Abstract
The global epidemic of tuberculosis, fuelled by acquired immune-deficiency syndrome, necessitates the development of a safe and effective vaccine. We have constructed a ΔRD1ΔpanCD mutant of Mycobacterium tuberculosis (mc26030) that undergoes limited replication and is severely attenuated in immunocompromised mice, yet induces significant protection against tuberculosis in wild-type mice and even in mice that completely lack CD4+ T cells as a result of targeted disruption of their CD4 genes (CD4–/– mice). Ex vivo studies of T cells from mc26030-immunized mice showed that these immune cells responded to protein antigens of M. tuberculosis in a major histocompatibility complex (MHC) class II-restricted manner. Antibody depletion experiments showed that antituberculosis protective responses in the lung were not diminished by removal of CD8+, T-cell receptor γδ (TCR-γδ+) and NK1.1+ T cells from vaccinated CD4–/– mice before challenge, implying that the observed recall and immune effector functions resulting from vaccination of CD4–/– mice with mc26030 were attributable to a population of CD4– CD8– (double-negative) TCR-αβ+, TCR-γδ–, NK1.1– T cells. Transfer of highly enriched double-negative TCR-αβ+ T cells from mc26030-immunized CD4–/– mice into naive CD4–/– mice resulted in significant protection against an aerosol tuberculosis challenge. Enriched pulmonary double-negative T cells transcribed significantly more interferon-γ and interleukin-2 mRNA than double-negative T cells from naive mice after a tuberculous challenge. These results confirmed previous findings on the potential for a subset of MHC class II-restricted T cells to develop and function without expression of CD4 and suggest novel vaccination strategies to assist in the control of tuberculosis in human immunodeficiency virus-infected humans who have chronic depletion of their CD4+ T cells.
Keywords: cytokines, T cells, tuberculosis, vaccine
Introduction
Even in the 21st century, the ancient scourge of tuberculosis (TB) remains an important international public-health concern. More than eight million new cases of TB are detected annually and two million people die of this disease each year.1 The convergence of the human immunodeficiency virus (HIV) and TB epidemics has exacerbated the global TB problem with over 15 million of the estimated 42 million people infected with HIV, being co-infected with Mycobacterium tuberculosis.2 Importantly, TB is currently the leading cause of death among people living with HIV. In these HIV-infected individuals, the destruction of CD4+ T cells by the virus accelerates progression to active TB in people with latent or recently acquired M. tuberculosis infections, which increases the risk of transmission to the general population.3 The overall TB burden in countries with advanced HIV epidemics has increased substantially in the past decade, especially in sub-Saharan Africa.4,5 In some countries of this region, escalating numbers of TB cases have overwhelmed the capacity of local public-health institutions to adequately respond to the co-epidemics. Clearly, improved preventive approaches, including more effective vaccines for use in both immunocompetent and HIV-infected people, are needed to curb this devastating global TB epidemic.
The limited success of international bacillus Calmette–Guérin (BCG) immunization programmes in controlling the TB epidemic has led to increased efforts to develop novel vaccines against TB. A promising new TB immunization strategy is the development of live attenuated M. tuberculosis vaccine strains.6 Since these vaccines are generated from homologous organisms, their overall potential to protect against tuberculous infections should exceed the effectiveness of M. bovis-derived vaccine strains including BCG. Previously, we have shown that live attenuated TB vaccines can be safely and effectively administered to wild-type and immunocompromised mice and to guinea-pigs.7–9 One of these live vaccine candidates (mc26030) contains attenuating mutations in genes required for pantothenic acid synthesis (panC and panD) as well as in the region of difference-1 (RD1) gene region. The RD1 locus is present in all M. tuberculosis strains but is absent in BCG strains, and removal of the RD1 region substantially reduces the virulence of M. tuberculosis.10–14 Recently, we demonstrated that the attenuated mc26030 strain is safer than BCG when administered to severe combined immunodeficient mice or to interferon-γ (IFN-γ) gene knockout mice, and that immunization with mc26030 protected wild-type and CD4–/– mice against an aerogenic M. tuberculosis challenge.15
To further evaluate the potential of the mc26030 vaccine, we investigated the mechanisms in immunocompromised mice that mediate the antituberculosis protective responses induced by immunization with this strain. Previous studies have shown that intracellular infections and live vaccines derived from intracellular organisms can stimulate T-cell receptor αβ-positive (TCR-αβ+) CD4– CD8– (double-negative, DN) T cells. Expansion of the DN T-cell populations has been reported in wild-type mice infected with Francisella tularensis, Mycobacterium bovis and M. tuberculosis.16–18 Furthermore, immunization of wild-type mice with the live vaccine strain of F. tularensis induced potent effector DN TCR-αβ+ T cells which controlled subsequent Francisella infections.18,19 While the role of DN T cells in human protective immunity is less clear, DN TCR-αβ+ T cells have also been shown to be expanded in the circulation of humans in response to infections caused by staphylococci and mycobacteria.20,21 In addition, multiple examples of human DN TCR-αβ+ T-cell lines reactive with mycobacterial lipid antigens presented by CD1 molecules and expressing antimycobacterial effector functions have been reported.22–24 Consistent with these observations, we have demonstrated in this study that the antituberculosis protective responses evoked by immunization of CD4–/– mice with the mc26030 strain are largely mediated by DN TCR-αβ+ T cells. Ex vivo studies of the M. tuberculosis-responsive population of T cells from mc26030-immunized mice, showed that these immune cells were primarily major histocompatibility complex (MHC) class II-restricted and recognized protein antigens of the bacilli. Our results provide support for the hypothesis that potent MHC class II-restricted effector cells can be present as part of a DN T-cell pool, and that the potential for these to serve as antimycobacterial effectors may be augmented through appropriate vaccination strategies. Immunization strategies targeting this DN T-cell population may yield improved vaccines for use in individuals with CD4 T-cell deficiencies, including people living with HIV.
Materials and methods
Animals
Specific pathogen-free CD4-deficient (B6.129S2-Cd4<tm1Mak>/J) and MHC class II-deficient (B6.SJL-ptprca/BoAiTac-Abbtm1N13) mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and Taconic Laboratories (Germantown, NY), respectively. The mice were maintained under barrier conditions and fed commercial mouse chow and water ad libitum. The mice were 6–8 weeks old at the time of vaccination.
Media and bacterial strains
Mycobacterium tuberculosis H37Rv and M. tuberculosis Erdman were obtained from the Trudeau Culture Collection (Saranac Lake, NY). When required, pantothenate (24 μg/ml) or hygromycin (50 μg/ml) were added. Stock strains were grown in Middlebrook 7H9 broth in roller bottles and harvested in mid-logarithmic growth phase before being stored in 1-ml vials at −70°.
Construction of M. tuberculosis ΔRD1 ΔpanCD (mc26030) deletion mutant
Specialized transduction was employed to disrupt the chromosomal copy of the panCD genes from the unmarked M. tuberculosis ΔRD1 mutant.25 The panCD and RD1 deletions were confirmed using polymerase chain reaction (PCR) and Southern blotting as described earlier.7,12
Vaccination studies
Mice (five per group) were vaccinated subcutaneously (s.c.) with 106 colony-forming units (CFU) of the mc26030 mutant. At 2–3 months after the initial immunization with mc26030, the mice were aerogenically challenged with approximately 100–200 CFU M. tuberculosis Erdman strain using a Middlebrook chamber (Glas Col, Terre Haute, IN). At 28 days following aerosol infection or when the mice became moribund, the mice were killed and the bacteria in the lung and spleen were enumerated by plating serial dilutions of the lung and spleen homogenates (50-μl aliquots) onto Middlebrook 7H11 agar (Difco, Detroit, MI) containing 10% Middlebrook OADC Enrichment (Becton Dickinson, Sparks, MD), 10 μg/ml ampicillin (Sigma, St Louis, MO), and 50 μg/ml cycloheximide (Sigma).26
Protein antigens and bacterial sonicates
The M. tuberculosis strain mc26030 was pelleted by centrifugation at 3000 g for 20 min, resuspended in 1·0 ml phosphate-buffered saline (PBS) and boiled for 10 min. Subsequently, bacteria were sonicated using a double-step Branson sonicator for 10 min (Duty cycle 30%, Output Control 1–2). The same method was used to generate a total sonicate of irradiated M. tuberculosis strain H37Rv. Purified protein derivative (PPD) was obtained from Statens Serum Institute (Copenhagen, Denmark). Antigen 85 (Ag85) Complex and Antigen 85 A purified proteins were obtained through the Colorado State TB Vaccine Testing and Research Materials Contract (HHSN266200400091c). Early secreted antigenic target-6 (ESAT-6), isocitrate lyase and malate synthase proteins (generously provided by Dr James Sacchetini, Texas A & M University), were produced as recombinant proteins in Escherichia coli and purified by fast protein liquid chromatography.
