Abstract
The cooperative role of CD4+ helper T (Th) cells has been reported for CD8+ cytotoxic T (Tc) cells in tumor eradication. However, its molecular mechanisms have not been well elucidated. We have recently demonstrated that CD4+ Th cells can acquire major histocompatibility complex/peptide I (pMHC I) complexes and costimulatory molecules by dendritic cell (DC) activation, and further stimulate naïve CD8+ T cell proliferation and activation. In this study, we used CD4+ Th1 and CD8+ Tc1 cells derived from ovalbumin (OVA)-specific T cell receptor (TCR) transgenic OT II and OT I mice to study CD4+ Th1 cell's help effects on active CD8+ Tc1 cells and the molecular mechanisms involved in CD8+ Tc1-cell immunotherapy of OVA-expressing EG7 tumors. Our data showed that CD4+ Th1 cells with acquired pMHC I by OVA-pulsed DC (DCOVA) stimulation are capable of prolonging survival and reducing apoptosis formation of active CD8+ Tc1 cells in vitro, and promoting CD8+ Tc1 cell tumor localization and memory responses in vivo by 3-folds. A combined adoptive T-cell therapy of CD8+ Tc1 with CD4+ Th1 cells resulted in regression of well-established EG7 tumors (5 mm in diameter) in all 10/10 mice. The CD4+ Th1’s help effect is mediated via the helper cytokine IL-2 specifically targeted to CD8+ Tc1 cells in vivo by acquired pMHC I complexes. Taken together, these results will have important implications for designing adoptive T-cell immunotherapy protocols in treatment of solid tumors.
Keywords: Th1, pMHC I, IL-2, apoptosis, Tc1 cell therapy
Introduction
CD8+ cytotoxic T (Tc) lymphocytes which are a major immunological effector cell population mediating resistance to cancer can eradicate the growth and metastasis of malignant tumor cells1. Effective cancer immunotherapy with adoptively transferred tumor-sensitized Tc cells has been well documented in animal models2,3. CD8+ Tc cells can be polarized to effector subsets with distinct cytokine production profiles (Tc1 cells producing IFN-γ and Tc2 cells secreting IL-4 and IL-5)4. Dobrzanski et al. previously reported that tumor-specific Tc1 cells were relatively more effective reduction of lung metastasis of OVA-transfected B16 melanoma than Tc2 cells5. Recently, they have further shown that these Tc1 and Tc2 can also cure intradermally transplanted OVA-transfected B16 melanomas, but only in their palpable sizes6. In clinical trials, only a limited number of patients have responded to T-cell therapy7,8 partly due to lacking of T cell helper arm and/or only very small fractions of transferred T cells accumulating in tumors9,10.
CD4+ T helper (Th) cells can also be subdivided into Th1 cells producing IL-2 and IFN-γ and Th2 cells secreting IL-4, IL-5 and IL-10. It has been reported that Th2 cells responsible for humoral immunity11 neither enhanced nor suppressed antitumor CD8+ cytotoxic T lymphocyte (CTL) responses12 whereas Th1 cells essential for cellular immunity play an important role in priming CTL-mediated antitumor responses9. The traditional explanation is that CD4+ Th1 cells provide IL-2 or helper to CD8+ Tc cells13. CD4+ Th cells have also been shown to have another role in induction of CD8+ Tc cell responses through DC activation by CD40/CD40L interactions14. Recently, it has also been reported that CD4+ Th cells are required in determining the magnitude and persistence of CTL responses15 and for CD8+ T cell infiltration of tumors16. However, the underlying immune mechanisms of these CD4+ Th's effects in adoptive CD8+ Tc-cell therapy are still largely unknown.
Stimulation of T cells by antigen-presenting cells (APCs) involves at least two signaling events: one elicited by T cell receptor (TCR) recognition of major histocompatibility complex/peptide (pMHC) complexes and the other one by costimulatory molecule signaling (e.g. T cell CD28/APC CD80)17. A consequence of such Ag-specific T cell–APC interactions is the formation an immunological synapse, comprising a central cluster of TCR-pMHC complexes and CD28–CD80 interactions surrounded by rings of engaged accessory molecules (e.g. complexed LFA-1-CD54)18,19. One important feature of synapse physiology is that APC-derived surface molecules are transferred to the Th cells during the course of their TCR internalization followed by recycling20,21. Recently, we have demonstrated that naive CD4+ T cells acquire DC molecules by DC activation and act as Th-APCs. These Th-APCs with acquired pMHC complexes and costimulatory molecules can stimulate naïve CD8+ T cell proliferation in vitro and in vivo and induce CTL responses and antitumor immunity22. However, the molecular mechanisms responsible for the functional effects of Th-APCs have not been well elucidated, and the critical role the acquired pMHC I complexes play in targeting CD4+ Th's effects to CD8+ T cells in vivo has not been clearly defined due to lacking the appropriate control cells such as CD4+ Th(pMHC I–/–) cells used in this study.
In this study, we developed a model system with a defined tumor antigen OVA using the OVA-transfected EG7 tumor cells and the OVA-specific TCR transgenic OT I and OT II mice with class I and II specificities, respectively,23. Based upon this model system, we investigated the help effects of OT II CD4+ Th1 cells in active CD8+ Tc1-cell immunotherapy of established solid EG7 tumors. We found that CD4+ Th1 cells prolonged active OT I CD8+ Tc1 cell survival and promoted active OT I CD8+ Tc1 cell tumor localization and memory responses. We further elucidated the molecular mechanisms responsible for their help effects in CD8+ Tc1 cell immunotherapy and disclosed the critical role of acquired pMHC I complexes in delivery of CD4+ T help effects to CD8+ Tc1 cells in vivo by using the recently established control CD4+ Th(pMHC I–/–) cells.
