71 Our method accounts for all sources of variability, including between-session (which can be substantial in fMRI data) and between-subject variability.
Results
Despite the partial failures due to subject motion, and complete failures due to noncompliance or the family moving away from the area, usable data was obtained on 30 out of the 34 subjects enrolled. The total number of data points potentially available for a subject is 500 (5 visits × 100 EPI time frames per visit). A substantial number of data points (i.e. frames not corrupted by motion) combined from all visits was available from most subjects (Table 1); 20 out of the 30 subjects had at least 100 data points, while 29 of the 30 had at least 50 data points. However, the number of usable points was much less than the theoretical maximum available (200 to 500, depending on date of study enrollment). Moreover, the number of total data points available for each subject varied widely across subjects, indicating the usefulness of a flexible data analysis technique able to handle the case of missing data. Salvaging all usable data is of special importance in a pediatric longitudinal study, where the failure rate is higher than in adult fMRI studies. With conventional processing methods, the presence of motion-corrupted frames in a single fMRI scan run would necessitate not only discarding the data from that run but also all other datasets acquired from the subject (as there would be a missing data point for the within-subject longitudinal analysis). A breakdown of subject age at the study entry, total number of visits, number of visits where the verb generation paradigm was successfully administered, and how many frames were available per scan run is given in Table 1. Failure to return for all 5 visits is due to factors such as later enrollment in the study or the family leaving the Greater Cincinnati area.
The average activation pattern associated with the verb generation task utilized in this study is presented in Figure 1. Prior to inclusion in the analysis, we visually inspected the fMRI results obtained on the left-handed and ambidextrous subjects. Their activation pattern did not differ from the activation pattern of the right-handed subjects hence their data was included. The composite activation map of the 29 subjects enrolled in this longitudinal study (Figure 1) is visually and qualitatively similar to the activation pattern seen in a cross-sectional sample of children ages 7–18 examined with the same task and scanner (Figure 1 in Holland et al., 2001, N = 17).35
