Abstract
In previous studies, we identified a gene product belonging to the silent information regulatory 2 protein (SIR2) family. This protein is expressed by all Leishmania species so far examined (L. major, L. infantum, L. amazonensis, L. mexicana) and found to be crucial for parasite survival and virulence. In the present study, we investigated whether a Leishmania SIR2 recombinant protein (LmSIR2) would affect T- and B-cell functions in a murine model. In vitro treatment of spleen cells from normal BALB/c mice with LmSIR2 showed increased expression of CD69 on B cells. This effect was not abolished by the addition of polymyxin B. Intravenous injection of LmSIR2 into BALB/c mice induced increased spleen B cell number by a factor of about ≈1·6, whereas no modification occurred at the level of CD4+ and CD8+ cells. Furthermore, intraperitoneal injection of LmSIR2 alone without adjuvant into BALB/c mice or nude mice triggered the production of elevated levels of LmSIR2-specific antibodies. The analysis of specific isotype profiles showed a predominance of immunoglobulin G1 (IgG1) and IgG2a antibody responses in BALB/c mice, and IgM in nude mice. Moreover, the anti-LmSIR2 mouse antibodies in the presence of complement induced the in vitro lysis of L. infantum amastigotes. In the absence of complement, the antibodies induced significant inhibition of amastigotes developpement inside macrophages. Together, the current study provides the first evidence that a Leishmania protein belonging to the SIR2 family may play a role in the regulation of immune response through its capacity to trigger B-cell effector function.
Keywords: Leishmania, SIR2, B cells, specific antibodies, leishmaniasis
Introduction
Protozoans of the genus Leishmania are obligate intracellular parasites causing a wide spectrum of human and veterinary diseases known as leishmaniasis. The disease causes a broad range of clinical symptoms, from a self-healing localized cutaneous form to the disseminated potential lethal infection, depending on the complex interaction between the infecting Leishmania species and the host immune response.1 Active visceral leishmaniasis (VL), caused by members of Leishmania donovani complex (L. donovani in the Old World, L. infantum in the South-east of Europe and Mediterranean area and L. chagasi in the New World) is a progressive infection characterized by fever, cachexia, hepatosplenomegaly and hypergammaglobulinaemia, symptoms that develop in both humans and dogs.2,3 The interaction between the parasite and its host led to a variety of disturbances in the immune system. One of the immunological hallmarks of VL is a profound suppression of parasite-specific T-cell mediated immune responses4 associated with a remarkable increase in the serum immunoglobulin levels as a result of polyclonal B-cell activation, which invariably produce large amounts of parasite non-specific antibodies with self autoreactivity particularly the immunoglobulin M (IgM) and IgG isotypes.5 Using a mechanism common to other intracellular pathogens, Leishmania spp. displays mitogenic-like activities that produce a non-specific general activation of lymphocytes. Thus, previous reports have characterized immunosupressor factors and mitogenic molecules within the parasite antigens.6,7 Thus, it seems that the persistence of infection could be related somehow to the immunosuppression phenomenon, the non-specific responses, and the development of autoimmune processes in relation to defined parasite molecules. Therefore, it seems reasonable to assume that induction of specific responses against ‘immunodominant’, ‘immunopathological’ or ‘protective’ antigens, which could interfere with some of the biological activities, might be among the most effective means to block the development of pathogenic processes.8
It is generally accepted that protective immunity against leishmaniasis is associated with a classical cell-mediated immune response, rather than a humoral one. This view has been interpreted in context of the T helper 1 (Th1)/Th2-biased responses in the mouse model of L. major. However, in visceral leishmaniasis, while a interleukin-12 (IL-12)-driven type 1 response is imperative for protection, type 2 responses (that support humoral immunity) also play a role, although minor, in promoting resistance.9 Although the real contribution of antibodies is still under debate, studies in different intracellular pathogens have shown that antibodies can also have a function in restricting the infection when the parasite is exposed to the extracellular milieu.10 In leishmaniasis, interaction of antibodies with the parasite could occur when the promastigote infects an individual or when the amastigotes are released from the infected macrophages.11 Moreover, their possible role in protection was shown by passive in vivo transfer of monoclonal antibodies.12
In previous reports we have characterized silent information regulatory 2 protein (SIR2) proteins from L. major (LmSIR2)13 and L. infantum (LiSIR2)14 while two other related protein sequences can be found in the Leishmania genome database (L. major sirtuin (CAB55543), L. major cobB (LmjF34.2140). Molecular and biological approaches allowed us to show the potential role of LmSIR2 and LiSIR2 genes in parasite survival.14,15 Furthermore, using LmSIR2 as a probe, immunological investigations demonstrated that LmSIR2 was highly immunogenic during natural human and canine infections.16
In the current study, we investigated whether LmSIR2 would trigger the activation of murine lymphocytes, and surprisingly murine B cells, but not T cells, respond strongly and directly to stimulation with LmSIR2. The B cells underwent differentiation in vivo as evidenced by the production of significant levels of specific antibodies. Our data suggest an immunoregulatory role of leishmania SIR2 protein during leishmaniasis.
Materials and methods
Leishmania strain and mice
A cloned line of L. infantum (MHOM/MA/67/ITMAP-263, wild type (WT)) was used for the infectious challenge. A cloned line of L. infantum expressing the luciferase (LUC) gene derived from WT17 was used in all in vitro experiments. Six to seven-week-old BALB/c male mice were obtained from Harlem Iberica (Spain). Seven-week-old BALB/c athymic (nude) mice were obtained from Charles River (L'Arbresle, France).
Purification of LmSIR2
The L. major SIR2 was obtained as recombinant protein containing six histidine residues at its N-terminal. The construction of plasmid and purification of protein have been described elsewhere.18 The purity of the recombinant protein (LmSIR2) was analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) stained with Coomassie blue. For biological assays, the protein was dialysed against phosphate-buffered saline (PBS) in decreasing concentrations of urea. The final dialysis was performed against PBS. The protein concentration was determined using the Folin procedure.19 To eliminate endotoxins, the recombinant protein was passed through an EndoTrap®red column (Profos, Germany) following manufacturer's instructions.
Mouse injections
In the case of BALB/c mouse experiments two different routes of administration were followed. One group of BALB/c mice was injected three times intraperitoneally (i.p.) at a 7-day interval with 50 µg of LmSIR2 in 300 µl of PBS, or 300 µl of PBS (control group). Two weeks after final immunization, spleens and sera were collected. Another group of BALB/c mice received one intravenous (i.v.) injection of 20 µg of LmSIR2 in 150 µl of PBS, or 150 µl of PBS (control group). After 72 hr, mice were killed and spleens collected. In the case of BALB.nude mice, two groups of five mice each were injected three times i.p. at a 7-day interval with either 50 µg of endotoxin-free LmSIR2 in 400 µl of PBS, or 400 µl of PBS (control group).