Materials and methods
Antibodies, cytokines, cell lines and animals
Biotin-conjugated antimouse MHC class I (H-2Kb) and II (Iab), CD4, CD8, CD11c, CD25, CD69 and Vβ5·1,5·2 TCR antibodies (Abs) were obtained from BD Pharmingen Inc. (Mississauga, Ontario, Canada). The FITC-conjugated avidin was obtained from Bio/Can Scientific (Mississauga, Ontario, Canada). PE-labeled H-2Kb/OVA257−264 (OVA I) tetramer and FITC-labeled anti-CD8 Ab were obtained from Beckman Coulter, Missisauga, Ontario, Canada. The anti-IL-2, -IL-4, -IFN-γ Abs, and the recombinant mouse granulocyte macrophage colony stimulating factor (GM-CSF), IL-2, IL-12 and interferon (IFN)-γ were purchased from R & D Systems (Minneapolis, MN). The anti-H-2Kb/OVA I (pMHC I) Ab was obtained from Dr R. Germain, National Institute of Health, Bethesda, MD24. The mouse B cell hybridoma cell line LB27 expressing Iab, thymoma cell line EL-4 and its derivative OVA-transfected cell line EG7 were obtained from American Type Culture Collection (ATCC), Rockville, MD. OVA I (SIINFEKL) and OVA II (ISQAVHAAHAEINEAGR) peptides were synthesized by Multiple Peptide Systems (San Diego, CA). Female C57BL/6 mice and OT I and OT II mice having transgenic Vα2Vβ5 TCRs specific for OVA257−264 (OVA II) epitope in the context of H-2Kb and OVA323−339 epitope in the context of Iab22,23, respectively, and H-2Kb, IL-2 and IFN-γ gene knockout (KO) mice on C57BL/6 background were obtained from the Jackson Laboratory (Bar Harbor, Maine). Homozygous OT II/H-2Kb–/–, OT II/IL-2–/– and OT II/IFN-γ–/– mice were generated by backcrossing the designated gene KO mice onto the OT II background for three generations; homozygosity was confirmed by polymerase chain reaction (PCR) according to Jackson laboratory's protocols. All mice were maintained in the animal facility at the Saskatoon Cancer Center and treated according to Animal Care Committee guidelines of University of Saskatchewan.
Preparation of dendritic cells
Bone marrow-derived dendritic cells (DCs) were generated using GM-CSF and IL-4 as described previously25. To generate OVA protein-pulsed DCs, DCs derived from wild-type C57BL/6 mice were pulsed overnight at 37° with 0·1 mg/ml OVA protein (Sigma, St. Louis, MO) and termed as DCOVA. DCOVA were capable of stimulating both OT II CD4+ and OT I CD8+ T cell proliferation in vitro26 indicating that OVA-pulsed DCOVA express both pMHC II and pMHC I complexes, respectively. DCOVA derived from C57BL/6 mice with H-2Kb gene KO were termed as (Kb–/–)DCOVA.
Preparation of active T cell subsets
OVA-specific CD4+ T and CD8+ T cells were isolated from the spleens and lymph nodes of OT-II and OT-I TCR-transgenic mice, enriched by passage through nylon wool columns (C & A Scientific Inc, Manassas, VA), and then, the CD4+ and CD8+ T cells were further fractionated by negative selection using antimouse CD8 (Ly2) and CD4 (L3T4) paramagnetic beads (DYNAL Inc., Lake Success, NY), respectively, according to the manufacturer's protocols. The OVA-specific T cell subsets (Th1 and Tc1 cells) were further generated by culturing naïve CD4+ and CD8+ T cells (3 × 105 cells/well) with irradiated (4000 rad) DCOVA (2 × 105 cells/well) in 96-well plate in the presence of IL-2 (20 U/ml), IL-12 (5 ng/ml) and anti-IL-4 Ab (5 µg/ml)22, respectively. In vitro-activated Th1 and Tc1 cell subsets were harvested after 4 days' culture and purified using Ficoll-Paque (Sigma, St. Louis, MO) density gradient centrifugation26 and followed by using CD8- and CD4-microbeads (Milttenyi Biotec, Auburn, CA), respectively22. These in vitro (Kb–/–)DCOVA-activated wild-type OT II CD4+ T cells were termed as Th1(pMHC I–/–), whereas wild-type DCOVA-activated CD4+ T cells from designated gene-deleted OT II (OT II/Kb–/–, OT II/IL-2–/– and OT II/IFN-γ–/–) mice under the same culture conditions were termed as CD4+ Th1(Kb–/–), Th1(IL-2–/–) and Th1(IFN-γ–/–), respectively. Con A-stimulated OT II CD4+ T (Con A-OT II) cells were generated and purified as previously described22.
Phenotypic characterization of active T cell subsets
The active T cell subsets were stained with a panel of antibodies and analyzed by flow cytometry27. Isotype-matched monoclonal Abs with irrelevant specificity were used as controls.
Cytokine secretion
Tc1 and Th1 subsets were re-stimulated by culturing T cells (0·5 × 106 cells/well) in flat-bottom 96-well plates (Costar Corp, Cambridge, MA) with irradiated (6000 rad) EG7 (0·6 × 105 cells/well) and OVAII-pulsed irradiated (4000 rad) LB27OVAII (0·6 × 105 cells/well), respectively26. The culture supernatants were harvested at 1 day for measurement of IFN-γ, IL-4, and IL-2 secretion by using enzyme-linked immunosorbent assay (ELISA) kits (Endogen, Woburn, MA). The results were normalized to the recombinant cytokine standard curves.
PMHC I complex transfer assay
Naïve CD4+ T cells (3 × 105 cells/well) derived from OT II mice with H-2Kb gene KO were cultured with irradiated (4000 rad) DCOVA (2 × 105 cells/well) in the presence of IL-2 (20 U/ml), IL-12 (5 ng/ml) and anti-IL-4 Ab (5 µg/ml) for 4 days. The active OT II T cells were analyzed using the antipMHC I Ab by flow cytometry.
Naïve CD8+ T cell proliferation assay
A constant number of naïve OT I CD8+ T cells (0·5 × 105 cells/well) were cultured with irradiated (4000 rad) stimulators including CD4+ Th1, Th1(pMHC I–/–), Th1(kb–/–) cells and DCOVA (0·4 × 105 cells/well), respectively, and their 2-fold dilutions. After 48 hr, all wells were pulsed for 12 h with 1 µCi of [3H]-thymidine (Amersham, Arlington Heights, IL) and then harvested onto glass fiber filters, respectively. Thymidine incorporation in each well was determined by liquid scintillation counting27.
Active Tc1 cell survival analysis
To examine CD8+ Tc1 cell survival in absence of IL-2 stimulation, in vitro-activated CD8+ Tc1 cells (0·4 × 105 cells/well) were incubated with or without irradiated (4000 rads) active CD4+ Th1 cells (0·4 × 105 cells/well) in RPMI 1640 plus 10% FCS in flat-bottom 96-well plates. To assess CD8+ Tc1 cell apoptosis formation, T cells were harvested after 4 days, stained with FITC-Annexin V (BD Pharmingen) and PE-anti-CD8 Ab, and analyzed by flow cytometry. In some experiments, each of a panel of neutralizing reagents (anti-IL-2 and -IFN-γ Abs) (each 15 µg/ml) was added to the culture system. To examine the effect of acquired pMHC I complexes on CD8+ Tc1 cell survival, Th1(pMHC I–/–) cells without acquired pMHC I complexes were used as control cells.
