Abstract
Colonization with commensal flora in very early life may profoundly influence intestinal lymphoid development and bias later immune responses. We defined gut-homing T cell phenotypes and the influence of flora on intestinal immune development in mice. Intestinal T cells were phenotyped and quantified in conventional (CV), germfree (GF) and conventionalized germfree (GF/CV) neonatal mice by immunohistochemistry. Mucosal adressin cell adhesion molecule 1 (MAdCAM-1) was expressed by mucosal vessels at birth in CV and GF mice and was more prevalent in CV than GF small intestine, but was distributed similarly and did not change with age. Less MAdCAM-1 was expressed in the colon; its distribution became restricted after weaning, with no difference between CV and GF mice. CD3+β7+ cells were present in similar numbers in CV and GF intestine at birth. They were CD62L– in CV mice and were accompanied by further CD3+β7+CD62L– T cells as development progressed, but in GF and GF/CV intestine they expressed CD62L and numbers did not change. IEL numbers increased at weaning in CV mice in both small and large intestine, but showed delayed development in GF intestine. Macrophages were present at high levels from birth in GF intestine, but dendritic cells did not develop until day 16. Thus, fetus-derived T cells seed the intestinal lamina propria before birth via β–MadCAM interactions. Their activation status depends on the microbiological status of the dam, and without a commensal flora they remain naive. We propose that these cells regulate antigen responsiveness of the developing mucosal T cell pool.
Keywords: germfree, microflora, neonate, T cell, weaning
Introduction
There is a growing acceptance that fetal and early neonatal exposure to antigen has a marked effect on the development of the mucosal immune system, to the extent that lifestyle factors which restrict full colonization with a balanced intestinal commensal flora predispose to atopic disease.1 Despite this awareness, little is known about the very early development of the mucosal immune system in the gut, or the influence of bacterial colonization on this development.
T cell immunity in the intestinal mucosa undergoes development during fetal and neonatal life and throughout the weaning period. The extent to which the intestine is populated by T cells at birth varies between species, driven presumably by type of placentation and maternal antigen experience affecting fetal development. Thus, in humans, the lamina propria of the intestine is populated by substantial numbers of T cells from 12 to 14 weeks of gestation and these can almost reach adult numbers by 22 weeks of gestation.2 The presence of putative lymphoid tissue precursor CD3–CD7+ cells in the human fetus and the observation that most T cells in the human gut at this early stage do not express the mucosal homing integrin, α4β7, have led to the proposal that many, if not most, T cells in the human fetal gut have developed in situ as a result of extrathymic differentiation.3 Indeed, we have shown that even after birth human intestinal T cells differentiate in situ.4 In mice, however, T cell development occurs relatively late − approximately day 18 of gestation5,6– with an influx of CD3+ cells into Peyer's patch anlagen, but there is no detailed information on in situ T cell development in the lamina propria or epithelium in the important period from just before birth until weaning.7
Crucial to the seeding of the developing gut mucosa with leucocytes, including T cells, is the interaction between the mucosal adressin cell adhesion molecule 1 (MAdCAM-1) and leucocyte integrin α4β7.8 In human fetuses, MAdCAM-1 is displayed on the vasculature of many peripheral tissues and is not restricted to the gut, but it gradually becomes restricted to mucosal vessels after birth.9 It has been proposed10 that the wide in utero distribution permits seeding of α4β7 integrin-expressing leucocytes and T cell precursors and permits access of naive T cells into fetal and neonatal tissues, whereas in the adult extravasation of circulating naive T cells is permitted only in organized lymphoid tissue. This may enable a mechanism for the peripheral induction of tolerance to self-antigens via presentation by tissue stromal cells, rather than professional antigen-presenting cells.11 A very similar pattern of expression of MAdCAM-1 has been described in the rat, with widespread expression on vasculature in peripheral as well as mucosal tissues, and restriction to mucosal tissues occurring only after birth.12 Again, fetal intestinal expression was much more widespread than expression in the adult gut. There have been no studies defining MAdCAM-1 expression during development of the large intestine. One possible influence on the extent of MAdCAM-1 expression in the gut during development is the rapid organization of the intestinal microflora, but this has not been investigated.