Generation of bone marrow-derived macrophages and dendritic cells
Bone marrow cells were flushed from femora and tibiae of mice and differentiated into macrophages (BMM) by incubation for 6 days in Dulbecco's modified Eagle's medium containing 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mm HEPES, 2·0 mm l-glutamine, 0·05 mm 2-mercaptoethanol, 0·1 mm minimal essential medium non-essential amino acids, 10% fetal calf serum (FCS) and 20% conditioned medium from a confluent culture of L-929 fibroblasts as a source of CSF-1 (LCCM). After removal of non-adherent cells, macrophages were recovered by washing plates with cold PBS plus 5 mm ethylene diamine tetraacetic acid (EDTA). Bone marrow cells were differentiated into dendritic cells (BMDC) according to the protocol of Lutz et al.27
For experiments using BMM derived from CD1–/–, β2-microglobulin–/– or MHC class II–/– mice, macrophages were assayed by flow cytometry using standard methods to verify the deletion phenotype. Antibodies used were anti-CD1d (clone 1B1), anti-H-2Dd (clone 34-5-8S), and anti-I-A/I-E (clone M5/114.15.2). BMM from C57BL/6 mice served as positive controls and unstained cells served as negative staining controls. Events were acquired on a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA) or a Becton Dickinson LSR II and were analysed using WinMDI (Windows Multiple Document Interface for Flow Cytometry).
T-cell isolation, culture and stimulation
Spleens from vehicle- or mc26030-immunized mice were mechanically dissociated and single-cell suspensions in RPMI-1640 medium containing 200 U/ml penicillin, 200 μg/ml streptomycin and 10% heat inactivated FCS were treated with Hybri-Max RBC lysing buffer according to the manufacturer's protocol (Sigma-Aldrich). Splenocytes were washed twice with medium and incubated for a minimum of 30 min at 4° with bidirectional rotation with Dynal Mouse T Cell Negative Isolation antibody mix containing monoclonal antibodies (mAbs) specific for CD11b, CD16/32, CD45R and Ter-119 and mouse depletion dynabeads (Dynal Biotech ASA, Oslo, Norway) in buffer containing PBS (without Ca2+ and Mg2+) plus 0·1% bovine serum albumin and 2 mm EDTA according to the manufacturer's protocol. After incubation, cells were washed and unlabelled cells were collected after placement of the cell suspension in a Dynal MPC magnet for a minimum of 2 min. The resulting T-cell suspensions were then washed and resuspended in complete medium (CM) consisting of RPMI-1640 media with 10 mm HEPES, 2·0 mm l-glutamine, 0·05 mm 2-mercaptoethanol, 0·1 mm MEM non-essential amino acids and 10% fetal bovine serum, to which was added 100 U/ml penicillin plus 100 μg/ml streptomycin. Harvested cell suspensions contained > 95% CD3+ cells by flow cytometry.
For measurement of antigen-specific responses of T cells in vitro, murine BMDC were plated in 96-well plates at a density of 1 × 105 cells/well in 100 μl CM. The following day, the medium was aspirated and cells were washed once with PBS. Washed viable mc26030 bacilli were then added to the wells at the indicated multiplicity of infection (MOI) in 100 μl infection medium (Dulbecco's modified Eagle's medium plus 10% fetal bovine serum). Infections were then allowed to proceed for 2 hr at 37°, after which the wells were washed once with medium followed by addition of T cells to a concentration of 5 × 104 cells/200 μl in CM with 10 μg/ml gentamicin. Supernatants were assayed for IFN-γ by enzyme-linked immunosorbent assay (ELISA) following incubations at 37° for the periods indicated in the text. The same protocol was used for experiments with BMM as antigen-presenting cells, except the postinfection culture medium used was CM with 100 μl/ml gentamicin containing 10% LCCM. For experiments in which antigen-presenting cells were pulsed with soluble antigens or bacterial extracts, the incubation time was 6 hr, unless otherwise indicated, in complete culture media.
IFN-γ ELISA and cell surface capture assays
IFN-γ concentrations were measured by a standard sandwich ELISA. Maxisorp plates (Nunc, Roskilde, Denmark) were coated with unconjugated anti-IFN-γ antibody (clone AN-18 or R4-6A2) and detection used the corresponding biotinylated mAb (clone R4-6A2 or XMG1.2, respectively). The plates were developed using Poly horseradish peroxidase–streptavidin (Pierce Endogen, Rockford, IL) and 1-Step Turbo TMB-ELISA (Pierce) as substrate. All conditions were performed in duplicate. Assays were standardized with murine recombinant cytokines (PharMingen, San Diego, CA).
Quantification of secreted IFN-γ from stimulated T cells was performed by flow cytometry using the Mouse IFN-γ secretion assay and following the manufacturer's protocol (Miltenyi Biotec, Auburn CA). Murine T cells were isolated and purified as above. Viable murine BMDC were plated in six-well plates at a density of 4 × 106 cells/well in 2 ml CM. The following day, antigen-specific in vitro stimulation was performed. Plating media were aspirated and cells were washed once with PBS. Washed mc26030 bacilli were then added to the wells at the indicated MOI in 2 ml of infection media. Infections were allowed to proceed for 2 hr at 37°. Next, cells were washed and viable T cells were added to the wells at a concentration of 2 × 106 cells/4 ml in CM with 10 μg/ml gentamicin and incubated for 12 hr (unless otherwise specified). T cells were then recovered, washed and labelled with mouse IFN-γ catch reagent for 5 min on ice, followed by incubation at 37° with slow rotation for 45 min. Cells were subsequently labelled with anti-mouse IFN-γ phycoerythrin-labelled detection antibody for 10 min on ice, and then with antibodies to specific markers of interest including NK1.1 (clone PK136), TCR-γδ (clone GL3), CD3ε (clone 145-2C11), TCR-β (clone H57-597),CD8α (clone Ly-2) and CD4 (clone GK1.5). All antibodies were purchased from PharMingen. DAPI staining was used for experiments where dead cell exclusion was noted. Unstained cells served as negative staining controls.
In vivo cell depletions using mAbs
Vaccinated CD4–/– mice were treated intraperitoneally (i.p.) with 0·5 mg anti-CD8 (clone 2.43), TCR-γδ (clone GL3), NK1.1 (clone PK.136) or Thy-1.2 (clone 30-H12) mAbs alone or in combination at 4 and 2 days before aerosol TB challenge and then once every 10 days until the mice were killed. The antibodies were produced as ascites in BALB/c nu/nu mice and precipitated with 50% ammonium sulphate. All antibody preparations contained < 10 EU/ml endotoxin. To evaluate the extent of depletion of the target cell populations, spleen cells were isolated from treated and untreated mice at the same time as organs were harvested for CFU determinations. Spleen cells (1 × 106) were incubated with anti-mouse CD16/CD32 (FcγIII/II receptor, clone 2.4G2) at 4° for 10 min in 50 μl PBS + 2% FCS. The cells were then stained for 30 min at 4° with antibodies against CD8 (rat anti-mouse CD8 fluorescein isothiocyanate antibody, clone 53-6.7), TCR-γδ (hamster anti-mouse phycoerythrin antibody, clone GL3), NK1.1 (anti-mouse phycoerythrin antibody, clone PK 136), TCR-β (hamster anti-mouse phycoerythrin antibody, clone H57-597) and Thy-1.2 (rat anti-mouse allophycocyanin antibody, clone 53-2.1) at 0·2 μg per 106 cells. After washing with PBS + 2% FCS, the cells were fixed with Cytofix/Cytoperm solution (PharMingen) at 4° for 30 min, washed with Perm/Wash solution (PharMingen), suspended in 1·0 ml FACS buffer (PBS + 2% FCS + 0·1% sodium azide) and analysed using an LSRII flow cytometer (Becton Dickinson) and FACSDiva software. Isotype controls for each antibody were used. All antibodies were obtained from PharMingen.
