Abstract
T-cell receptor (TCR) stimulation is both central to homeostatic maintenance of CD4+ CD25+ regulatory T cells (Treg cells) in vivo and a prerequisite for the initiation of suppression by Treg cells, both in vivo and in vitro. However, TCR-independent stimulation of Treg cells, e.g. with superagonistic CD28-specific monoclonal antibodies (CD28-SA), not only expands these cells in vivo but, as we show here, also mediates large-scale expansion of rat Treg cells in vitro. Interestingly, CD28-SA stimulation plus interleukin (IL)-2 was even superior to conventional costimulation plus IL-2 in promoting Treg cell growth in vitro. Despite their highly activated phenotype suppression by Treg cells expanded in the absence of TCR stimulation remained fully dependent on TCR-triggering for initiation and cell contact was required to exert suppression. With regard to the regulation of suppression by CD28 stimulation we observed that neither the presence of a conventional anti-CD28 monoclonal antibody nor a CD28-SA generally rendered conventional T cells resistant to suppression by preactivated Treg cells. Taken together, we provide a novel protocol for long-term propagation of Treg cells in vitro and our data are the first to reveal a difference in the signals required for activation and expansion of Treg cells and those, involving the TCR, necessary for the initiation of suppression.
Keywords: regulatory T cells (Treg), co-stimulation/costimulatory molecules, anergy/suppression/tolerance, proliferation
Introduction
CD4+ CD25+ regulatory T cells (Treg cells) are key players in the protection against autoimmunity and maintenance of immunologic self-tolerance.1 These ‘naturally occurring’, self-reactive2 Treg cells arise in the thymus under the control of the forkhead-winged transcription factor FoxP3.3–6 Peripheral Treg cells continue to express FoxP3 independent of CD25 expression or other ‘activation markers’, like CD152 or CD69, which are also induced on conventional T cells (Tconv cells) after stimulation in vivo. FoxP3, thus, is the most reliable marker for regulatory T cells in vivo. Reduced Treg cell numbers or functional impairment of Treg cells were found to cause autoimmunity in several animal models,7 indicating that CD4+ CD25+ Treg cells control other autoreactive T cells. Importantly, correlative deficiencies in either Treg cell quality or quantity also characterize many human autoimmune diseases, like multiple sclerosis,8 rheumatoid arthritis9 or type 1 diabetes.10
Factors promoting proliferation, survival and activation of Treg cells in vivo are autoantigen recognition,11,12 triggering of CD28 on Treg cells13,14 and CD28-induced interleukin-2 (IL-2) production by conventional autoreactive CD4+ CD25low T cells.15–17
To study Treg cells, a number of protocols have been established for in vitro culture of these cells using either antigen-pulsed dendritic cells,18 allogeneic antigen-presenting cells (APC)19 or anti-CD3/anti-CD28 monoclonal antibody (mAb)-coated beads and IL-2.20,21 Moreover, consecutive expansion of Treg cells in vitro and adoptive transfer of expanded Treg cells into, for example, non-obese diabetic mice or into recipients of allogeneic T cells in vivo mediated protection from diabetes18,20 or graft-versus-host disease,19 respectively.
We have recently shown that superagonistic anti-CD28 antibodies (CD28-SA) are capable of activating rat regulatory T cells both in vitro22 and in vivo,22,23 and of strongly expanding Treg cells in vivo.22,23 Of clinical significance, in vivo activation of Treg cells by CD28-SA directly translated into protection from experimental autoimmune encephalomyelitis in two independent models.23
In this study we followed up on our previous in vitro data by establishing long-term cultures of rat Treg cells using a CD28 superagonist (CD28-SA) and IL-2. Further, we analysed CD28-SA/IL-2-expanded rat Treg cells both phenotypically based on marker protein expression and functionally in surrogate in vitro suppression assays.
Materials and methods
Animals
Normal Lewis rats and C57Bl/6 mice were bred at the animal facility of the Institute for Virology and Immunobiology, University of Würzburg, and used for experiments between 6 and 12 weeks of age. All experiments were performed according to the Bavarian state regulations for animal experimentation and approved by the responsible authorities.
Purification of CD4+ CD25+ (Treg cells) and CD4+ CD25– T cells (Tconv cells)
Routinely, single-cell suspensions were prepared form inguinal, axillary, cervical, mesenteric and paraortic lymph nodes of normal Lewis rats and T-cell subsets were purified essentially as described.22 In brief, lymph node cells were first depleted of B cells and CD8+ cells prior to separation of CD4+ cells into CD4+ CD25+ and CD4+ CD25– cells using magnetic-activated cell sorting (MACS) beads (MACS®, Miltenyi Biotec, Bergisch Gladbach, Germany) and MACS® separation columns. Cell purities of regulatory CD25+ T cells and conventional CD25– T cells were on average 85% and 95%, respectively.
