Abstract
Higher susceptibility of newborns to infections has been attributed to the hypo-responsiveness of their cellular immune system. Here we compared the activation status and expression of cytokines and cytotoxic molecules of cord versus maternal peripheral blood mononuclear cells in an African population. Human leucocyte antigen-DR was expressed on a lower percentage of cord compared to maternal γδ and CD3+ T cells. Similarly, a lower proportion of cord versus maternal γδ and CD3+ T cells displayed perforin, granzyme B and cytokine activity either ex vivo or following non-specific stimulation in vitro. In contrast, comparable proportions of cord and maternal CD94+ CD3− natural killer (NK) cells showed perforin and granzyme B expression ex vivo. We conclude that cord blood γδ and CD3+ T cells are functionally hypo-responsive as reflected by reduced numbers of such cells expressing either an activation marker, T helper 1 (Th1) and Th2 cytokines or cytotoxic effector molecules. The similarity in numbers of cord and maternal CD94+ CD3− cells expressing cytotoxic effector molecules suggests that neonatal Africans' NK cells may be functionally mature.
Keywords: neonate, innateimmunity, cytokines, cordblood, perforin
Introduction
Infectious diseases are a major cause of child mortality especially in developing countries and often cause morbidity and mortality already in the neonatal period.1
The cellular immune system of newborns is generally considered to be immature and hypo-responsive in comparison to that of adults. This is reflected in clinical observations showing that neonates are more highly susceptible to infection than adults and exhibit more severe or prolonged symptoms when infected.2 However there is still controversy about the factors underlying this hypo-responsiveness. The dogmatic view that the neonatal immune system has intrinsically less immunological capacity per se than that of adults has recently been challenged by reports suggesting that neonatal cells are naive but nevertheless immunologically competent, that the observed differences are of a quantitative nature and that neonatal cells have the capacity to mount adult-like responses under appropriate conditions of stimulation.3–5
Because immune cells mediate many of their functions by secretion of cytokines, differences in cytokine production capacity help to characterize functional differences in neonatal and adult immune responses. Several studies have compared cytokine production by neonatal and adult cells in non-African populations.6–8 Most studies directly compare cord blood to adult peripheral blood cells collected in different settings without taking into account the specific situation of pregnancy and delivery during which the levels of several immunomodulatory substances, including cortisol, prolactin, prostaglandin E2, oestrogens, are known to be altered in maternal and cord blood.9–12 There are, in addition, substantial differences with the respect to global immune status in African versus European populations in general that are considered to be the result both of genetic differences as well as of environmental influences.13–15 The latter include infectious diseases such as malaria.
The study described here therefore aimed to characterize the activation status and cytokine production capacity of Gabonese cord blood mononuclear cells (CBMC) in comparison to corresponding mothers' peripheral blood mononuclear cells (PBMC) collected shortly after delivery, in an attempt to further elucidate factors contributing to the perceived immunological immaturity of the neonate. We focused our analyses in particular on cells comprising components of the innate immune system. These include γδ T cells that represent 1–5% of the lymphocytes in the peripheral blood of adults, but a lower percentage in cord blood, and are cells that are involved in immune responses to several micro-organisms, including Mycobacterium tuberculosis, Listeria monocytogenes and Brucella abortus.16–20 Natural killer (NK) cells are also important in this context, comprising approximately 15% of all circulating lymphocytes, and capable of lysing target cells as well as secreting cytokines, via which they activate antigen-presenting cells and direct the T helper (Th) cell type in the Th1 direction.21 NK cells are usually defined by expression of CD56 and by a lack of CD3 on their surface. The CD56bright subset of NK cells is characterized by a high expression of NK receptor CD94.22
Subjects and methods
Study population
A total of 120 pregnant women and their 126 newborns were recruited shortly after delivery in the maternity unit of the Albert Schweitzer Hospital, Lambaréné, Gabon, a town of ∼20 000 inhabitants situated in a rural tropical rainforest area, close to the equator, where malaria is hyperendemic and perennially transmitted. Mothers were of mixed ethnicity but predominantly from the locally most prevalent groups, i.e. Fang, Kele, Myene. Samples of maternal peripheral venous and of cord blood were taken into heparinized tubes after written informed consent to participate in the study had been obtained. For technical reasons, including low blood volumes and/or low recovery of viable cells as well as lower limits set for inclusion of flow cytometry-derived data (see below), variable numbers of samples (as indicated in the figures) were tested and analysed. Because in vitro rather than ex vivo assays were expected to provide the most relevant information, the former were favoured over the latter. Restricted availability of cells consequently resulted in a maximum of 10 maternal and 31 cord blood samples being used for ex vivo assays, and, for non-specific stimulation of cells in vitro, of 48 maternal and 74 cord blood samples, and 20 cord blood as well as 10 maternal blood samples for isopentenylpyrophosphate (IPP) stimulation. The study was approved by the ethics committee of the International Foundation of the Albert Schweitzer Hospital in Lambaréné.