Adoptive transfer with double-negative T cells
Double-negative T cells were purified from the spleens of CD4–/– mice vaccinated 2 months earlier with mc26030 and treated i.p. with 0·5 mg anti-CD8, anti-TCR-γδ and anti-NK1.1 mAbs, 4 and 2 days before harvesting the spleens. A single cell suspension was achieved by passing the spleen homogenates through a 70-μm nylon cell strainer (Becton Dickinson) and collecting the cells by centrifugation. The erythrocytes were removed by treating this preparation with ACK buffer (Quality Biological, Inc., Gaithersburg, MD). The leucocytes were washed with PBS + 2% FCS (PBS/FCS), pooled into one tube in 5 ml PBS/FCS, counted and the volume was adjusted to achieve a cell concentration of 1·1 × 108 cells/ml (90 μl per 107 cells total). Anti-B220 magnetic beads were then added to the cells (10 μl per 90 μl volume) and incubated at 4° for 15 min, followed by washing with PBS/FCS. The cells were then applied to a depletion column (Miltenyi Biotec). Cells that failed to bind (i.e. B220-negative) were collected by washing the column with PBS/FCS during application of the magnetic field, and then were resuspended, counted and adjusted to 1·1 × 108 cells/ml in PBS/FCS. Next, 10 μl anti-Thy-1.2 magnetic beads were added per 90 μl volume of cell suspension, and the cells were then incubated at 4° for 15 min and added to a separation column. After washing the column, the column was removed from the magnetic field and then bound (i.e. Thy-1.2+) cells were eluted, pelleted, resuspended in 1·0 ml PBS/FCS, and counted. For the adoptive transfer studies, four million purified Thy-1.2+ B220– cells were injected intravenously per CD4–/– mouse. After 1 hr, the mice were aerogenically challenged with ∼200 CFU M. tuberculosis Erdman.
Early cytokine production in lung leucocytes after vaccination and challenge
Cytokine messenger RNA levels were evaluated as described previously.28 Briefly, CD4–/– mice were vaccinated with the mc26030 strain, and, 6 weeks later, were treated i.p. with 0·5 mg anti-CD8, anti-TCR-γδ and anti-NK1.1 mAbs twice, at 4 and 2 days before being aerogenically challenged with M. tuberculosis. The mice were killed 8 days after the aerosol infection and the lung cells were harvested from vaccinated and non-vaccinated mice by shredding the lung tissue with razor blades and treating with dispase (2 mg/ml) (Invitrogen, Carlsbad, CA) for 1 hr at 37°. A single cell suspension of leucocytes was achieved by passage through a 70 μm strainer, treatment with ACK buffer and washing and resuspension in PBS/FCS. An aliquot of cells was used for flow analysis to verify that the target cell populations had been depleted. The remainder of the cells was used for DN T-cell isolation as described above using antibody-conjugated magnetic beads. RNA from the DN T cells was extracted and purified using an RNAqueous-4PCR kit (Ambion, Austin, TX) and reverse transcribed using a SuperScript First-Strand Synthesis kit (Invitrogen). The same amount of RNA (1 μg) from each group was used for the cDNA synthesis reaction. The cDNA was used as a template for real-time PCR using the following probe and primers specific for IFN-γ: forward primer (5′-AGCAACAGCAAGGCGAAAA), reverse primer (5′-CTGGACCTGTGGGTTGTTGA), and probe (5′ FAM-CCTCAAACTTGGCAATACTCATGAATGCATCC-TAMRA). TaqMan reagents for assessing the mRNA levels for tumour necrosis factor-α, interleukin-2 (IL-2) and IL-12p40 were obtained from Applied Biosystems (Foster City, CA). The PCR amplifications were completed with an ABI Prism 7000 sequence detection system (Applied Biosystems). Glyceraldehyde phosphate dehydrogenase (GAPDH) Taqman reagents (Applied Biosystems) were used to measure GAPDH mRNA levels as an internal standard and the level of cytokine mRNA relative to GAPDH mRNA was calculated using the following equation: relative mRNA expression = 2−(Ctof cytokine − Ctof GAPDH), where Ct is the threshold cycle.
Histopathology assessments
For histopathological analysis of infected animals, lung tissues were perfused and fixed with formalin in PBS and then embedded in paraffin for sectioning. The lung sections were stained with haematoxylin & eosin or with Ziehl–Neelsen acid-fast stain and were evaluated by light microscopy.
Results
Protection of CD4–/– mice against an aerogenic tuberculous challenge by immunization with M. tuberculosis mc26030
We have previously shown that immunization with an M. tuberculosis specific DNA vaccine or BCG can induce substantial antituberculosis protective immunity in CD4–/– mice.29 To further evaluate the cellular mechanisms associated with the protective immune responses induced by TB vaccines in CD4-deficient mice, vaccination/challenge studies were initiated with the ΔRD1ΔpanCD (mc26030) attenuated mutant of M. tuberculosis. In initial experiments, the contributions of T cells to the vaccine-induced protective responses were identified by depletion with an antibody that recognizes the Thy-1.2+ T-cell marker. CD4–/– mice were immunized with mc2,6030 and then, two months later, groups of these mice were depleted of Thy-1.2+ cells before an aerogenic M. tuberculosis challenge. As seen in Table 1, immunization with mc26030 evoked significant antituberculosis protective responses in CD4–/– mice. At 28 days postchallenge, reductions in mycobacterial CFU (compared to naive animals) of 1·87 log10 in the lungs and 1·89 log10 in the spleens were detected. In contrast, treatment with the anti-Thy-1.2 antibody completely abrogated vaccine-induced immunity. In fact, significantly higher lung CFU values were seen in the anti-Thy-1.2-treated mice relative to non-immunized mice.
Table 1.
Protective immunity against tuberculosis induced in CD4–/– mice after vaccination with the mc26030 vaccine with or without depletion of Thy-1.2+ cells
| Log10 CFU of M. tuberculosis (mean ± SEM)2 | ||
|---|---|---|
| Group1 | Lung | Spleen |
| Naive | 8·0 ± 0·17 | 6·56 ± 0·17 |
| mc26030 | 6·13 ± 0·07 | 4·67 ± 0·08 |
| (−1·87)*** | (−1·89)*** | |
| + anti-Thy-1.23 | 8·80 ± 0·05 | 6·90 ± 0·09 |
| (+0·80)** | ||
CD4–/– mice were immunized s.c. with 106 CFU of the mc26030 vaccine. Two months later, some of the vaccinated mice were treated with anti-Thy-1.2 mAb before being aerogenically challenged and subsequently treated every 10 days.
Mean bacterial CFU (log10) (± SEM) in the lungs and spleens of mice 28 days following an aerosol infection with 200 CFU M. tuberculosis Erdman per mouse (n = 5). The values in parentheses represent the reduction in bacterial burden relative to the naive control mice. Statistical significance compared to controls:
P < 0·01
P < 0·001.
0·5 mg of anti-Thy-1.2 (clone 30-H12) were injected intraperitoneally at 4 days before, the day before, and every 10 days after the aerosol infection.
Characterization of T cells stimulated by immunization with the mc26030 strain
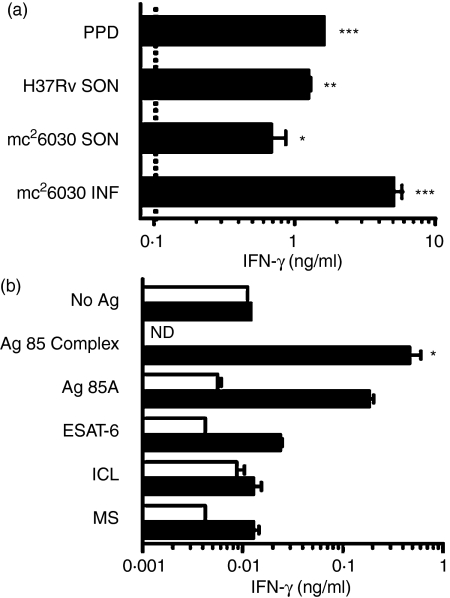
To more fully characterize the T-cell responses evoked by immunization with the mc26030 mutant, CD4–/– mice were primed s.c. with 106 live mc26030 and then boosted intravenously (i.v.) with the same dose 13 months later. We have previously shown that immunization with a live attenuated mycobacterial vaccine induced substantial protective immunity against tuberculosis for at least 1 year postvaccination.28 Two weeks after boosting, T cells were purified from the spleens of primed and boosted mice as well as from primed animals and naive controls. IFN-γ production from T cells was assessed after the immune cells had been cultured with BMM that had been either infected with the mc26030 mutant, pulsed with sonicated extracts of mc26030 or M. tuberculosis H37Rv, or incubated with PPD. Naive T cells or mc26030-primed T cells did not produce detectable levels of IFN-γ in response to these mycobacterial antigens (data not shown). However, T cells isolated from primed and boosted CD4-deficient mice did produce IFN-γ in response to stimulation by each of these preparations. The highest IFN-γ levels were seen in primed and boosted T cells cultured with macrophages infected with the live mc26030 strain (Fig. 1a).