Results from our parallel studies (Probert et al. submitted) show that by 5 days of age the conventional (CV) mouse mucosal T cell compartment has established a polyclonal T cell receptor (TCR) repertoire, whereas germfree (GF) mice of the same age have an oligoclonal repertoire, which remains oligoclonal throughout weaning. These results can be interpreted as either an influx into the CV mouse gut by polyclonal T cells from the fetus, or that an oligoclonal fetus-derived population becomes expanded rapidly by an influx of polyclonal T cells immediately after birth under the influence of the rapidly expanding commensal microflora. We have examined this hypothesis by semiquantitative analysis of T cell phenotypes entering the CV and GF neonatal mouse gut.
Methods
Mice
BALB/cdm2 mice were bred and maintained under CV conditions in the Department of Clinical Veterinary Science, University of Bristol, UK. BALB/cdm2 GF mice were caesarian-derived and maintained in plastic isolators in the Gnotobiology Laboratory, Czech Academy of Science. Faecal samples from GF mice were cultured under both aerobic [Sabouraud broth, Schaedler broth, blood agar (all Difco, Detroit, MI, USA)] and anaerobic [Sabouraud broth, Schaedler broth under Anaerogen (Oxoid, Basingstoke, UK)] conditions on a weekly basis to validate continued sterility of the colony. Cultures were also smeared for differential fluorescent detection.13 For detection of segmented filamentous bacteria (SFB) and Bacteroides, faecal samples were probed by fluorescence in situ hybridization (FISH).14 Helicobacter spp. were excluded by polymerase chain reaction (PCR) screening15. Some GF litters were conventionalized at birth [conventionalized germfree (GF/CV)] with a defined faecal flora comprising Bacillus spp., Lactobacillus lactis, Acinetobacter spp., Staphylococcus sciuri, L. delbrukii sp. bulgaricus, L. fermentum, L. catenaforme, Bacteroides distasonis, B. thetaiotomicron, Peptostreptococcus micros and P. asaccharolyticus (Hannover Central Institute for Animal Breeding) and SFB. Composition of the diet was equivalent for both colonies. Pups were housed with their mothers and weaned at 21 days old. Both UK and Czech ethical authorities approved experiments.
Tissue samples were taken from mid-colon and mid–small intestine, embedded in optimal cutting temperature compound (OCT), snap-frozen in liquid nitrogen-cooled isopentane and stored at − 20°.
Antibodies
Primary antibodies were rat monoclonal anti-mouse antibodies: CD3 (IgG2a, clone KT3); CD4 (IgG2b, clone YTS191·1); CD8 (IgG2a, clone YTS105·18); CD11b (IgG2b, clone M1/70); F4/80 (IgG2b, clone CI:A3-1); MAdCAM-1 (IgG2a, clone MECA-367); CD62L (IgG2a, clone MEL-14) (all Serotec, Oxford, UK); β7 integrin (IgG2a, clone M293); B220 (IgG2a, clone RA3–6B2); and biotinylated hamster anti-mouse CD11c (IgG1, clone HL3) (all BD PharMingen, San Jose, CA, USA). Secondary antibody for rat primaries was biotinylated affinity purified goat anti-rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Conjugated fluorochromes were streptavidin–Texas red, streptavidin–fluorescein isothiocyanate (FITC) and avidin–aminomethylcoumarin (AMCA) (all Vector Laboratories, Peterborough, UK).
Immunohistochemistry and image analysis
Frozen sections were cut at 5 µm, air-dried and fixed in acetone. Sections were blocked with 10% normal goat serum. Primary antibodies were applied at room temperature for 3 hr, and secondary antibody and fluorochomes for 1 hr each at room temperature. Sections were mounted in Vectashield (Vector Laboratories). Controls included substitution of primary antibodies with isotype-matched antigen-irrelevant antibodies, or detection system only.
For both colon or small intestine immunohistochemical sections, cell counting was performed on tissues from at least four mice per group, using five to seven crypt or crypt–villus units from three to four distinct well-orientated representative fields of view, at × 200 or × 400 magnification on a Leica DMR microscope with a triple filter and mercury lamp, using the Image Pro-Plus capture and analysis software (Media Cybernetics UK, Berkshire, UK). Cell counts were made from measured areas and numbers of cells were equated to cells/mm2. Cells were counted only in diffuse lamina propria or epithelium and organized lymphoid tissue was not counted.
Statistics
Data were separated into groups on a preweaning (days 0–20)/peri-weaning (days 21–25)/post-weaning (> day 25) basis. Groups were compared using analysis of variance (anova) (spss for Windows version 11·5). Significance was defined as P < 0·05.
