Abstract
We have previously shown that normal human peripheral blood polymorphonuclear neutrophils (PMNs) contain cytoplasmic ‘stores’ of three key molecules normally associated with antigen presentation and T-cell costimulation, i.e. major histocompatibility complex class II (DR) antigen, CD80 (B7-1) and CD86 (B7-2). These cytoplasmic molecules were found to translocate to the cell surface within a few minutes following cross-linking (X-L) of Mac-1: an early neutrophil activation signal. In this study we have compared X-L of Mac −1 in parallel with four other well documented in vitro neutrophil activators: phorbol myristate acetate, N-formyl methionyl leucyl phenylalanine, lipopolysaccharide, and phagocytosis of immunoglobulin G–Latex particles. In addition, we have used paired samples of neutrophils obtained from peripheral blood (as a control) and synovial fluid from patients with rheumatoid arthritis as a source of in vivo activated cells. With the exception of phagocytosis, all activators resulted in the rapid (within 30 min) generation of two populations of activated neutrophils (designated P1 and P2) based on flow-cytometry measurements of size, granularity and phenotype. Significant up-regulation of DR and costimulatory molecules was observed, predominantly on P2 cells, with all activators except phagocytosis. CD80 and CD86 were noted to respond to the various activation signals in a different pattern suggesting that their intracellular granule location may be different. Dual-staining confocal laser microscopy studies showed that CD80 is largely confined to secretory vesicles (SVs) while CD86 appears to have a much wider distribution being found in SVs and within secondary (specific) and primary (azurophilic) granules. Increased surface expression of these antigens was also observed on P2 synovial fluid neutrophils appearing as large heterogeneous clusters on the cell surface when visualized by confocal laser microscopy.
Keywords: costimulatory molecules, MHC class II antigen, neutrophils
Introduction
Polymorphonuclear neutrophils (PMNs) have been shown to contain many different types of receptor molecule preformed within cytoplasmic granules and in particular within secretory vesicles (SVs).1,2 These cytoplasmic ‘reservoirs’ may then rapidly translocate to the cell surface thereby providing an immediate change in cell phenotype and function; a desirable feature for cells required for rapid immune responses. Active synthesis of receptor molecules may also occur later thereby providing for sustained receptor expression on the cell surface.3
We have recently shown that normal human peripheral blood PMNs contain cytoplasmic stores of major histocompatibility complex (MHC) class II (DR) antigen and two important costimulatory molecules, CD80 (B7-1) and CD86 (B7-2). These molecules are not constitutively expressed on the surface of circulating PMNs4 but may appear when PMNs are pretreated with certain cytokines in vitro and have been demonstrated in vivo on a subpopulation of peripheral blood PMNs obtained from patients with active Wegener's granulomatosus.5,6 It therefore seems likely that activated PMNs may function as antigen-presenting cells (APCs)7 and may even play a role in T-cell regulation within sites of inflammation.
In a previous study,4 we have shown by dual-staining confocal laser microscopy and immunoelectron microscopy that normal human peripheral blood PMNs contain cytoplasmic reservoirs of CD80 within SVs Because intracellular CD80 was found to colocalize with CD86 and MHC class II antigen it was assumed that all three of these molecules reside primarily within SVs. It was also shown that these molecules were rapidly, within a few minutes, deployed to the PMN cell surface following in vitro cross-linking of the Mac-1 heterodimer (CD11b + CD18).4 This observation is therefore consistent with translocation of preformed receptor molecules from SVs with subsequent incorporation of these molecules into the plasma membrane.
In this study we have investigated the possibility that rapid (within 30 min) translocation of costimulatory molecules, from preformed cytoplasmic stores, may also occur when PMNs are activated via other pathways using a variety of well documented in vitro neutrophil activation signals: phorbol myristate acetate (PMA), lipopolysaccharide (LPS), N-formyl methionyl leucyl phenylalanine (fMLP), and phagocytosis of immunoglobulin G (IgG)–Latex particles. Furthermore, LPS and fMLP have been shown to preferentially stimulate release of molecules from neutrophil SVs when used at very low concentrations, while PMA also stimulates release of molecules from secondary (neutrophil specific) and tertiary granules.8,9 By comparing these activation signals in parallel we therefore hoped to obtain further information regarding the precise intracellular location of these molecules.
In this study we also utilized synovial fluid PMNs aspirated from the inflamed knee joints of patients with rheumatoid arthritis (RA) as a source of in vivo activated neutrophils. The mechanisms involved in in vivo activation are complicated and not completely understood. As circulating neutrophils pass through the endothelium en route to the site of inflammation they bind to adhesion molecules on the vascular endothelium, e.g. intracellular adhesion molecule-1 (ICAM-1), utilizing receptor molecules like leucocyte function-associated antigen-1 and Mac−1. These interactions may serve to activate neutrophils as they move into the site of infection.10 Similarly, synovial fluid contains soluble immune complexes and a wide variety of molecules, e.g. leukotriene Β4, C5a and granulocyte–macrophage colony-stimulating factor all of which have the potential to activate PMNs in situ.11,12 It is therefore not surprising that large numbers of activated and primed PMNs have been reported in RA synovial fluid.13 Phenotyping studies have shown that many synovial fluid neutrophils show increased expression of CD66, and CD64 (FcγRI) relative to circulating cells.14,15 Up-regulation of these molecules on the surface of PMNs is therefore widely regarded as an indicator of cell activation. Cross et al.16 have also shown that synovial neutrophils, purified by density gradient centrifugation, can be ‘persuaded’ to express cell surface MHC class II antigen when incubated at 37° for 20 hr in culture medium alone, but expression of B7 costimulatory molecules (CD80 and CD86) was however, comparatively weak.