Figure 1.
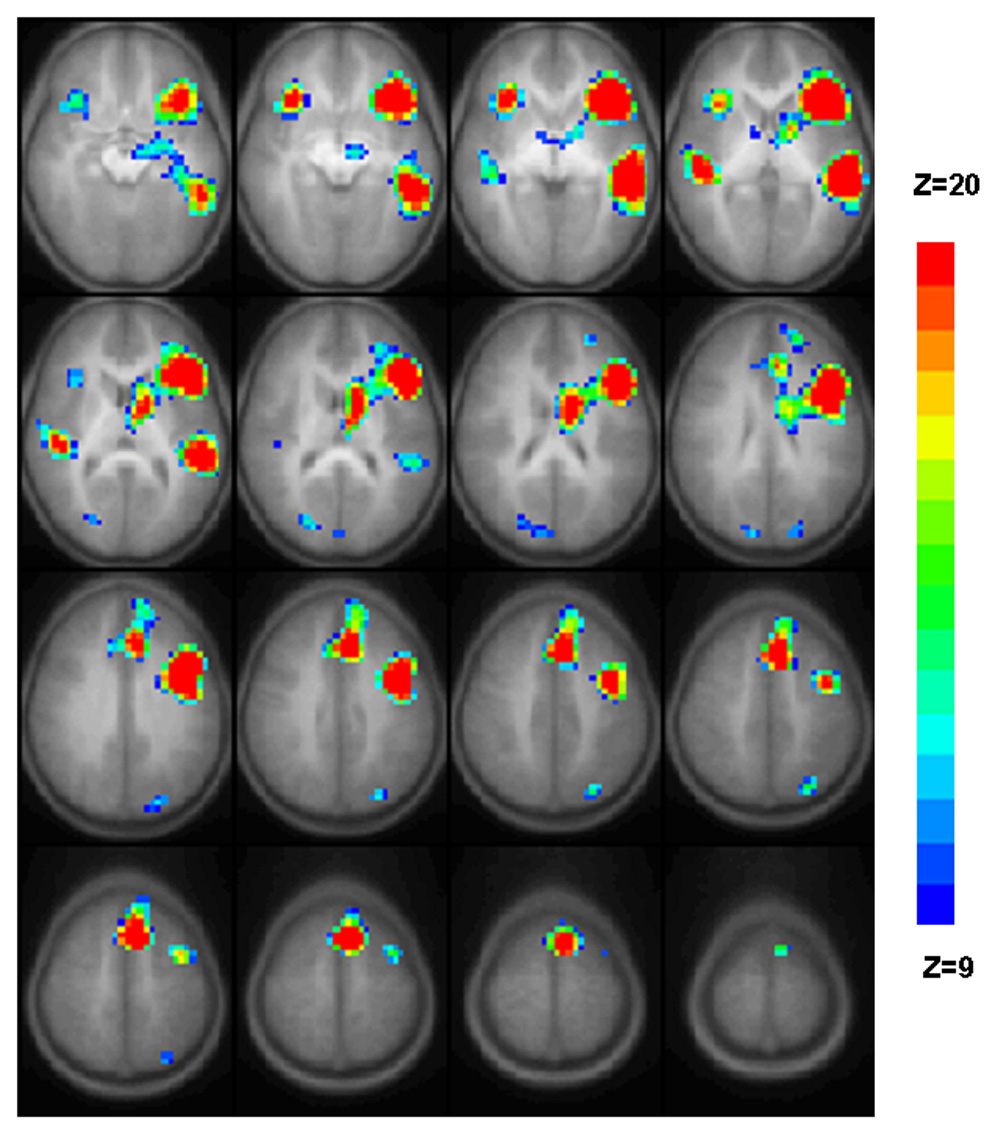
Regions with significant activation in response to the verb generation task for healthy children enrolled in this study. GLM composite map presented in radiological convention (left on the picture corresponds to the right hemisphere) incorporates all 29 subjects and all annual visits for which useable data points were obtained. Activated voxels have nominal Z = 9 (blue) to Z = 20 (red) are superimposed on average T1-weighted image generated from all T1-weighted images from all subjects/all sessions. All regions are significant with p < 0.05 corrected for multiple voxel comparisons. 16 axial slices selected for display (slice range in Talairach coordinates: z = −10 to + 65 mm). For exact location of the BOLD signal changes see Table 2.
Using the data analysis approach detailed above, regions with statistically significant BOLD signal increases (Figure 2A) with age were found in right lingual and inferior temporal gyri, left medial temporal gyrus, left inferior/medial frontal gyrus (Broca’s area), and left angular gyrus. Several cortical and subcortical areas (left posterior insula, left superior frontal gyrus, left thalamus, and right anterior cingulate gyrus) showed decreases in BOLD signal associated with age (Figure 2B). A detailed listing of the Talairach coordinates of the activation foci appearing in Figure 2, and the Brodmann’s areas, are given in Table 2. Further analysis of the fMRI data revealed linear relationship between age and percentage of BOLD increases in the left inferior frontal region (t(5) = 2.64, p < 0.05 double-tailed; Figure 3). The χ² value of the fit (χ²(5) = 7.31; p = 0.2) does not indicate a significant deviation from linearity, despite the outlying point at 10 years of age. The 5 year age span of the current study may be insufficient to detect nonlinear trends, but they may be present when the age range is extended beyond the age of 11.
Figure 2.