BALB/c mice immunization and infection
For immunization, one group of BALB/c mice was injected three times i.p. at a 7-day interval with 50 µg of LmSIR2 in 200 µl of sterile PBS. A control group was injected with 200 µl of sterile PBS in a similar schedule. Fifteen days after the last immunization both groups were challenged with 108 stationary-phase WT promastigotes ressuspended in 200 µl of sterile PBS.
Spleen B-cell isolation
After cervical dislocation, the spleens were removed and homogenized in a Petri dish to obtain a single-cell suspension. After two washes in RPMI-1640 culture medium (Cambrex, Saint Beauzine, France), the cells were adjusted to 107/ml in RPMI-1640 culture medium supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 20 mm HEPES. Spleen B-cell isolation was carried out using a B Cell Isolation Kit, MACS® (Miltenyi Biotec, Auburn, CA) as described by the manufacturer. The effectiveness of B-cell purification was determined by double labelling with specific monoclonal antibodies (mAb; anti-µ+, anti-CD4+ and anti-CD8+) and further fluorescence-activated cell sorting (FACS) analysis. After purification, we obtained over 97% B-cell purity.
Flow cytometry determinations
Spleen cells or isolated B cells from normal or LmSIR2 immunized mice and their respective controls were prepared in order to obtain single-cell suspensions. The cells were washed by centrifugation and ressuspended in PBS supplemented with 2% FCS. A total of 106 viable cells were incubated for 30 min at 4° with saturating concentrations of phycoerythrin (PE)-conjugated mAb to CD69 plus fluorescein isothiocyanate (FITC)-conjugated mAbs to either CD4, CD8α or IgM (anti-µ) from BD Pharmingen (San Diego, CA). After two washing steps with PBS−2% FCS, the cells were analysed by flow cytometry in a FACS Scan equipped with CellQuest Pro software (Becton Dickinson, San Jose, CA). Lymphocytes were selected on the basis of forward scatter/side scatter values and dead cells were excluded from all samples by propidium iodide labelling.
To evaluate the binding of antibodies to LUC-recombinant WT amastigotes, 2·5 × 105 parasites were washed twice in PBS containing 0·5% of bovine serum albumin (PBS–BSA) and incubated for 30 min at 4° with mouse anti-SIR2 immune serum diluted 1 : 10 in PBS–BSA. After two washes with PBS–BSA, the parasites were incubated for 30 min at 4° with FITC-conjugated goat anti-mouse IgG diluted 1 : 5000 in PBS–BSA. Labelled amastigotes were washed twice and resuspended in 500 µl of PBS–BSA followed by flow cytometry analysis. A preimmune serum was used as control. Results were expressed as mean fluorescent intensity (MFI).
Enzyme-linked immunosorbent assays (ELISA) for immunoglobulins
Ninety-six-well flat-bottomed microtitre plates (Greiner, Laborchnik, Solingen, Germany) were coated overnight at 4° with one of the following reagents in 0·01 m carbonate/bicarbonate buffer pH 8·5: unlabelled goat anti-mouse immunoglobulin (5 µg/ml), total L. infantum protein extract (10 µg/ml), LmSIR2 (5 µg/ml), whale skeletal muscle type II myoglobulin (MYO, 5 µg/ml), keyhole limpet haemocyanin (KLH, 5 µg/ml), LmS3a (5 µg/ml). The plates were washed with PBS containing 0·1% Tween-20 (PBS-T) and blocked with PBS 1% gelatine (200 µl/well) for 1 hr at 37°. The plates were incubated at 37° with serial dilutions (for total titres) or 1 : 100 dilutions in triplicate (for specific antibody reactivity) of each serum for 2 hr. After washing with PBS-T, the plates were incubated for 30 min at 37° with peroxidase-labelled goat anti-mouse immunoglobulin isotypes (anti-IgM, anti-IgG, anti-IgG1, anti-IgG2a) and developed with 0·5 mg/ml of o-phenylenediamine dihydrochloride (OPD, Sigma, St Louis, MO) with 10 µl of H2O2 in citrate buffer. The reactions were stopped by the addition of 50 µl of 3 m HCl to each well. The concentration of non-specific antibody was determined by comparison to a standard curve generated with unlabelled purified isotypes. Absorbance values were read at 492 nm in an automatic ELISA reader.
Western blot
The SDS–PAGE (12%) was run with 50 µg of LUC-L. infantum amastigotes total protein extract in each lane. After migration proteins were transferred onto a nitrocellulose membrane, using transfer buffer (10% of Tris-glycine 10×, 20% of ethanol 100%). Transfer was performed at 80 mA for 75 min at room temperature (RT). Membranes were then saturated for 1 hr with a solution of PBS (0·01 m pH 7·4) complemented with 5% fat dehydrated milk, during 1 hr at RT. The first antibody (sera from LmSIR2-treated mice, sera from PBS-treated mice or preimmune sera) was incubated overnight at 4°, with agitation, and then washed three times in 0·05% PBS–Tween for 15 min at RT and two times in PBS for 10 min. Horseradish peroxidase (HRP)-labelled goat antibodies to mouse IgG were then added at a dilution of 1 : 5000 and membranes were incubated for 1 hr at RT with agitation, followed by washing procedures as above. At last, it was revealed with ECL Western blotting analysis system.
Immunofluorescence assays
L. infantum axenic amastigotes were fixed with 4% paraformaldehyde in PBS for 1 hr at room temperature. After several washes, the parasites were permeabilized with 0·1% (v/v) Triton-X-100 in PBS. Parasites were then incubated with a mouse immune serum to LmSIR2 or preimmune serum (control) diluted 1 : 100 in PBS and containing 1% bovine serum albumin (PBS–1% BSA). The secondary antibody used was Alexa Fluor 488 goat anti-mouse IgG diluted 1 : 100 in PBS–1% BSA (Molecular Probes, Eugene, OR). Washed parasites were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA) and analysed with a fluorescent microscope (Axioskop-Carl Zeiss, Jena, Germany) at 1000× magnification and images captured with a digital camera (Spot 2-Diagnostic Instruments, Sterling Heights, MI, USA) and the software Spot 3.1 (Diagnostic Instruments, USA).