In this study we have used a simple whole blood (or synovial fluid) flow-cytometry method to study the effects of in vitro and in vivo neutrophil activation on expression of MHC class II antigen and costimulatory molecules.
Materials and methods
Healthy donors
With ethical consent, samples of blood were taken from 10 members of hospital staff. These donors consisted of five males and five females, average age = 49 years. All donors were well at the time of testing with no history (or family history) of known allergies, or autoimmune disease and were not on any medication.
Patients
With ethical consent, samples of blood and synovial fluid (from knee aspirates) were taken simultaneously from nine patients with rheumatoid arthritis (diagnosed according to criteria of the American College of Rheumatology 1987) attending the out patients clinic at Gartnavel General Hospital, Glasgow. Samples of synovial fluid were transported rapidly from the clinic to the laboratory and the assay commenced typically within one hour of collection in order to ensure maximum viability of cells (>95% cells viable as assessed by uptake of propidium iodide and exclusion of phycoerythrin (r-PE)-conjugated anti-myeloperoxidase (MPO) antibody).
Eight of these patients were receiving anti-inflammatory therapy at the time of testing – non-steroidal anti-inflammatory drugs (NSAIDs) and/or disease-modifying anti-rheumatic drug (DMARD) therapy as shown in Table 1.
Table 1.
Patient details and therapy
| Patient | Age/sex | NSAIDs | DMARD |
|---|---|---|---|
| 1 | 81/M | Ibuprofen 400 mg tid | no |
| 2 | 65/F | no | no |
| 3 | 64/F | Naproxen 500 mg bd | Sulfasalazine 4·5 g Methotrexate 20 mg/week Hydroxychloroquine 200 mg |
| 4 | 61/F | no | Sulfasalazine 2 g Methotrexate 17·5 mg/week Hydroxychloroquine 200 mg |
| 5 | 61/M | Indometacin 25 mg | Sulfasalazine 3 g |
| 6 | 77/F | Etodolac 600 mg | Sulfasalazine 4 g |
| 7 | 55/F | Co-proxamol, as needed | Hydroxychloroquine, 200 mg |
| Etodolac, 600 mg | Sulfasalazine, 2·5 g | ||
| 8 | 66/F | Naproxen, 500 mg | Hydroxychloroquine, 200 mg Sulfasalazine, 4·5 g Methotrexate, 20 mg/wkly Adalimumab, 40 mg/2 wkly |
| 9 | 78/F | Etodolac, 600 mg | Penicillamine, 250 mg |
Whole-blood (fluid) assay for the study of neutrophil activation
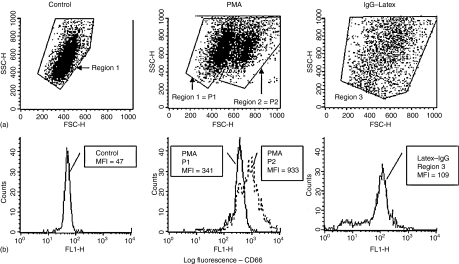
In a previous study the effect of cross-linking neutrophil CD11b on surface expression of MHC class II antigen (DR), CD80 and CD86 was investigated using a whole blood assay. This assay (previously discussed in full)4 has many advantages over other methods where isolated neutrophils are used. For example, it is rapid, inexpensive, requires only small volumes of blood (or fluid) and more importantly does not result in spontaneous activation of neutrophils. For the purposes of this study a similar whole blood method was adopted for the study of other neutrophil activators. The optimum concentration of each activator was established by pretitration based on the total number of large ‘activated’ neutrophils generated (gated as P2 cells using dot-plots of forward versus side scatter – see Fig. 1), surface expression of CD66 and viability as assessed by the uptake of propidium iodide.
Figure 1.
In vitro activation of neutrophils: effect of PMA and phagocytosis of IgG–Latex. (a) Flow cytometry density-plots of forward scatter – FSC (cell size) versus side scatter – SSC (granularity) showing control unstimulated neutrophils compared with neutrophils activated in vitro with PMA or with IgG–Latex. Control cells were observed within a fairly homogeneous population and were gated as region 1 for analysis. Following activation with PMA, two distinct populations of neutrophils were evident – population 1 (P1) was essentially similar to control cells but population 2 (P2) cells showed increased forward scatter. In general, P2 cells had a forward scatter value of >600 and P1 cells <600 but to allow for instrument and individual donor variations these two regions were simply gated by eye for analysis in each experiment. This two-population pattern was also observed following in vitro activation of neutrophils with lipopolysaccharide, fMLP and following cross-linking of neutrophil CD11b. In contrast, activation of neutrophils following ingestion of IgG–Latex particles resulted in a dramatic increase in SSC (settings had to be adjusted in order to visualize these cells) and a slight increase in FSC. However, phagocytosis did not result in the formation of two clearly identifiable populations of cells and for this reason all neutrophils were gated as a single population (region 3) for analysis. (b) All neutrophils express cell surface CD66 as shown in this example by flow-cytometry histogram analysis. The mean fluorescence intensity values (MFI) for cell surface CD66 expression were found to increase dramatically following in vitro activation with PMA with much higher levels observed on the P2 cells. Increased surface expression of CD66 was also observed to a lesser extent on latex-IgG activated neutrophils. Note: This figure illustrates a typical experiment and was designed to show how cells were gated for analysis and how MFI values for cell surface CD antigen expression were obtained. Mean MFI values for multiple experiments are shown in Table 2.