Tc1 cell tumor infiltration
Detection of tumor infiltration of transferred active CD8+ Tc1 cells in vivo was performed as previously described27. Briefly, in vitro DCOVA-activated CD8+ Tc1 cells (5 × 106 cells/mouse) together with or without in vitro DCOVA-activated CD4+ Th1, Th1(pMHC I–/–), Th1(Kb–/–), Th1(IL-2–/–) and Th1(IFN-γ–/–) cells (2 × 106 cells/mouse) were i.v. injected into C57BL/6 mice bearing EG7 tumors with ∼5 mm in diameter, respectively. At different days subsequent to T cell injection, tumors were removed. Cell suspensions were prepared from these EG7 tumors by mincing them into small pieces and pressing them through a fine mesh16. Red cells were lysed by using 0·84% ammonium chloride. T cells were then purified from these cell suspensions by using the CD3 microbeads (Mittenyi Biotec), stained using PE-labeled H-2Kb/OVA I tetramer (PE-tetramer) and FITC-labeled anti-CD8 Ab (FITC-CD8) for flow cytometric analysis.
Adoptive Tc1 cell immunotherapy model
Mice (10 per group) received s.c. injections of 1 × 106 EG7 tumor cells in their thighs. At 10–12 days postinoculation, tumors became around 5 mm in diameter. To study the help effect of CD4+ Th1 cells, tumor-bearing mice were injected i.v. with 5 × 106 CD8+ Tc1 cells alone, or in conjunction with different amounts of CD4+ Th1 cells, respectively. To study the molecular mechanism of CD4+ Th1’s help effect in CD8+ Tc1-cell therapy, tumor-bearing mice were also injected i.v. with 5 × 106 of CD8+ Tc1 cells in conjunction with 2 × 106 CD4+ Th1(pMHC I–/–), Th1(Kb–/–), Th1(IL-2–/–) and Th1(IFN-γ–/–) cells, respectively. Animal mortality and tumor growth or regression were monitored daily for up to 10 weeks; for humanitarian reasons, all mice with tumors that achieved a size of 1·5 cm in diameter were sacrificed.
Assessment of CD8+ Tc1 cell memory responses
Naïve C57BL/6 mice were i.v. injected with active CD8+ Tc1 cells (5 × 106 cells) alone or in conjunction with CD4+ Th1 or Th1(pMHC I–/–) cells (2 × 106 cells). Tetramer staining assay was performed to examine the presence of OVA-specific CD8+ memory T (Tm) cells in mouse peripheral blood 3 months after the adoptive Tc1 cell transfer. The mouse tail blood samples were incubated with PE-tetramer, FITC-CD8 and ECD-anti-CD44 (ECD-CD44) Abs (Beckman Coulter, Mississauga, Ontario, Canada). The erythrocytes were then lysed using lysis/fixed buffer (Beckman Coulter) and the samples were analyzed by flow cytometry according to the company's protocol. In one set of experiments, the immunized mice were boosted with i.v. injection of irradiated (4000 rad) DCOVA (1 × 106 cells). Four days after the boost, the mouse tail blood samples were tested for tetramer staining as described above. In another set of experiments, the immunized mice were challenged with s.c. injection of 1 × 106 or 3 × 106 EG7 tumor cells. Animal mortality and tumor growth or regression were monitored daily for up to 10 weeks as described above.
Results
Characterization of active CD4+ Th1 and CD8+ Tc1 cells
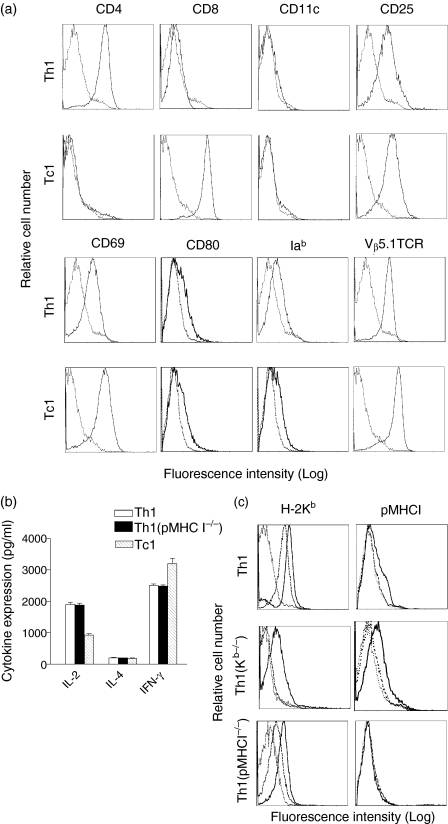
Two different T cell subsets (CD4+ Th1 and CD8+ Tc1 effector cells) were generated in vitro from OVA-specific TCR transgenic OT II and OT I mice, respectively, as described in Materials and Methods. Both CD4+ and CD8+ T cells displayed their T cell subset marker (CD4 or CD8), Vα2Vβ5+ TCR and active T cell markers CD25 and CD69 (Fig. 1a), indicating that they are OVA-specific active CD4+ and CD8+ T cells. They also expressed some MHC class II and CD80 molecules, which may be acquired from DC through synapse-composed molecule internalization/recycling18,19,22. In addition, there was no CD11c-positive DC population existing in these purified active T cells (Fig. 1a). This is because that any survival of irradiated DCOVA cells and potential small amount of contamination of spleen DC or B cells with the original naïve OT II CD4+ and OT II CD8+ T cell preparations, which might have picked up OVA peptides from irradiated DCOVA in the culture, would be eliminated by the killing activity of these activated Th and Tc cells expressing perforin22,27,28. These activated Th and Tc cells secreted abundant IFN-γ (2·5 ng/ml/106 cells/24 h and 3·2 ng/ml/106 cells/24 hr) and IL-2 (1·9 ng/ml/106 cells/24 h and 0·9 ng/ml/106 cells/24 hr), respectively, but very little IL-4 (∼50–60 pg/ml/106 cells/24 hr) in their culture supernatants (Fig. 1b), indicating that they are type 1 CD4+ Th1 and CD8+ Tc1 cells, respectively.
Figure 1.