In vitro expansion of Treg and Tconv cells
Purified Treg and Tconv cells were resuspended to a density of 5 × 104−5 × 105 cells/ml in x-vivo 15 medium™ (Bio Whittaker, Verviers, Belgium) supplemented with 15% heat-inactivated fetal calf serum, 1 mm sodium pyruvate, non-essential amino acids, 100 U/ml penicillin and streptomycin, 30 µm mercaptoethanol and 2 mm l-glutamine (all from Gibco, Gaithersburg, MD) and cultured in flat-bottomed plates coated with sheep anti-mouse-immunoglobulin (0.5 mg/ml in 15 mm Na2CO3/35 mm NaHCO3, pH 9.6). Five µg/ml mAb JJ316 and 300 U/ml recombinant human (rh) IL-2 (Chiron, Amsterdam, The Netherlands) were added in solution to stimulate the T cells. For costimulation, anti-TCR mAb R73 (5 µg/ml) was immobilized on sheep anti-mouse immunoglobulin-coated plates and conventional anti-CD28 mAb JJ319 (0.2 µg/ml) was added in solution. Proliferation was determined by [3H]thymidine incorporation (Amersham Biosciences Europe, Freiburg, Germany) for the last 16 hr of culture. The DNA of [3H]thymidine pulsed cells was harvested onto fibreglass filters and radioactive content quantitated using a β-scintillation counter.
For long-term culture, cells were propagated at densities between 5 × 104 and 2 × 106 cells/ml and restimulated on a weekly basis. Long-term costimulation was performed with soluble anti-TCR and anti-CD28 mAbs in the presence of coated sheep anti-mouse immunoglobulin.
In vitro suppression assays
To test for suppressor function, fresh indicator T cells were cocultured with different numbers of Treg cells. In case of stimulation with concanavalin A (Con A, 2 µg/ml, Sigma-Aldrich, Taufkirchen, Germany), irradiated (20 Gy) lymph node or spleen cells were added as APC. Proliferation was either measured by determining carboxyfluorescein succinimidyl ester diacetate (CFSE) dye dilution (5 µm; MoBiTec GmbH, Göttingen, Germany) among conventional T cells or by measuring [3H]thymidine incorporation during the final 16 hr of a 3-day culturing period. Counts per minute (c.p.m.) are given as means ± SD.
Transwell cultures
Five × 105 CD28-SA-expanded Treg cells were cocultured with 5 × 105 nylon wool non-adherent (NWNA) cells together in the upper well of a transwell chamber (24-well plate with millicell® culture plate insert; Millipore, Bedford, MA) and a further 5 × 105 NWNA cells were cultured in the lower well and stimulated with Con A. After 2 days, cells in both chambers were resuspended and aliquots transferred as triplicates into 96-well round bottom plates before [3H]thymidine was added.24
Co-cultures of mouse lymph node cells and rat Treg cells
CFSE-labelled mouse lymph node cells were stimulated by adding αCD3 mAb alone (clone 145-2C11; Pharmingen) or αCD3 mAb together with αCD28 mAb (clone 37.51; Pharmingen). Co-cultured rat Treg cells were stimulated either with anti-rat TCR mAb alone (R73) or with anti-rat TCR plus anti-rat CD28 (JJ319).
Fluorescence-activated cell sorting (FACS) analysis
The following mAbs were used: anti-rat CD4–CyChrome™ (clone OX35, BD Pharmingen); anti-rat CD25–fluoroscein isothiocyanate (FITC) or –biotin (clone Ox39; Serotec); anti-rat CD152 (cytotoxic T lymphocyte-associated antigen 4, CTLA-4)-biotin (clone WKH203) and anti-mouse FoxP3 (clone FJK-16s, both eBioscience, San Diego, CA).
Staining was performed with up to 1 × 106 cells in 50 µl of phosphate-buffered saline (PBS)/0.1% bovine serum albumin (BSA)/0.02% NaN3. Fc-receptors were blocked by incubation with 20 µg/ml of normal mouse immunoglobulin (Sigma-Aldrich). After the blocking step FITC-, phycoerythrin- and CyChrome™-conjugated or biotinylated mAbs were added (15 min, 4°). Bound biotinylated antibodies were detected by incubation with either CyChrome™ or allophycocyanin-conjugated streptavidin (Pharmingen). The cells were analysed on either a FACScan™ or FACSCalibur™ flow cytometer using Cell Quest™ software (all Becton Dickinson, San Jose, CA). Dot plots and histograms are shown as log10 fluorescence intensities on a four-decade scale.
For intracellular staining of FoxP3 and CD152 cells were fixed for 30 min at room temperature with fixation buffer (eBioscience) prior to permeabilization (permeabilization buffer, eBioscience). The cells were blocked with rat serum before staining with anti-CD152 mAb and anti-FoxP3 mAb for 30 min at room temperature. Specificity of anti-CD152 staining was verified by blockade with 100 µg/ml unconjugated anti-CD152 mAb (WKH203).