Whole blood assay
To 100 μl of heparinized whole blood (or synovial fluid) was added 25 μl Dulbecco's modified Eagle's medium (DMEM), i.e. background control or 25 μl of activator at the appropriate concentration: PMA (obtained from Sigma, Poole, UK and used at 250 ng/ml blood), fMLP (Sigma; 0·25 ng/ml blood), LPS (Sigma; 25 ng/ml blood) and IgG latex particles (RapiTex beads obtained from Dade Behring, Germany and used at 25 μl per 100 μl whole blood).
Samples were then incubated for 30 min at 37° in a non-shaking water bath, and washed in 2 ml phosphate-buffered saline (PBS) by centrifugation at 200 g for 5 min. The supernatant was discarded and 5 μl of fluoroscein isothiocyanate (FITC)-conjugated mouse monoclonal antibody (all IgG1) was added. The optimum concentration for each antibody was determined by pretitration.
FITC-conjugated antibodies used were as follows: mouse IgG1 (clone MOPC 21; BD Pharmingen, Oxford, UK) used as a negative control; anti-CD66abce (clone Kat4c; Dako Cytomation, Denmark) as positive control; anti-MHC class II (DR) – clone TU149, anti-CD80 (clone MEM233) and anti-CD86 (clone BU63) from Caltag Laboratories, Towcester, UK.
Following incubation at 4° for 30 min cells were incubated for 5 min at room temperature in 2 ml fluorescence-activated cell sorting (FACS) lysing solution (Becton-Dickinson, San Jose, CA) and centrifuged at 200 g for 5 min. The supernatant was discarded and the cell pellet fixed in 200 μl 1% paraformaldehyde. Cells (10 000) were acquired on a Becton-Dickinson FACSCalibur flow-cytometer utilizing an argon laser at 488 nm. Data analysis was conducted using Cell Quest software. Neutrophils were gated for analysis by their characteristic position on dot-plots of forward scatter (cell size) versus side scatter (granularity) and by expression of CD66. Binding of fluorochrome conjugated monoclonal antibodies to PMNs was measured using single histogram analysis or from dot-plots of forward scatter versus log fluorescence. Binding levels were recorded as the mean fluorescence intensity (MFI) for each monoclonal antibody used. Lymphocytes, monocytes and eosinophils also present in whole blood (data not shown) were gated according to their characteristic position in dot plots of forward versus side scatter and on the basis of phenotype (as described previously) were also analysed in parallel for comparison.
Confocal microscopy
Surface staining
Cells were prepared according to the above protocol for flow cytometry. 120 μl aliquots were then cytocentrifuged (150 g, 5 min) onto 3-amino-propyl-tri-ethoxysilone (APES)-coated microscope slides, air-dried for 5 min, mounted in Vectashield mounting medium (Vector laboratories, Peterborough, UK) supplemented with DAPI (0·2 mg/ml 4′,6-diamidino-2-phenylindole) and the cover-slips sealed with nail varnish.
Cells were viewed using a Leica SP2 confocal microscope. Optimum excitation wavelengths for DAPI (350 nm), FITC (488 nm) and r-PE (543 nm) were used. Emission wavelengths for FITC and r-PE were only collected within peak areas (FITC: 500–550 nm; r-PE: 550–650 nm) in order to exclude ‘bleed-through’ emission. Each cell was viewed at the Z-level that delivered an optimal image. Merged images (i.e. DAPI & FITC/r-PE) were obtained using LCS Lite confocal software. Background noise was reduced and the brightness/contrast of each image optimized using Adobe Photoshop v7.0.
Cytoplasmic staining and co-localization of CD86
Cytoplasmic staining was performed as described above but using fixed and permeabilized cells (formaldehyde + di-ethylene glycol) in place of viable cells. This method has previously been described in full.4 Briefly, FITC or r-PE conjugated anti-CD80 or anti-CD86 (Caltag Medsystems Ltd, Towcester, UK) was used in combination with markers specific for the four main types of neutrophil granule.
Granule-specific markers used were as follows:
Primary granules: r-PE-anti-myeloperoxidase (mouse monoclonal – clone H-43-5, Caltag).
Secondary granules: r-PE-anti-lactoferrin (mouse monoclonal – clone 3C5, Caltag).
Tertiary granules: unconjugated anti-MMP9 (gelatinase) – mouse monoclonal – clone GE213, Serotec Laboratories, Oxford, UK + FITC-rabbit anti-mouse IgG F(ab′)2– Dako Ltd, Ely, UK).
Secretory vesicles
FITC-anti-human serum albumin (rabbit polyclonal; Dako) used at a dilution of 1/5 in PBS.
Results
Translocation of neutrophil cytoplasmic CD antigens from cytoplasmic stores onto the cell surface following in vitro activation
Neutrophil activation was monitored by flow cytometry by measuring the change in the size and/or granularity of cells using dot-plots of forward versus side angle light scatter and for up-regulation of the granulocyte-specific antigen CD66. Non-specific background binding of monoclonal antibodies was monitored by including an appropriate mouse IgG–isotype control in all experiments. Five neutrophil activators were used in this study as described in Methods, i.e. PMA, cross-linking (X-L) of Mac-1, LPS, fMLP and phagocytosis of IgG–Latex particles. A typical example, showing the effect of activators on PMNs, is shown in Fig. 1. In vitro activation of neutrophils resulted in the generation of two distinct populations of cells – gated as P1 and P2, respectively, both of which showed a significant increase in cell-surface CD66. It was also evident that the larger P2 neutrophils showed much higher levels of expression than the smaller P1 cells – see Table 2. The proportion of neutrophils classified as P2 cells did vary considerably depending on the activator used – mean values for percentage total neutrophils classified as P2 in all experiments conducted were as follows:
Table 2.