Phenotypic characterization of in vitro DCOVA-activated CD4+ Th1 and CD8+ Tc1 cells.(a) The in vitro DCOVA-activated CD4+ Th1 and CD8+ Tc1 cells as described in Materials and Methods were stained using a panel of Abs for analysis of CD4, CD8, CD11c, CD25, CD69, CD80, Iab and Vβ5·1TCR (solid lines). The isotype-matched irrelevant Abs were used as controls (dotted lines).(b) The supernatants of these CD4+ Th1, Th1(pMHC I–/–) and CD8+ Tc1 cells were assayed for IFN-γ, IL-4 and IL-2 secretion by ELISA, respectively. The values presented represent the means of triplicate cultures from three distinct experiments.(c) Transfer of H-2Kb and pMHC I molecules onto CD4+ Th1 cells by DC activation. The active CD4+ Th1 or CD4+ Th1(Kb–/–) cells (solid lines) derived from activation of naïve OT II CD4+ T cells or CD4+ T(Kb–/–) cells of OT II/H-2Kb–/– mice by DCOVA, and the original naïve CD4+ T or CD4+ T(Kb–/–) cells (thick dotted lines) as well as the active CD4+ Th1(pMHC I–/–) cells (solid lines) derived from activation of naïve OT II CD4+ T cells by (Kb–/–)DCOVA, and the original naïve OT II CD4+ T cells (thick dotted lines) were stained with a panel of Abs for H-2Kb and pMHC I and analyzed by flow cytometry. The isotype-matched irrelevant Abs were used as controls (thin dotted lines). One representative experiment of three in the above different experiments is shown.
CD4+ Th1 cells acquire pMHC I complexes by DC activation
We have previously shown that active CD4+ Th cells can acquire DC molecules such as MHC I and II and costimulatory molecules by DC activation22. In this study, we demonstrated that active CD4+ Th1 cells expressed large amount of H-2Kb and some pMHC I complexes detected by the antipMHC I Ab (Fig. 1c). To confirm the acquisition of pMHC I complexes, we used CD4+ T cells derived from OT II mice with H-2Kb gene KO to rule out the possibility of its endogenous H-2Kb picking up OVA I peptide released from DCOVA in the culture. As shown in Fig. 1(d), naïve CD4+ Th1(Kb–/–) cells themselves did not display any endogenous H-2Kb expression, but the active CD4+ Th1(Kb–/–) cells derived from naïve CD4+ Th1(Kb–/–) cells activated by DCOVA did express both H-2Kb and pMHC I. As a control, the active CD4+ Th1(pMHC I–/–) cells derived from activation of naïve OT II CD4+ T cells with (Kb–/–)DCOVA did express H-2Kb, but did not express pMHC I (Fig. 1c), indicating that the pMHC I complexes on active CD4+ Th1 cells are acquired from DCOVA, but not derived from uptaking the DCOVA-derived OVA protein and self-loading OVA I peptide onto the self H-2Kb molecules. In addition, the active CD4+ Th1(pMHC I–/–) cells with acquired pMHC I complexes displayed a similar cytokine profiles as the active CD4+ Th1 cells with acquired pMHC I complexes (Fig. 1b). Therefore, these two types of CD4+ Th1 cells became useful reagents in studying the role of acquired pMHC I complexes in delivery of CD4+ Th1’s effect to CD8+ Tc1 cells in vivo.
CD4+ Th1 cells stimulate naïve CD8+ T cell proliferation via acquired pMHC I complexes
We have previously shown that active CD4+ Th cells with acquired DC molecules can stimulate naïve CD8+ T cell proliferation and activation22. To confirm that the stimulation of naïve CD8+ T cells is mediated via acquired pMHC I on CD4+ Th1 cells, we repeated T cell proliferation assays using CD4+ Th1(pMHC I–/–) cells with similar cytokine profiles [secreting IFN-γ (∼2·6 ng/ml/106 cells/24 hr) and IL-2 (∼2·2 ng/ml/106 cells/24 hr)] as CD4+ Th1 cells, but without acquired pMHC I complexes. As shown in Fig. 2(a), the positive control DCOVA strongly induced OT I cell proliferation. Active CD4+ Th1 cells with acquired DC molecules also stimulated proliferation of naïve OT I CD8+ T cells, but in a less extent, possibly due to its less costimulatory molecules compared with DCOVA. Interestingly, CD4+ Th1(pMHC I–/–) cells without acquired pMHC I complexes failed in stimulation of naïve CD8+ T cell proliferation, whereas CD4+ Th1(Kb–/–) cells lacking self H-2Kb, but with acquired pMHC I complexes had similar effect as CD4+ Th1 cells, thus confirming that activation of naïve CD8+ OT I T cells is critically mediated via acquired pMHC I complexes.
Figure 2.
Functional characterization of in vitro DCOVA-activated CD4+ OT II Th1 cells.(a) Naïve OT I CD8+ T cell proliferation assay. Varying numbers of stimulators including the irradiated CD4+ Th1, Th1(Kb–/–), Th1(pMHC I–/–) cells and DCOVA were cocultured with a constant number of naïve OT I CD8+ T cells. After two days, the proliferative responses of the CD8+ T cells were determined by 3H-thymidine uptake assays. (b) Apoptosis formation assay. Active CD8+ Tc1 cells were harvested 4 days after in vitro DCOVA activation, and cultured with or without CD4+ Th1 cells in the medium without IL-2 for 4 days. T cells were harvested, stained with FITC-Annexin V and PE-anti-CD8 Ab, and analyzed by flow cytometry. In some experiments, each of a panel of neutralizing reagents (anti-IL-2 and -IFN-γ Abs) (each 15 µg/ml) was added to the culture system. Th1(pMHC I–/–) cells without acquired pMHC I complexes were used as control cells in the above assay. *P < 0·05 (Student t-test) vs. cohorts of Th1 plus Tc1 cells. One representative experiment of two in the above different experiments is shown.
CD4+ Th1 cells prolong active CD8+ Tc1 cell survival in vitro via IL-2 secretion
T cell death or apoptosis formation can be derived from lymphokine withdrawal29. As shown in Figs 2(b) and 95% of active CD8+ Tc1 cells expressed Annexin V (early apoptosis marker) in medium without IL-2 after 4 days incubation. The CD8+ Tc1 cell apoptosis formation dramatically dropped to only 32% in presence of CD4+ Th1 cells, indicating that CD4+ Th1 cells can inhibit apoptosis formation of active Tc1 cells. CD4+ Th1(pMHC I–/–) with similar level of IL-2 secretion, even without acquired pMHC I, can still prolong CD8+ Tc1 survival in vitro. Interestingly, our data showed that Th1’s protection was significantly reduced (P < 0·05) in presence of anti-IL-2 Ab, but not in presence of anti-IFN-γ Ab, indicating that Th1’s help effect is mediated via its IL-2 secretion.