Results
Short-term proliferative response of CD4+ CD25+ regulatory T cells after superagonistic anti-CD28 stimulation in vitro
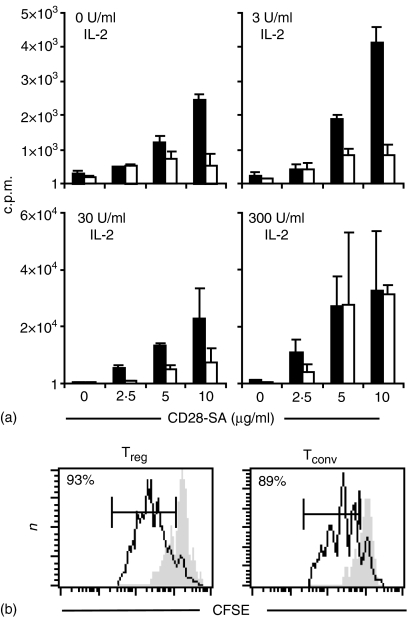
For immunotherapy with in vitro expanded Treg cells large-scale expansion is necessary. Therefore, we tried to optimize culture conditions for the expansion of Treg cells with the CD28-SA JJ316 and IL-2. First, we isolated Treg and Tconv cells form normal Lewis rats and cultured them for 3 days with different dosages of JJ316 in the absence or presence of exogenous IL-2. Under all these conditions the CD28-SA induced significant proliferation of Treg and also Tconv cells as measured by [3H]thymidine incorporation (Fig. 1a). However, Treg cell proliferation clearly was superior to the proliferation of conventional T cells when the cells were stimulated with 10 µg/ml of JJ316 with little or no IL-2-supplementation. Analysis of CFSE dye dilution among CD28-SA-stimulated Treg and Tconv cells revealed that the vast majority, if not all, regulatory and conventional T cells could be induced to proliferate upon superagonistic anti-CD28 stimulation (Fig. 1b). Therefore, superagonistic anti-CD28 stimulation in vitro induces a strong proliferative response in CD4+ CD25+ Treg cells that is clearly superior to that induced in conventional T cells.
Figure 1.
In vitro proliferation of Treg and Tconv cells after stimulation with the CD28-SA mAb JJ316 in the presence of different amounts of rhIL-2. (a)[3H]thymidine incorporation by 104 freshly purified Treg cells (black bars) or Tconv cells (white bars) stimulated for 3 days with the indicated amounts of CD28-SA JJ316 and rhIL-2. The bars indicate means of triplicate cultures ± SD. (b) CFSE-labelled Treg cells (left) and CFSE-labelled Tconv cells (right) were cultured for 3 days together with irradiated splenic APCs in the presence (black line) or absence (grey shadow) of the CD28-SA added in solution.
Long-term in vitro expansion of Treg cells
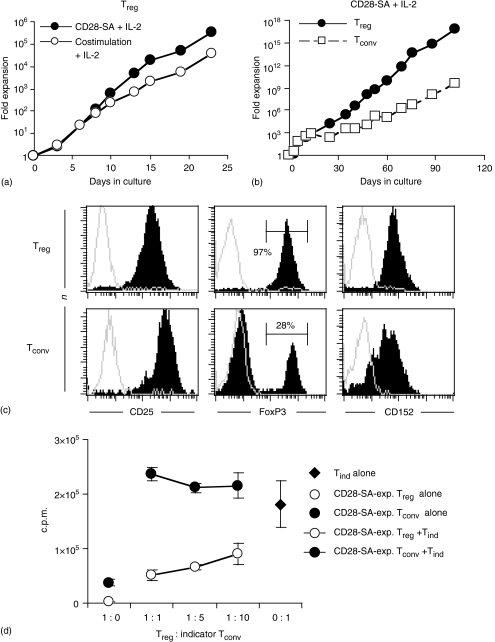
To explore whether superagonistic CD28 stimulation is suitable for long-term and large-scale expansion of Treg cells, we compared cell growth obtained with CD28-SA and IL-2 to that obtained with anti-TCR/anti-CD28 stimulation plus IL-2. Within 23 days, CD28-SA/IL-2 stimulation led to a more than 105-fold expansion while costimulation/IL-2 expanded Treg cells 10-fold less (Fig. 2a). To further assess the potential of CD28-SA for long-term Treg cell culture freshly isolated Treg and Tconv cells were cultured for up to 110 days in the presence of the CD28-SA and IL-2 (Fig. 2b). Treg cells were preferentially expanded over conventional T cells leading to expansion rates of up to 6 × 1016-fold for Treg cells and 5 × 109-fold for Tconv cells within 102 days in culture (Fig. 2b). To phenotypically characterize CD28-SA/IL-2-expanded Treg and Tconv cells (see Fig. 2b), we analysed the expression of CD25, CD152 (CTLA-4) and FoxP3 marker proteins by flow cytometry. CD28-SA-expanded Treg and Tconv cells cultured for more than 95 days displayed high expression levels of CD25 and CD152 (Fig. 2c). Importantly, all CD28-SA-expanded Treg cells expressed FoxP3 protein (Fig. 2c), identifying them as bona fide Treg cells. FoxP3 expression was, however, not confined to the progeny of Treg cells as also 28% of CD28-SA/IL-2-expanded Tconv cells expressed FoxP3 (Fig. 2c). Western blot analysis confirmed FoxP3 expression by CD28-SA-expanded Treg and Tconv cells (data not shown). To test whether long-term cultured Treg cells had also functionally retained their suppressor phenotype we performed a standard in vitro suppression assay using CD28-SA-expanded Treg cells after 78 days in culture and freshly isolated CD4+ CD25– T cells as indicator cells. Indeed, these long-term cultured Treg cells significantly inhibited the proliferation of indicator cells upon coculture (Fig. 2d). Tconv cells, however, expanded in parallel cultures displayed no suppressive activity (Fig. 2d), despite expression of FoxP3 by 70% of these cells (data not shown). In line with the missing regulatory T cell activity of CD28-SA/IL-2-expanded Tconv cells expressing FoxP3, CFSE-tracking experiments using freshly isolated CD4+ CD25– cells, indeed, indicated induction of ‘aberrant’ FoxP3 expression in FoxP3– Tconv cells, rather than outgrowth of pre-existing FoxP3+ Treg cells (data not shown). Therefore, FoxP3 expression by the progeny of rat Tconv cells does not indicate conversion to a Treg cell phenotype, thus resembling in vitro-cultured human CD4+ CD25– T cells which also express FoxP3 upon activation, but without becoming suppressive.25 Taken together, CD28-SA-stimulation in conjunction with IL-2 is superior to costimulation plus IL-2 in propagating Treg cell growth in vitro and is also suitable for large-scale and long-term expansion of Treg cells.