Effect of various in vitro activators on neutrophil surface antigen expression
| PMA N = 9 | LPS N = 9 | X-L N = 9 | fMLP N = 6 | PHAG N = 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antigen | C | P1 | P2 | C | P1 | P2 | C | P1 | P2 | C | P1 | P2 | C | R3 | |
| M-IgG Negative control | Mean | 2·2 | 2·9 | 7·7 | 2·2 | 2·4 | 8 | 2·2 | 5 | 19·6 | 2·3 | 2·5 | 11 | 2 | 2 |
| SEM | 0·2 | 0·3 | 0·7 | 0·2 | 0·2 | 1 | 0·5 | 1·2 | 3·8 | 0·5 | 0·3 | 4·1 | 0 | 0·6 | |
| CD66 Positive control | Mean | 68·7 | 310·6 | 772·3 | 61·4 | 150·3 | 314·6 | 61·4 | 285 | 533·7 | 61·3 | 110·2 | 194·7 | 83·3 | 156·7 |
| SEM | 9·8 | 43·5 | 114·3 | 10·8 | 19·2 | 62·6 | 18·4 | 144·1 | 252·5 | 18·8 | 22·5 | 34·3 | 9·8 | 38·6 | |
| CD80 | Mean | 6·6 | 9·6 | 39·4 | 5·6 | 7·4 | 29·6 | 5·6 | 17·9 | 72·7 | 5·8 | 5·8 | 23·7 | 8·3 | 9·3 |
| SEM | 0·7 | 1·3 | 5·6 | 0·8 | 0·9 | 5·2 | 1·9 | 6·0 | 12·7 | 1·1 | 0·8 | 5·5 | 1·7 | 2·2 | |
| CD86 | Mean | 9·9 | 16·9 | 39·2 | 7·0 | 20 | 56·7 | 7 | 13·9 | 46·4 | 8 | 20 | 63·7 | 10·7 | 10·7 |
| SEM | 0·6 | 5·7 | 8·3 | 0·9 | 4 | 5·7 | 2·1 | 1 | 3·8 | 0·9 | 6·8 | 9·7 | 1·9 | 2·6 | |
| MHC class II Antigen | Mean | 5·2 | 7·4 | 33·3 | 5 | 6·2 | 75 | 5 | 9·6 | 60·6 | 4·2 | 5·2 | 51·3 | 6 | 12 |
| SEM | 0·3 | 0·9 | 3·4 | 0·6 | 0·9 | 17·7 | 1·2 | 1·3 | 8·9 | 0·4 | 0·7 | 12 | 0·6 | 4·4 | |
Activators: PMA, phorbol myristate acetate; LPS, lipopolysaccharide; X-L, cross-linking of CD11b; fMLP, N-formyl-methiony-leucyl-phenylalanine; PHAG, phagocytosis of IgG–Latex particles.
C, baseline control where no activator was present. Number (N) of individual experiments conducted for each activator is indicated.
Controls: In all experiments an appropriate negative mouse IgG isotype control was included and the granulocyte specific antigen CD66 provided a positive control.
Mean fluorescence intensity (MFI) values, derived from flow-cytometry analysis, are shown for neutrophils. Following activation, neutrophils were subdivided into P1 or P2 populations according to their size and granularity as shown in Fig. 1. All neutrophils were gated as region 3 (R3) following phagocytosis of IgG-latex.
Values significantly (P < 0·05) elevated relative to baseline control and to the appropriate mouse IgG isotype control are indicated in bold – Wilcoxon two sample assay for paired data. In all cases the MFI value for the P2 subpopulation was significantly higher than the MFI for the P1 population.
(1) Control cells (no activator) 1%
(2) PMA-activated cells 50%
(3) X-L activated cells 20%
(4) LPS activated cells 24%
(5) fMLP activated cells 16%
Because two distinct populations of cells were not evident following phagocytosis of IgG–Latex, all neutrophils activated in this way were gated (region 3) and analysed as a single population. Ingestion of these particles resulted in increased side angle light scatter and consequently the instrument settings had to be adjusted in order to visualize these cells – see Fig. 1(a). Significant up-regulation of CD66 was however, observed indicating that activation of neutrophils did indeed occur following phagocytosis – see Fig. 1(b) and Table 2.
As shown in Table 2, significant up-regulation of CD66 (known to be present predominantly within neutrophil secondary/tertiary granules) was observed with all five activators in the order PMA > X-L > LPS > fMLP > phagocytosis.
MHC class II (DR) antigen and CD80 (previously demonstrated predominantly within neutrophil SVs) were significantly elevated only on P2 neutrophils with four activators in the order of X-L > LPS > fMLP > PMA with no significant up-regulation observed following phagocytosis. Some weak (but significant) up-regulation of CD80 was also observed on P1 neutrophils following X-L CD11b. In contrast, CD86 was significantly elevated on both neutrophil subpopulations with higher levels of expression found on the larger P2 cells in the order fMLP > LPS > X-L > PMA with no up-regulation evident following phagocytosis.