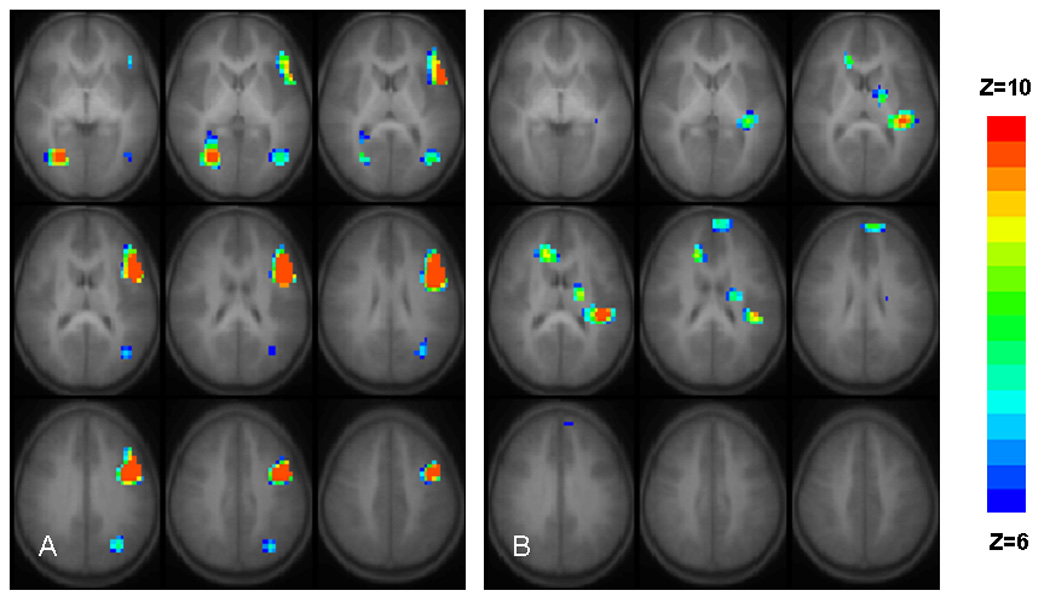
Regions with significant longitudinal positive (A) or negative (B) BOLD signal changes with subject age for 29 normal children performing the task of covert verb generation are presented in radiological convention (left on the picture corresponds to the right hemisphere). Activated voxels have nominal Z = 6 (blue) to Z = 10 (red) are superimposed on average T1 weighted image generated from T1-weighted image generated from all subjects/all sessions. All regions are significant with p < 0.05 corrected for multiple voxel comparisons. Nine axial slices selected for display (slice range: Z = 0 to + 40 mm, Talairach coordinates). For exact location of the BOLD signal changes see Table 2.
Table 2.
| Location | BA | X, Y, Z |
|---|---|---|
| Localization of BOLD signal changes in all healthy subjects (Figure 2) | ||
| R. Inferior Frontal Gyrus | 47 | 30, 23, −5 |
| L. Medial Temporal Gyrus | 21/22 | −50, −33, 5 |
| R. Medial Temporal Gyrus | 21/22 | 42, −29, 5 |
| L. Caudate | −10, −5, 20 | |
| L. Lentiform | −10, −13, −5 | |
| L. Inferior Frontal Gyrus | 44 | −46, 15, 25 |
| R. Medial Occipital Gyrus | 19 | 18, −81, 15 |
| L. Precuneus | 7 | −26, −73, 45 |
| L. Middle Frontal Gyrus | 6 | −6, 11, 50 |
| L. Middle Frontal Gyrus | 8 | −6, 35, 35 |
| Positive BOLD signal changes with age (Figure 3A) | ||
| Right and Left Inferior Temporal Gyrus | 18/19 | −26/26, −61, 0 |
| Left Medial Temporal Gyrus | 39 | −42, −65, 10 |
| Left Inferior/Medial Frontal Gyrus | 44/9 | −42, 3, 30 |
| Left Angular Gyrus | 39 | −30, −65, 30 |
| Negative BOLD signal changes with age (Figure 3B) | ||
| Left Posterior Insula (extrastriate cortex) | −42, −29, 15 | |
| Right Anterior Cingulate Gyrus | 24/32 | 14, 27, 15 |
| Left Superior Frontal Gyrus | 9 | −10, 55, 25 |
| Left Thalamus | −18, −9, 15 | |
Figure 3.