Figure 2. Long-term expansion of bona fide Treg cells. (a) The potency of CD28-SA (5 µg/ml) and rhIL-2 (300 U/ml) to expand freshly isolated Treg cells in vitro was compared to that of anti-TCR mAb (R73; 2.5 µg/ml) plus conventional anti-CD28 mAb (JJ319; 2.5 µg/ml) and rhIL-2 (300 U/ml). (b) Freshly isolated Treg and Tconv cells were stimulated with CD28-SA and rhIL-2 for up to 102 days. The cultures were re-stimulated on a weekly basis. The fold cell expansion is given on a logarithmic scale.
(c) Expression of CD25, CD152 and FoxP3 by CD28-SA-expanded Treg and Tconv cells after more than 95 days in culture was determined by triple staining. Specific stainings are depicted in black. Grey shadows show, in the case of CD25 and FoxP3, staining with an isotype-matched control antibody and, in the case of CD152, staining after preincubation with unconjugated anti-CD152 mAb. (d) After 78 days in culture CD28-SA-expanded Treg cells (open circles) or Tconv cells (filled circles) were cultured in the absence or presence of 5 × 104 freshly isolated CD4+ indicator T cells (Tind cells) and stimulated with Con A plus APC. Filled diamond: Proliferation of Tind cells in the absence of CD28-SA-expanded cells. Detached circles: Proliferation of CD28-SA-expanded Treg cells (open) or Tconv (filled) without Tind cells.
Functional characterization of CD28-SA-expanded Treg cells
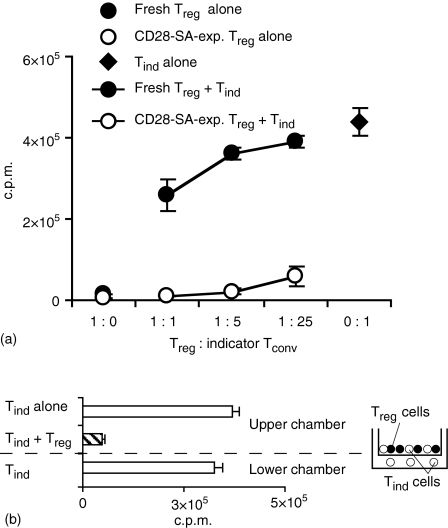
To carefully assess the suppressor qualities of CD28-SA/IL-2-expanded Treg cells, we made a side-by-side comparison of their effector function and that of freshly isolated regulatory T cells. CD28-SA-expanded Treg cells displayed greatly enhanced suppressive activity as compared to freshly isolated Treg cells (Fig. 3a), almost completely inhibiting indicator T-cell proliferation at a Treg to indicator T-cell ratio of as little as 1 : 25 (Fig. 3a).
Figure 3.
Characterization of suppression by Treg cells after CD28-SA-mediated expansion. (a) After a 12 day culturing period using CD28-SA plus IL-2 Treg cells (open circles; 800-fold expansion) and freshly isolated Treg cells (filled circles) were cocultured with freshly isolated CD4+ CD25– Tind cells at the given Treg to Tind cell ratios. Filled diamond: Proliferation of Tind cells in the absence of Treg cells. Detached circles: Proliferation of Treg cells without Tind cells. Cultures were stimulated for 3 days with Con A and proliferation was measured by [3H]thymidine incorporation. (b) CD28-SA-expanded Treg cells (53 days in culture; 5.3 × 108-fold expansion) were cultured with nylon wool non-adherent indicator T cells (Tind) of which half had direct cell-contact and the other half was separated from the Treg cells by a millicell® cell-culture insert.
In vitro suppression by ‘naturally occurring’ regulatory T cells, unlike that of inducible IL-10-producing Tr1 cells26 or transforming growth factor-β-producing TH3 cells,27 is limited to settings where Treg cells are in direct contact with the cells they suppress.28 In coculture experiments using transwell-chambers only the proliferation of indicator cells with direct cell contact to CD28-SA-expanded Treg cells was suppressed, whereas indicator cells separated from the Treg cells by a membrane were not inhibited in their proliferation (Fig. 3b).