Demonstration of cytoplasmic costimulatory molecules by confocal laser microscopy
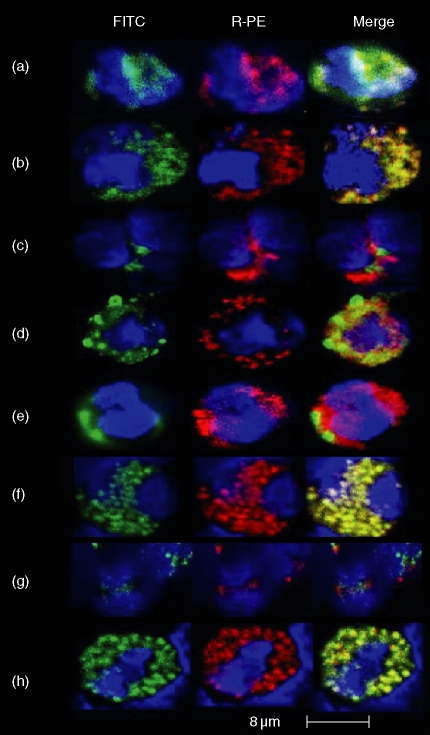
Previous studies have shown that CD80 is located almost exclusively within neutrophil SVs CD80 and CD86 were observed to colocalize suggesting that CD86 is also found in SVs. The differing responses to various activation signals observed between the two costimulatory molecules in this study suggested that CD86 may be more abundant within SVs and/or may be found within other types of neutrophil cytoplasmic granule. For this reason, dual-staining confocal laser microscopy studies were conducted in an attempt to clarify this point.
In good agreement with our previous findings4 CD80 appeared to be confined to SVs (Fig. 2a, c, e, g) whereas CD86 was observed within SVs, secondary (specific) granules and primary (azurophilic) granules (Fig. 2b, d, f, h).
Figure 2.
Dual staining confocal laser microscopy of fixed and permeabilized human peripheral blood neutrophils. Various combinations of FITC- (green) and RPE (red)-conjugated mouse monoclonal antibodies were used to demonstrate intracellular expression of CD80 or CD86 colocalizing with various molecules known to be specific for the four major types of neutrophil granule as described in methods. In all experiments the neutrophil nucleus was counterstained with DAPI (blue). In this study many hundreds of confocal images were obtained. For practical reasons the images shown in this figure were therefore selected to illustrate the authors conclusions based on detailed inspection of multiple images. Row A/B: Secretory vesicles (SVs): FITC-conjugated anti-human serum albumin (HSA) was used as a marker for SVs in combination with RPE-conjugated anti-CD80 (Row A) or CD86 (Row B). Individual fluorochromes are shown in order to observe the similarities in intracellular localization and the merged image (yellow) indicates colocalization. Row C/D: tertiary granules: FITC-conjugated anti-human CD80 or CD86 (green) were used in combination with gelatinase (MMP-9) – shown in red to indicate tertiary granules. Individual and merged images show that neither molecule colocalizes with gelatinase. Row E/F: secondary granules: FITC-conjugated anti-human CD80 or CD86 (green) were used in combination with RPE-anti-lactoferrin (red) to indicate neutrophil-specific (secondary) granules. Individual and merged images show that CD80 (row E) does not colocalize with lactoferrin but CD86 (row F) does. Row G/H: primary granules: FITC-conjugated anti-human CD80 or CD86 (green) were used in combination with RPE-anti-myeloperoxidase (red) to indicate azurophilic (primary) granules. Individual and merged images show that CD80 (row G) does not colocalize with myeloperoxidase but CD86 (row H) does.
Translocation of neutrophil cytoplasmic CD antigens from cytoplasmic stores onto the cell surface following in vivo activation
Neutrophils obtained from synovial fluid aspirated from the knee joint of patients with rheumatoid arthritis (RA) were used as a source of in vivo activated cells. Paired blood samples from the same patients obtained at the same time and tested in parallel provided control values in these experiments.
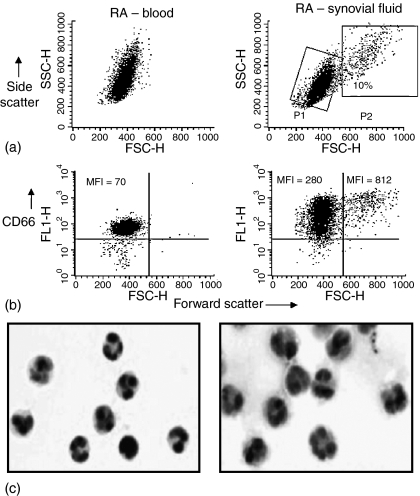
As shown in Fig. 3(a), some large P2 neutrophils were observed in RA synovial fluid but not in the paired blood samples. The mean percentage of neutrophils classified as P2 cells for nine patients was 2 ± 0·9% for blood and 17 ± 6·4% for synovial fluid. Both P1 and P2 cells obtained from synovial fluid showed significantly increased levels of cell surface CD66 expression relative to blood cells – see example in Fig. 3(b) and Table 3.
Figure 3.
In vivo activation of neutrophils: paired samples of peripheral blood and synovial fluid (SF) neutrophils obtained from patients with rheumatoid arthritis (RA). (a) Flow cytometry dot-plots of forward scatter versus side scatter showing distinct neutrophil subpopulations – designated P1 and P2 in RA synovial fluid but not in the paired blood sample tested in parallel. In this example 10% of synovial fluid neutrophils were gated as P2 cells. The P2 subpopulation was observed with 7/9 synovial fluid samples. For the nine RA patients included in this study the mean ± SEM proportion of synovial fluid neutrophils classified as P2 cells was 17 ± 6·4% compared with 2 ± 0·9% for the corresponding paired RA peripheral blood neutrophils. (b) Flow cytometry dot-plots of forward scatter versus log florescence (FITC anti-CD66) show that virtually all cells gated according to their forward versus side scatter profile shown above express the granulocyte specific antigen CD66. Up-regulation of CD66 on SF cells relative to blood is evident as indicated by increased mean fluorescence intensity (MFI) values on both P1 and P2 populations of synovial fluid neutrophils with P2 values being higher than P1. These results show that all synovial fluid neutrophils appear to be activated with the small P2 subpopulation (‘effective responders’)18 showing much higher levels of expression of this particular activation marker. (c) Cytospin preparations of paired peripheral blood and synovial fluid neutrophils show that a proportion of individual SF cells appear to be significantly larger than their blood counterparts with a more voluminous cytoplasm. Clumping of cells was not observed. Haematoxylin & eosin stain.