CD4+ Th1 cells promote CD8+ Tc1 cell tumor infiltration in vivo
We next tested whether CD4+ Th1 cells can promote CD8+ Tc1 cell tumor infiltration in vivo by flow cytometry. Our data showed that the amount of transferred CD8+ Tc1 cells reached peak at 3 days in tumors, but dramatically dropped to a minimal level of detection at 5 days subsequent to T cell injection (Fig. 3a). At day 3 subsequent to Tc1 cell injection, the transferred OVA-specific CD8+ Tc1 cells detected in tumors of mice with injection of CD8+ Tc1 and CD4+ Th1 cells is 0·52% of the total CD8+ T cells, which is around 3-folds more than that (0·16%) detected in tumors of mice with injection of CD8+ Tc1 cells alone (Fig. 3b). These results clearly indicate that OVA-specific CD4+ Th1 cells can also promote tumor infiltration of OVA-specific CD8+ Tc1 cells in vivo. In addition, the numbers of OVA-specific CD8+ Tc1 cells detected in EG7 tumors reduced by nearly 42% and 60% in the mice with cotreatment of Th1(IL-2–/–) and Th1(pMHC I–/–) cells, respectively, but were not affected with Th1(IFN-γ–/–) and Th1(Kb–/–) cells (Fig. 3c). Our data thus indicate that the degree of CD8+ Tc1 cell tumor infiltration is greatly affected by the helper cytokine IL-2, but not IFN-γ, and critically dependent on the OVA-specific delivery to CD8+ Tc1 cells in vivo via acquired pMHC I complexes on active CD4+ Th1 cells.
Figure 3.
Enumeration of OVA-specific CD8+ Tc1 cells in tumors. Active CD8+ Tc1 cells (5 × 106 cells/mouse) alone or together with in vitro-activated Th1 cells (2 × 106 cells/mouse) were i.v. injected into EG7 tumor-bearing C57BL/6 mice.(a) Tumors were removed for preparation of cell suspensions as described in Materials and Methods at different days subsequent to T cell injection. T cells were purified from the cell suspensions by using anti-CD3 Ab-coated magnetic beads and analyzed by flow cytometry using PE-tetramer and FITC-labeled anti-CD8 Ab.(b) The tumor-bearing mice were injected i.v. with 5 × 106 Tc1 cells alone or in conjunction with 2 × 106 Th1 cells. Tumors were removed for preparation of cell suspansions 3 days after T cell injection. Plots display the percentages of FITC-CD8 and PE-tetramer positive CD8+ Tc1 cells in the total CD8+ T cell population.(c) The tumor-bearing mice were injected i.v. with 5 × 106 Tc1 cells in conjunction with 2 × 106 Th1, Th1(IL-2–/–), Th1(IFN-γ–/–), Th1(pMHC I–/–) and Th1(Kb–/–) cells. Tumors were removed for preparation of cell suspensions 3 days after T cell injection. *P < 0·05 vs. cohorts of injection of Tc1 plus Th1 cells (Student's t-test). Data shown are representative of three separate experiments with three to five mice per experimental group.
CD4+ Th1 cells enhance adoptive CD8+ Tc1 cell therapy in vivo
To assess CD4+ Th1's help effects in vivo, we conducted animal studies using in vitro-activated CD8+ Tc1 cells for the passive transfers in combination with different amounts of in vitro-activated CD4+ Th1 cells. As shown in Fig. 4, CD4+ Th1 cells alone did not induce any tumor growth reduction. A combined adoptive T-cell therapy of CD8+ Tc1 (5 × 106 cells per mouse) with CD4+ Th1 (2 × 106 cells per mouse) resulted in regression of all 10 established EG7 tumors (∼5 mm in diameter), indicating that OVA-specific CD4+ Th1 cells can greatly enhance CD8+ Tc1-cell immunotherapy. The therapeutic efficiency is dose-dependent, since CD8+ Tc1 cells (5 × 106 cells per mouse) in conjunction with less CD4+ Th1 cells (1 × 106 and 0·5 × 106 cells per mouse) only cured 7/10 (70%) and 4/10 (40%) tumor bearing mice, respectively.
Figure 4.
Impact on tumor growth and mortality rates of cotransfer of Tc1 with Th1 cell subset in adoptive T-cell immunotherapy of established tumors. Mice bearing established (i.e. ∼5 mm in diameter) EG7 tumors were given i.v. injections of 5 × 106 CD8+ Tc1 in conjuction with different amounts of CD4+ Th1 cells. (a) Tumor growth was monitored and the tumor size (diameter) measured daily using an engineering caliper. The evolution of the tumors in individual mice is depicted, as are the fractions of mice in each treatment group that were tumor-free at 60 days post-treatment. (b) The readout in this figure represents the long-term mortality among the animals, as determined by daily assessments across 60 days post-treatment. The data closely mirror that in (a) with a conclusion that the combined CD4+ Th1 and CD8+ Tc1 cell immunotherapy provides a significant therapeutic advantage over the single use of Tc1 cells alone. One representative experiment of two is shown.
Targeted delivery of Th1’s IL-2 to CD8+ Tc1 cells in vivo via acquired pMHC I
To study the mechanism of Th1's effect, mice bearing tumors were i.v. injected with 5 × 106 Tc1 cells in conjunction with 2 × 106 CD4+ Th1(IL-2–/–) and CD4+ Th1(IFN-γ–/–) cells with respective cytokine deficiency. As shown in Fig. 5, the Th1’s help effect was substantially lost in mice with administration of CD4+ Th (IL-2–/–) cells, but not affected in mice injected with CD4+ Th1(IFN-γ–/–) cells, indicating that the Th1’s help effect is mainly dependent upon its IL-2, but not IFN-γ. CD4+ Th1(Kb–/–) cells with acquired pMHC I complexes had similar help effect as CD4+ Th1 cells. Interestingly, the CD4+ Th1’s help effect was completely lost in mice with administration of CD4+ Th1(pMHC I–/–) cells with similar cytokine profiles as CD4+ Th1, but without acquired pMHC I complexes, indicating the critical importance of acquired pMHC I complexes in targeted delivery of CD4+ Th1's helper cytokine IL-2 to CD8+ Tc1 cells in vivo.
Figure 5.
Animal study using CD4+ Th1 cells with respective gene deficiency. The mice bearing tumors were injected i.v. with 5 × 106 of Tc1 cells in conjunction with 2 × 106 Th1, Th1(IL-2–/–), Th1(IFN-γ–/–), Th1(Kb–/–) and Th1(pMHC I–/–) cells. Tumor growth was monitored and the tumor size (diameter) measured daily using an engineering caliper. The evolution of the tumors in individual mouse is depicted, as are the fractions of mice in each treatment group that were tumor-free at 60 days post-treatment.