Thus, the functional characterization of CD28 superagonist/IL-2-expanded Treg cells revealed a much stronger, but also cell contact-dependent, suppressive activity of these cells as compared to freshly isolated Treg cells.
Suppression by CD28-SA-expanded Treg cells is TCR-dependant, compatible with CD28-SA stimulation and high concentrations of IL-2
Suppression by Treg cells is only initiated after stimulation of their TCR, but not restricted to Tconv cells sharing the same TCR specificity with the Treg cells.29 Furthermore, it was postulated that ‘over-stimulation’ of Tconv cells by IL-2 or via CD28 constitutes a general mechanism for Tconv cells to escape suppression by Treg cells.30–32 However, both CD2813,14 and IL-2 are also pivotal for Treg cell homeostasis in vivo33 and IL-2 is known to be a strong activator of Treg cell effector functions both in vivo and in vitro.31–34
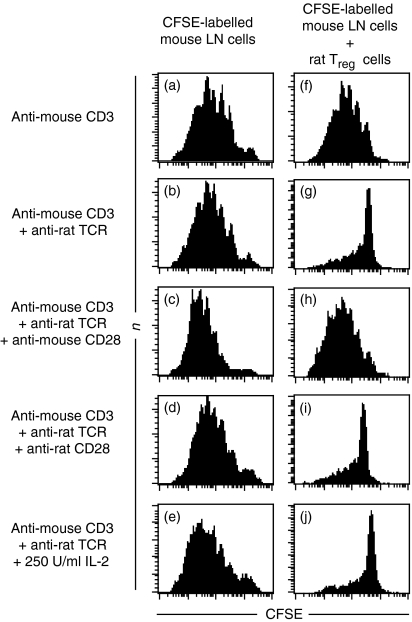
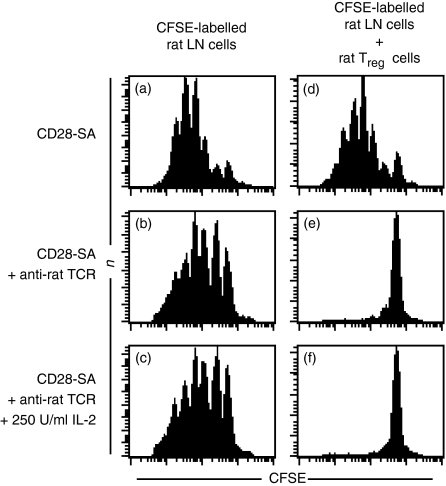
Co-cultures of mouse lymph node cells and CD28-SA/IL-2-expanded rat regulatory T cells allowed us to stimulate the TCR complexes and CD28 molecules of indicator and regulatory T cells independently of each other. Neither addition of rat CD28-SA-expanded Treg cells (Fig. 4f) nor anti-rat TCR mAb (Fig. 4b) inhibited mouse CD4+ cell proliferation induced by anti-mouse CD3 mAb (Fig. 4a). Suppression was only achieved when rat regulatory T cells were added together with anti-rat TCR mAb (Fig. 4g). Addition of IL-2 or an anti-mouse CD28 mAb did not greatly enhance proliferation of indicator CD4+ T cells in the absence of CD28-SA-expanded Treg cells (Fig. 4c, e). However, suppression by rat CD28-SA-expanded Treg cells was completely abrogated in the presence of anti-mouse CD28 mAb (Fig. 4h), but not after addition of IL-2 (Fig. 4j). Addition of conventional anti-rat CD28 mAb in the absence of CD28-SA-expanded Treg cells did not hamper mouse indicator T-cell proliferation (Fig. 4d) and did not interfere with suppression (Fig. 4i). These data map the inhibition of suppression elicited by strong CD28 stimulation to the side of the indicator T cells and suggest that, in contrast to Tconv cells, Treg cells cannot be ‘over-stimulated’.
Figure 4.
Impact of the TCR, CD28 and IL-2 on suppression by CD28-SA-expanded Treg cells. CFSE-labelled lymph node cells (1 × 105) of C57Bl/6 mice were stimulated with anti-CD3 mAb (0.5 µg/ml) added in solution and cultured either alone (a–e) or in the presence of 5 × 104 CD28-SA-expanded rat Treg cells (f–j). Anti-rat TCR mAb (1 µg/ml), conventional anti-rat CD28 mAb (0.5 µg/ml), anti-mouse CD28 mAb (0.5 µg/ml) or rhIL-2 (250 U/ml) were added where indicated. CFSE-dye dilution was assessed after 4 days in culture.