Table 3.
In vivo neutrophil activation: Comparison of neutrophil surface antigen expression on paired samples of blood and synovial fluid obtained from nine patients with rheumatoid arthritis
| Antigen | RA blood | RA synovial fluid P1 | RA synovial fluid P2 | |
|---|---|---|---|---|
| CD66 Positive control | Mean | 82·4 | 162·5 | 264·6 |
| SEM | 8·7 | 18·4 | 55·8 | |
| CD80 | Mean | 4·9 | 18·9 | 53·5 |
| SEM | 0·4 | 7·9 | 15·1 | |
| CD86 | Mean | 8·4 | 26·7 | 100·3 |
| SEM | 1·5 | 8·5 | 34 | |
| MHC class II antigen | Mean | 8·2 | 24·4 | 121·1 |
| SEM | 3·1 | 9·3 | 34 |
In all experiments the granulocyte specific antigen CD66 provided a positive control. Mean fluorescence intensity (MFI) values, derived from flow-cytometry analysis, are shown for peripheral blood and synovial fluid neutrophils. Synovial fluid samples were found to contain, to a variable extent, a subpopulation (P2) of large neutrophils as shown in Fig. 3. This P2 population was not observed in blood samples. Values significantly (P < 0·05) elevated relative to the paired blood MFI values are shown in bold – Wilcoxon two sample test for paired data. In all cases the MFI values observed for P2 cells was significantly greater than the corresponding P1 value.
Morphologically, many of the synovial fluid neutrophils appeared larger than their peripheral blood counterparts with a more voluminous cytoplasm – see Fig. 3(c).
Mean fluorescence intensity (MFI) values for MHC class II antigen, CD80 and CD86 were all observed to be significantly elevated on the surface of viable synovial fluid neutrophils relative to paired blood samples with higher levels evident on the larger P2 cells – see Table 3.
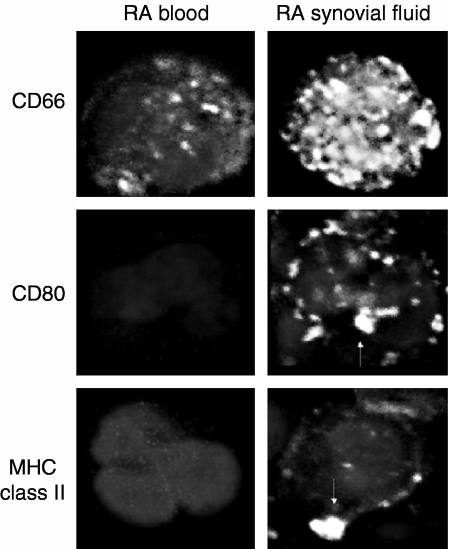
This up-regulation of CD66, MHC class II antigen and costimulatory molecules on synovial fluid cells relative to blood cells was visualized by confocal laser microscopy as shown in Fig. 4. Class II antigen and costimulatory molecules were found in large heterogeneous clusters on the surface of synovial fluid neutrophils but were not observed on their peripheral blood counterparts.
Figure 4.
Confocal laser microscopy of viable peripheral blood neutrophils using paired samples of blood and synovial fluid from patients with rheumatoid arthritis (RA). Paired preparations of viable (as assessed by exclusion of propidium iodide and failure to bind anti-myeloperoxidase antibody) neutrophils obtained from peripheral blood and synovial fluid were inspected by confocal laser microscopy using cell surface staining for CD66 as a positive control. The characteristic shape of the neutrophil nucleus, stained with DAPI, was used to identify these cells in combination with expression of the granulocyte specific marker CD66 as shown. Significant up-regulation of CD66 on synovial fluid neutrophils relative to blood was evident in large heterogeneous clusters and, based on images taken at different Z-levels (not shown), appeared to cover the entire surface of the cell. The staining pattern for CD66 observed on RA-peripheral blood neutrophils was virtually identical to the that observed on neutrophils obtained from healthy donors (not shown). Co-stimulatory molecules (CD80/CD86) and MHC class II (DR) antigen were not observed on the surface of RA peripheral blood neutrophils but were clearly visible within large heterogeneous clusters on the surface of synovial fluid neutrophils as indicated by the arrows.