Results
MAdCAM-1
Because the architecture of mucosal tissues changes with age of development, we used two separate parameters to assess MAdCAM-1 expression in intestinal tissues − percentage of depth of tissue expressing MAdCAM-1 (from muscularis mucosa to villus tip for small intestine and muscularis mucosa to luminal surface in the large intestine), and overall area of tissue expressing MAdCAM-1 as a percentage of total area of tissue in a tissue section.
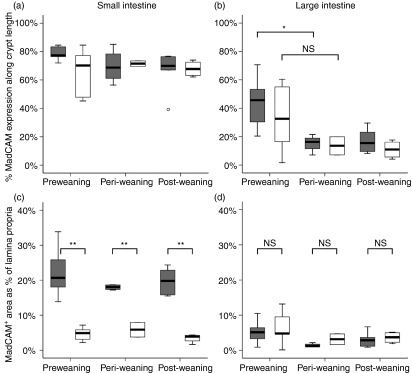
In the small intestine of both GF and CV mice, the depth of mucosa over which MAdCAM-1 was expressed was 70–80% from muscularis to lumen, with no difference between GF and CV and no significant change with increasing age (Fig. 1a). The area of mucosa expressing MAdCAM-1 was always significantly greater in CV mice (approximately 20%) than in GF mice (approximately 5%)(Figs 1c and 2), but this did not change at any age in either group of mice (individual measurements were made from days 5 to 31). This indicates that in GF mice, vessels expressing MAdCAM-1 were confined to the centre of the characteristically narrow villi, whereas in CV mice MAdCAM-1+ vessels expanded to fill the laterally expanded villi (Fig. 2a). Thus, the vascular architecture in the small intestine is more extensive from birth in CV mice than in GF mice and MAdCAM-1 expression simply reflects this and is unaffected by age of development, or by increasing complexity of the flora.
Figure 1.
Distribution of mucosal adressin cell adhesion molecule 1 (MAdCAM-1) in small and large intestine of conventional (CV) and germfree (GF) mice before and after weaning. The distribution (a,b) and quantity (c,d) of MAdCAM-1 expressed by mucosal vasculature in small intestine and large intestine were determined in digitized images of tissues stained by immunofluorescence. Open boxes, GF; shaded boxes, CV. Horizontal bar, median; box, interquartile range; whiskers, extreme range; o, ordinary outlier (1·5–3 box lengths from lower part of box). *P < 0·05; **P <0·01; NS, not significantly different.
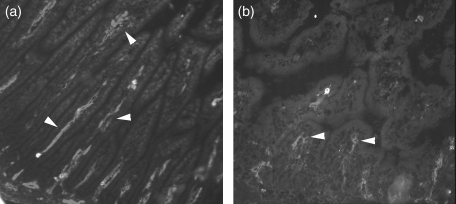
Figure 2.
Representative images defining mucosal adressin cell adhesion molecule 1 (MAdCAM-1) expression (arrowheads) in conventional (CV, a) and germfree (GF, b) mouse small intestine at 10 days after birth.
In the large intestine, however, depth of mucosa expressing MAdCAM-1 was approximately 50% in CV mice, significantly decreasing to 10–20% at weaning, with no further change after weaning (Fig. 1b). In GF mice a similar trend was observed, with reductions in MAdCAM-1 distribution from a median of 33% before weaning to 10–20% after weaning, but because of high variance in the preweaning samples this difference was not significant. Area of expression in the colon was low, at approximately 5% of total tissue at all time-points, with no significant difference between CV and GF (Fig. 1d). Thus, MAdCAM-1+ vessels became more concentrated at the base of the colonic mucosa at weaning in both sets of mice. As the trend was the same in both sets of mice, this indicates that this change was driven either by dietary or developmental factors and not by changes in the gut flora.