Complement mediated lysis assay (CML)
CML assay was done according to Norris et al.20 with some modifications. Briefly, cultured LUC-recombinant promastigotes or amastigotes were washed and resuspended in RPMI-1640 culture medium with 10% of BSA at 107/ml. A 50-µl portion of this suspension was incubated with a mouse anti-LmSIR2 immune serum for 1 hr, washed and incubated with rabbit complement (1 : 10) for an additional hour. All incubations were carried out at 37°. The complement lysis was determined by measuring the luciferase activity of the remaining intact parasites after washing. The percentage of lysis was determined as follows: killing percentage = 100 − [(luciferase activity after antibodies plus complement/luciferase activity after antibody plus inactivated complement) × 100].
In vitro macrophage infections
Peritoneal macrophages obtained from 6–8-week-old healthy male BALB/c mice were washed with prewarmed RPMI-1640 medium supplemented with 10% FCS, 2 mm glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin and cultured at 2 × 104 macrophages/well in 96-well test plates (TPP, Trasadingen, Switzerland). Non-adherent cells were removed by washing twice with prewarmed RPMI medium and macrophages were infected with cultured LUC-recombinant amastigotes (pretreated or not with sera for 1 hr at 37° with 5% CO2) at a ratio of 5 : 1 amastigotes per macrophage for 2 hr at 37° with 5% CO2. Non-internalized parasites were removed by gently washing, and culture was maintained for 24 hr. The sera were decomplemented by incubation at 56° for 1 hr in a water bath.
Luciferase activity
The luciferase activity of the LUC-recombinant parasites in the CML assay or in intracellular amastigotes isolated from infected macrophages was determined essentially as described elsewhere.21 Values were expressed as relative light units (RLU).
Statistical analysis
The data were analysed using Student's t-test.
Results
B cells, but not T cells, express CD69 in response to LmSIR2 treatment
In previous studies we have examined the antibody response during human and canine L. infantum infections using defined recombinant Leishmania proteins (LmS3a, LmSIR2 and LimTXNPx16). The LmSIR2 was found to be among highly immunoreactive antigens. Thus, we though it was reasonable to examine the effect of LmSIR2 on the cells of the immune system using a murine model.
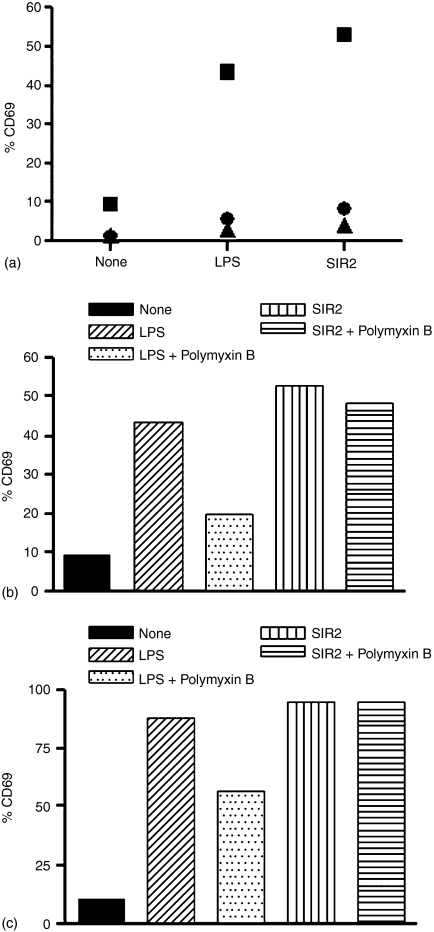
To identify the cell populations responding to LmSIR2, we analysed the in vitro membrane expression of CD69, an early activation marker, by CD4+, CD8+ and B cells. As shown in Fig. 1(a), normal spleen cells from BALB/c mice, cultured in the presence of LmSIR2 for 20 hr, showed increased expression of CD69 marker on B cells compared to the unstimulated cells. Interestingly, no significant increase in CD69 surface expression was observed in the case of CD4+ and CD8+ T-cell populations when subjected to the same treatment. As a positive control, lipopolysaccharide (LPS) induced high expression of CD69 by B cells (Fig. 1a). Therefore, in order to rule out the possibility that LmSIR2 biological activity could be linked to endotoxin contaminants, additional experiments where polymyxin B was added to the culture were conducted. As shown in Fig. 1(b), the presence of polymyxin B was able to significantly abrogate the LPS activity whereas no effect on LmSIR2-induced up-regulation of CD69 expression by spleen cells could be demonstrated.
Figure 1.
B cells express CD69 in response to LmSIR2. Expression of CD69 activation marker in spleen cells from BALB/c (a and b) and purified spleen B cells isolated from BALB/c mice (c) after culture with LmSIR2. A total of 2·5 × 105 cells per well were cultured in the presence of LmSIR2 (10 µg/ml) or LPS (10 µg/ml). In some experiments 10 µg of polymixin B per ml was added to LmSIR2 and LPS. To determine the percentage of CD69 in B (▪) cells or CD4 (•) or CD8 (▴) T cells, the different cell populations were positively gated. The results are from a representative experiment of three carried out independently.
To further explore whether the activation of B cells by LmSIR2 requires T-cell dependent mechanisms, complementary experiments were done using BALB/c purified B splenocytes. LmSIR2-treated purified B cells showed significant increase of CD69 surface expression (95% CD69 positive B cells) when compared to the control non-treated cells (10% CD69 positive B cells). Positive control test using LPS as a triggering agent showed increased expression of CD69 on B cells. Interestingly, polymyxin B partially prevents LPS but not LmSIR2-induced CD69 expression (Fig. 1c). These data allow excluding the potential involvement of contaminating endotoxin in LmSIR2-induced up-regulation of CD69 expression by B cells. Similar results were obtained when using spleen cells from athymic-BALB/c mice (nude mice; data not shown). It is thus reasonable to assume that the LmSIR2 preferentially triggers the direct activation of B cells.