Figure 1.
In vitro T-cell responses in mc26030-immunized mice. (a) T-cell responses to infected macrophages or crude mycobacterial antigen preparations. IFN-γ production by purified splenic T cells from a CD4–/– mouse that was primed and boosted with mc26030. T cells were stimulated for 4·5 days by coculture with syngeneic bone marrow-derived macrophages (BMM) which had been infected for 2 hr with mc26030 at an MOI of 10 : 1 (mc26030 INF), or pulsed for 10 hr with sonicates of either mc26030 (mc26030 SON) or H37Rv bacilli (H37Rv SON) or PPD. IFN-γ levels were assayed in culture supernatants by ELISA. For this experiment, priming was performed 13 months before killing by s.c. injection in the flank of 106 live mc26030 in 200 μl vehicle (PBS + 0·05% Tween-80), and boosting was 2 weeks before killing by i.v. injection of 106 live mc26030 in vehicle. The concentration of mc26030 sonicate used for pulsing BMM was adjusted to give the equivalent of a bacterial load corresponding to MOI of 10 : 1. H37Rv sonicate was used at 1·0 mg/ml based on dry weight of sonicated bacteria, and PPD was used at a total protein concentration of 100 μg/ml. IFN-γ production was below the level of detection (∼0·01 ng/ml, indicated by dotted line) in the absence of infection or antigen pulsing of BMM. Asterisks indicate statistically significant increases in IFN-γ production over the background levels observed with T cells from naive mice (*P < 0·05; **P < 0·01; ***P < 0·001; one-way anova, Bonferroni post-test).(b) Responses to purified and recombinant M. tuberculosis protein antigens. T cells purified from spleens of a naive CD4–/– mouse (open bars) or an mc26030-primed and boosted CD4–/– mouse (filled bars) were assayed for responses to the indicated antigens by measurement of IFN-γ secretion. Levels of IFN-γ are shown for T cells stimulated for 5 days by BMM in the presence of the indicated antigens at 10 μg/ml. For this experiment, priming was performed by s.c. injection of 106 live mc26030 in vehicle (PBS + 0·05% Tween-80), and 6 months later boosting was by i.v. injection of 106 live mc26030. Naive mice were sham primed and boosted on the same schedule with vehicle only. Mice were killed and splenic T cells were isolated 2 weeks after boosting. ICL, isocitrate lyase; MS, malate synthase; ND, not determined. *P < 0·05 compared to no antigen control (one-way anova, Bonferroni post-test).
The cellular immune responses in vaccinated CD4-deficient mice were further investigated by evaluating whether specific mycobacterial proteins could induce IFN-γ production from immune T cells. As shown in Fig. 1(b), significantly higher levels of IFN-γ were generated when immune T cells were cultured with BMM in the presence of antigen 85 complex proteins. In contrast, the mycobacterial antigens, ESAT-6, isocitrate lyase and malate synthase did not activate T cells to produce IFN-γ in these in vitro assays.
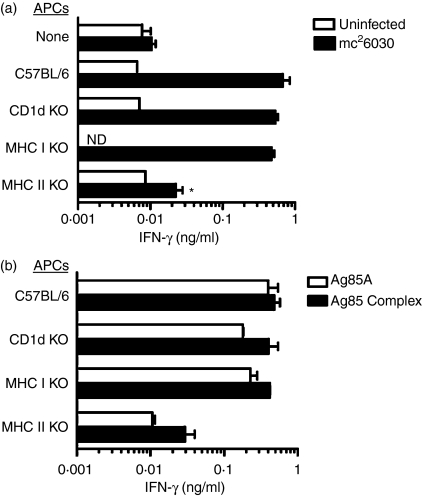
To more completely address the mechanisms of antigen presentation, immune T cells were cultured with BMM isolated from wild-type C57BL/6 mice or from gene knockout mice deficient in either CD1d, MHC class I or MHC class II molecules. A previous study had surprisingly shown that the CD8 T-cell population in CD4–/– mice contained a substantial percentage of class II restricted cells in addition to conventional class I restricted cells.30Figure 2(a) shows that antigen-presenting cells, which were infected with mc26030 after isolation from CD1d-deficient or MHC class I-deficient mice, stimulated immune T cells to produce levels of IFN-γ that were similar to that detected when the T cells were stimulated with wild-type C57BL/6 BMM. However, significantly decreased levels of IFN-γ were generated when mc26030-infected BMM from MHC class II-deficient mice were used for antigen presentation. Furthermore, similar results were obtained when the BMM were pulsed with antigen 85 complex proteins (Fig. 2b). The levels of IFN-γ were essentially equivalent when immune T cells from CD4–/– mice were incubated with either antigen 85 protein-pulsed antigen-presenting cells from C57BL/6, CD1d-deficient or MHC class I-deficient mice. In contrast, about 10-fold lower concentrations of IFN-γ were detected when the antigen 85 proteins were presented in the absence of class II molecules. Taken together, these in vitro data suggest that immune T cells from mc26030 vaccinated CD4-deficient mice primarily recognize mycobacterial protein antigens in the context of MHC class II.
Figure 2.
MHC class II restriction of responses to mycobacterial antigens by T cells from mc26030 immunized CD4–/– mice.(a) MHC class II restriction of responses to mycobacteria-infected BMM. Purified T cells from an mc26030-primed and boosted CD4–/– mice were either cultured without APCs, or with BMM from wild-type C57BL/6 or the indicated knockout mouse strains as APCs. Replicate cultures were either left uninfected (open bars) or infected with mc26030 (MOI 10 : 1) for 2 hr followed by washing to remove uningested bacteria. Supernatant levels of IFN-γ were measured after 5 days of culture. The mouse used for T-cell isolation in this experiment was primed and boosted as in Fig. 1(b), and killed 2 weeks after boosting. Asterisk indicates statistically significant reduction with MHC class II knockout APCs compared to wild-type C57BL/6 APCs (*P < 0·05; one-way anova, Bonferroni post-test).(b) MHC class II restriction of responses to purified M. tuberculosis protein antigens. Purified T cells from a CD4–/– mouse that was primed and boosted with mc26030 as in (a) were cultured with BMMs from wild-type or the indicated knockout mouse strains as APCs. The BMMs were pulsed with 10 μg/ml purified antigen 85 A (open bars), antigen 85 complex (filled bars), or no antigen (not shown) for 6 hr before the addition of T cells. IFN-γ levels were determined as in (a); levels were below the limit of detection (∼0·01 ng/ml) for cultures containing T cells purified from naive mice, or in the absence of antigen stimulation.
Vaccination/challenge studies in MHC class II-deficient mice supported the in vitro antigen presentation results. As seen in Table 2, moderate but statistically significant decreases in lung bacterial burdens, compared to naive controls, were seen in MHC class II–/– mice immunized with the mc26030 mutant within the first month postchallenge. Moreover, MHC class II–/– mice vaccinated with the mc26030 strain survived 12 days longer postchallenge (mean time to death = 33 ± 1·5 days, P < 0·05) than the highly susceptible naive class II-deficient animals (mean time to death = 21 ± 0·2 days). However, the vaccine-induced protective immune responses detected in MHC class II-deficient mice were modest relative to the protection seen in immunized CD4–/– mice. Substantially decreased lung bacterial burdens were detected in vaccinated CD4–/– mice (−1·8 log10) (Table 1) 4 weeks after challenge and the survival period was extended about 150 days postchallenge in vaccinated CD4–/– mice relative to naive controls. Clearly, the decreased protection seen in the MHC class II–/– mice relative to CD4–/– mice supports the MHC class II restriction data and suggests that the protective immunity elicited by vaccination of with the mc26030 mutant is primarily mediated by MHC class II-restricted T cells.
Table 2.
TB vaccination/challenge studies in MHC class II–/– mice
| Log10 CFU of M. tuberculosis (mean ± SEM)2 | ||
|---|---|---|
| Group1 | Lung | Spleen |
| Naive | 6·88 ± 0·05 | 6·00 ± 0·17 |
| mc26030 | 6·27 ± 0·04 | 5·8 ± 0·20 |
| (−0·61)* | ||
| + anti-Thy-1.2 | 8·77 ± 0·04 | 6·76 ± 0·06 |
| (+1·89)* | (+0·76)* | |
MHC Class II–/– mice were vaccinated s.c. with 106 CFU of the mc26030 vaccine. Two months after vaccination, some of the vaccinated mice were treated i.p. with anti-Thy-1.2 (clone 30-H12) twice before challenge and every 10 days subsequent to being challenged.