Mitogens and antigens
The combination of phorbol 12-myristate 13-acetate (PMA), and ionomycin (both from Sigma, Deisenhofen, Germany) was used for mitogenic stimulation of mononuclear cells (see below). For specific stimulation of the γδ T-cell subset, IPP (Sigma), was used.
PBMC/CBMC cultures
All blood samples were processed within 4 hr of collection. Maternal PBMC or CBMC were isolated from heparinized blood using routine density centrifugation methods with Ficoll (Amersham Biosciences, Uppsala, Sweden). For stimulation cells were cultured at a concentration of 1·25 × 106/ml in medium (Ultra Culture, BioWhittaker, Walkersville, MD) supplemented with l-glutamine (2 mm), 2-mercaptoethanol (3·5 µl/l, Sigma) and gentamicin (170 µg/ml). A mixture of PMA (10 ng/ml) and ionomycin (1·25 µm) was used to stimulate cells for 4 hr at 37° in 5% CO2 in the presence of brefeldin A (10 µg/ml, Sigma). IPP (10 µg/ml) was used as a specific stimulus of γδ T cells for 18 hr and in this case brefeldin A (10 µg/ml) was added for the last 12 hr of incubation.
At the end of culture periods, cells were harvested on ice, washed twice in phosphate-buffered saline (PBS) and fixed with a 2% solution of formaldehyde (Merck, Darmstadt, Germany) in PBS for 20 min, again washed twice in PBS and then stored in Hank's balanced salt solution (HBSS, containing 0·1% sodium azide, both Sigma) supplemented with 0·3% bovine serum albumin (Serva, Heidelberg, Germany) at 4° in the dark until used for staining. These procedures follow those originally described by Winkler and colleagues.23
Flow cytometric analysis
For immunostaining, cells were washed once in PBS followed by surface staining in PBS at room temperature for 25 min. Cells were then permeabilized with PBS/0·1% saponin (Sigma). Intracellular staining was carried out in PBS/0·1% saponin for 30 min at room temperature, using appropriate combinations of the antibodies described below, followed by washing in PBS/0·1% saponin and PBS. Four-colour staining was performed and 2 × 105 cells were acquired on a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany). Gates were set according to the profiles obtained with antibody isotype controls. Data derived from samples from which insufficient cells (i.e. <100 γδ T cells, <2000 CD3+ T cells) could be acquired were discarded. Data were analysed using CellQuest 3.3 software (Becton Dickinson).
Antibodies and reagents
The following monoclonal antibodies (mAb) were used: fluoroscein isothiocyanate (FITC)–anti-interferon-γ (IFN-γ) mAb, FITC–anti-perforin mAb, FITC–anti-human leucocyte antigen (HLA)-DR mAb, phycoerythrin (PE)–anti-CD69 mAb, PE–anti-interleukin (IL)-13 mAb, PE–anti-IL-10 mAb, PE–anti-IL-5 mAb, PerCP–anti-CD3 mAb, allophycocyanin (APC)–anti-T-cell receptor (TCR) γδ mAb, APC–anti-CD94 mAb (all Becton Dickinson/Pharmingen), PE–anti-granzyme B mAb (Caltag, Hamburg, Germany). Isotype controls were included as appropriate.
Because cord blood NK cells may lack CD56 expression,24 and also for technical reasons, the combination of CD3– CD94+ was used to gate NK cells.