T cells
Lamina propria T cells
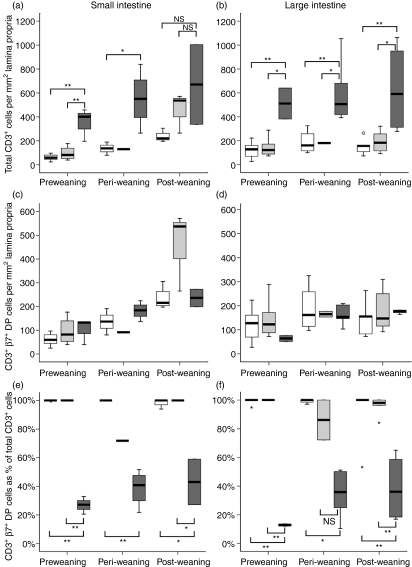
As expected, in CV mice there were more CD3+ T cells in the lamina propria of intestinal mucosa − both small and large intestine − than in GF mice at all stages of development (Fig. 3a,b), although the difference between CV and GF was not significant in the small intestine after weaning because of high variance in the CV group (Fig. 3a). When CD3+ T cells were counted in preweaning samples of CV mice – and samples from days 5, 10 and 15 were analysed together as preweaning samples − there were approximately four times as many cells (approximately 400/mm2 in the small intestinal mucosa) than in GF mice (approximately 100/mm2). However, this difference between CV and GF mice did not emerge until day 15 after birth, with no significant difference between CV and GF small intestinal CD3+ cell count at days 5 or 10 (100–200 cells/mm2). Samples from GF mice retained this level of CD3+ cells in the lamina propria throughout the period of study, whereas CD3+ cell numbers increased significantly with age in CV mice. In the small intestine, the density of the lamina propria CD3+ cell infiltration in GF/CV mice was no different from GF mice at pre- and peri-weaning time-points, but at the post-weaning time-point the median total CD3+ cell count in GF/CV mice was no different from CV mice, although the increased variance at this age caused the conventionalized group to be not significantly different from GF mice. This trend towards an increase in lamina propria CD3+ cells in GF/CV mice was not apparent in the colon.
Figure 3.
Lamina propria T cell development in small and large intestine of young mice, expressed as total CD3+ cell frequency (a,b), CD3+α4β7+ double positive frequency (c,d), and frequency of α4β7 expression within the CD3+ cell population (e,f). Tissues were double-stained for CD3 and β7 and frequencies determined by image analysis. Open boxes, germfree (GF); dark shaded boxes, conventional (CV); light shaded boxes, conventionalized germfree (GF/CV). Horizontal bar, median; box, interquartile range; whiskers, extreme range; o, ordinary outlier (1·5–3 box lengths above or below box); small star, extraordinary outlier (> 3 box lengths above or below box) *P < 0·05; **P < 0·01; NS, not significantly different.
Within this CD3+ population, the number of cells co-expressing CD3 and the β chain of the mucosa-homing integrin, α4β7, did not change significantly over the period of study in CV, GF or GF/CV mouse small intestine or colon (Fig. 3c,d), although there was a trend towards a greater number of CD3+β7+ cells in the small intestine of GF/CV mice at the post-weaning time-point. Thus, the frequency of intestinal lamina propria T cells co-expressing α4β7 was essentially 100% in GF and GF/CV mice throughout the ages studied, whereas in CV mice it remained constant at around 20–40% of total CD3+ cells (Fig. 3e,f).
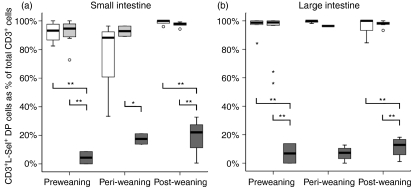
Very few CD3+ cells in CV mouse small intestine and colon co-expressed l-selectin, but almost all CD3+ cells in small intestine and colon of GF mice were l-selectin+(Fig. 4a,b). The differences were significant in both small and large intestine, with the exception of the peri-weaning samples in the small intestine, at which point the increased variance in l-selectin expression in GF mice may indicate a transient perturbation due to weaning stress.
Figure 4.
Frequency of CD3+ lamina propria cells co-expressing CD62L (l-selectin) in the small and large intestine of conventional (CV), germfree (GF) and conventionalized germfree (GF/CV) mice. Open boxes, GF; dark shaded boxes, CV; light shaded boxes, GF/CV. Horizontal bar, median; box, interquartile range; whiskers, extreme range; o, ordinary outlier (1·5–3 box lengths above or below box); small star, extraordinary outlier (> 3 box lengths above or below box) *P < 0·05; **P < 0·01; NS, not significantly different.
In summary, all groups of mice had a similar small number of CD3+β7+ T cells in the intestinal lamina propria immediately after birth; in CV mice, these fetus-derived T cells did not express l-selectin, but in GF or GC/CV mice l-selectin was retained; in CV mice, by days 10–15 this birth population became overlaid with increasing numbers of CD3+β7+CD62L– antigen-experienced T cells; in the absence of flora, the naive (CD62L+) birth population of T cells was retained to beyond weaning; in conventionalized GF mice, there was some evidence of recruitment of more T cells, particularly into the small intestine, but without loss of CD62L.