Discussion
Takano et al.17 previously demonstrated rapid translocation of CD64 (FcγRI) from cytoplasmic stores onto the surface of PMNs within 20 min following cross-linking of either the CD18 or CD11b components of the B2-integrin molecule Mac-1. This was not observed following cross-linking of CD11a or CD11c. Using a whole blood modification of this method we were able to show that MHC class II antigen and the costimulatory molecules, CD80 and CD86, can also be rapidly translocated onto the surface of normal human peripheral blood PMNs.4 This assay probably mimics the in vivo interaction between neutrophil Mac-1 and ICAM-1 on vascular endothelial cells and therefore represents a very early PMN activation signal. In this study we have used a similar whole blood assay to study the effect of other well-documented PMN activators on translocation of these three key molecules from pre-existing cytoplasmic stores onto the cell surface. All activators (except phagocytosis) used in this assay resulted in the production of two distinct PMN subpopulations which were gated as P1 and P2 on dot-plots of forward versus side scatter. This effect has been observed by others using isolated human PMNs.18 Both subpopulations were found to be activated based on various criteria including their ability to generate a respiratory burst with the larger P2 population always producing the greater response. These activated neutrophil subpopulations were therefore categorized by Vuorte et al.18 as ‘moderate’ (P1) or ‘effective’ (P2) responders. The proportion of P2 cells was found to vary depending on the activator used and the individual donor concerned but in general this population represents between 20 and 50% of all neutrophils. In good agreement with this concept of two distinct populations of activated PMNs, we have shown that CD66 is significantly elevated on all activated PMNs with higher levels of expression evident with the larger P2 cells (see Table 2). This up-regulation occurred irrespective of the type of activator used but it was clear that the highest levels of CD66 expression were achieved following activation with PMA. These findings are therefore in good agreement with others who have shown that PMA, a C-kinase activator, translocates molecules from SVs, secondary and tertiary granules.9 Up-regulation of MHC class II antigen and CD80 was found mainly on the surface of activated P2 cells (see Table 2) with cross-linking (X-L) of Mac-1, LPS and fMLP being more effective than PMA, i.e. X-L > LPS > fMLP > PMA. This observation is therefore consistent with previous studies that have shown that, when used at low concentrations, fMLP preferentially stimulates release of molecules from SVs.9
Although phagocytosis of IgG–Latex particles significantly up-regulated CD66 no distinct P2 population was generated following this particular activation signal nor was there any significant up regulation of MHC class II antigen, CD80 or CD86. Four in vitro activators showed significant up-regulation of CD86 on both populations of PMNs and showed a slightly different activation pattern from CD80 in the general order – fMLP > LPS > X-L > PMA. Despite the fact that both costimulatory molecules interact with the same T-cell ligands, i.e. CD28 and CD152 (CTLA-4) there are many well documented differences between these molecules. For example, it is known that the levels of CD86 tend to be higher than CD80 on antigen presenting cells and show different kinetics of expression following activation.19 CD86 is thought to more important in the initiation phase of the immune response with CD80 being required for sustaining the response.20 In our previous study4 we showed that CD80 was found mainly within SVs. In this study we have shown that CD86 appears to have a wider cytoplasmic distribution being found in SVs, secondary and somewhat surprisingly also within primary azurophilic granules. This wider granule distribution may therefore, at least in part, explain the differences in cell expression and activation profiles noted in this study. It seems unlikely that CD86 translocates onto the cell surface from primary granules following activation since the contents of these granules are not generally released unless the PMNs are ‘self destructing’. It is of interest to note that Rodeberg et al.21 have shown that PMN primary granules also appear to contain CD14. These workers proposed that CD14 may be released into the fluid phase as an immunomodulatory molecule within sites of infection following ‘frustrated phagocytosis’. This process is thought to occur within the joints of RA patients as PMNs try to engulf IgG bound to collagen or large insoluble immune complexes.22 It is therefore possible that soluble CD86 may be released from PMN primary granules, by a similar mechanism, within the joint space in RA and could conceivably play an immunoregulatory role by interfering with normal APC CD86 interactions with CD28 on naive T cells or with CD152 on activated T cells or Treg cells. This possibility requires elucidation by further study.
Up-regulation of CD66, MHC class II antigen, CD80 and CD86 was also observed on in vivo activated PMNs (P1 and P2 cells) found as the predominant cell type in RA synovial fluid – see Table 3. Although the data is not shown here we did also observe a further significant increase in surface expression of these molecules on RA synovial fluid PMNs following incubation at 37° for 30 min in Dulbecco's minimal essential medium supplemented with 10% heat-inactivated fetal calf serum. This ‘spontaneous activation’ was not observed with normal donor or RA blood PMNs, suggesting, as others have done,22 that many of the neutrophils in RA synovial fluid are primed for activation. Addition of the various in vitro activators, used in this particular study, to RA synovial fluid PMNs produced no further increase in expression of these antigens relative to the level of spontaneous up-regulation. This observation suggests that PMNs in RA synovial fluid are refractory to these stimuli, i.e. they are already primed and/or activated and cannot be stimulated further.
Increased expression of these antigens on RA synovial fluid PMNs was visualized by confocal laser microscopy as large heterogeneous clusters – see Fig. 4. The reason for this is not known but may reflect the known clustering of molecules which occurs on APCs and thought to be required for immune synapse formation. This mechanism may be useful when the number of cell surface molecules required for antigen presentation and costimulation is low on APCs relative to the number reciprocal molecules on the surface of T cells.23,24 It is also of interest to note that the level of cell surface expression of MHC class II antigen and costimulatory molecules induced on activated PMNs (in vitro and in vivo) was equivalent to or greater than the level expressed on monocytes or B cells measured in parallel in peripheral blood or in synovial fluid (data not shown).
Iking-Konert et al.25 have also recently shown that activated neutrophils within RA synovial fluid may express cell surface antigens normally associated with dendritic cells, e.g. MHC class II antigen and CD83. This up-regulation probably results from active synthesis of these molecules and depends on neutrophil activation by T-cell derived cytokines. It was therefore proposed that these phenotypically altered neutrophils play an important role in perpetuating the local inflammatory process within the joint in RA.
In conclusion, we have shown that MHC class II antigen, CD80 and CD86 may be expressed rapidly on the surface of human PMNs following in vitro or in vivo activation. A distinct subpopulation of enlarged PMNs exhibit the highest levels of expression of these antigens. Because these levels are comparable with, and in some instances exceed, the level expressed on known APCs this study provides further support for the hypothesis that neutrophils have the capacity to function as APCs. This may be of particular significance in RA where the neutrophil is the predominant cell type found in synovial fluid. Activated PMNs may therefore play a role in T-cell activation and proliferation within the joint. Furthermore, the discovery of CD86 within primary granules also raises the possibility of soluble regulators of the immune response being released into the joint space following ‘frustrated phagocytosis’.