Statistical analyses
Statistical analyses were performed using the Statview software programme version 5.0.1 (SAS Institute, Cary, NC). Because the numbers of paired mother–cord samples varied and was frequently small, e.g. only six pairs for ex vivo analyses, only unpaired comparisons of continuous variables were made using the non-parametric Mann–Whitney U-test where a value of P < 0·05 was considered significant.
Results
In this study CBMC and maternal PBMC were compared with respect to expression of activation markers, perforin and granzyme B expression ex vivo, as well as to cytokine production and perforin/granzyme B expression after stimulation with PMA/ionomycin or IPP.
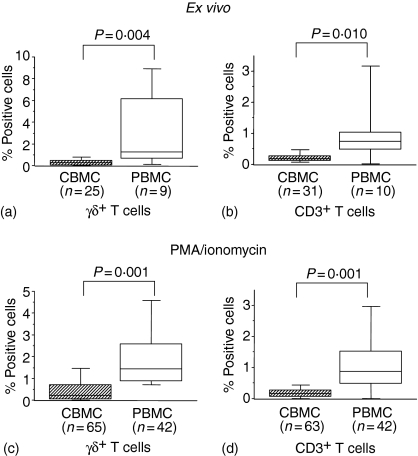
CD69 and the major histocompatibility complex class II molecule HLA-DR are considered early and late T-cell activation markers, respectively, the latter playing a role in antigen presentation. Ex vivo analyses showed a lower percentage of HLA-DR+ cord γδ and CD3+ T cells in CBMC compared to PBMC (Fig. 1a, b). This pattern was identical following stimulation with PMA/ionomycin (Fig. 1c, d). Expression of CD69 was not detected on γδ and CD3+ T cells ex vivo with the exception of a small number of CBMC and PBMC samples in which low percentages of CD69+ cells were detected (data not shown).
Figure 1.
Expression of HLA-DR on CBMC and maternal PBMC ex vivo (a, b) or stimulated with PMA/ionomycin for 4 h in the presence of brefeldin A (c, d), fixed and stained for CD3, TCR-γδ, HLA-DR. A gate was set on CD3+TCRγδ+ cells (a, c) or on CD3+ cells (b, d). Box-whisker plots show medians of percentages of cells expressing either HLA-DR with 25th and 75th percentiles in boxes and 10th/90th percentiles as whiskers. P-values refer to significance of differences between percentages considered as continuous variables and compared between groups using the non-parametric Mann–Whitney U-test.
Perforin and granzyme B are important effector molecules of cytotoxic T and NK cells that mediate target cell lysis and induction of apoptosis.25 Expression of these molecules was examined both ex vivo and after non-specific stimulation. Maternal γδ and CD3+ T cells expressed both perforin and granzyme B ex vivo but expression by cord γδ or CD3+ T cells was minimal (Table 1). This pattern was unaffected by stimulation with PMA/ionomycin, although a certain amount of degranulation was suggested by the slightly (but not statistically significantly) lower proportions of maternal γδ T cells with detectable granzyme B after stimulation (Table 1). In ex vivo samples of CBMC and PBMC the proportions of NK cells (CD94+ CD3– cells) expressing perforin and granzyme B did not show statistically significant differences, but after PMA/ionomycin stimulation a higher proportion of maternal CD94+ CD3– cells expressed granzyme B compared to cord cells (Table 1).
Table 1.