Intraepithelial T cells
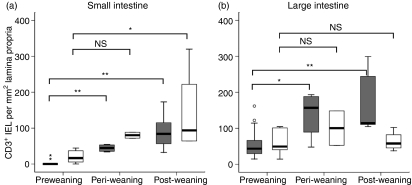
Intraepithelial T cells (IEL) were rare at birth in both CV and GF mice, both in the small intestine and colon (Fig. 5a,b). In the small intestine, their numbers did not start to increase until day 16 after birth. Significant increases over the birth population in the small intestine were reached by weaning in CV mice and by day 31 in GF mice, at which time-point there was no significant difference between numbers in CV and GF tissues (Fig. 5a). In the colon, numbers of IEL in CV mice increased significantly at weaning, but in GF mice numbers did not increase above birth levels with age (Fig. 5b).
Figure 5.
Intraepithelial CD3+ cells during development in conventionalized (CV) and germfree (GF) mouse small and large intestine. Open boxes, GF; dark shaded boxes, CV. Horizontal bar, median; box, interquartile range; whiskers, extreme range; o, ordinary outlier (1·5–3 box lengths above or below box); small star, extraordinary outlier (> 3 box lengths above or below box). *P < 0·05; **P < 0·01; NS, not significantly different.
Myeloid and B cells in GF mice
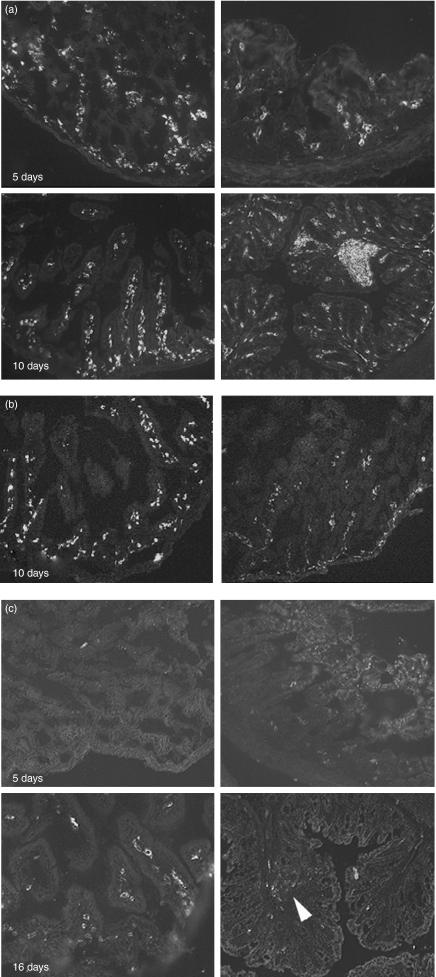
A striking feature of the intestine of GF mice was that, from birth, the lamina propria was highly cellular. As we had determined that very few T cells infiltrated the tissue at early time-points, we investigated the phenotype of infiltrating cells (Table 1, Fig. 6). The leucocyte origin of the cells was confirmed by the high frequency of CD45+ cells immediately after birth (Fig. 6a). Within these CD45+ cells, F4/80+CD11b+ macrophages were the most common phenotype, with high numbers present from the earliest time-points examined in both small intestine and colon (Fig. 6b). CD11c+ cells were rare in the small intestine and colon at day 5, but increased rapidly in frequency in the small intestine, reaching adult levels comparable to frequency seen in CV tissues by day 16 (Fig. 6c). In the colon, CD11c+ cells were still rare in the lamina propria by day 16, but were present in the colon at this age in large numbers in isolated lymphoid follicles (Fig. 6c).
Table 1.
Myeloid cell development in the lamina propria of the intestine of germfree mice with age. Cell frequency was subjectively scored in tissues stained for myeloid cell differentiation antigens
| B220 | IgM | CD11b | CD11c | F4/80 | |
|---|---|---|---|---|---|
| 5 days | |||||
| SI | +/− | +/− | + + + | +/− | + + + |
| LI | +/− | +/− | + + | +/− | + + |
| 10 days | |||||
| SI | +/− | +/− | + + + | + | + + + |
| LI | +/− | +/− | + + + | +/− | + + + |
| 16 days | |||||
| SI | +/− | + | + + + | + + + | + + + |
| LI | +/− | + | + + + | +/− | + + + |
| 21 days | |||||
| SI | + | + | + + + | + + + | + + + |
| LI | + | + | + + + | + | + + + |
LI: large intestine; SI: small intestine.