The TCR-dependency of suppression by CD28-SA-expanded Treg cells was confirmed in syngeneic suppression assays, where no suppression was detectable upon superagonistic anti-CD28 stimulation (compare Fig. 5a and d). Importantly, addition of by itself weakly mitogenic anti-TCR mAb (data not shown) to the CD28-SA, both in solution, allowed very profound suppression by CD28-SA-expanded Treg cells (compare Fig. 5b and e). The slight reduction in the proliferation of indicator CD4+ cells (compare Fig. 5a and b) upon addition of anti-TCR mAb to the CD28-SA can be attributed to the 10% Treg cells usually found within CD4+ cells. Addition of IL-2 to these cocultures of syngeneic indicator and Treg cells also did not abrogate suppression, confirming the data we had obtained in the xenogenic system (Fig. 4j). Thus, we conclude that CD28-SA/IL-2-expanded and -activated Treg cells strictly depended on triggering of their TCR to exert suppression, which is abrogated by strong costimulation through CD28 but not by addition of IL-2. This, further, implies that strong anti-CD28 costimulation does not abrogate suppression by inducing IL-2 production in Tconv cells.31
Figure 5.
CD28-SA and anti-TCR mAb in solution allow strong suppression by CD28-SA-expanded Treg cells. (a) CFSE-labelled rat lymph node cells (1 × 105) were either stimulated with CD28-SA alone (10 µg/ml) (b) a combination of CD28-SA (10 µg/ml) and anti-TCR mAb (1 µg/ml) or (c) with 250 U/ml of rhIL-2 in addition the stimuli also contained in (b). (d–f) CD28-SA-expanded Treg cells (5 × 104) were added to cultures otherwise set up as in (a–c), respectively. CFSE-dye dilution among CD4+ lymph node cells was determined after 3 days.
CD28-SA/IL-2-expanded Treg cells do not confer infectious tolerance to conventional T cells
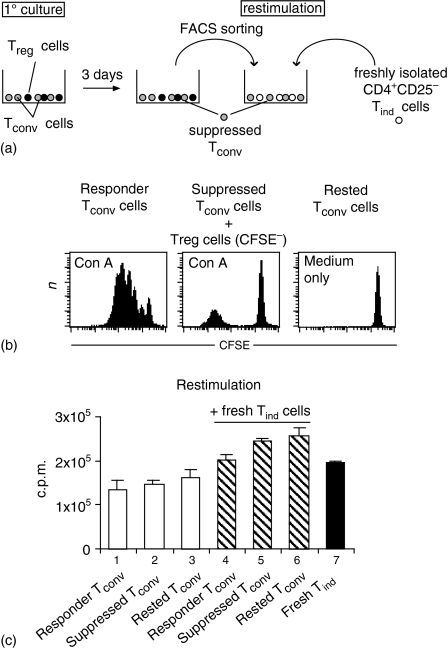
In a number of experimental settings in vivo35 and, also, in vitro24,36 induction of T-regulatory activity in conventional T cells by ‘naturally occurring’ Treg cells has been described. Co-culture of mouse CD4+ CD25+ Treg cells and T helper 2 (Th2)-primed Tconv cells, however, induced only anergy in the Th2 cells but no suppressor phenotype.37 To test whether CD28-SA/IL-2-expanded regulatory T cells render conventional T cells anergic and/or suppressive, we first cocultured CD28-SA-expanded Treg cells with CFSE-labelled conventional T cells in the presence of APC and Con A. After 3 days CFSE+ suppressed Tconv cells were separated from CFSE– Treg cells by FACS. Responder (Fig. 6b, left), suppressed (Fig. 6b, middle) and rested (Fig. 6b, right) Tconv cells, were re-stimulated with Con A in the presence or absence of fresh CD4+ CD25– Tind cells. As depicted in Fig. 6(c), all re-stimulated Tconv cells (columns 1–3) responded equally to Con A stimulation, but altogether less than freshly isolated CD4+ CD25– cells (column 7). Importantly, also the cocultures of each population of preactivated Tconv cells, including suppressed Tconv cells, with freshly isolated Tind cells produced similar levels of overall proliferation (Fig. 6c, columns 4–6).
Figure 6.
CD28-SA-expanded Treg cells do not induce ‘infectious tolerance’ in conventional T cells. (a) Schematic diagram of the experiment. (b) CFSE-labelled CD4+CD25– Tconv cells were stimulated with Con A in the presence (‘suppressed Tconv’) or absence (‘responder Tconv’) of an equal number of CD28-SA-expanded Treg cells or were left in medium only (‘rested Tconv’). (c) After 3 days, CFSE+ cells were sorted by flow cytometry and fresh CD4+ CD25– Tconv cells (Tind) were purified in parallel. Suppressed Tconv, responder Tconv and rested Tconv cells were re-stimulated with Con A either in the absence (lanes 1–3) or presence (lanes 4–6) of an equal number of Tind cells. Lane 7: Stimulation of Tind cells alone. Proliferation was assayed by [3H]thymidine incorporation.
These data indicate that the anergy induced in Tconv cells by CD28-SA/IL-2-expanded Treg cells is limited to the actual coculturing period and that there is no conversion of conventional T cells into regulatory T cells.