CD4+ Th1 cells promote CD8+ Tc1 cell memory responses in vivo
We next assessed Th1's effect on CD8+ Tm cell responses by tetramer staining assays. Active CD8+ Tc1 cells can become long-lived memory Tm cells after adoptive transfer in vivo30. Three months after adoptive transfer of 5 × 106 active CD8+ Tc1 cells, there were 1·32% OVA-specific CD8+ T cells with CD44 expression (Tm marker)31 in the total host CD8+ T cell population (Fig. 6a), indicating that they became CD8+ Tm cells. The number of detected OVA-specific CD8+ Tm cells increased nearly by 3-folds to 3·86% in mice with transfer of both CD8+ Tc1 and CD4+ Th1 cells, indicating that CD4+ Th1 cells greatly promote CD8+ Tc1 cell memory responses. In the mice with transfer of CD8+ Tc1 and CD4+ Th1(pMHC I–/–) cells, however, the number of detected OVA-specific CD8+ Tm cells remained the same as that in the mice with transfer of CD8+ Tc1 cells alone, indicating that the acquired pMHC I complexes play an important role in targeting Th1’s effect to the promotion of CD8+ Tc1 cell memory responses in vivo. These CD8+ Tm cells still remained functional because they could be stimulated for expansion (∼10 folds) by DCOVA stimulation (Fig. 6b). Interestingly, transfer of CD8+ Tc1 cells alone can completely protect 8/8 mice from a challenge of 1 × 106 EG7 tumor cells, but only 4/8 mice from a challenge of 3 × 106 EG7 tumor cells (Fig. 6c). However, all (8/8) mice with transfer of both CD8+ Tc1 and CD4+ Th1 cells survived even with a challenge of 3 × 106 EG7 tumor cells, indicating that CD4+ Th1 cells can promote CD8+ Tc1 cell memory responses leading to an enhanced antitumor immunity.
Figure 6.
CD4+ Th1 cells promote active CD8+ Tc1 cell memory responses.(a) In vitro DCOVA-activated CD8+ Tc1 cells can become long-lived memory T cells. In vitro DCOVA-activated CD8+ Tc1 cells (5 × 106 cells/mouse) alone or in conjuction with active CD4+ Th1 or Th1(pMHC I–/–) cells (2 × 106 cells/mouse) were i.v. injected into C57BL/6 mice. Three months later, tail blood samples were taken from the mice, and a triple staining for PE-Kb/OVA tetramer, FITC-anti-CD8 and ECD-anti-CD44 Abs were conducted to determine the percentage of OVA-specific triple staining-positive CD8+ T cells in the total CD8+ population indicated in each right upper plot. The T cell population with PE-tetramer and FITC-CD8 double positivity was also the respective population with PE-tetramer and ECD-CD44 double positivity by flow cytometric analysis.(b) Three months later, the immunized mice were boosted by i.v. injection of 1 × 106 irradiated DCOVA. Four days after the boost, tail blood samples were taken from the boosted mice, and a double staining for PE-Kb/OVA tetramer and FITC-anti-CD8 Ab were conducted to determine the percentage of OVA-specific double staining-positive CD8+ T cells in the total CD8+ population indicated in each right upper plot. Naive C57BL/6 mice were also boosted with DCOVA and used as control.(c) Three months later, the immunized mice were also s.c. challenged with 1 × 106 or 3 × 106 EG7 tumor cells. Mouse survival was monitored daily. The results presented are representative of two separate experiments with 4 mice (a & b) or 8 mice (c) per group.
Discussion
The cooperative role of CD4+ T cells has been extensively reported for CD8+ CTLs in tumor eradication in animal models11,15,16. However, the potential immune mechanisms underlying enhancement of CD4+ Th1 cells in adoptive CD8+ Tc1-cell therapy have not been well elucidated. In this study, we developed a model system with a defined tumor Ag OVA using the OVA-transfected EG7 tumor cells and the OVA-specific TCR transgenic OT I and OT II mice with class I and II specificities, respectively23,24. These transgenic mice provide a virtually monoclonal source of T cells with known specificity, where the respective role of OVA-specific CD4+ Th1 cells in OVA-specific CD8+ Tc1 cell-mediated antitumor immunity can thus be feasibly evaluated. In previous animal tumor models using TCR transgenic CD8+ T cells, the treatment of only lung tumor metastasis or solid tumors in small palpable size has been investigated5,6. In clinical settings, however, patients usually have well-established tumors. In this study, we demonstrated that a combined adoptive transfer of CD8+ Tc1 cells with CD4+ Th1 cells resulted in regression of well-established tumors (5 mm in diameter), which more mimics the clinical situations, in all 10/10 mice, compared to only 40% curing rate when using adoptive transfer of CD8+ Tc1 cells alone, indicating the critical help effect of CD4+ Th1 cells in CD8+ Tc1-cell immunotherapy of established solid tumors. Active CD8+ Tc1 cells can become long-lived CD8+ Tm cells after adoptive transfer in vivo30. In this study, we also demonstrated that CD4+ Th1 cells greatly promoted CD8+ Tc1 cell memory responses by 3-folds. Interestingly, mice with transfer of CD8+ Tc1 cells alone can only protect 50% (4/8) of them from a challenge of 3 × 106 EG7 tumor cells, whereas all (8/8) mice with transfer of both CD8+ Tc1 and CD4+ Th1 cells survived, indicating that CD4+ Th1 cells can promote CD8+ Tc1 cell memory responses leading to an enhanced antitumor immunity.
Interleukin-2 (IL-2) support of transferred Tc cells in vivo can be critical to T-cell therapeutic efficiency. IL-2 administration after T cell transfer was shown to augment the antitumor therapy and increase transferred Tc cell persistance32,33. The potential mechanism by which IL-2 treatment may augment adoptive Tc cell therapy in vivo by promoting the survival and proliferation of transferred Tc cells34,35. Although the systemic use of IL-2 could enhance T-cell therapy efficiency in mouse models, the results from clinical trials in treatment of melanomas and renal cell carcinomas were very disappointed, showing no improvement in T-cell therapeutic efficiency36,37. In addition, the systemic administration of IL-2 also induced severe side-effects such as the vascular leak syndrome38. An alternative is the use of CD4+ Th cells providing IL-2 help to CD8+ Tc cells13. However, the molecular mechanism by which the CD4+ Th cell's IL-2 is specifically delivered to CD8+ Tc cells in vivo is unclear. T cell death or apoptosis formation can be derived from lymphokine withdrawal29. In this study, we demonstrated that CD4+ Th1 cells can reduce apoptosis formation of active CD8+ Tc1 cells by 3-folds in the culture medium without IL-2 stimulation, indicating that this help effect may be mediated via its secreted helper cytokine IL-2. In addition, we also demonstrated that (i) the CD4+ Th1 cells greatly augmented the in vivo CD8+ Tc1 cell-therapy in treatment of established tumors, and (ii) the Th1's help effect was substantially lost in mice with administration of CD4+ Th1(IL-2–/–) cells, indicating that the in vivo Th1's help effect is dependent upon its IL-2.