Figure 6.
Myeloid cell phenotype in the developing germfree (GF) mouse gut. Representative images of small intestine (left) and large intestine (right) stained for CD45 (a), F4/80 (b) or CD11c (c) at the ages shown. Arrowhead, isolated lymphoid follicle.
Except for primitive Peyer's patch structures in the small intestine, B220+ and/or IgM+ B cells were rare throughout development in GF tissues (data not shown).
Discussion
The mucosal addressin, MAdCAM-1, has been shown to be responsible for facilitating access of naive B and T cells into intestinal Peyer's patches and MLN, via HEV, and of effector T cells into mucosal lamina propria via the endothelium of mucosal vasculature.16,17 It is able to interact with both naive and armed T cells using CD62L–mucin interactions and CAM–integrin interactions (with the mucosa-homing integrin, α4β7), respectively. During development, MAdCAM-1 also has a role in seeding peripheral lymph nodes with CD3–CD4+β7+ lymphoid progenitor cells10 and it is expressed in the developing rat gut from day 16 of gestation.12 However, there have been no perinatal studies of its distribution in the mouse gut, or of its regulation by the rapidly developing bacterial flora in the postnatal period. In this study, the distribution of MAdCAM-1-expressing vessels in the small intestine did not change with age from birth until after weaning and was not affected by the developing microflora. However, the amount of vasculature expressing MAdCAM-1 in CV small intestine was always greater than in GF intestine. These data indicate that postnatal developmental events do not influence MAdCAM-1 expression per se, but that colonization with flora probably enhances gut angiogenesis,18 although as this difference was present immediately after birth, environmental effects are probably mediated during fetal life. However, in the colon, although the amount and distribution of MAdCAM-1+ vasculature was always significantly less than in the small intestine, its distribution was more restricted at weaning than at birth, becoming more concentrated in the lamina propria at crypt bases, without a clear effect of bacterial colonization. Thus, although there is an environmental effect on MAdCAM-1 expression in the small intestine, the lower level expression in the colon indicates that the influence of gut bacteria is not simply quantitative, but probably involves maternal antigen experience as well as organ-specific factors.
It has been noted previously that bacterial colonization of the gut has a dramatic effect on mucosal T cell numbers,19 so it was not surprising that, even before weaning, total CD3+ cells in CV gut exceeded those in GF gut. From birth, however, equal frequencies of CD3+ T cells were found in both CV and GF gut, suggesting that both are seeded with an equivalent number of T cells during fetal life. Significantly, these fetus-derived T cells expressed α4β7 in all three groups of mice, suggesting that the quantitative difference observed in small intestine MAdCAM-1 expression between CV and GF mice does not significantly affect homing of these fetal T cells. Expression of CD3 by these cells indicates that they are not the CD3–CD4+ lymphoid progenitor cells which have been shown to seed peripheral tissues during early development.10 In GF mice, and in GF/CV mice over the period studied, these fetus-derived CD3+β7+ T cells expressed the naive T cell selectin, CD62L. In these mice, there was no significant recruitment of further T cells with development, except for a possible post-weaning influx of CD3+β7+CD62L+ cells in GF mice given a faecal flora. However, in mice derived from conventional dams and raised in a conventional environment, these fetus-derived T cells did not express l-selectin and were overlaid by 15 days after birth with increasing numbers of CD3+β7+CD62L– cells. Although in the adult, naive T cells gain access to peripheral organized lymphoid tissue only and not to peripheral non-lymphoid tissue, there is growing evidence that during the neonatal period peripheral non-lymphoid tissues are seeded with naive T cells.20 However, in so far as l-selectin is a marker of naivety, the data suggest that this does not take place in the gut colonized with normal flora. Moreover, studies in human fetal tissue2 show that a substantial proportion, if not all, gut mucosal T cells take on an activated phenotype before birth. Together, these data provide powerful evidence that the activation status of T cells present in the gut mucosa at birth is a product of maternal antigen experience and that, in the GF maternal environment, there is no antigen to drive activation of these fetal cells. What would be the selective advantage of this small population of fetus-derived T cells in the neonatal animal? Although the function of naive T cells in those peripheral tissues which do receive them neonatally is not known, it has been proposed that, through antigen presentation in a non-professional antigen-presenting cell (APC) environment, they may be involved in induction of peripheral tolerance11. This has obvious relevance to current thoughts regarding the effects of early environment on immune biasing and later susceptibility to immune-mediated mucosal allergic and inflammatory disease,1 and this ‘starter’ population of mucosal T cells may be involved in regulating the repertoire of subsequent mucosal T cell immigrants, based on maternal antigen experience. Changes in the neonatal environment not corresponding to maternal antigen experience may result in defective regulation of the neonatal mucosal T cell repertoire and immune biasing. However, confirmation of any regulatory function of these fetus-derived cells must await further phenotypic and functional studies on late fetus and early infant T cells.