Perforin and granzyme B expression in CBMC and PBMC
| Ex vivo | PMA + Ionomycin stimulation | |||||
|---|---|---|---|---|---|---|
| Cord blood (n = 24–31) | Maternal peripheral blood (n = 5–9) | P-value2 | Cord blood (n = 60–74) | Maternal peripheral blood (n = 35–48) | P-value | |
| Perforin | ||||||
| γδ T cells | 0·1 (0·5)1 | 14·2 (33·1) | 0·005 | 0·2 (0·5) | 5·8 (12·7) | <0·001 |
| CD3+ T cells | 0·1 (0·2) | 0·6 (6·3) | 0·01 | 0·1 (0·2) | 0·4 (0·8) | <0·001 |
| CD94+ CD3– cells | 30·1 (53·1) | 24·2 (34·6) | 0·45 | 19·7 (26·5) | 18·7 (19·8) | 0·62 |
| Granzyme B | ||||||
| γδ T cells | 0·4 (1·2) | 60·6 (43·9) | <0·001 | 0·4 (1·0) | 32·2 (16·6) | <0·001 |
| CD3+ T cells | 0·2 (0·4) | 11·4 (16·6) | <0·001 | 0·3 (0·5) | 7·6 (7·7) | <0·001 |
| CD94+ CD3– cells | 20·7 (32·5) | 55·8 (59·4) | 0·25 | 34·6 (38·7) | 74·4 (17·0) | <0·001 |
Values are medians (interquartile ranges) of percentage cells detected with perforin/granzyme B activity.
Mann–Whitney U-test (cord blood versus maternal peripheral blood mononuclear cell).
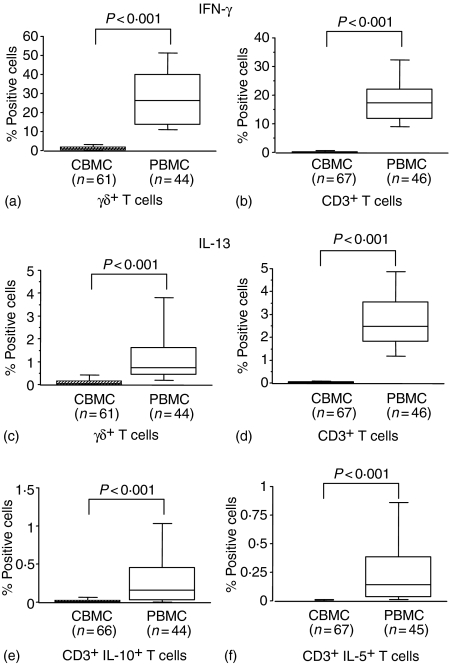
Following stimulation with PMA/ionomycin the proportions of CBMC γδ and CD3+ T cells expressing either IFN-γ or IL-13 were lower than in PBMC, indicating a stronger capacity of adult versus neonatal cells for production of both Th1- and Th2-type cytokines after polyclonal activation (Fig. 2a–d). A smaller proportion of CBMC CD3+ T cells expressed IL-10 compared with PBMC CD3+ T cells after stimulation (Fig. 2e), and expression of IL-5 was detected in only low numbers of CBMC CD3+ T cells (Fig. 2f).
Figure 2.
The percentages of IFN-γ- (a, b), IL13- (c, d), IL-10- (e) and IL-5-containing (f) CBMC and maternal PBMC detected by flow cytometry after stimulation with PMA/ionomycin for 4 hr in the presence of brefeldin A, followed by fixation, surface and intracellular staining and gating on CD3+ TCRγδ+ cells (a, c) or on CD3+ cells (b, d, e, f). Box-whisker plots show medians of percentages with 25th and 75th percentiles in boxes and 10th/90th percentiles as whiskers. P-values refer to significance of differences between percentages considered as continuous variables and compared between groups using the non-parametric Mann–Whitney U-test.
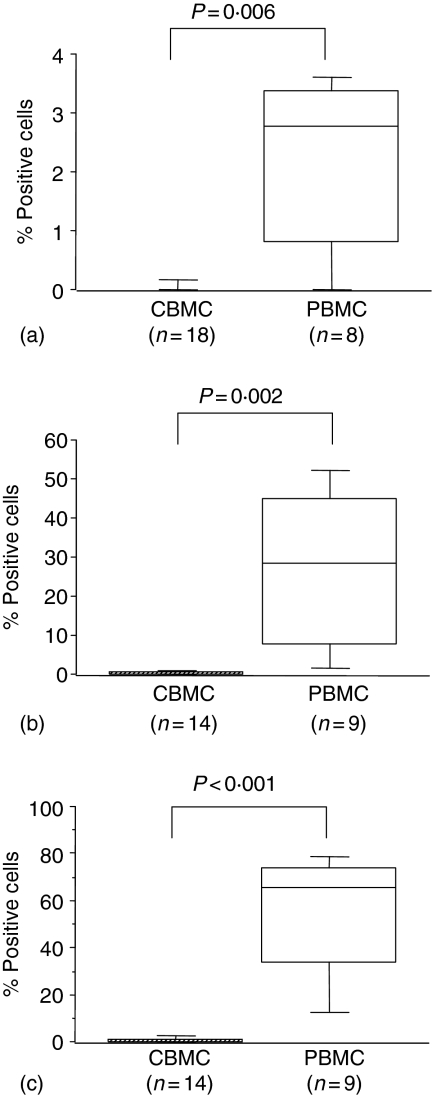
After stimulation with IPP significantly fewer CBMC γδ T cells expressed perforin and granzyme B compared to those in PBMC and expression of IFN-γ was not observed in cord γδ T cells (Fig. 3a–c). Similarly low proportions of γδ T cells expressed IL-13 after IPP stimulation, with no difference between CBMC and PBMC (data not shown).