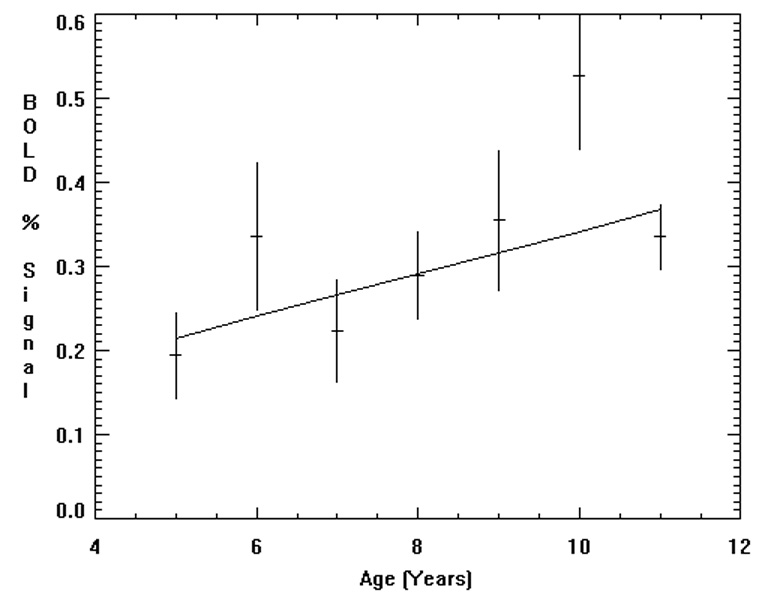
Plot of percentage of BOLD signal change (mean +/− SEM) for subjects in each age group for the left inferior frontal gyrus.
The general increase in BOLD signal with age offers assurance that the use of a finger-tapping control operated as intended during the scan session. Although this type of dual verbal-motor performance is more difficult that doing either activity alone,58–60 it is possible that continued verb generation while subjects are engaged in finger tapping could reduce the difference in BOLD effect between experimental and control epochs. However, we would expect that children would become more proficient in this type of simultaneous verbal-motor output with age,60 such that an age-dependent reduction in the overall BOLD signal would result. This was not the case. Instead, the predominant change in BOLD signal reflected an increase with age, suggesting that the control task was providing an appropriate contrast to verb generation.
Discussion
In this longitudinal fMRI study we examined the anatomical underpinnings of language development in children between the ages of 5 and 11. Our major findings are that the inferior and middle frontal, middle temporal and angular gyri of the left hemisphere and the lingual and inferior temporal gyri of the right hemisphere increase their contribution to semantic/lexical language processes in healthy children while the contribution of the left posterior insula/extrastriate cortex, left superior frontal and right anterior cingulate gyri, and the left thalamus diminishes with age (Figure 2A&B and Table 2). The results suggest that the development of language representation in the brain reflects qualitative rather than simple quantitative changes with age.
In anatomical studies of language, critical regions have shown asymmetries favoring the left hemisphere; these asymmetries have been thought to provide a bias towards left hemisphere language lateralization.9–11 However, there has been some question concerning whether this initial structural bias leads to a functional left lateralization that is stable or changes with time. The verb generation task used in this study is well known to lateralize to the left hemisphere.35,37,49 Cross sectional study of an independent sample of children suggested increasing left lateralization with age.35 The current longitudinal sample suggests that these changes in lateralization are driven by increasing activation with age of regions in the left hemisphere closely associated with language functions.3 This would be consistent with the idea of progressive change in brain development. This, in turn, runs counter to the notion that the early bias conferred by brain structure may be diminished as children become more proficient at language-based skills. Instead, our findings suggest that the development continues to build on the initial left hemisphere bias conferred by anatomical asymmetries such that functional asymmetries become stronger with age. This pattern was strongest for frontal regions (including Broca’s area) and posterior temporal areas (including parts of Wernicke’s area and the angular gyrus). Interestingly, the planum temporale did not show age related change, despite the fact that this area also contributes to the overall pattern of left structural asymmetry of language cortex. This may be because this subregion within the broader Wernicke’s area seems to respond to acoustic and phonetic aspects of words, a minor component in the verb-generation paradigm.