Mean bacterial CFU (log10) (± SEM) in the lungs and spleens of mice 24 days following an aerosol infection with about 200 CFU of M. tuberculosis Erdman per mouse (n = 5). The values in parentheses represent the difference in bacterial burden relative to the naive control mice.
P < 0·05.
Secretion of IFN-γ from CD8+ and double-negative T cells after priming and boosting CD4–/– mice with the mc26030 vaccine
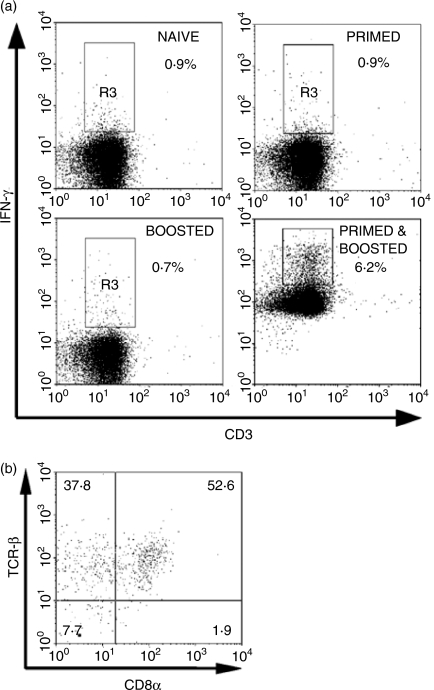
The T-cell populations in vaccinated CD4–/– mice that were stimulated to produce IFN-γ were assessed by flow cytometry (Fig. 3). For this study, animals were primed s.c. with 106 CFU of the mc26030 strain and boosted i.v. with the same dose of the mutant 6 months later. After 2 weeks, T cells isolated from these mice were stimulated for 12 hr with mc26030-infected BMM. Following stimulation, the cells were stained with antibodies recognizing T-cell markers and were then analysed for IFN-γ using a surface capture protocol. Similar low levels of IFN-γ-producing cells (0·7–0·9%) were detected in naive CD4–/– mice, and also in T cells isolated from the spleens of mice that had received either a single s.c. immunization 6 months before (primed) or a single i.v. immunization 2 weeks before (boosted) being killed. However, mice that had been immunized s.c. with mc26030 6 months earlier and then boosted i.v. 2 weeks before being killed (primed and boosted), showed a considerably higher fraction of T cells (6·2%) secreting IFN-γ. Phenotypic analysis of IFN-γ-positive T cells from these primed and boosted mice, revealed that more than 90% were TCRβ+ while less than 10% expressed the TCR-γδ (Fig. 3b, and data not shown). Interestingly, although about 53% of the IFN-γ-positive cells were CD8 T cells, nearly 38% of the IFN-γ-positive, TCRβ+ cells were DN T cells.
Figure 3.
Frequency and phenotype of IFN-γ-secreting T cells in the spleens of mc26030-immunized CD4–/– mice. (a) CD4–/– mice were immunized either with vehicle only (naive), one injection s.c. of 106 live mc26030 at 6 months (primed) or i.v. at 2 weeks (boosted) before killing, or both s.c. injection of 106 live mc26030 at 6 months followed by i.v. injection at 2 weeks before killing (primed and boosted) as indicated. T cells were purified by negative selection and stimulated for 12 hr in vitro with BMDC previously infected for 2 hr with mc26030 (MOI 10 : 1). Following stimulation, cells were analysed for IFN-γ secretion by surface capture protocol and FACS as described. Dot plots display events gated for live lymphocytes, based on FSC/SSC and DAPI exclusion. (b) A separate experiment showing the phenotype of splenic T cells pooled from three CD4–/– mice primed s.c. with 106 live mc26030 and boosted i.v. with 106 live mc26030 6 months later. Mice were killed 2 weeks after boosting. Splenocytes were stimulated in vitro and stained for surface IFN-γ capture as in (a), and with mAbs specific for TCR-β and CD8. The dot plot shows events gated as lymphocytes based on FSC/SSC and positive for IFN-γ staining, revealing enrichment of CD4– CD8– T cells among the population of cells showing antigen-induced IFN-γ secretion. Percentages of total IFN-γ+ gated lymphocytes in each quadrant are indicated.
Role of TCR-αβ+ DN T cells in mediating protective immunity induced by immunization of CD4–/– mice
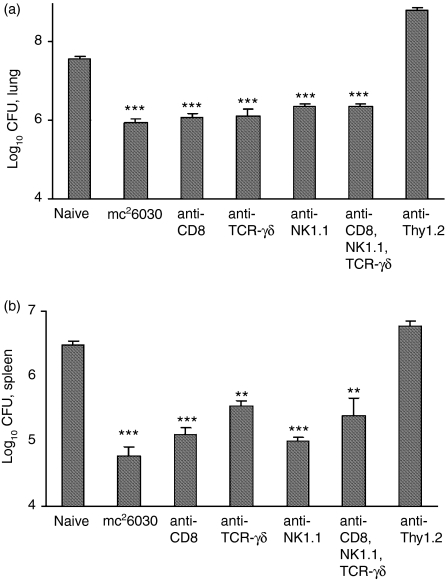
The immune cells contributing to the vaccine-induced protective immunity in CD4–/– mice were characterized using in vivo antibody depletion experiments. The significant numbers of IFN-γ-positive CD8+, DN, and TCR-γδ+ T cells detected in the flow cytometry studies suggested that one or more of these T-cell subsets was responsible for the antituberculosis protective response in the absence of CD4+ T cells (Fig. 3). Additionally, because natural killer (NK) cells and NK T cells can produce IFN-γ, the effect of in vivo depletion of these cells was evaluated (Fig. 4). In this study, significant decreases in pulmonary mycobacterial growth were again detected in mc26030-vaccinated mice relative to naive controls at 28 days postchallenge. Surprisingly, CD8+ T-cell depletion had only a minimal effect on the vaccine-induced pulmonary protective response. The injection of antibodies recognizing either the NK1.1 surface antigen or TCR-γδ into immunized CD4–/– mice also did not impact vaccine effectiveness in the lung. Moreover, substantial postchallenge antituberculosis protective immunity was still detected in the lungs and spleens of vaccinated CD4–/– mice following depletion of > 95% of the CD8+, NK1.1+ and γδ+ T cells by concurrent administration of all three antibodies. By contrast, depletion of Thy-1.2+ cells by injecting anti-Thy-1.2 antibody ablated vaccine-induced protection. Significantly higher mycobacterial lung CFU values were detected in anti-Thy-1.2-treated mice compared to naive controls (P < 0·01). Depletion of CD8+ T cells by antibody treatment also had a minimal impact on the mc26030 vaccine-induced protective responses in the spleen. However, treatment of CD4–/– mice with the anti-TCR-γδ antibody did significantly increase (P < 0·05) the bacterial burden in the spleen at 1 month postchallenge relative to untreated immunized CD4–/– mice. Although the levels of mycobacteria were still significantly lower than in naive CD4–/– mice, the anti-TCR-γδ antibody treatment did increase the bacterial numbers in the spleens of vaccinated mice by about 0·7 log10 CFU.
Figure 4.
mc26030-induced antituberculosis protection was retained in CD4–/– mice that were depleted of CD8+, TCR-γδ+, and NK1.1+ cells. CD4–/– mice were vaccinated with the mc26030 vaccine strain and 60 days later were treated i.p. with 0·5 mg of a mAb against CD8 (clone 2.43), TCR-γδ (clone GL3), NK1.1 (clone PK-136) or Thy-1.2 (clone 30-H12) molecules alone or in combination twice before an Erdman aerogenic challenge and every 10 days subsequent to challenge. Mice were killed 1 month following challenge, and the lung (a) and spleen (b) bacterial CFU were determined. Flow analysis of spleen cells showed that the mAbs depleted > 95% of the target cell populations. 1, naïve; 2, mc26030-immunized; 3–7 were vaccinated and then antibody depleted; **P < 0·01; ***P < 0·001.