T cell-to-T cell (T-T) Ag presentation, dependent upon CD4+ T cells first acquiring MHC and costimulatory molecules from APCs, and then becoming Th-APCs with the capacity in stimulation of other T cells, is increasingly attracting attention39,40. Recently, Brandes et al. have shown that active human T cells expressed a similar phenotype as APC and can act as Th-APCs in stimulation of CTL responses after directly pulsed with soluble Ag41. Although these active CD4+ T cells pulsed with Ag can act as T-APCs in stimulation of CTL responses, they are different from the concept of CD4+ Th-APCs as recently described22. We have recently demonstrated that in vitro DC-activated CD4+ T cells with acquired DC membrane molecules by DC activation can act as Th-APCs in stimulation of CTL responses and antitumor immunity22 and CD4+ Th(pMHC I–/–) cells without acquired pMHC I complexes lost its antitumor protection (data not shown). In addition, we also found that in vivo DC-activated CD4+ T cells can also acquire APC membrane molecules by DC activation, and induce antitumor immunity (data not shown), indicating the physiological significance of APC membrane molecule acquisition of CD4+ T cells in vivo. Kennedy et al. have also demonstrated that CD4+ T cells can acquire APC membrane molecules in vivo, and induce memory CTL responses42 further confirming the physiological significance of the above concept. In this study, in addition to the acquired costimulatory molecules22 we further demonstrated that CD4+ T cells acquired pMHC I complexes by using a specific antibody recognizing pMHC I complexes. More importantly, for the first time, we elucidated the molecular mechanism on targeted delivery of CD4+ T cell help to active CD8+ Tc cells in vivo by using the recently established control CD4+ Th1(pMHC I–/–) cells. We showed that it is the acquired pMHC I complex on active CD4+ Th1 cells that mediate the specific delivery of the CD4+ Th cytokine IL-2 to CD8+ Tc1 cells in vivo, leading to an enhanced Tc1-cell immunotherapy of established EG7 solid tumors. In addition, we also found that the DCOVA-activated CD4+ Th1 cells with acquired pMHC I complexes were able to stimulate naive CD8+ T cell proliferation in vitro and in vivo, and induce antitumor immunity22 whereas CD4+ Th1(pMHC I–/–) cells with the same cytokine profiles as CD4+ Th1 cells, but without acquired pMHC I complexes completely lost its stimulatory effect (data not shown), thus confirming the importance of acquired pMHC I complexes in targeting CD4+ Th1's helper effects to both naïve or active CD8+ Tc cells in vivo.
The importance of successful Tc-cell immunotherapy of Tc-cell tumor infiltration is increasingly being recognized2,26,43. The systemic administration of IL-2 has been shown to enhance CD8+ T cells tumor infiltration10. However, the severe side-effects38 derived from the systemic administration of IL-2 limit its use in clinic trials. It has also been shown that the transferred CD8+ T cells infiltrated into tumors only in presence CD4+ T cell help by immunohistochemical analysis16. In this study, we kinetically and quantitatively examined CD8+ Tc1 cell tumor infiltration by flow cytometric analysis. Our data showed that a 3-fold more of the transferred OVA-specific CD8+ Tc1 cells can be detected in EG7 tumors of mice with treatment of both CD4+ Th1 and CD8+ Tc1 cells, indicating that CD4+ Th1 cells can significantly promote CD8+ Tc1 cell tumor infiltration. Interestingly, we also clearly demonstrated that the in vivo CD4+ Th1 cell's helper effect in promoting CD8+ Tc1 cell tumor infiltration is mediated by its IL-2. More importantly, we further demonstrated that the critical role of acquired pMHC I complexes on CD4+ Th1 cells in targeting its helper effect in promoting CD8+ Tc1 cell tumor infiltration. In addition, some other factors may also be involved in enhanced CD8+ Tc1 cell tumor infiltration, including (i) an augmented CD8+ Tc1 cell pool in the mice, thereby allowing for enhanced CD8+ T cell infiltration of tumors, and (ii) a prolonged survival of tumor infiltrating CD8+ Tc1 cells in tumors. In addition, some other mechanisms may also be involved. For example, it has been reported that the costimulatory signals41,42,44 and MHC class II molecules45 were involved in survival of the responding cells such as T cells. Therefore, the active Th1 cells with acquired CD80 and pMHC II may also provide its helper effects to active Tc1 cells in vivo through these mechanisms.
Taken together, our results provide clear evidence that CD4+ Th1 cells are capable of enhancing adoptive CD8+ Tc1-cell immunotherapy by prolonging CD8+ Tc1 cell survival in vitro and promoting CD8+ Tc1 cell tumor localization and memory responses in vivo. The CD4+ Th1’s effect is mainly mediated by its own helper cytokine IL-2 specifically targeted to CD8+ Tc1 cells in vivo via acquired pMHC I complexes. Therefore, these results will have important implications for designing adoptive T-cell immunotherapy protocols in treatment of established solid tumors.
Acknowledgments
This work was supported by a research grant (MOP 67230) from Canadian Institute of Health Research (CIHR), a Bridge Research Fund of the Saskatchewan Health Research Foundation (SHRF) and a Hazel Constance Broker Research Fund. Siguo Hao was supported by Postdoctoral Fellowship of Saskatchewan Health Research Foundation. We appreciated Mark Boyd for help in flow cytometric analysis.