Discussion
In this study we assessed the potential of CD28-SA together with IL-2 to induce large-scale and long-term expansion of rat regulatory T cells in vitro. By carefully titrating the amount of the CD28-SA and IL-2 in a short-term proliferation assay (Fig. 1), CD28 could be identified to substitute for the TCR in providing the ‘first signal’ and IL-2 the ‘second’, costimulatory, signal. This suggests that CD28-SA are a super-mimic of physiological CD80/CD86–CD28 interactions, thus inducing Treg cell proliferation by strongly activating a signalling cascade which, in vivo, is critically involved in Treg cell homeostasis.13,14
On a long-term basis, Treg cells could be expanded dramatically with CD28-SA and IL-2 and kept in culture for more than 3 months (Fig. 2). The long-term expansion of Treg cells was clearly superior to that of freshly purified Tconv cells (Fig. 2b), thereby minimizing the risk of outgrowing contaminating Tconv cells in Treg cell cultures, as has been observed with other protocols.20 However, both Treg cells and Tconv cells showed better expansion upon CD28-SA/IL-2 stimulation than after costimulation plus IL-2 (Fig. 2a and data not shown). We assume that this difference is, on the one hand, the result of the absence of a strong, pro-apoptotic, TCR signal and, on the other hand, mediated by the induction of anti-apoptotic molecules, like BCLXL, upon CD28-SA stimulation.38 Importantly, long-term culture of freshly isolated Treg cells using CD28-SA and IL-2 gave rise to populations of pure FoxP3+ cells with high CD25 and CD152 expression. Similar to other protocols used for mouse Treg cell expansion in vitro,20,29 this activated phenotype was indicative of the very strong suppressive activity exerted by rat Treg cells after CD28-SA/IL-2 stimulation as compared to freshly isolated Treg cells (Fig. 3).22 As far as the mode of suppression was concerned, suppression by CD28-SA/IL-2-activated Treg cells, unlike mouse Treg cells expanded with anti-CD3 mAb, APCs and IL-229 remained dependent on ‘cell-contact’ as determined in transwell cell culture systems (Fig. 3). However, the distances between Treg cells and Tconv cells were presumably too big in these transwell cell cultures to rule out that the scavenging of IL-2 by Treg cells32,34 substantially contributes to suppression by CD28-expanded Treg cells.32 However, even when there were no restraints on the proximity of CD28-SA-expanded Treg cells and indicator T cells39 the Treg cells had to be activated through their TCR in order to become suppressive (Fig. 4), while IL-2 scavenging also occurs in the absence of TCR stimulation.34 Therefore, our data generated with CD28-SA-activated rat Treg cells add to the work of others,31,34 suggesting that suppression by Treg cells in vitro is not mediated by mere competition for IL-2. Moreover, the strict dependence of CD28-SA-expanded Treg cells on TCR stimulation for suppression reveals that the molecular programme inducing proliferation and preactivation of Treg cells and that licensing Treg cells for suppression by TCR/CD3 stimulation can be separated.
In contrast to TCR-derived signals, strong CD28 stimulation has been described as a mechanism for Tconv cells to escape suppression by Treg cells.30,31 However, by providing a, by itself, weakly mitogenic TCR stimulus we could show that suppression occurs even in the presence of strong CD28 stimulation, mediated either by a CD28-SA (Fig. 5) or by a conventional anti-CD28 mAb (data not shown). In accordance with published data,30,31 suppression in the context of strongly mitogenic anti-CD3 stimulation (Fig. 4) was not compatible with additional triggering of CD28 on Tconv cells with mAbs. Taken together, these findings suggest that the nature and/or the strength of the TCR signal in Tconv cells determines whether additional costimulatory signals transduced by CD28 (Fig. 4),30,31 and probably also the glucocorticoid-induced tumour necrosis factor receptor40 or OX4041 render Tconv cells refractory to suppression by Treg cells. Physiological stimulation of T cells by peptide/major histocompatibility complexes also generates a comparatively weak signal, which relies on costimulation via CD28 for full T-cell activation.42 Therefore, suppression assays using weak TCR stimulation probably resemble the physiological setting more closely than assays employing strongly mitogenic concentrations of anti-CD3 mAb.
Apart from CD28-mediated signals IL-2 has also been described to partially counter anergy of CD4+ CD25– indicator T cells during suppression assays31 or to completely abrogate suppression by freshly isolated mouse Treg cells in vitro.32 In contrast, functional analysis of CD28-SA/IL-2-activated Treg cells using either syngeneic (Fig. 5) or xenogeneic indicator cells (Fig. 4) revealed no abrogation of suppression in the presence of high concentrations of IL-2. This, in turn, indicates that it is not induction of IL-2 synthesis by strong costimulation via CD28,31 which allows conventional T cells to escape suppression by CD28-SA expanded Treg cells (Fig. 4). In vivo the cellular source of IL-2 are putatively pathogenic Tconv cells that supply the IL-2 to keep Treg cells metabolically fit.15 Despite continuous IL-2 production by freshly isolated Tconv cells cocultured with Treg cells34, freshly isolated Treg cells are very capable of suppressing these Tconv cells. Therefore, our data strengthen the notion that the primary role of IL-2 in vivo is to place Treg cells ‘ahead’ of putatively pathogenic Tconv cells.