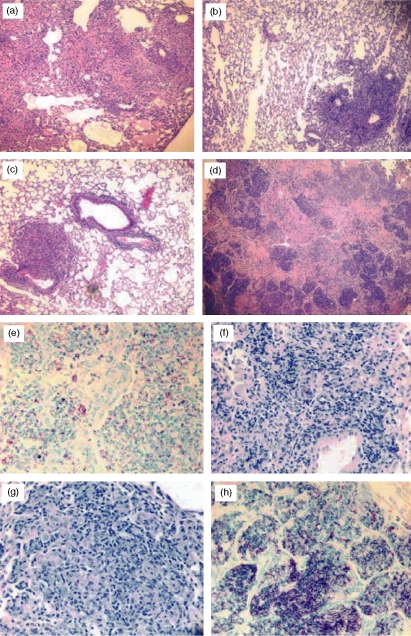
The lung pathology seen in the CD4–/– mice depleted with specific antibodies supported the bacterial CFU protection findings. Histologically, significant differences were observed between the vaccinated mice and the naive controls. The naive mice developed severe lung lesions with multiple large inflammatory nodules, some of which coalesced and spread to create areas of extensive diffuse pneumonia. The inflammatory response consisted of macrophages, numerous neutrophils, and low numbers of lymphocytes (Fig. 5a,e). In contrast, mice vaccinated with mc26030 showed reduced severity of lung lesions. These mice had scattered distinct lesions, adjacent to airways and localized, which remained smaller in diameter than in non-vaccinated mice. These multifocal lesions were composed largely of macrophages and numerous lymphocytes (Fig. 5b). There was also a marked reduction in the number of acid-fast organisms compared to naive controls (Fig. 5f). The histopathology of the vaccinated and CD8-, NK1.1- and TCR-γδ-depleted CD4–/– mice was remarkably similar to the immunized, non-treated mice and was characterized by reduced inflammation (compared to naive mice) and lymphocyte-rich granulomatous lesions (Fig. 5c). Furthermore, considerably reduced numbers of acid-fast organisms were seen in lung tissues of these antibody-treated mice relative to naive animals (Fig. 5g). Consistent with the bacterial CFU values, the anti-Thy-1.2 mAb treatment of vaccinated CD4–/– mice resulted in substantially worse lung pathology. In lung sections of the anti-Thy-1.2 antibody-treated animals, numerous large necrotic lesions, severe pneumonia and significantly elevated numbers of acid-fast bacilli were seen (Fig. 5d,h).
Figure 5.
Lung histopathology from the naive, vaccinated, and antibody-treated CD4–/– mice showed substantially worsening pathology only in vaccinated mice that were depleted of Thy-1.2+ cells and not in immunized CD4–/– mice that had been depleted of CD8+, TCR-γδ+ and NK1.1+ cells. Lung tissue was obtained from each group at the time of death, fixed in formalin, and embedded in paraffin. The lung sections were stained with haematoxylin & eosin (a–d) or with Ziehl–Neelsen acid-fast stain (e–h) and evaluated by light microscopy. Stained lung sections from naive (a, e), mc26030-vaccinated (b, f), mc26030-vaccinated and anti-CD8, NK1.1, and TCR-γδ mAb-treated (c, g), and mc26030-vaccinated and anti-Thy-1.2 mAb-treated (d, h) mice are shown above.
Protective effects of adoptively transferred immune DN TCR-αβ+ T cells
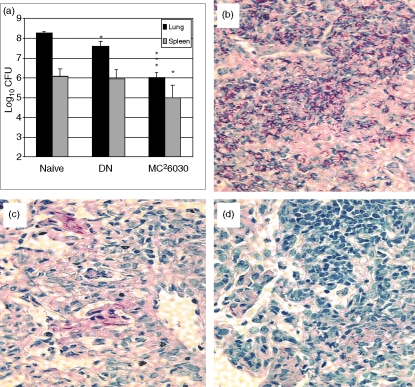
The antibody treatment experiments, which showed that CD8+, NK1.1+, and TCR-γδ+ T-cell depletions did not diminish the antituberculosis pulmonary protective responses, strongly suggested that DN T cells were primarily responsible for the vaccine-induced protective immunity. To more directly demonstrate that postimmunization protective responses were mediated primarily by DN T cells, splenocytes were isolated from vaccinated CD4–/– mice that had been treated with anti-CD8, NK1.1 and TCR-γδ antibodies. Following depletion of B cells using anti-B220 antibody conjugated to magnetic beads, the remaining T cells were positively selected using anti-Thy-1.2 magnetic beads and then adoptively transferred into naive CD4–/– mice. Flow cytometric analysis indicated that most of the separated cells were DN TCR-αβ+ T cells (83% TCR-αβ+, 99% CD8– and 93% Thy-1.2+). About 1 hr after adoptive transfer of the purified cells by intravenous injection, the naive CD4–/– recipients were aerogenically challenged with about 200 CFU virulent M. tuberculosis. Twenty-eight days later, the mycobacterial growth and lung pathology in the adoptively transferred mice, mc26030 immunized animals, and naive controls were compared. While differences in the spleen mycobacterial CFU were not detected, significant reductions (0·7 log10 relative to naive controls) were seen in the growth of M. tuberculosis in the lungs of the recipients of the adoptively transferred lymphocytes (Fig. 6a). Importantly, acid-fast staining of the lung sections from the mice were consistent with the bacterial CFU data; numerous acid-fast rods were present in lung sections of naive mice (Fig. 6b), while few acid-fast bacilli were seen in pulmonary sections taken from adoptively transferred (Fig. 6c) or mc26030 vaccinated mice (Fig. 6d). Additionally, haematoxylin & eosin staining of relevant lung sections showed substantial differences in the pathology at 28 days postchallenge (data not shown). While necrotic foci and extensive pneumonia were present in the naive lungs, no necrosis, considerably less pneumonia, and decreased overall inflammation were seen in the lungs of the adoptively transferred mice and the mc26030-vaccinated controls. Overall, these antibody depletion and adoptive transfer studies suggest that vaccination with the live mc26030 strain stimulates DN T cells that contribute to the antituberculosis protection seen in CD4–/– mice.
Figure 6.
Adoptive transfer of immune CD4– CD8– Thy-1.2+ cells protected CD4–/– mice against an aerogenic tuberculous challenge. Two months after immunizing 20 CD4–/– mice with mc26030 (mc2), the mice were treated with anti-CD8 (clone 2.43), anti-TCR-γδ (GL3) and anti-NK1.1 (PK 136) mAbs twice before harvesting the spleens. The spleens were removed and the B cells were depleted from the spleen cells by adding anti-B220 microbeads and then passing the cells through a depletion column. Thy-1.2+ cells were positively selected by adding anti-Thy-1.2+ microbeads to the cells and then passing the cells through a separation column. Flow cytometry used fluorochrome-conjugated anti-CD8 (clone 53-6.7), anti-Thy-1.2 (53-2.1), anti-TCR-γδ (GL3), anti-TCR-β (H57-597), anti-B220 (RA3-6B2) and anti-NK1.1 (PK 136) mAbs; 4 × 106 cells were adoptively transferred into CD4–/– mice by intravenous injection, and 1 hr later these and the naive and mc26030-vaccinated control mice were challenged with 200 CFU of M. tuberculosis Erdman. One month later, the organ bacterial burden and lung pathology were evaluated.(a) CFU were reduced in the lungs of mice receiving DN T cells and mc26030-vaccinated CD4–/– mice relative to naive controls (*P < 0·05; **P < 0·01; ***P < 0·001). Post-challenge lung pathology for naive (b), adoptively transferred(c), and mc26030-vaccinated(d) CD4–/– mice is shown.
Production of elevated levels of IFN-γ and IL-2 messenger RNA by activated DN T cells isolated from vaccinated and challenged CD4–/– mice
We have recently shown that vaccine-induced cellular responses could be detected in the mouse model of pulmonary tuberculosis between 1 and 2 weeks after a low-dose aerogenic challenge.31 To investigate the specific immune mechanisms associated with vaccine-induced protection by DN T cells from CD4-deficient mice, naive and immunized CD4–/– mice were treated with a mixture of anti-CD8, NK1.1 and TCR-γδ antibodies and were then infected by the aerosol route with virulent M. tuberculosis. Eight days after infection, the mice were killed and pulmonary DN T cells were isolated as described above. In this experiment, 99% of the Thy-1.2+ pulmonary cells from vaccinated, antibody treated CD4–/– mice were CD8–, TCR-γδ–, NK1.1–. Post-challenge cytokine mRNA levels in these cells were evaluated by real-time RT-PCR. The DN T cell IFN-γ mRNA levels from vaccinated mice were significantly higher (17-fold, P < 0·05) than the IFN-γ mRNA values from DN T cells taken from naive mice. In addition, significantly increased concentrations of IL-2 mRNA (4·5-fold, P = 0·05) were detected from DN cells. While the levels of tumour necrosis factor-α and IL-12p40 message were generally about two-fold higher than controls in lung DN cells isolated from immunized CD4–/– mice, these differences did not reach statistical significance.