LmSIR2 induces in vivo increased number of spleen B cells
Although LmSIR2 was able to stimulate the expression of CD69 on B cells, we failed to show in vitro LmSIR2-induced proliferation of B cells. Indeed, no proliferation of total spleen cells or purified B cells could be evidenced upon incubation with LmSIR2. This may suggest that, besides the LmSIR2 activity, others signals might be necessary to provide a fully B-cell activation and proliferation.22
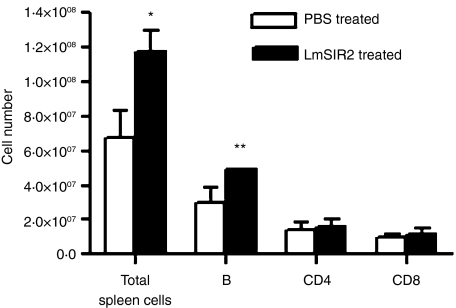
In order to analyse the in vivo effect of LmSIR2 on the cell populations, the protein was injected intravenously (i.v.) into BALB/c mice and 72 hr later the levels of cell populations in the spleen were determined by FACS analysis using mAb against cell surface markers. As shown in Fig. 2, large increases in total splenocytes were seen in the case of LmSIR2-injected mice when compared to the PBS-injected mice. Analysis of subpopulations of splenocytes showed striking increase of B-cell number (1·6-fold increase compared to the control mice, P < 0·05) whereas no significant difference in total CD4 or CD8 cells could be evidenced when compared to the control mice. Because of the fact that the total increase in the number of spleen cells is much greater than that of B cells, we can not rule out that other cell populations (i.e. macrophages, dendritic cells) are responding to LmSIR2. It is also possible that the increased number of splenic B cells might be the result of migration of B cells from lymph nodes or peripherical blood, not cell division. Taken together these observations strengthen the notion that B cells are targets of LmSIR2.
Figure 2.
In vivo injection of LmSIR2 into BALB/c mice induced spleen B-cell proliferation. The percentage of B cells, CD4 and CD8 T cells in the spleen of untreated and LmSIR2 (20 µg/mouse)-treated BALB/c mice 72 h after the LmSIR2 i.v. injection were determined by flow cytometric analysis. The number of each spleen cell subpopulation was calculated based on the total viable cells determined by trypan blue exclusion and the percentage of cell bound mAb to the specific cell surface marker (anti-CD4, anti-CD8 and anti-µ). The data represent the mean and the standard deviations of three animals analysed individually and are representative of two independent experiments. Statistically significant differences between untreated and LmSIR2 treated mice are indicated: *P < 0·01; **P < 0·05.
LmSIR2 injection into BALB/c mice promotes a strong humoral response with specific immunoglobulin secretion
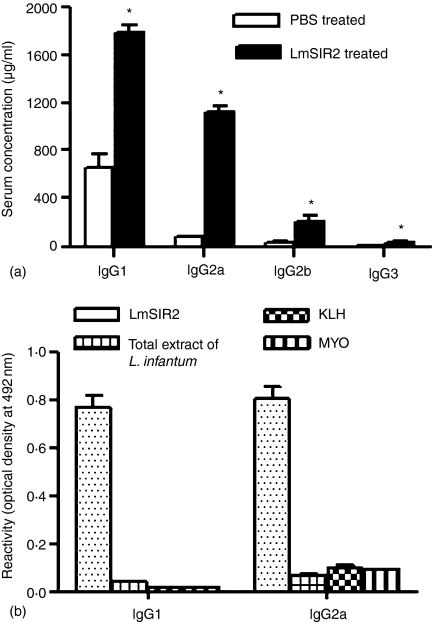
To further examine the effects of LmSIR2 on the B-cell response, we determined the levels of the different isotypes in the sera of mice that received three i.p. injections of LmSIR2 at 7 days interval, and control mice, which received PBS. A strong humoral response, as evidenced by increased levels of total immunoglobulins, was found in the sera of BALB/c mice injected with LmSIR2 compared to the sera from PBS-treated mice (Table 1). Statistical analysis showed significant differences between PBS and LmSIR2-treated mice only in the case of IgG antibodies (P = 0·005). Moreover, analysis of IgG antibodies showed significant increase in all IgG isotypes, being the higher increases in the IgG1 and IgG2a subclasses in the sera of mice which received LmSIR2 protein compared to the controls (P < 0·05, Fig. 3a).
Table 1.
Total serum immunoglobulins of BALB/c and BALB.nude mice 14 days after the last LmSIR2 injection
| Total serum Ig (µg/ml) | |||
|---|---|---|---|
| IgM | IgG | ||
| BALB/c | PBS-treated | 301·5 ± 70·3 | 1929·6 ± 61·4 |
| LmSIR2-treated | 649·0 ± 197·1 | 4830·9 ± 520·9 | |
| P = 0·1436 | P = 0·0050 | ||
| BALB/c nude | PBS-treated | 380·1 ± 33·1 | 464·4 ± 42·9 |
| LmSIR2-treated | 570·4 ± 79·1 | 453·5 ± 11·3 | |
| P = 0·0014 | P = 0·9670 | ||
Figure 3.
Humoral immune response of BALB/c mice 15 days after the last LmSIR2 injection. Groups of three mice were immunized i.p. with LmSIR2 as indicated in Materials and methods. (a) Levels of total IgG1, IgG2a, IgG2b and IgG3 in the sera of LmSIR2-treated and untreated BALB/c mice were quantified by ELISA in comparison to standard curves using purified mouse IgG1, IgG2a, IgG2b and IgG3, respectively. The asterisk indicates a statistically significant difference (P < 0·05) between untreated and LmSIR2-treated mice. (b) IgG1 and IgG2a antibodies levels in the sera of LmSIR2-treated BALB/c mice reacting against LmSIR2, L. infantum total extract, keyhole limpet hemocyanin (KLH) and myoglobulin (MYO) were determined by ELISA. Sera were diluted 1/100 in PBS−1% gelatin. The data represent the mean and the standard deviations of three animals analysed individually and are representative of two independent experiments.
Further, we explored the specificity of the immune response. As indicated in Fig. 3(b), mice treated with LmSIR2 developed IgG1 and IgG2a antibodies which recognized specifically LmSIR2. No reactivity could be seen when using heterologous antigens (i.e. KLH or myosin) or another recombinant Leishmania ribosomal protein namely LmS3a. However, very low reactivity was observed when using L. infantum total extracts in the ELISA test. This is not surprising given the specificity of the serum and that SIR2 protein is expressed at low levels whatever the Leishmania strain extract used (data not shown).
These observations indicate that LmSIR2 when injected without adjuvant in BALB/c mice induces a strong B-cell activation and differentiation leading to a strong humoral response with specific antibodies secretion.
LmSIR2 induce B-cell proliferation by a T-cell independent mechanism
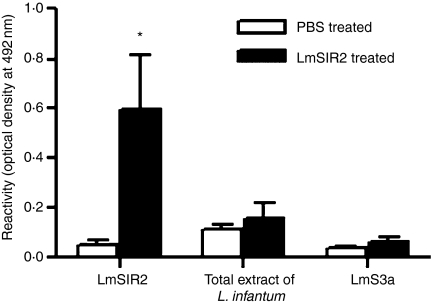
The above data therefore suggested that LmSIR2 when administrated in vivo to BALB/c mice stimulates the B, but not the T lymphocytes, inducing B-cell differentiation. To further confirm that LmSIR2 was activating B cells, but not T cells, similar experiments were performed in nude mice using LmSIR2 that was passed through an EndoTrap®red column to eliminate residual endotoxin contaminants. The data shown in Table 1 demonstrated that nude mice responded to LmSIR2 injection by producing significant levels of IgM antibodies when compared to PBS-injected nude mice (P = 0·0014). Moreover, the IgM antibodies when reacted in ELISA test showed specific recognition of LmSIR2 (Fig. 4). Altogether, these data suggest that LmSIR2 triggered significant B-cell differentiation and secretion of specific antibodies.