One explanation for increasing activation with age in the left inferior/medial frontal and middle temporal gyri is the inability of the younger and less mature brain to proficiently process language - a function that improves with age. This is probably why one previous study showed that, although the activation patterns for a verbal fluency task (somewhat similar to the verb generation task used in this study) in children and adults are similar, there is a more widespread distribution of the frontal activation in children than in adults.27 This coalescence of BOLD signal changes and increases with age can be explained by an ongoing process of synaptic pruning and a decrease in neuronal/synaptic density that are likely offset by increasing myelination of the white matter tracts that continue through the first decades of life.23 In our sample, the greatest age-related increases occurred in frontal, temporal, and parietal lobes—regions that also show extended periods of maturation.17,18,31,32 Microscopic studies showed that the synaptic pruning and dendritic myelination are delayed in the frontal and prefrontal brain regions in comparison to other brain areas which may also contribute to the changes seen in this study.72
In addition to the regions of increased activation, we also observed areas that decreased in activation with age. Thus, the overall pattern of physiologic change provided support for the notion of both progressive and regressive change in development. However, unlike regions of increasing activation, those that showed a pattern of decreasing activation occurred outside of classic language cortex. It is likely that the decreased activity in the posterior insula/extrastriate cortex (Figure 2B) is related to a decreased recruitment of compensatory brain areas as the use of primary language areas becomes more efficient with age.28 The increases in BOLD signal seen in our study in the left prefrontal and parietal areas may also be related to attention and visuospatial working memory skills that improve with age as similar increases in activation were seen previously in a cross-sectional study of 9–18 year olds performing fMRI working memory task.73 Therefore, as children became more proficient in performing the task with age, they appeared to rely less of additional systems that may mediate language functions. Similar findings have already been seen in cross-sectional developmental language studies using verb generation and other language tasks.26,28 These authors explained their findings as “alternative strategies” used by the immature brain to process lexical information in absence of fully functional inferior frontal lobe.
The other area showing decreased task-related activation is the anterior cingulate. This area of the brain has been implicated in attention required for task performance.49 Therefore, another explanation to the “alternative strategies” for age-related decrease in the anterior cingulate activation could be that with increasing age task performance becomes more automatic and, therefore, requiring less attention. Although pediatric data in support of this hypothesis are not available, data from adult fMRI studies of language support the idea of decreasing with age effectiveness of cognitive control and associate that with a decrease in BOLD signal in the anterior cingulate.74 On the microscopic level, our and their findings can be explained by myelination that increases with age and improved organization of the frontal white matter.18,30
Alternative explanations for the increases and decreases in BOLD signal with age as the children process semantic/lexical information are that either different areas of the brain may be involved in the processing of early vs. late learned words or that words used frequently may be processed by different brain areas than words used infrequently.75,76 It has been long known that words learned early in life are processed differently than words that are learned later in development.77 This fact has been recently confirmed by a fMRI study conducted in 9–18 year old children and adolescents.76 These authors showed that word age of acquisition modulates brain areas that are not affected by word frequency; early learned words led to brain activity changes in bilateral precuneus and left temporal operculum, while later learned words activated mainly left inferior frontal and insular regions (similar to our posterior insula/extrastriate region). The other plausible explanation of the age related changes in brain activation is the effect of word frequency that was examined in another fMRI study.75 This study found increased activation in the left prefrontal region when subjects performed semantic judgment of low- and high-frequency words. While the effects of age-of-acquisition and word frequency on brain activation are obvious, we do not think that the these factors played a major role in the age-related activation changes seen in our study, as the task used by us was designed to be relatively simple and based on concrete nouns found in the lexicon of the average 5 year old.
The covert nature of the verb generation task is a limitation of the study. Even though we found no significant differences in code word recollection between younger and older children outside of the scanner,61 this does not exclude the possibility of performance differences in the scanner. We are therefore unable to reject the alternative hypothesis that our results may, to some extent, represent age-related differences in performance. Although it is not clear whether or to what extent BOLD signal changes are related to the maturity of the language skills and language performance during the task, at least one recent study found no correlation between performance and language lateralization.34 These authors did find a positive correlation between out of scanner performance and number of activated voxels in a manually generated large left frontal ROI that was based on anatomical boundaries and included medial frontal and subcortical gray matter. Therefore, comparison of their findings to ours is difficult as our analysis was not based on ROI but rather on a general linear model modified to investigate developmental changes on a pixelwise basis. The above findings also contradict the findings by Chee et al. of decreasing activation in the left frontal language ROI with increasing language proficiency and increasing right homologue with decreasing language proficiency.78 Since we did not observe stable and age-independent out-of-scanner performance, we do not think that our findings are related to differences in performance or language proficiency.