Discussion
HIV-infected individuals are extremely susceptible to severe infections by vaccine-preventable pathogens, and routine immunizations continue to be recommended for most children living with HIV infections.32 Although immune responses within this population to some vaccines may be suboptimal, clinically effective protective responses against specific pathogens have been demonstrated in immunized HIV-infected individuals.33–35 Since CD4 T cells are reduced, often dramatically, in individuals with advanced HIV infection, it is important to determine whether other remaining T-cell populations in these people may be able to provide useful protective immunity when targeted by appropriate vaccines. In the current study, we have shown that in CD4–/– mice, a model of severe, longstanding CD4+ T-cell deficiency, the activation of DN T cells contributed to protection against M. tuberculosis induced by a novel live attenuated vaccine strain, mc26030. Antibody depletion of CD8+, NK1.1+ and TCR-γδ+ cells in CD4–/– mice that had been vaccinated with mc26030 did not eliminate the pulmonary protective responses evoked by immunization. Moreover, adoptive transfer of highly enriched DN TCR-αβ+ T cells from these vaccinated CD4–/– mice protected naive CD4–/– mice against an aerosol M. tuberculosis challenge, as measured by reduced pulmonary mycobacterial growth and improved lung pathology. Our ex vivo studies and the reduced protection seen in MHC class II–/– mice suggest that these DN T cells are primarily MHC class II restricted. Taken together, our results strongly implicate a population of DN TCR-αβ+ T cells as the principal cell type responsible for the protective recall response generated by vaccination of CD4–/– mice with mc26030. It should be noted that treatment with the anti-γδ antibody significantly reduced the splenic vaccine-induced protective effect. This TCR-γδ+Τ-cell depletion result and our failure to protect against dissemination of the tuberculous challenge to the spleen in the adoptive transfer experiment suggest that TCR-γδ+ T cells, as well as DN T cells, may be necessary to limit mycobacterial growth in the spleens of mc26030-vaccinated CD4–/– mice.
Expanded populations of mature DN TCR-αβ+ T cells in peripheral lymphoid organs have been described previously in TCR transgenic mouse models36 and in certain strains of mice with autoimmune lymphoproliferative disorders37 but these studies did not clearly imply a physiological role for such T cells in the normal immune system. Using CD4–/– mice as we have done in the current study, other investigators have demonstrated the presence of an easily detectable population of MHC class II-restricted DN TCR-αβ+ T cells which can comprise about 10–20% of the peripheral T-cell pool despite the complete absence of CD4 co-receptor expression.30 A few studies have shown that these T cells from immunodeficient mice can contribute to protective immune responses in models of intracellular infection including leishmaniasis38 and tuberculosis.39
Although the DN TCR-αβ+ population is clearly expanded in CD4–/– mice, a variety of studies indicate that a smaller but readily detectable number of mature peripheral DN TCR-αβ+ T cells is present in normal animals. In the spleen or lymph nodes, these typically comprise < 1% of the T cells, although in certain tissues, such as the lung and the female genital tract, they have been reported to be much more frequent.17,40 In contrast to the predominantly MHC class II-restricted DN T cells that we identified in our current study, it is clear that a substantial fraction of mouse DN TCR-αβ+ T cells are CD1d-restricted NK T cells that recognize predominantly or exclusively lipid antigens.41 Furthermore, DN TCR-αβ+ T cells have been identified that appear to be neither MHC class II-restricted nor CD1d-restricted, such as those described recently by Elkins and colleagues in their studies of DN TCR-αβ+ T cells that mediate potent control of intracellular growth of F. tularensis and M. tuberculosis.18 The specific antigens and antigen-presenting molecules for these T cells are currently unknown but they have been isolated from wild-type as well as MHC class II–/– and β2-microglobulin–/– mice, suggesting a novel mode of pathogen recognition.18,19 Thus, it is clear that DN TCR-αβ+ T cells exist in wild-type mice, and that these comprise a diverse population with regard to the nature of their specific antigens and the antigen-presenting molecules they recognize.
The immune mechanisms contributing to the protective responses evoked by the DN cells in mc26030 vaccine-induced CD4–/– mice remain unclear. A primary mode of action for the DN TCR-αβ+ T cells is likely to be cytokine secretion, because considerably elevated levels of IFN-γ and IL-2 mRNA were detected in this T-cell population at 8 days postchallenge. Also, purified DN T-cell populations produced IFN-γ in response to TB-infected cells ex vivo. Interestingly, more than 13% of DN cells expressed granzyme B at 60 days postvaccination with the mc26030 mutant (I.M. Orme, unpublished results). These disparate results indicate that the DN population may be heterogeneous. One fraction of these cells seems to produce IFN-γ and IL-2 and so may resemble CD4 cells. Another subset of this population may be CD8-like because they express granzyme B. To clarify some of these mechanistic issues, more detailed analyses of the vaccine-induced DN T cells is ongoing.
While the role of the DN T cells in human protective immunity to infectious diseases is less clear, recent studies indicate that DN TCR-αβ+ T cells are expanded in humans in response to pathogens. Carulli et al.20 reported the increase of CD3+ CD4– CD8– TCR-αβ+ T cells in an individual with staphylococcal toxic shock syndrome. The percentage of CD3+ CD4– CD8– TCR-γδ– T cells has also been shown to be increased in HIV disease, especially in people with low numbers of CD4+ cells.42 Among HIV-infected patients, expansion of these DN T cells is frequently seen in individuals with disseminated M. avium infections.43 These studies are encouraging because they suggest that the DN T-cell populations may be relatively spared or even expanded in patients with advanced HIV infection, raising the possibility that their antimicrobial activities might be significantly augmented through appropriate vaccinations. Interestingly, a variety of mycobacterial lipids including mycolic acids, the major component of the mycobacterial cell wall, can stimulate CD1-restricted DN T cells in the peripheral blood of healthy humans.44 Mycobacterial lipid antigen presentation may not be a prominent target of DN TCR-αβ+ T-cell responses in mice, which lack orthologues of the human group 1 CD1 proteins that present these antigens. However, in humans it is possible that this capacity of lipid molecules derived from mycobacteria to activate DN T cells may even more significantly augment the protection induced by live vaccine strains in individuals with severe depletion of CD4+ T cells.
Although the stimulation of DN T cells by immunization with the mc26030 strain was clearly demonstrated, our failure to detect vaccine-induced protective CD8 responses that could be depleted with specific antibody was surprising because more than 50% of the TCR-β+ IFN-γ-producing cells had the CD8 phenotype. In an earlier study, we showed that the depletion of CD8+ cells in CD4–/– mice immunized with an efficacious DNA vaccine cocktail completely abrogated the antituberculosis protective immunity evoked by immunization.29 Using a similar CD4–/– model of immunodeficiency, Wang et al.39 reported that antibody depletion of CD8 cells partially, but not completely, eliminated the protective responses induced by BCG immunization. While the reasons for the seemingly different levels of CD8 activation detected in our experiments and the Wang et al. studies are uncertain, the use of different vaccines and different mouse strains or the relative insensitivity of the biological protection assay could explain the apparent inconsistencies. However, the results of both studies indicate that a significant component of protective antituberculosis responses induced in CD4–/– mice by immunization with live attenuated mycobacterial vaccines was not mediated by CD8 cells. The activation of DN T cells by live vaccines and the absence of DN T-cell activation by a highly effective DNA vaccine cocktail suggest that live vaccines may uniquely stimulate this T-cell population instead of CD8+ cells. It should be noted that following live bacterial or viral infections of CD4-deficient mice, defective CD8 memory cells are often generated that do not mount competent recall responses and cannot effectively respond to a secondary challenge with live organisms.45,46 The presence of CD4 help seems to promote protective CD8 memory development. Moreover, even though substantial increases in antigen-specific CD8 T cells have been detected in vaccination/challenge studies using CD4–/– mice, the expanded CD8 cells typically have reduced functional capacities and restricted TCR repertoires.47,48 Therefore, these data are consistent with our CD8 depletion results in suggesting that the CD8 T cells induced by immunization with the mc26030 strain in CD4–/– mice were relatively non-functional and played only a minor role in protecting against a tuberculous challenge.