The data regarding IEL development are consistent with the limited data available,7 such that numbers of CD3+ IEL begin to increase at around day 15. Data from the rat21 and pig22 show that IEL development lags behind lamina propria T cell development, with final maturation not taking place until well after weaning. There are no data concerning the effect of microflora on neonatal IEL development in the mouse gut. The available data for rats23 and mice24 relate to colonization of adult animals and show a marked effect of bacteria on infiltration of the epithelium by αβ T cell subsets. Although we have analysed only CD3+ IEL and have not investigated subsets within these T cells, our results show that even in the absence of flora, IEL numbers increased significantly in the small intestine but not in the colon. Possession of a commensal flora from birth, however, clearly had an inductive effect and this presumably reflects increases in the αβ subset.
Although the lamina propria and epithelial T cell compartments were severely compromised in the neonatal GF gut, the mucosa was by no means acellular. In fact, a feature of the GF lamina propria of small intestine and colon was its cellularity. Expression of CD45 by these cells confirmed their leucocyte phenotype. Further analysis showed the majority to be tissue macrophages. The high frequency of these innate cells in the GF gut from birth results probably from release from the normal homeostatic regulatory mechanisms because of the lymphopoenic nature of the tissue. It is known that the commensal gut flora has a close relationship with innate immune mechanisms and is able to modulate them,18,25 so it is not surprising that innate cell infiltration is disturbed in the GF tissue. However, this was not a general innate cell phenomenon, evidenced by the observation that CD11c+ cells were rare at birth and reached adult levels only at day 16 in small intestine and remained at low frequency in the colonic lamina propria. Although CD11c is expressed by other cell types,26 the subepithelial location in the small intestine and clustering in colonic lymphoid follicles suggests that these were dendritic cells. There are no published data describing neonatal development of gut DC, but it is clear from the current study that their homing to, or maturation to, a CD11c+ phenotype, in gut tissues is independent of commensal signals. Also, it seems likely that antigen sampling in the gut in the early days of life is not carried out by cell types which we associate with professional antigen presentation. Again, the implications of this to induction of peripheral tolerance are obvious, but informed comment awaits a more detailed study of antigen handling during the neonatal phase.
Acknowledgments
This work was funded by Biotechnology and Biological Sciences Research Council grant no. 7/D13513; Wellcome Trust Biomedical Research Collaboration grant no. AL069896; Nanna Svartz Foundation; and Vetenskapsrådet grant no. K2002–06X-14233.