Figure 3.
The percentages of IFN-γ- (a), Perforin- (b) and granzyme B-containing (c) CBMC and maternal PBMC detected by flow cytometry after stimulation with IPP for 18 hr in the presence of brefeldin A (12 hr), followed by fixation, surface and intracellular staining and gating on CD3+ TCRγδ+ cells. Box-whisker plots show medians of percentages with 25th and 75th percentiles in boxes and 10th/90th percentiles as whiskers. P-values refer to significance of differences between percentages considered as continuous variables and compared between groups using the non-parametric Mann–Whitney U-test.
Discussion
Neonates are more susceptible to infection than adults and suffer from more severe or prolonged symptoms when infected. This has been attributed to an immature cellular immune system in newborns that is relatively hypo-responsive compared to that in adults.2 The immaturity of the adaptive immune response in newborns is explained by lack of antigen contact and the high number of naïve neonatal T cells.5,7
Here, we aimed to compare the activation status and cytokine production capacities of neonatal in comparison with maternal mononuclear cells. Flow cytometry was used to examine different cell populations: CD3+ T cells as part of the adaptive immune system, NK cells and γδ T cells as part of the innate immune system. The latter might be of special importance in neonates as they constitute the first line of defence against pathogens. Differences between Europeans and Africans with respect to immune status have been reported by others13,15 that might result from different quantity and quality of in utero or postnatal antigen exposure. Here, we chose to focus only on an African population.
The expression of the early activation marker CD69 was equally low in both PBMC and CBMC, whereas the late activation marker HLA-DR was more frequently expressed on maternal γδ and CD3+ T cells, possibly reflecting more frequent recent activation in mothers than in newborns. This finding is consistent with a study showing higher HLA-DR expression on Ethiopian adult CD4+ and CD8+ T cells than on those of neonates.26 We extend this observation to the γδ T-cell population and to the context of pregnancy and delivery. In contrast to our observation the study by Hodge and colleagues did not find significant differences in HLA-DR expression on T cells or NK cells of Australian donors' PBMC and CBMC either ex vivo or after stimulation.2 We speculate that this difference reflects the relatively higher exposure of sub-Saharan African populations to endemic parasitic infections for example, that, in the population studied here, include malaria and a variety of helminths.
Perforin and granzyme B are two effector molecules of cytotoxic cells. We found that higher proportions of maternal γδ T and CD3+ T cells expressed these molecules, implying a higher capacity to lyse target cells than cord cells. γδ T cells of newborns are the main cellular effectors combating extracellular pathogens, but the cytolytic and proliferative activity of cord blood γδ T-cell clones is decreased compared with that of adult γδ T cell clones27 a finding consistent with our own. Berthou and colleagues found no constitutive expression of perforin in cord blood γδ or CD4+ T cells as opposed to adult blood cells, whereas cord blood CD8+ T cells expressed perforin.25 We therefore conclude that the perforin expressing CD3+ T cells we detected here belong to the CD8+ T cell subset.