Figure 4.
Levels of specific IgM anti-LmSIR2 in the sera of BALB.nude mice. Sera from LmSIR2 and PBS injected mice were reacted in ELISA assay against L. infantum total extract, LmS3a or LmSIR2. Statistically significant differences between sera from LmSIR2-treated and control mice were observed: *P < 0·005.
Anti-LmSIR2 antibodies bind to the amastigote surface
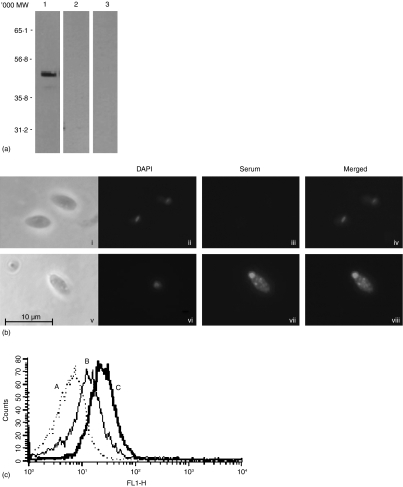
The above observations demonstrated that LmSIR2 immunization of mice induced a strong specific antibody response. Thus, we examined the reactivity of anti-LmSIR2 mice immune serum against L. infantum axenic amastigotes carrying a luciferase encoding gene (LUC-L. infantum). As shown in Fig. 5(a), sera from mice immunized with LmSIR2 recognized the corresponding antigen (MW ≈ 52 000) in LUC-L.infantum extracts whereas no reactivity could be seen when using preimmune sera or the sera from PBS-injected mice. Moreover, indirect immunofluorescence assays showed a positive labelling in vesicles and in the flagellar pocket zone (Fig. 5b), that may indicate protein secretion from the parasite.23
Figure 5.
(a) Western blot of LUC-L. infantum amastigotes total protein extract reacted with sera from LmSIR2-treated BALB/c mice (1); sera from PBS-treated mice (2); preimmune sera (3). (b) Immunofluorescence using a preimmune serum (ii–iv) and serum from LmSIR2-treated mice (vi–viii) reacted with L. infantum axenic amastigotes. Parasites were photographed at 1000×. Contrast phase pictures of the preparations are also included (i, v). (c) FACS analysis of anti-LmSIR2 antibodies binding to LUC-L. infantum amastigote surface. Parasites were incubated with mouse immune serum to LmSIR2, followed by FITC-conjugated goat antimouse IgG (C). As controls, amastigotes were incubated with FITC-conjugated goat antimouse IgG (A) or preimmune serum followed by FITC-conjugated goat antimouse IgG (B).
We then analysed by FACS whether a binding of the anti-LmSIR2 antibodies was detectable. The results shown in Fig. 5(c) indicated that indeed, anti-LmSIR2 bound to the amastigote. The level of binding was approximately three times higher when using sera from LmSIR2-immunized mice compared to preimmune sera (MFI = 45 and 15, respectively).
Anti-LmSIR2 antibodies induce complement mediated lysis and inhibit amastigote developpement inside macrophages in vitro
The above data indicate that LmSIR2 epitopes are expressed on the parasite surface and are accessible to the antibodies. Therefore, we thought it was reasonable to determine the ability of sera from LmSIR2-immunized mice to lyse in vitro LUC-L. infantum axenic amastigotes. As shown in Table 2, the percentage of killing of amastigotes was between 21 and 45% depending on the concentration of serum used. As a control, preimmune serum was unable to support lysis of the parasites even at high concentration. This was also the case for a IIIG4 mAb against LmSIR2,14 which showed no capacity to induce parasite lysis. This might be the result of the fact that IIIG4 mAb, being of the IgG1 subclass, fixes complement poorly. Furthermore, the anti-LmSIR2 immune serum was also found to be able to induce the lysis of LUC-L. infantum promastigotes (data not shown).
Table 2.
Complement-mediated lysis of L.infantum axenic amastigotes
| % Lysis at a dilution ofa | ||
|---|---|---|
| Treatment | 1 : 5b | 1 : 10b |
| Anti-LmSIR2 mouse immune serum | 45 | 21 |
| Monoclonal Anti-LmSIR2 (IIIG4) | 2 | 1 |
| Pre-immune serum | 0 | 0 |
The percentage of lysis is the mean of triplicate determinations of a representative experiment from three carried out independently.
A optimized 1 : 10 dilution of rabbit complement was used with different anti-LmSIR2 immune serum dilutions.
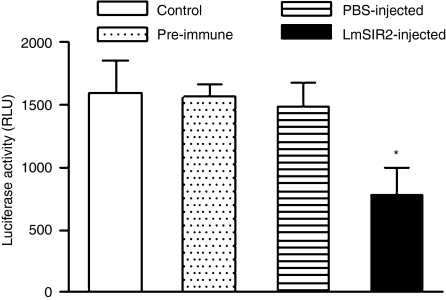
We also tested whether the anti-LmSIR2 immune sera were able to modulate in vitro amastigote–macrophage interaction. Mouse peritoneal macrophages were used as in vitro model to measure infection with LUC-L.infantum axenic amastigotes in the presence of heat-inactivated anti-LmSIR2 immune serum. Thus, peritoneal macrophages were incubated with LUC-L. infantum axenic amastigotes at a ratio of five parasites per macrophage in the presence of 1 : 10 dilution of preimmune serum or sera from LmSIR2 and PBS-injected mice. Twenty-four hr later, the level of infection was determined. As shown in Fig. 6 there was a significant inhibition of amastigote development inside macrophages in the presence of heat-inactivated anti-LmSIR2 immune serum when compared to the control preimmune sera or sera from PBS-injected mice. These data suggest that anti-LmSIR2 sera in the absence of complement can reduce intracellular parasite development.
Figure 6.