As expected from our previous studies in a cross-sectional cohort of normally developing children performing an identical verb generation fMRI task, we found increasing with age activation in the left inferior frontal gyrus. The increases found in the posterior temporal and parietal regions were bilateral and restricted to angular gyrus and lingual gyrus rather than the superior temporal gyrus (BA 22) as might have been predicted. Furthermore, unanticipated decreased correlations with age were found in the longitudinal sample in several regions. This new finding can be attributed in part to increased sensitivity to age-related changes in brain activity obtained in this longitudinal study in a relatively large number of normally developing children coupled with new data analysis methods that preserve and fully utilize available data.
Three of our subjects were left-handed, as verified via the Edinburgh Handedness Inventory.54 While it is well-known that atypical language hemispheric dominance is associated with left-handedness in adults, there is less evidence for this in children; we are currently in the process of investigating the magnitude of this effect and its interaction with age (Jacola et al., unpublished data). We constructed a composite map based on only the data from the three left-handed subjects, and the activation in Broca’s area was highly left-hemispheric dominant (data not shown). We also computed LIs based on a previously published method,35 and two out of the three subjects exhibited left-dominance (LI > 0.2), while the third showed bilateral representation. Hence we do not believe that the inclusion of left-handed subjects biased our results.
Finally, the methods used in this study demonstrate that longitudinal studies of childhood development are feasible. In this study, a novel approach to data analysis permitted us to compensate for the difficulty in obtaining movement-free scans from young children. This procedure, paired with a child-adapted task, revealed age-related changes in task performance within the same sample of children over time.
Acknowledgements
This study was presented in part at the International Society for Magnetic Resonance in Medicine Meeting, Miami, FL, USA 5/05. Support was provided by NIH RO1 HD38578.
BIBLIOGRAPHY
- 1.Broca P. Remarques sur le siege de la faculte du langage articule; suivies d'une observation d'aphemie. Bull Soc Anat Paris. 1861;6:398–407. [Google Scholar]
- 2.Wernicke C. The symptom of complex aphasia. In: Church AE, editor. Diseases of the nervous system. New York: Appleton; 1911. pp. 265–324. [Google Scholar]
- 3.Geschwind N. Behavioral change in temporal lobe epilepsy. Arch Neurol. 1977;34:453. doi: 10.1001/archneur.1977.00500200013002. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 1985;42:428–459. doi: 10.1001/archneur.1985.04060050026008. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 6.Lansdell H. Verbal and nonverbal factors in right-hemisphere speech: relation to early neurological history. J Comp Physiol Psychol. 1969;69:734–738. doi: 10.1037/h0028306. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- 8.Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitsky W, Geschwind N. Asymmetries of the right and left hemisphere in man. Trans Am Neurol Assoc. 1968;93:232–233. [PubMed] [Google Scholar]
- 10.Falzi G, Perrone P, Vignolo LA. Right-left asymmetry in anterior speech region. Arch Neurol. 1982;39:239–240. doi: 10.1001/archneur.1982.00510160045009. [DOI] [PubMed] [Google Scholar]
- 11.Albanese E, Merlo A, Albanese A, Gomez E. Anterior speech region. Asymmetry and weight-surface correlation. Arch Neurol. 1989;46:307–310. doi: 10.1001/archneur.1989.00520390073019. [DOI] [PubMed] [Google Scholar]
- 12.Foundas AL, Leonard CM, Gilmore R, et al. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 13.Foundas AL, Leonard CM, Gilmore RL, et al. Pars triangularis asymmetry and language dominance. Proc Natl Acad Sci U S A. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- 15.Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- 16.Durston S, Hulshoff Pol HE, Casey BJ, et al. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Schneider JF, Il'yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- 18.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagawa H, Iwasaki S, Kichikawa K, et al. Normal myelination of anatomic nerve fiber bundles: MR analysis. AJNR Am J Neuroradiol. 1998;19:1129–1136. [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard C, LJ L, Mercado L. Cerebral asymmetry and cognitive development in children: A magnetic resonance imaging study. Psychol Science. 1996;7:89–95. [Google Scholar]