No significant differences between CBMC and PBMC with respect to the proportion of NK cells containing perforin or granzyme B were observed, indicating that these innate immune cells may have similar cytotoxic potential in newborns and adults. In line with this a separate study reported no significant difference in neonatal versus maternal NK cell cytotoxicity.28 Other studies have reported neonatal NK cell cytotoxicity to be low6,25,29 but capable of being enhanced in the presence of exogenous IL-224 and to reach levels comparable to adults upon addition of IL-12.6,25
We found that, following stimulation, lower proportions of CBMC expressed cytokines of both the Th1 and Th2 types compared with maternal PBMC, a finding that we assume to be an indication of their decreased functional capacity. This observation is broadly in accordance with other studies of neonatal and adult cells in non-African populations2,6–8 although it should be noted that comparability is hampered somewhat by the varying methods of stimulation used in these different studies. Our study, in addition, took into account the immunological changes associated specifically with pregnancy and delivery. Krampera and colleagues compared PMA/ionomycin-stimulated CBMC and PBMC of Italian donors and reported no differences in T cell Th2-type (IL-5, IL-10, IL-13) cytokine activity.30 This is clearly inconsistent with the results we report here. As discussed already above, we speculate that the higher capacity of African adult cells' for such cytokine production that we observed simply reflects the prevalence of, for example, intestinal and other helminth infections in this population. These data are consistent with those reported by Wilfing and colleagues.15 One recent study reported significantly elevated expression of IL-13 specifically by naïve cord blood CD8+ T cells of Dutch donors.31 Interestingly, IL-13 promotes Th2 cell differentiation in mice32 and it is thought that the neonatal immune system is biased towards Th2-type responses unless exposure to infectious agents redirects it to Th1.31,33 It should be noted that such exposure –in utero or postnatally – is more frequent in developing countries. Both here and in a separate study in the same site34 we have observed lower proportions of CBMC T cells containing IL-13 following either specific or non-specific stimulation compared with maternal PBMC, but we have not measured IL-13 protein secretion during extended stimulation and culture of cells in vitro. One recent study of Kenyan mothers and their offspring, however, did not detect differences in IL-13 production in vitro following parasite antigen-specific stimulation.35 A bias in favour of IL-10 production by CBMC following either specific or nonspecific stimulation has also been reported35,36 and may reflect fetal exposure in African populations in which in utero sensitization to certain parasite antigens is common.35,37,38
Clearly, however, cytokine production capacity alone cannot explain the differences observed in immune responses between adult and neonatal mononuclear blood cells. The expression of the corresponding cytokine receptors must also influence the biological effect exerted by cytokines. In this context, it has indeed been shown that a range of cytokine receptors are expressed at a lower level on CBMC.39
In conclusion we show that African cord blood γδ and CD3+ T cells exhibit reduced expression of an activation marker, of Th-1- and Th-2-type cytokines as well as cytotoxic effector molecules compared to corresponding maternal cells, reflecting their functional hypo-responsiveness, whereas cord CD94+ CD3– cells contain levels of cytotoxic effector molecules comparable to maternal cells.
Acknowledgments
The authors are indebted to the mothers for their participation in this study, and to the staff of the maternity unit at the Albert Schweitzer Hospital in Lambaréné for their cheerful and diligent assistance. We thank Christian Menzel and Maxi Margos for technical assistence. The study received financial assistance through grant support from the German DFG-BMZ Programme (Contract Lu 812/1-3) and from the fortüne Programme of the Medical Faculty of the University of Tübingen.