Effect of anti-LmSIR2 antibodies on amastigote development inside macrophages. A total number of 2 × 104 BALB/c peritoneal macrophages were infected at a ratio of 5 : 1 LUC-amastigotes/macrophage and the number of intracellular amastigotes was determined after 24 h by measuring luciferase activity. Prior to infection, the amastigotes were incubated with indicated sera samples (1 : 10 dilution) or control (no serum). The asterisk indicates a statistically significant difference (*P < 0·01) in comparison with the values obtained using normal serum. The results are from a representative experiment of three carried out independently.
In preliminary immunization experiments, two groups of four mice each were injected i.p. with or without 50 µg of LmSIR2 in 200 µl of PBS three times at 7 day intervals. Fifteen days later, they were infected i.p. with 108 WT promastigotes. At 15 days postinfection, a significant decrease (≈1·5-log reduction; P = 0·03) of parasite load was observed in the spleen of LmSIR2-immunized mice in comparison to the control (PBS-injected mice).
Discussion
In the current study, we demonstrated that the Leishmania cytosolic nicotinamide adenine dinucleotide-dependent deacetylase, LmSIR2, could directly activate B lymphocytes (but not T cells) from normal mice. However, although we were unable, though, to show the in vitro proliferation of spleen cells or purified B cells upon incubation with LmSIR2, in vivo administration of LmSIR2 i.v. into BALB/c mice triggered the differentiation of B cells, demonstrating therefore that LmSIR2 could serve as an important activation signal in vivo. Indeed, in vivo injection of LmSIR2 alone without adjuvant into BALB/c mice or nude mice induced the synthesis of IgG and IgM antibodies, respectively, thereby indicating that B cells were able to undergo differentiation into antibody-secreting cells. Several experiments demonstrated that the B lymphocytes were responding directly to LmSIR2 and not to endotoxin contaminants. The production of antibodies in nude mice that lack T cells is direct evidence of the capacity of LmSIR2 to induce a humoral response by a T-cell independent mechanism. Because the nude mice are athymic, these animals cannot produce T-cell cytokines for isotype switching of antibodies. Indeed, in the case of BALB/c mice, an immunoglobulin isotype switch occurs, as shown by the high production of all IgG isotypes. Taken into account that T-cell independent antigens stimulate mainly the IgM production, being the IgG secretion in lower amounts, restricted to the IgG3 isotype24 it is tempting to speculate that LmSIR2 has dual functions in vivo, being able to act as a T-cell independent or a T-cell dependent antigen, which could explain its enhanced immunogenicity. However, the classic division of antigens in these two categories is not absolute. In fact, previous studies in hepatitis B and vesicle stomatites virus have already reported the existence of antigens that can induce B-cell activation and proliferation through both T-dependent and -independent mechanisms.25,26 However, other possible interpretations can be made. It has been proposed that in immunocompetent mice, after activating the B cells, some T-independent antigens require a ‘second signal’ in order to develop an IgG secretory response27 and this could also be the case for LmSIR2. However, so far the support for the ‘second signal hypothesis’ has been elusive. This may be due to the high number of candidates that are capable of giving this potential second signal.27 Thus, these responses might be dependent on differentiation-inducing factors produced by antigen non-specific cells.28 Natural killer cells and macrophages were already been referred to be involved in this process, as much for the release of cytokines after activation as for the mobilization of T cells and thereby their derived cytokines.29 Recently, two tumour necrosis factor (TNF) family ligands, BAFF (B-cell activation factor of the TNF family) and APRIL (a proliferation-inducing ligand) were implicated in several immunological phenomena, such as peripheral B-cell survival, T-cell independent antibody isotype switching, and the induction of self-reactive B cells.30 These ligands are expressed in macrophages, dendritic cells and T cells, and their up-regulation caused by cytokines produced through the activation of the immune system provides survival signals, and also may contribute to class switch recombination in B cells activated by T-independent antigens.31 Thus, so far, the mechanism by which LmSIR2 is capable of inducing immunoglobulin isotype switching in the presence of T cells but not in their absence (BALB/c mice and BALB.nude mice, respectively) is still unknown. Further studies will have to investigate in detail the mechanisms leading to the LmSIR2-induced isotype switching in vivo.
It is well known that Leishmania spp. release a large number of molecules that could act as mitogenic substances inducing polyclonal lymphocyte responses and consequently a general lack of specificity of antibodies and T-cell responses during the infection. Indeed, soluble parasite-derived antigens from L. major and L. donovani are mitogenic and trigger the production of immunoglobulins with autoantibody activity.6 Thus, crude extracts of L. donovani contains components that cause strong in vitro polyclonal activation of hamster spleen cells.7 Moreover, an excreted factor derived from the culture medium of L. major was found to suppress concanavalin A-induced polyclonal activation of mouse T cells.32 Furthermore, in previous reports, we have identified a Leishmania gene encoding a protein sharing significant homology to mammalian ribosomal proteins S3a named LmS3a exhibiting dual activity being stimulatory and inhibitory towards T and B cells, respectively.33 The investigations on the immunogenicity of LmS3a have revealed that in vivo, a single injection of the recombinant protein without any adjuvant into mice induced a quick increase in the number of B cells and the production of high levels of immunoglobulins, mainly of IgM isotype. The IgM response was mostly unrelated to the antigens present in the total parasite extracts or to the protein itself. These observations contrasted with the in vivo LmSIR2 activity on B cells. Indeed, although the protein triggered B-cell differentiation, the antibodies produced reacted specifically against SIR2 and not other heterologous antigens such as myosin or KLH, and were able to induce the complement-mediated killing of amastigotes and inhibition of their multiplication inside macrophages. Indirect immunofluorescence assays of L. infantum axenic amastigotes with sera from LmSIR2-immunized mice showed the presence of SIR2 protein in vesicles and in the flagellar pocket zone, already known to be filled with secretory material;23 this being therefore in agreement with our previous observations.18 Moreover, using a highly sensitive radiolabelled immunoprecipitation technique, we observed that SIR2 is among the parasite excreted-secreted antigens (unpublished data). Results from the in vitro macrophage infections with LUC-L. infantum amastigotes, the presence of anti-LmSIR2 sera may indicate a potential role of SIR2 in the binding, internalization and/or multiplication of the parasite in the macrophage.
Studies on the molecular mechanisms of parasite entry into macrophages have led to the identification of several candidate receptors facilitating multiple routes of entry.34 Indeed, internalization of promastigotes into macrophages has been shown to be mediated by macrophage membrane proteins such as the mannose receptor,35 the fibronectin receptor,36 the Fc receptor (FcR),37 and the complement receptors such as CR1 (CD35) and CR3 (CD11b/CD18).35,38 Thus, the in vitro modulation of macrophage infection by anti-LmSIR2 antibodies may suggest possible roles of SIR2 in the internalization process of the amastigote and/or further multiplication of the parasite.