References
- 1.Rook GAW, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54:317–20. doi: 10.1136/gut.2004.053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howie D, Spencer J, deLord D, et al. Extrathymic T cell differentiation in the human intestine early in life. J Immunol. 1998;161:5862–72. [PubMed] [Google Scholar]
- 3.Gunther U, Holloway JA, Gordon JG, et al. Phenotypic characterization of CD3-7+ cells in developing human intestine and an analysis of their ability to differentiate into T cells. J Immunol. 2005;174:5414–22. doi: 10.4049/jimmunol.174.9.5414. [DOI] [PubMed] [Google Scholar]
- 4.Williams AM, Bland PW, Phillips AC, et al. Intestinal αβ T cells differentiate and rearrange antigen receptor genes in situ in the human infant. J Immunol. 2004;173:7190–9. doi: 10.4049/jimmunol.173.12.7190. [DOI] [PubMed] [Google Scholar]
- 5.Adachi S, Yoshida H, Kataoka H, et al. Three distinctive steps in Peyer's patch formation of murine embryo. Int Immunol. 1997;9:507–14. doi: 10.1093/intimm/9.4.507. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H, Kawamoto H, Santee SM, et al. Expression of α4β7 integrin defines a distinct pathway of lymphoid progenitors committed to T cells, fetal intestinal lymphotoxin producer, NK, and dendritic cells. J Immunol. 2001;167:2511–21. doi: 10.4049/jimmunol.167.5.2511. [DOI] [PubMed] [Google Scholar]
- 7.Steege JC, Buurman WA, Forget PP. The neonatal development of intraepithelial and lamina propria lymphocytes in the murine small intestine. Dev Immunol. 1997;5:121–8. doi: 10.1155/1997/34891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlin C, Berg EL, Briskin MJ, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 9.Salmi M, Alanen K, Grenman S, et al. Immune cell trafficking in uterus and early life is dominated by the mucosal addressin MAdCAM-1 in humans. Gastroenterology. 2001;121:853–64. doi: 10.1053/gast.2001.27968. [DOI] [PubMed] [Google Scholar]
- 10.Mebius RE, Streeter PR, Michie S, et al. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3– cells to colonize lymph nodes. Proc Natl Acad Sci USA. 1996;93:11019–24. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold B, Schuler T, Hammerling GJ. Control of peripheral T-lymphocyte tolerance in neonates and adults. Trends Immunol. 2005;26:406–11. doi: 10.1016/j.it.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Iizuka T, Tanaka T, Suematsu M, et al. Stage-specific expression of mucosal addressin cell adhesion molecule-1 during embryogenesis in rats. J Immunol. 2000;164:2463–71. doi: 10.4049/jimmunol.164.5.2463. [DOI] [PubMed] [Google Scholar]
- 13.Fazzi P, Ciancaglini E, Sorforza G. Differential fluorescent staining method for detection of bacteria in blood cultures, cerebrospinal fluid and other clinical specimens. Eur J Clin Microbiol Infect Dis. 2002;21:373–8. doi: 10.1007/s10096-002-0731-3. [DOI] [PubMed] [Google Scholar]
- 14.Saltzman NH, deJong H, Paterson Y, et al. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002;148:3651–60. doi: 10.1099/00221287-148-11-3651. [DOI] [PubMed] [Google Scholar]
- 15.Fox JG, Gorelick PL, Kullberg MC, et al. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun. 1999;67:1757–62. doi: 10.1128/iai.67.4.1757-1762.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streeter PR, Berg EL, Rouse BT, et al. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 17.Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363:461. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 18.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host–microbial relationships in the intestine. Science. 2001;291:881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 19.Stepankova R, Sinkora J, Hudkovic T, et al. Differences in development of lymphocyte subpopulations from gut-associated lymphatic tissue (GALT) of GF and conventional rats: effect of aging. Folia Microbiol (Praha) 1998;43:531–4. doi: 10.1007/BF02820814. [DOI] [PubMed] [Google Scholar]
- 20.Alferink J, Tafuri A, Vestweber D, et al. Control of neonatal tolerance to tissue antigens by peripheral T cell trafficking. Science. 1998;282:1338–41. doi: 10.1126/science.282.5392.1338. [DOI] [PubMed] [Google Scholar]
- 21.Helgeland L, Brandtzaeg P, Rolstad B, et al. Sequential development of intraepithelial γ/δ and α/β T lymphocytes expressing CD8αβ in neonatal rat intestine: requirement for the thymus. Immunology. 1997;92:447–56. doi: 10.1046/j.1365-2567.1997.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothkötter HJ, Ulbrich H, Pabst R. The postnatal development of gut lamina propria lymphocytes: number, proliferation, and T and B cell subsets in conventional and germ-free pigs. Pediatr Res. 1991;29:237–42. doi: 10.1203/00006450-199103000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Helgeland L, Vaage JT, Rolstad B, et al. Regional phenotype specialization of intraepithelial lymphocytes in the rat intestine does not depend on microbial colonization. Scand J Immunol. 1997;46:349–57. doi: 10.1046/j.1365-3083.1997.d01-133.x. [DOI] [PubMed] [Google Scholar]
- 24.Umesaki Y, Setoyama H, Matsumoto S, et al. Expansion of αβ T-cell receptor-bearing intestinal intraepithelial lymphocytes after microbial colonization in germ- free mice and its independence from thymus. Immunology. 1993;79:32–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–12. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 26.Huleatt JW, Lefrancois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J Immunol. 1995;154:5684–93. [PubMed] [Google Scholar]