As a result of suppression by mouse or human Treg cells, Tconv cells can be converted into IL-10 producing regulatory Tr1 cells.24,26,36 Such a form of ‘infectious tolerance’35 unleashes a regulatory cascade originating from CD4+ CD25+ Treg cells. However, even complete suppression of rat indicator T-cell proliferation by CD28-SA/IL-2-stimulated Treg cells did not induce stable anergy or even Treg-cell activity in conventional T cells (Fig. 6). This, together with the licensing of CD28-SA-expanded Treg cells through antigen recognition, could be important to achieve specific suppression of autoimmunity in vivo while preserving a pool of conventional effector T cells capable of responding to foreign antigen.
In humans, direct activation of Treg cells in vivo or adoptive immunotherapy with cultured Treg cells will certainly turn out to be both feasible and beneficial in, at least, some clinical settings. Therefore, protocols for the expansion of human Treg cells in vitro have started to emerge,21 including superagonistic anti-human CD28 mAb, which are also capable of strongly expanding human Treg cells in vitro without loss of function (unpublished data).
Finally, we believe that the availability of CD28-SA-expanded rat Treg cells and the thorough characterization of their suppressive properties in vitro will facilitate future research into the underlying mechanisms of CD28-SA therapy in vivo, as well as the nature and physiology of Treg cells in general.
Acknowledgments
The authors like to thank Christian Linden for conducting cell sorting procedures and Margarita Friedrich for expert technical assistance. This work was funded by a joint grant from TeGenero ImmunoTherapeutics AG and the Bayerische Forschungsstiftung (forimmun).
Glossary
Abbreviations
- APC
antigen-presenting cell
- BSA
bovine serum albumin
- CD28-SA
superagonistic anti-CD28 mAb
- CFSE
carboxyfluorescein succinimidyl ester diacetate
- Con A
concanavalin A
- c.p.m.
counts per minute
- IL
interleukin
- mAb
monoclonal antibody
- NWNA
nylon wool non-adherent
- PBS
phosphate-buffered saline
- rh
recombinant human
- Tconv cell
conventional T cell
- TCR
T-cell receptor
- Tind cells
indicator T cells
- Treg cell
regulatory T cell
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 2.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 6.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S. Animal models of autoimmunity and their relevance to human diseases. Curr Opin Immunol. 2000;12:684–90. doi: 10.1016/s0952-7915(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 8.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4 (+) CD25 (+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 11.Seddon B, Mason D. Peripheral autoantigen induces regulatory T cells that prevent autoimmunity. J Exp Med. 1999;189:877–82. doi: 10.1084/jem.189.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garza KM, Agersborg SS, Baker E, Tung KS. Persistence of physiological self antigen is required for the regulation of self tolerance. J Immunol. 2000;164:3982–9. doi: 10.4049/jimmunol.164.8.3982. [DOI] [PubMed] [Google Scholar]
- 13.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+ CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 15.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3 (+) CD25 (+) CD4 (+) regulatory T cells by interleukin (IL) -2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 17.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–62. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 18.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–77. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4 (+) CD25 (+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 20.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–65. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4 (+) CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 22.Lin CH, Hunig T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003;33:626–38. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- 23.Beyersdorf N, Gaupp S, Balbach K, et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:445–55. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25 (+) regulatory T cells convey suppressor activity to conventional CD4 (+) T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allan SE, Passerini L, Bacchetta R, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–84. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–8. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 30.Thornton AM, Shevach EM. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge. IL-2 is critically required for the in vitro activation of CD4+ CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 32.de la Rosa M. Rutz S., Dorninger H. & Scheffold A. Interleukin-2 is essential for CD4+ CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–8. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 33.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4 (+) regulatory T cell function. J Exp Med. 2002;196:851–7. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O'Garra A, Stockinger B. CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol. 2005;17:279–88. doi: 10.1093/intimm/dxh207. [DOI] [PubMed] [Google Scholar]
- 35.Waldmann H, Cobbold S. Exploiting tolerance processes in transplantation. Science. 2004;305:209–12. doi: 10.1126/science.1099538. [DOI] [PubMed] [Google Scholar]
- 36.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4 (+) CD25 (+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196:247–53. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stassen M, Jonuleit H, Muller C, Klein M, Richter C, Bopp T, Schmitt S, Schmitt E. Differential regulatory capacity of CD25+ T regulatory cells and preactivated CD25+ T regulatory cells on development, functional activation, and proliferation of Th2 cells. J Immunol. 2004;173:267–74. doi: 10.4049/jimmunol.173.1.267. [DOI] [PubMed] [Google Scholar]
- 38.Kerstan A, Hunig T. Cutting edge. distinct TCR- and CD28-derived signals regulate CD95L, Bcl-xL, and the survival of primary T cells. J Immunol. 2004;172:1341–5. doi: 10.4049/jimmunol.172.3.1341. [DOI] [PubMed] [Google Scholar]
- 39.Scheffold A, Huhn J, Hofer T. Regulation of CD4+ CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur J Immunol. 2005;35:1336–41. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- 40.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+ CD25+ T cells. J Immunol. 2004;173:5008–20. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 41.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4 (+) CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–51. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 42.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–51. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]