Evasion from the host complement system is one of the strategies used by Leishmania parasites to avoid host immune defence.39 Indeed, metacylic promastigotes and amastigotes are relatively resistant to direct serum killing.40 However, previous studies have shown that antibodies that were able to bind to living parasites and lyse them in conjunction with complement were associated with host protection.20 Thus, one can assume that, the production of antibodies capable of enhancing complement cytotoxicity towards the amastigote stage might be, working in co-operation with the cellular immunity, an important requirement for effective antiparasitic immunity.41 Given that the anti-LmSIR2 sera induced complement-mediated lysis of amastigotes and promastigotes, one may speculate that a LmSIR2 immunization can be seen as capable of protecting the host against infection and disease progression.
Although it has been reported that the antibodies may play a role in the host protection mechanisms against experimental leishmaniasis12 a more recent study has shown that antibodies could exert deleterious effects on the host. In fact, by using B cell-mutant mice and genetically modified mice lacking circulating antibodies infected with L. amazonensis and L. pifanoi, the authors reported that these mice developed barely detectable lesions compared to control BALB/c mice.42 Reconstitution of the B cell-mutant with the immune anti-Leishmania serum increased the pathological processes in the otherwise non-susceptible mice. Therefore, preliminary BALB/c immunization experiments were conducted using LmSIR2 protein. A significant decrease of parasite load in the spleen of LmSIR2-immunized mice was observed in comparison to non-immunized control mice at 2 weeks postchallenge infection. Thus, it is reasonable to suggest that anti-LmSIR2 antibodies may in part play a role in protective immune mechanisms rather than exacerbating the disease. Further studies using different doses and routes of immunization are needed to explore further the protective role of the LmSIR2 molecule.
The LmSIR2 protein belongs to a highly conserved family of closely related proteins in both prokaryotic and eukaryotic species.43 Historically, the biological significance of SIR2-like proteins was attributed to the histones' deacetylation, leading to chromatin condensation and transcriptional silencing.44 However, diverse cellular localizations were found among SIR2 homologues, suggesting that these enzymes have other physiological substrates than histones and thus several biological functions inside the different organisms. Indeed, several roles have been attributed to SIR2-related family of proteins including cell cycle progression and chromosome stability,45 DNA-damage repair,46 life span extension in yeast47 and in Caenorhabditis elegans.48 To our knowledge, this report is the first description of a protein belonging to this large family, which is among the Leishmania cytosolic and secretory products that proved to have a role in the regulation of the immune response through its capacity to trigger preferentially B-cell effector functions. The high degree of homology within this family of proteins, and the fact that homologues have been found in mouse49 and human50 has led us to perform additional experiments in order to verify the possibility of the existence of SIR2 cross-reactive epitopes on the mouse and human cells that could be recognized by the sera from LmSIR2-immunized mice. Using different approaches (Western blot, ELISA, immunoprecipitation of [35S]methionine-labelled mouse spleen cells) no such cross-reactivity was found, arguing for the Leishmania specificity of the antibodies produced by LmSIR2 immunization (data not shown).
In summary, our present results show that LmSIR2 is a potent B-cell modulatory factor both in vitro and in vivo. Following the injections of LmSIR2 without adjuvant a selective B-cell response was induced resulting in a surprisingly parasite-specific production of antibodies, which were lytic in co-operation with the complement, and restrain the capacity of the parasites to infect macrophages. There is strong evidence that an ideal vaccine, especially against visceral leishmaniasis, may require the induction of both humoral and cellular branches of the immune system, for maximum efficiency.11 In that view, one can consider LmSIR2 to be among vaccination candidate, taking into account the safety and the strong response when delivered in a low dose. Overall, these data add LmSIR2 to the list of Leishmania antigens that could specifically stimulate the immune system of the host and suggest that LmSIR2 could be among the parasite molecules to be used to design an optimal multicomponent vaccine to control Leishmania infection.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (FCT) and FEDER, grant POCTI/CVT/39257/2001 and POCI/CVT/59840/2004, INSERM and IRD UR008. R.S. and J.T. are supported by fellowships from FCT and FEDER number SFRH/BD/13120/2003 and SFRH/BD/18137/2004, respectively.
Abbreviations
- VL
visceral leishmaniasis
- CML
complement-mediated lysis
- FCS
fetal calf serum
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- i.p.
intraperitoneally
- i.v.
intravenously
- MFI
mean fluorescence intensity
- ELISA
enzyme-linked immunosorbent assays
- RLU
relative light units
- WT
wild type
- FACS
fluorescence-activated cell sorter
References
- 1.Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, Teixeira R, Johnson WD. New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–11. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 2.Ashford RW. Leishmaniasis reservoirs and their siginificance in control. Clin Dermatol. 1996;14:523–32. doi: 10.1016/0738-081x(96)00041-7. [DOI] [PubMed] [Google Scholar]
- 3.Desjeux P. Leishmaniasis: public health aspects and control. Clin Dermatol. 1996;14:417–23. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho EM, Teixeira RS, Johnson WD. Cell-mediated immunity in American visceral leishmaniasis: reversible immunosupression during acute infection. Infect Immun. 1981;33:498–502. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galvao-Castro B, Ferreira JAS, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Polyclonal B cell activation, circulating immune complexes and autoimmunity in American visceral leishmaniasis. Clin Exp Immunol. 1984;56:58–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Bohme MW, Evans DA, Miles MA, Holborow EJ. Occurrence of autoantibodies to intermediate filament proteins in human visceral leishmaniasis and their induction by experimental polyclonal B-cell activation. Immunology. 1986;59(4):583–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Bunn-Moreno MM, Madeira ED, Miller K, Menezes JA, Campos-Neto A. Hypergammaglobulinaemia in Leishmania donovani infected hamsters. possible association with a polyclonal activator of B cells and with supression of T cell function. Clin Exp Immunol. 1985;59(2):427–34. [PMC free article] [PubMed] [Google Scholar]
- 8.Reina-San-Martin B, Cosson A, Minoprio P. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol Today. 2000;16:62–7. doi: 10.1016/s0169-4758(99)01591-4. [DOI] [PubMed] [Google Scholar]
- 9.McMahon-Pratt D, Alexander J. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniasis or the visceral disease? Immun Rev. 2004;201:206–24. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 10.Casadevall A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998;6:102–7. doi: 10.1016/s0966-842x(98)01208-6. [DOI] [PubMed] [Google Scholar]