- 21.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 22.Quartz SR, Sejnowski TJ. The neural basis of cognitive development: a constructivist manifesto. Behav Brain Sci. 1997;20:537–556. doi: 10.1017/s0140525x97001581. discussion 556–596. [DOI] [PubMed] [Google Scholar]
- 23.Huttenlocher P. Synaptic density in human frontal cortex - developmental changes and effects of age. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 24.Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth JR, Burman DD, Meyer JR, et al. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 26.Brown TT, Lugar HM, Coalson RS, et al. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- 27.Gaillard WD, Hertz-Pannier L, Mott SH, et al. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- 28.Schlaggar BL, Brown TT, Lugar HM, et al. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- 29.Schapiro MB, Schmithorst VJ, Wilke M, et al. BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klingberg T, Vaidya CJ, Gabrieli JD, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 31.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 32.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 33.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 34.Wood AG, Harvey AS, Wellard RM, et al. Language cortex activation in normal children. Neurology. 2004;63:1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- 35.Holland SK, Plante E, Weber Byars A, et al. Normal fMRI Brain Activation Patterns in Children Performing a Verb Generation Task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 36.Gaillard WD, Sachs BC, Whitnah JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. doi: 10.1002/hbm.20177. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byars AW, Holland SK, Strawsburg RH, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17:885–890. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Booth JR, Burman DD, Meyer JR, et al. Development of brain mechanisms for processing ortographic and phonologic representations. J Cogn Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plante E, Schmithorst V, Holland S, Byars A. Sex differences in the activation of language cortex during childhood. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2005.08.016. in press. [DOI] [PubMed] [Google Scholar]
- 41.Schmithorst V, Holland S, Plante E. Cognitive modules utilized for narrative comprehension in children: A functional Magnetic Resonance Imaging study. NeuroImage. doi: 10.1016/j.neuroimage.2005.07.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellis N, Large B. The early stages of reading: A longitudinal study. Applied Cognitive Psychol. 1988;2:47–76. [Google Scholar]
- 43.Baciu MV, Rubin C, Decorps MA, Segebarth CM. fMRI assessment of hemispheric language dominance using a simple inner speech paradigm. NMR Biomed. 1999;12:293–298. doi: 10.1002/(sici)1099-1492(199908)12:5<293::aid-nbm573>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Benson RR, FitzGerald DB, LeSueur LL, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- 45.Cuenod CA, Bookheimer SY, Hertz-Pannier L, et al. Functional MRI during word generation, using conventional equipment: a potential tool for language localization in the clinical environment. Neurology. 1995;45:1821–1827. doi: 10.1212/wnl.45.10.1821. [DOI] [PubMed] [Google Scholar]
- 46.Hertz-Pannier L, Gaillard WD, Mott SH, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003–1012. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- 47.Lehericy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- 48.Loring DW, Meador KJ, Allison JD, Wright JC. Relationship between motor and language activation using fMRI. Neurology. 2000;54:981–983. doi: 10.1212/wnl.54.4.981. [DOI] [PubMed] [Google Scholar]