Acknowledgments
Co-author K.H. was supported by a scholarship from the Health Foundation. The authors would also like to thank Ms Margaret Oprey, Beatson Institute for Cancer Research, Glasgow, UK, for help in obtaining the confocal microscopy images presented in this study.
References
- 1.Borregaard N, Cowland JB. Granules of the human neutrophilic polmorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 2.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microb Infect. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Grenier A, Dehoux M, Boutten M, Arce-Vicioso M, Durand G, Gougerot-Pociadalo M-A, Chollet-Martin S. Oncostatin M production and regulation by human polymorponuclear neutrophils. Blood. 1999;93:1413–21. [PubMed] [Google Scholar]
- 4.Sandilands GP, Ahmed Z, Perry N, Davison M, Lupton A, Young B. Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation. Immunology. 2005;114:354–68. doi: 10.1111/j.1365-2567.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosselin EJ, Wardwell K, Rugby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFNγ and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 6.Iking-Konert C, Vogt S, Radsak M, Wagner C, Hansch GM, Andrassy K. Polymorphonuclear neutrophils in Wegener's granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001;60:2247–62. doi: 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 7.Ashtekar AR, Saha B. Poly's plea. membership to the club of APCs. Trends Immunol. 2003;24(9):485–90. doi: 10.1016/s1471-4906(03)00235-7. [DOI] [PubMed] [Google Scholar]
- 8.Feuk-Lagerstedt E, Jordan ET, Leffler H, Dahlgren C, Karlsson A. Identification of CD66a and CD66b as the major galectin 3 receptor candidates in human neutrophils. J Immunol. 1999;163:5592–8. [PubMed] [Google Scholar]
- 9.Sengelov H, Boulay F, Kjeldsen L, Borregaard N. Subcellular localization and translocation of the receptor for N-formylmethionyl-leucyl-phenylalanine in human neutrophils. Biochem J. 1994;99(2):473–9. doi: 10.1042/bj2990473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nourshargh S, Marelli-Berg FM. Transmigration through venular walls. A key regulator of leukocyte phenotype and function. Trends Immunol. 2005;26:157–65. doi: 10.1016/j.it.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Pillinger MH, Abrahamson SB. The neutrophil in rheumatoid arthritis. Rheum Dis Clin N Am. 1995;21:691–713. [PubMed] [Google Scholar]
- 12.Edwards SW, Hallet MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–4. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 13.Watson F, Robinson JJ, Phelan M. Bucknall RC Edwards SW. Receptor expression in synovial fluid neutrophils from patients with rheumatoid arthritis. Ann Rheum Dis. 1993;52:354–9. doi: 10.1136/ard.52.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honig M, Peter HH, Jantscheff P, Grunert F. Synovial PMN show a coordinated up-regulation of CD66 molecules. J Leukoc Biol. 1999;66:429–36. doi: 10.1002/jlb.66.3.429. [DOI] [PubMed] [Google Scholar]
- 15.Quayle JA, Watson F, Bucknall RC, Edwards SW. Neutrophils from synovial fluid of patients with rheumatoid arthritis express the high affinity immunoglobulin G receptor, FcγRI (CD64): role of immune complexes and cytokines in induction of receptor expression. Immunology. 1997;91:2660–273. doi: 10.1046/j.1365-2567.1997.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross A, Bucknall RC, Cassatella MA, Edwards SW, Moots RJ. Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis Rheum. 2003;48:2796–806. doi: 10.1002/art.11253. [DOI] [PubMed] [Google Scholar]
- 17.Takano K, Kaganoi J, Yamamoto K, Takahashi A, Kido T, Sasada M. Rapid and prominent up-regulation of high-affinity receptor for immunologlobulin G (FcγRI) by cross-linking of β2 Integrins on polymorphonuclear leukocytes. Int J Hematol. 2000;72:48–54. [PubMed] [Google Scholar]
- 18.Vuorte J, Janson SE, Repo H. Standardisation of a flow cytometric assay for phagocyte respiratory burst activity. Scand J Immunol. 1996;43:329–34. doi: 10.1046/j.1365-3083.1996.d01-45.x. [DOI] [PubMed] [Google Scholar]
- 19.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 co-stimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 20.Liang L, Sha WC. The right place at the right time: novel B7 family members regulate effector T cell responses. Curr Opin Immunol. 2002;14:384–90. doi: 10.1016/s0952-7915(02)00342-4. [DOI] [PubMed] [Google Scholar]
- 21.Rodeberg DA, Morris RE, Babcock GF. Azurophilic granules of human neutrophils contain CD14. Infect Immun. 1997;65(11):4747–53. doi: 10.1128/iai.65.11.4747-4753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cross A, Bakstad D, Allen JC, Thomas L, Moots RJ, Edwards SW. Neutrophil gene expression in rheumatoid arthritis. Pathophysiology. 2005;12:191–202. doi: 10.1016/j.pathophys.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Bromley SK, Burak WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–96. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 24.Friedl P, Gunzer M. Interaction of T cells with APCs: the serial encounter model. Trends Immunol. 2001;22:187–91. doi: 10.1016/s1471-4906(01)01869-5. [DOI] [PubMed] [Google Scholar]
- 25.Iking-Konert C, Ostendorf B, Sander O, Jost M, Wagner C, Joosten L, Schneider M, Hansch GM. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis. evidence for activation of T cells. Ann Rheum Dis. 2005;64:1436–42. doi: 10.1136/ard.2004.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]