Abbreviations
- APC
antigen-presenting cells
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- PCR
polymerase chain reaction
- pMHC
major histocompatibility complex/peptide
- TCR
T cell receptor
- Tc
CD8+ cytotoxic T
- Th
CD4+ helper T
References
- 1.Tanaka H, Yoshizawa H, Yamaguchi Y, et al. Successful adoptive immunotherapy of murine poorly immunogenic tumor with specific effector cells generated from gene-modified tumor-primed lymph node cells. J Immunol. 1999;162:3574–82. [PubMed] [Google Scholar]
- 2.Mukai S, Kjaergaard J, Shu S, Plautz GE. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. 1999;59:5245–9. [PubMed] [Google Scholar]
- 3.Kjaergaard J, Shu S. Tumor infiltration by adoptively transferred T cells is independent of immunologic specificity but requires down-regulation of 1-selectin expression. J Immunol. 1999;163:751–9. [PubMed] [Google Scholar]
- 4.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996;8:336–42. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 5.Dobrzanski MJ, Reome JB, Dutton RW. Therapeutic effects of tumor-reactive type 1 and type 2 CD8+ T cell subpopulations in established pulmonary metastases. J Immunol. 1999;162:6671–80. [PubMed] [Google Scholar]
- 6.Dobrzanski MJ, Reome JB, Dutton RW. Type 1 and type 2 CD8+ effector T cell subpopulations promote long-term tumor immunity and protection to progressively growing tumor. J Immunol. 2000;164:916–25. doi: 10.4049/jimmunol.164.2.916. [DOI] [PubMed] [Google Scholar]
- 7.Chang AE, Yoshizawa H, Sakai K, Cameron MJ, Sondak VK, Shu S. Clinical observations on adoptive immunotherapy with vaccine-primed T-lymphocytes secondarily sensitized to tumor in vitro. Cancer Res. 1993;53:1043–50. [PubMed] [Google Scholar]
- 8.Ogawa M, Umehara K, Yu WG, et al. A critical role for a peritumoral stromal reaction in the induction of T-cell migration responsible for interleukin-12-induced tumor regression. Cancer Res. 1999;59:1531–8. [PubMed] [Google Scholar]
- 9.Goedegebuure PS, Eberlein TJ. The role of CD4+ tumor-infiltrating lymphocytes in human solid tumors. Immunol Res. 1995;14:119–31. doi: 10.1007/BF02918172. [DOI] [PubMed] [Google Scholar]
- 10.Pockaj BA, Sherry RM, Wei JP, et al. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclophosphamide and correlation with response. Cancer. 1994;73:1731–7. doi: 10.1002/1097-0142(19940315)73:6<1731::aid-cncr2820730630>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 12.Fernando GJ, Stewart TJ, Tindle RW, Frazer IH. Th2-type CD4+ cells neither enhance nor suppress antitumor CTL activity in a mouse tumor model. J Immunol. 1998;161:2421–7. [PubMed] [Google Scholar]
- 13.Fearon ER, Pardoll DM, Itaya T, et al. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 14.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 15.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59:1071–9. [PubMed] [Google Scholar]
- 16.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major ‘post-licensing’ role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–55. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 17.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 18.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285(5425):221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 19.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283(5402):680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 20.Huang JF, Yang Y, Sepulveda H, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286(5441):952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 21.Hwang I, Huang JF, Kishimoto H, et al. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–48. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- 23.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge. CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–32. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 24.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–26. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Chen Z, Li F, Kamencic H, Juurlink B, Gordon JR, Xiang J. Tumour necrosis factor-alpha (TNF-alpha) transgene-expressing dendritic cells (DCs) undergo augmented cellular maturation and induce more robust T-cell activation and anti-tumour immunity than DCs generated in recombinant TNF-alpha. Immunology. 2003;108:177–88. doi: 10.1046/j.1365-2567.2003.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, Bi XG, Yuan JY, Xu SL, Guo XL, Xiang J. Combined CD4+ Th1 effect and lymphotactin transgene expression enhance CD8+ Tc1 tumor localization and therapy. Gene Ther. 2005;12:999–1010. doi: 10.1038/sj.gt.3302486. [DOI] [PubMed] [Google Scholar]
- 27.Huang H, Li F, Gordon JR, Xiang J. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res. 2002;62:2043–51. [PubMed] [Google Scholar]
- 28.Li Y, Wang MN, Li H, et al. Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med. 2002;195:1575–84. doi: 10.1084/jem.20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis – immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 30.Wang LX, Kjaergaard J, Cohen PA, Shu S, Plautz GE. Memory T cells originate from adoptively transferred effectors and reconstituting host cells after sequential lymphodepletion and adoptive immunotherapy. J Immunol. 2004;172:3462–8. doi: 10.4049/jimmunol.172.6.3462. [DOI] [PubMed] [Google Scholar]
- 31.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 32.Cheever MA, Greenberg PD, Fefer A, Gillis S. Augmentation of the anti-tumor therapeutic efficacy of long-term cultured T lymphocytes by in vivo administration of purified interleukin 2. J Exp Med. 1982;155:968–80. doi: 10.1084/jem.155.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu SY, Rosenberg SA. Adoptive immunotherapy of newly induced murine sarcomas. Cancer Res. 1985;45:1657–62. [PubMed] [Google Scholar]
- 34.Cheever MA, Thompson DB, Klarnet JP, Greenberg PD. Antigen-driven long term-cultured T cells proliferate in vivo, distribute widely, mediate specific tumor therapy, and persist long-term as functional memory T cells. J Exp Med. 1986;163:1100–12. doi: 10.1084/jem.163.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Cheever MA. Donor T cells can be induced to grow and survive long term in vivo without previous host immunosuppression. J Immunol. 1994;152:4767–74. [PubMed] [Google Scholar]
- 36.Rosenberg SA, Yannelli JR, Yang JC, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–66. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 37.Schwartzentruber DJ, Hom SS, Dadmarz R. White DE, Yannelli JR, Steinberg SM, Rosenberg SA, Topalian SL. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. J Clin Oncol. 1994;12:1475–83. doi: 10.1200/JCO.1994.12.7.1475. [DOI] [PubMed] [Google Scholar]
- 38.Oldham RK, Blumenschein G, Schwartzberg L, Birch R, Arnold J. Combination biotherapy utilizing interleukin-2 and alpha interferon in patients with advanced cancer: a National Biotherapy Study Group Trial. Mol Biother. 1992;4:4–9. [PubMed] [Google Scholar]
- 39.Tatari-Calderone Z, Semnani RT, Nutman TB, Schlom J, Sabzevari H. Acquisition of CD80 by human T cells at early stages of activation: functional involvement of CD80 acquisition in T cell to T cell interaction. J Immunol. 2002;169:6162–9. doi: 10.4049/jimmunol.169.11.6162. [DOI] [PubMed] [Google Scholar]
- 40.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II. peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101:2704–10. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 41.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309(5732):264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy R, Undale AH, Kieper WC, Block MS, Pease LR, Celis E. Direct cross-priming by th lymphocytes generates memory cytotoxic T cell responses. J Immunol. 2005;174:3967–77. doi: 10.4049/jimmunol.174.7.3967. [DOI] [PubMed] [Google Scholar]
- 43.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 44.Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA. CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol. 1996;157:3909–17. [PubMed] [Google Scholar]
- 45.Vella AT, Mitchell T, Groth B, Linsley PS, Green JM, Thompson CB, Kappler JW, Marrack P. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long-term survival in vivo. J Immunol. 1997;158:4714–20. [PubMed] [Google Scholar]