- 49.Petersen SE, Fox PT, Posner MI, et al. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 50.Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- 51.Xiong J, Rao S, Gao JH, et al. Evaluation of hemispheric dominance for language using functional MRI: a comparison with positron emission tomography. Hum Brain Mapp. 1998;6:42–58. doi: 10.1002/(SICI)1097-0193(1998)6:1<42::AID-HBM4>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 53.Szaflarski JP, Binder JR, Possing ET, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 54.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 55.Carrow-Woolfolk E. Oral and Written Language Scales. Circle Pines, MN: American Guidance Service, Inc; 1996. p. 241. [Google Scholar]
- 56.Flynn JR. IQ gains over time. In: RJS, editor. Encyclopedia of human intelligence. New York: Macmillan; 1994. pp. 617–623. [Google Scholar]
- 57.Flynn JR. Massive IQ gains in 14 nations: What IQ tests really measure. Psychol Bull. 1987;101:171–191. [Google Scholar]
- 58.Friedman A, Polson MC, Dafoe CG, Gaskill SJ. Dividing attention within and between hemispheres: testing a multiple resources approach to limited-capacity information processing. J Exp Psychol Hum Percept Perform. 1982;8:625–650. doi: 10.1037//0096-1523.8.5.625. [DOI] [PubMed] [Google Scholar]
- 59.Kemper S, Herman RE, Lian CH. The costs of doing two things at once for young and older adults: talking while walking, finger tapping, and ignoring speech or noise. Psychol Aging. 2003;18:181–192. doi: 10.1037/0882-7974.18.2.181. [DOI] [PubMed] [Google Scholar]
- 60.Hiscock M, Kinsbourne M, Samuels M. Effects of speaking upon the rate and variability of concurrent finger tapping in children. J Exp Child Psychology. 1985;40:486–500. [Google Scholar]
- 61.Chiu C, Schmithorst J, Brown R, et al. Making memories: A cross-sectional investigation of episodic memory encoding in children using fMRI. Developmental Neuropsychology. 2005 doi: 10.1207/s15326942dn2902_3. in press. [DOI] [PubMed] [Google Scholar]
- 62.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 64.Duewell S, Wolff SD, Wen H, et al. MR imaging contrast in human brain tissue: assessment and optimization at 4 T. Radiology. 1996;199:780–786. doi: 10.1148/radiology.199.3.8638005. [DOI] [PubMed] [Google Scholar]
- 65.Thevenaz P, Unser M. A pyramid approach to sub-pixel registration based on intensity. IEEE Trans Image Processing. 1998;Vol. 7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- 66.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- 67.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 68.Friston K, Stephan K, Lund T, et al. Mixed-effects and fMRI studies. Neuroimage. 2005;24:244–252. doi: 10.1016/j.neuroimage.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 69.Beckmann C, Jenkinson M, Smith S. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 70.Foulley J, Jaffrezic F, Robert-Granie C. EM-REML estimation of covariance parameters in Gaussian mixed models for longitudinal data analysis. Genet Sel Evol. 2000;32:129–141. doi: 10.1186/1297-9686-32-2-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ledberg A, Akerman S, Roland P. Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage. 1998;8:113–128. doi: 10.1006/nimg.1998.0336. [DOI] [PubMed] [Google Scholar]
- 72.Rivkin MJ. Developmental neuroimaging of children using magnetic resonance techniques. Ment Retard Dev Disabil Res Rev. 2000;6:68–80. doi: 10.1002/(SICI)1098-2779(2000)6:1<68::AID-MRDD9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 73.Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci. 2002;14:1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- 74.Persson J, Sylvester CY, Nelson JK, et al. Selection requirements during verb generation: differential recruitment in older and younger adults. Neuroimage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 75.Chee MW, Hon NH, Caplan D, et al. Frequency of concrete words modulates prefrontal activation during semantic judgments. Neuroimage. 2002;16:259–268. doi: 10.1006/nimg.2002.1061. [DOI] [PubMed] [Google Scholar]
- 76.Fiebach CJ, Friederici AD, Muller K, et al. Distinct brain representations for early and late learned words. Neuroimage. 2003;19:1627–1637. doi: 10.1016/s1053-8119(03)00227-1. [DOI] [PubMed] [Google Scholar]
- 77.Gilhooly K, Logie R. Word age-of-acquisition and lexical decision making. Acta Psychol. 1982;50:21–34. [Google Scholar]
- 78.Chee MW, Hon N, Lee HL, Soon CS. Relative language proficiency modulates BOLD signal change when bilinguals perform semantic judgments. Blood oxygen level dependent. Neuroimage. 2001;13:1155–1163. doi: 10.1006/nimg.2001.0781. [DOI] [PubMed] [Google Scholar]