- 11.Ravindran R, Nahid A. Progress in vaccine research and possible effector mechanism in visceral leishmaniasis. Curr Mol Med. 2004;4:697–709. doi: 10.2174/1566524043360212. [DOI] [PubMed] [Google Scholar]
- 12.Monjour L, Berneman A, Vouldoukis I, Domurado M, Guillemin MC, Chopin C, Alfred C, Roseto A. Monoclonal antibodies against defined Leishmania antigens protect mice against infection by different species of Leishmania. CR Acad Sci III. 1985;300(9):395–8. [PubMed] [Google Scholar]
- 13.Yahiaoui B, Taibi A, Ouaissi A. A Leishmania major protein with extensive homology to silent information regulator 2 of Saccharomyces cerevisae. Gene. 1996;169:115–8. doi: 10.1016/0378-1119(95)00785-7. [DOI] [PubMed] [Google Scholar]
- 14.Vergnes B, Sereno D, Madjidian-Sereno N, Lemesre JL, Ouaissi A. Cytoplasmic SIR2 homologue overexpression promotes survival of Leishmania parasites by preventing programmed cell death. Gene. 2002;296:139–50. doi: 10.1016/s0378-1119(02)00842-9. [DOI] [PubMed] [Google Scholar]
- 15.Vergnes B, Sereno D, Tavares J, et al. Targeted disruption of cytosolic SIR2 deacetylase discloses its essential role in Leishmania survival and proliferation. Gene. 2005;363:85–96. doi: 10.1016/j.gene.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Cordeiro-da-Silva A, Cardoso L, Araújo N, et al. Identification of antibodies to Leishmania silent information regulatory 2 (SIR2) protein homologue during canine natural infections: pathological implications. Immunol Lett. 2003;68:155–62. doi: 10.1016/s0165-2478(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 17.Sereno D, Roy G, Lemesre JL, Papadopoulou B, Ouellette M. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antmicrob Agents Chemother. 2001;45:1168–73. doi: 10.1128/AAC.45.4.1168-1173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zemzoumi K, Sereno D, Francis C, Guilvard E, Lemesre JL, Ouaissi A. Leishmania major. Cell type dependent distribution of a 43 kDa antigen related to silent information regulatory-2 protein family. Biol Cell. 1998;90:239–45. doi: 10.1016/s0248-4900(98)80020-8. [DOI] [PubMed] [Google Scholar]
- 19.Lowry OH, Rosebrough NJ, Farr L, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:267–9. [PubMed] [Google Scholar]
- 20.Norris KA, Hearth G, So M. Purification of a Trypanosoma cruzi membrane glycoprotein which elicits lytic antibody. Infect Immun. 1989;57:2372–7. doi: 10.1128/iai.57.8.2372-2377.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy G, Dumas C, Sereno D, et al. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol. 2000;110:195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]
- 22.Baumgarth N. A two-phase of B-cell activation. Immunol Rev. 2000;176:171–80. doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 23.Pimenta PFP, Saraiva EMB, Sacks D. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Exp Parasitol. 1991;72:191–204. doi: 10.1016/0014-4894(91)90137-l. [DOI] [PubMed] [Google Scholar]
- 24.Bjorklund M, Coutinho A. Isotype commitment in the in vivo immune responses. II. Polyclonal plaque-forming cell responses to lipopolysaccharide in the spleen and bone marrow. Euro J Immunol. 1983;13:44–50. doi: 10.1002/eji.1830130111. [DOI] [PubMed] [Google Scholar]
- 25.Freer G, Burkhart C, Ciernik I, Bachmann MF, Hengartner H, Zinkernagel RM. Vesicular stomatitis virus Indiana glycoprotein as a T-cell-dependent and – independent antigen. J Virol. 1994;68:3650–5. doi: 10.1128/jvi.68.6.3650-3655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986;234:1398–401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- 27.Vos Q, Lees A, Wu Z, Snapper CM, Mond JJ. B-cell activation by a T-cell independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganism. Immunol Rev. 2000;176:154–70. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 28.Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–7. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 29.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–54. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 30.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Castigli E, Wilson SA, Scott S, et al. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–9. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimaldi GJ, Tesh RB. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbial Rev. 1993;6(3):230–50. doi: 10.1128/cmr.6.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cordeiro-da-Silva A, Borges MC, Guilvard E, Ouaissi A. Dual role of the Leishmania major ribosomal protein S3a homologue in regulation of T- and B-cell activation. Infect Immun. 2001;69:6588–96. doi: 10.1128/IAI.69.11.6588-6596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 35.Wilson ME, Pearson RD. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988;56:363–9. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brittingham A, Chen G, McGwire BS, Chang KP, Mosser DM. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect Immun. 1999;67:4477–84. doi: 10.1128/iai.67.9.4477-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Love DC, Kane MM, Mosser DM. Leishmania amazonensis: the phagocytosis of amastigotes by macrophages. Exp Parasitol. 1998;88(3):161–71. doi: 10.1006/expr.1998.4232. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez M, Torano A. Immune adherence-mediated opsonophagocytosis. mechanism of Leishmania infection. J Exp Med. 1999;189:25–35. doi: 10.1084/jem.189.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bogdan C, Röllinghoff M. The immune response to Leishmania: mechanism of parasite control and evasion. Int J Parasitol. 1998;28:121–34. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 40.Hall BF, Joiner KA. Strategies of obligate intracellular parasites for evading host defenses. Parasitol Today. 1991;12:A22–A7. doi: 10.1016/S0167-5699(05)80007-6. [DOI] [PubMed] [Google Scholar]
- 41.Hoover DL, Berger M, Oppenheim MH, Hockmeyer WT, Meltzer MS. Cytotoxicity of human serum for Leishmania donovani amastigotes: antibody facilitation of alternate complement pathway-mediated killing. Infect Immun. 1985;47(1):247–52. doi: 10.1128/iai.47.1.247-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191(6):1063–7. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–8. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 44.Gasser SM, Cockell MM. The molecular biology of the SIR proteins. Gene. 2001;279:1–16. doi: 10.1016/s0378-1119(01)00741-7. [DOI] [PubMed] [Google Scholar]
- 45.Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 46.Tsukamoto Y, Kato J, Ikeda H. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997;388:900–3. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- 47.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–80. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tissenbaum HA, Guarente L. Increased dosage of a Sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–30. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 49.Yang YH, Chen YH, Zhang CY, Nimmakayalu MA, Ward DC, Weissman S. Cloning and characterization of two mouse genes with homology to the yeast Sir2 gene. Genomics. 2000;69:355–69. doi: 10.1006/geno.2000.6360. [DOI] [PubMed] [Google Scholar]
- 50.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: SIR2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–9. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]