Abbreviations
- CBMC
cord blood mononuclear cells
- NK cells
natural killer cells
- IPP
isopentenylpyrophosphate
- PMA
phorbol 12-myristate 13-acetate
References
- 1.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226–34. doi: 10.1016/S0140-6736(03)13779-8. [DOI] [PubMed] [Google Scholar]
- 2.Hodge S, Hodge G, Flower R, Han P. Cord blood leucocyte expression of functionally significant molecules involved in the regulation of cellular immunity. Scand J Immunol. 2001;53:72–8. doi: 10.1046/j.1365-3083.2001.00845.x. [DOI] [PubMed] [Google Scholar]
- 3.Hermann E, Truyens C, Alonso-Vega C, et al. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood. 2002;100:2153–8. [PubMed] [Google Scholar]
- 4.Seghaye MC, Heyl W, Grabitz RG, Schumacher K, von Bernuth G, Rath W, Duchateau J. The production of pro- and anti-inflammatory cytokines in neonates assessed by stimulated whole cord blood culture and by plasma levels at birth. Biol Neonate. 1998;73:220–7. doi: 10.1159/000013980. [DOI] [PubMed] [Google Scholar]
- 5.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–5. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SB, Perez-Cruz I, Fallen P, Gluckman E, Madrigal JA. Analysis of the cytokine production by cord and adult blood. Hum Immunol. 1999;60:331–6. doi: 10.1016/s0198-8859(98)00126-8. [DOI] [PubMed] [Google Scholar]
- 7.Fadel S, Sarzotti M. Cellular immune responses in neonates. Int Rev Immunol. 2000;19:173–93. doi: 10.3109/08830180009088504. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–8. [PubMed] [Google Scholar]
- 9.Faist E, Schinkel C, Zimmer S. Update on the mechanisms of immune suppression of injury and immune modulation. World J Surg. 1996;20:454–9. doi: 10.1007/s002689900071. [DOI] [PubMed] [Google Scholar]
- 10.Giltay EJ, Fonk JC, von Blomberg BM, Drexhage HA, Schalkwijk C, Gooren LJ. In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J Clin Endocrinol Metabol. 2000;85:1648–57. doi: 10.1210/jcem.85.4.6562. [DOI] [PubMed] [Google Scholar]
- 11.Bouyou-Akotet MK, Adegnika AA, Agnandji ST, Ngou-Milama E, Kombila M, Kremsner PG, Mavoungou E. Cortisol and susceptibility to malaria during pregnancy. Microbes Infect. 2005;7:1217–23. doi: 10.1016/j.micinf.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clin Exp Immunol. 2005;139:287–96. doi: 10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassu A, Tsegaye A, Petros B, et al. Distribution of lymphocyte subsets in healthy human immunodeficiency virus-negative adult Ethiopians from two geographic locales. Clin Diagn Lab Immunol. 2001;8:1171–6. doi: 10.1128/CDLI.8.6.1171-1176.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clerici M, Butto S, Lukwiya M, et al. Immune activation in africa is environmentally-driven and is associated with upregulation of CCR5. Italian–Ugandan AIDS Project. AIDS. 2000;14:2083–92. doi: 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 15.Wilfing A, Winkler S, Schrattbauer K, et al. African–European differences in the capacity of T-cell cytokine production. Am J Trop Med Hyg. 2001;65:504–9. doi: 10.4269/ajtmh.2001.65.504. [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Zhou D, Qiu L, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dieli F, Troye-Blomberg M, Ivanyi J, et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis. 2001;184:1082–5. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 18.Ottones F, Dornand J, Naroeni A, Liautard JP, Favero J. V gamma 9V delta 2 T cells impair intracellular multiplication of Brucella suis in autologous monocytes through soluble factor release and contact-dependent cytotoxic effect. J Immunol. 2000;165:7133–9. doi: 10.4049/jimmunol.165.12.7133. [DOI] [PubMed] [Google Scholar]
- 19.Nomura A, Matsuzaki G, Takada H, Hiromatsu K, Nabeshima S, Nakamura T, Kishihara K, Nomoto K. The role of gammadelta T cells in induction of bacterial antigen-specific protective CD8+ cytotoxic T cells in immune response against the intracellular bacteria Listeria monocytogenes. Immunology. 1998;95:226–33. doi: 10.1046/j.1365-2567.1998.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladel CH, Blum C, Kaufmann SH. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by gamma/delta T lymphocytes. Infect Immun. 1996;64:1744–9. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott P, Trinchieri G. The role of natural killer cells in host–parasite interactions. Curr Opin Immunol. 1995;7:34–40. doi: 10.1016/0952-7915(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 22.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 23.Winkler S, Willheim M, Baier K, Schmid D, Aichelburg A, Graninger W, Kremsner PG. Reciprocal regulation of Th1- and Th2-cytokine-producing T cells during clearance of parasitemia in Plasmodium falciparum malaria. Infect Immun. 1998;66:6040–4. doi: 10.1128/iai.66.12.6040-6044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradstock KF, Luxford C, Grimsley PG. Functional and phenotypic assessment of neonatal human leucocytes expressing natural killer cell-associated antigens. Immunol Cell Biol. 1993;71:535–42. doi: 10.1038/icb.1993.59. [DOI] [PubMed] [Google Scholar]
- 25.Berthou C, Legros-Maida S, Soulie A, Wargnier A, Guillet J, Rabian C, Gluckman E, Sasportes M. Cord blood T lymphocytes lack constitutive perforin expression in contrast to adult peripheral blood T lymphocytes. Blood. 1995;85:1540–6. [PubMed] [Google Scholar]
- 26.Tsegaye A, Wolday D, Otto S, et al. Immunophenotyping of blood lymphocytes at birth, during childhood, and during adulthood in HIV-1-uninfected Ethiopians. Clin Immunol. 2003;109:338–46. doi: 10.1016/j.clim.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Morita CT, Parker CM, Brenner MB, Band H. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol. 1994;153:3979–88. [PubMed] [Google Scholar]
- 28.Eisenthal A, Hassner A, Shenav M, Baron S, Lifschitz-Mercer B. Phenotype and function of lymphocytes from the neonatal umbilical cord compared to paired maternal peripheral blood cells isolated during delivery. Exp Mol Pathol. 2003;75:45–52. doi: 10.1016/s0014-4800(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 29.Baley JE, Schacter BZ. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985;134:3042–8. [PubMed] [Google Scholar]
- 30.Krampera M, Tavecchia L, Benedetti F, Nadali G, Pizzolo G. Intracellular cytokine profile of cord blood T-, and NK- cells and monocytes. Haematologica. 2000;85:675–9. [PubMed] [Google Scholar]
- 31.Ribeiro-do-Couto LM, Boeije LC, Kroon JS, Hooibrink B, Breur-Vriesendorp BS, Aarden LA, Boog CJ. High IL-13 production by human neonatal T cells: neonate immune system regulator? Eur J Immunol. 2001;31:3394–402. doi: 10.1002/1521-4141(200111)31:11<3394::aid-immu3394>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie GJ, Emson CL, Bell SE, et al. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9:423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 33.Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, Holt PG. Transplacental priming of the human immune system to environmental allergens. universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–7. [PubMed] [Google Scholar]
- 34.Brustoski K, Kramer M, Moller U, Kremsner PG, Luty AJF. Neonatal and maternal immunological responses to conserved epitopes within the DBL-gamma3 chondroitin sulfate A-binding domain of Plasmodium falciparum erythrocyte membrane protein 1. Infect Immun. 2005;73:7988–95. doi: 10.1128/IAI.73.12.7988-7995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra I, Mungai P, Muchiri E, Ouma J, Sharma S, Kazura JW, King CL. Distinct Th1- and Th2-type prenatal cyto-kine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect Immun. 2005;73:3462–70. doi: 10.1128/IAI.73.6.3462-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han P, Hodge G. Intracellular cytokine production and cytokine receptor interaction of cord mononuclear cells: relevance to cord blood transplantation. Br J Haematol. 1999;107:450–7. doi: 10.1046/j.1365-2141.1999.01696.x. [DOI] [PubMed] [Google Scholar]
- 37.Brustoski K, Moller U, Kramer M, et al. IFN-gamma and IL-10 mediate parasite-specific immune responses of cord blood cells induced by pregnancy-associated Plasmodium falciparum malaria. J Immunol. 2005;174:1738–45. doi: 10.4049/jimmunol.174.3.1738. [DOI] [PubMed] [Google Scholar]
- 38.Brustoski K, Moller U, Kramer M, Hartgers FC, Kremsner PG, Krzych U, Luty AJF. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum Infection. J Infect Dis. 2006;193:146–54. doi: 10.1086/498578. [DOI] [PubMed] [Google Scholar]
- 39.Zola H, Fusco M, Macardle PJ, Flego L, Roberton D. Expression of cytokine receptors by human cord blood lymphocytes: comparison with adult blood lymphocytes. Pediatr Res. 1995;38:397–403. doi: 10.1203/00006450-199509000-00021. [DOI] [PubMed] [Google Scholar]