To summarize, the surprising capacity of the mc26030 vaccine to activate DN TCR-αβ+ T cells, which protected CD4-deficient animals against TB, suggests that the stimulation of DN T cells may be a unique mechanism to provide protective immunity against infectious pathogens for individuals living with HIV. Based on these results, evaluation of this mc26030 mutant in other animal models of TB, particularly simian immunodeficieny virus-infected non-human primates, is clearly warranted. Additionally, the further development of vaccine preparations for HIV-infected populations that are designed to target the DN T-cell population should be encouraged.
Abbreviations
- APC
antigen-presenting cells
- BCG
bacillus Calmette–Guérin
- BMDC
bone-marrow-derived dendritic cells
- BMM
bone-marrow-derived macrophages
- CFU
colony-forming units
- DN
double-negative
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HIV
human immunodeficiency virus
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneal
- i.v.
intravenous
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- MOI
multiplicity of infection
- NK
natural killer
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PPD
purified protein derivative
- s.c.
subcutaneous
- TB
tuberculosis
- TCR
T-cell receptor
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglone MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.McShane H. Co-infection with HIV and TB. Double trouble. Int J STD AIDS. 2005;16:95–100. doi: 10.1258/0956462053057576. [DOI] [PubMed] [Google Scholar]
- 3.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–20. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 4.Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362:887–99. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. http://www.who.int/mediacentre/factsheets. Fact Sheet no. 104.
- 6.Kamath AT, Fruth U, Brennan MJ, et al. New live mycobacterial vaccines: the Geneva consensus on essential steps toward clinical development. Vaccine. 2005;23:3753–61. doi: 10.1016/j.vaccine.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Sambandamurthy VK, Wang X, Chen B, Russell RG, Derrick S, Collins FM, Morris SL, Jacobs WR. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat Med. 2002;8:1171–4. doi: 10.1038/nm765. [DOI] [PubMed] [Google Scholar]
- 8.Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, Jacobs WR. Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect Immun. 2005;73:1196–203. doi: 10.1128/IAI.73.2.1196-1203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR, Bloom BR, Hondalus MK. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun. 2004;72:3031–7. doi: 10.1128/IAI.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 11.Mostovy S, Cousins D, Brinkman J, Aranaz A, Behr MA. Genomic deletions suggest a phylogeny for the Mycobacterium tuberculosis complex. J Infect Dis. 2002;186:74–80. doi: 10.1086/341068. [DOI] [PubMed] [Google Scholar]
- 12.Hsu T, Hingley-Wilson SM, Chen B, et al. The primary mechanism of attenuation of BCG is a loss of secreted lytic function required for invasion. Proc Natl Acad Sci USA. 2003;100:12420–5. doi: 10.1073/pnas.1635213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. Deletion of RD1 from Mycobacterium tuberculosis mimics Bacille Calmette–Guérin attenuation. J Infect Dis. 2003;187:117–23. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol. 2002;46:709–17. doi: 10.1046/j.1365-2958.2002.03237.x. [DOI] [PubMed] [Google Scholar]
- 15.Sambandamurthy VK, Derrick SC, Hsu T, et al. Mycobacterium tuberculosisΔRDI ΔpanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompressed mice against experimental tuberculosis. Vaccine. 2006;24:6309–20. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 16.Yee D, Rhinehart-Jones TR, Elkins KL. Loss of either CD4+ or CD8+ T cells does not affect the magnitude of the protective immunity to an intracellular pathogen, Francisella tularenisis LVS. J Immunol. 1996;158:1217–5048. [PubMed] [Google Scholar]
- 17.Kawakami K, Teruya K, Tohyama M, Kudeken N, Yonamine Y, Saito A. Mac1 discriminates unusual CD4–CD8– double negative T cells bearing αβ antigen receptor from conventional ones with either CD4 or CD8 in murine lung. Immunol Lett. 1995;46:143–52. doi: 10.1016/0165-2478(95)00034-3. [DOI] [PubMed] [Google Scholar]
- 18.Cowley SC, Hamilton E, Frelinger JA, Su J, Forman J, Elkins KL. CD4–CD8– T cells control intracellular bacterial infections both in vitro and in vivo. J Exp Med. 2005;202:309–19. doi: 10.1084/jem.20050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley SC, Elkins KL. Multiple T cell subsets control Francisella tularensis LVS intracellular growth without stimulation through macrophage interferon-γ receptors. J Exp Med. 2003;198:379–89. doi: 10.1084/jem.20030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carulli G, Lagomarsini G, Azzara A, Testi R, Riccioni R, Petrini M. Expansion of TcRαβ+CD3+CD4–CD8–(CD4/CD8 double-negative) T lymphocytes in a case of staphylococcal toxic shock syndrome. Acta Haematol. 2004;111:163–7. doi: 10.1159/000076526. [DOI] [PubMed] [Google Scholar]
- 21.Moreau JF, Taupin JL, Dupon M, et al. Increases in CD3+CD4–CD8– T lymphocytes in AIDS patients with disseminated Mycobacterium avium complex infection. J Infect Dis. 1996;174:969–76. doi: 10.1093/infdis/174.5.969. [DOI] [PubMed] [Google Scholar]
- 22.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4–8– T lymphocytes to a microbial antigen. Nature. 1992;360:593–7. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 23.Moody DB, Ulrichs T, Muhlecker W, et al. CD1c-mediated T cell recognition of isoprenoid glycolipids in M. tuberculosis infection. Nature. 2000;404:884–8. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- 24.Brigl M, Brenne MB. CD1 antigen presentation and T cell function. Ann Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 25.Bardarov S, Bardarov S, Pavelka MS, et al. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–17. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- 26.Delogu G, Li A, Repique C, Collins F, Morris SL. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induced sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect Immun. 2002;70:292–302. doi: 10.1128/IAI.70.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutz MB, Kukutsch N, Ogilvie AJ, Rößner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 28.Derrick SC, Yang AL, Morris SL. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine. 2004;23:780–8. doi: 10.1016/j.vaccine.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Derrick SC, Repique C, Snoy P, Yang AL, Morris SL. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect Immun. 2004;72:1685–92. doi: 10.1128/IAI.72.3.1685-1692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyznik AJ, Sun JC, Bevan MJ. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J Exp Med. 2004;199:559–65. doi: 10.1084/jem.20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goter-Robinson C, Derrick SC, Yang A, Jeon BY, Morris SL. Protection against an aerogenic Mycobacterium tuberculosis infection in BCG-immunized and DNA-vaccinated mice is associated with early type I cytokine responses. Vaccine. 2006;24:3522–9. doi: 10.1016/j.vaccine.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Obaro SK, Pugatch D, Luzuriaga K. Immunogenicity and efficacy of childhood vaccines in HIV-1 infected children. Lancet Infect Dis. 2004;4:510–18. doi: 10.1016/S1473-3099(04)01106-5. [DOI] [PubMed] [Google Scholar]
- 33.Cooper CL, Davis HL, Angel JB, Morris ML, Eifer SM, Seguin I, Kreig AM, Cameron DW. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19:1473–9. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 34.Madhi SA, Kuwanda L, Cutland C, Holm A, Kayhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis. 2005;24:410–16. doi: 10.1097/01.inf.0000160942.84169.14. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka H, Teruya K, Tanaka M, Kikuchi Y, Takahashi T, Kimura S, Oka S. Efficacy and immunologic responses to influenza vaccine in HIV-infected patients. J Acquir Immune Defic Syndr. 2005. pp. 167–73. the HIV/Influenza Vaccine Study Team. [PubMed]
- 36.Blank C, Brown I, Marks R, Nishimura H, Honjo T, Gajewski TF. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative T cells. Immunology. 2003;179:4574–81. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzawa A, Shimizu M, Takeda Y, Nagase H, Sayama K, Kimura M. Significant role of Fas ligand-binding but defective Fas receptor (CD95) in lymph node hyperplasia composed of abnormal double-negative T cells. Immunology. 2002;106:470–5. doi: 10.1046/j.1365-2567.2002.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science. 1993;261:1448–51. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Santosuosso M, Ngai P, Zganiacz A, Xing Z. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J Immunol. 2004;173:4590–7. doi: 10.4049/jimmunol.173.7.4590. [DOI] [PubMed] [Google Scholar]
- 40.Johansson M, Lycke N. A unique population of extrathymically derived αβTCR+CD4–CD8– T cells with regulatory functions dominates the mouse female genital tract. J Immunol. 2003;170:1659–66. doi: 10.4049/jimmunol.170.4.1659. [DOI] [PubMed] [Google Scholar]
- 41.Godfrey DI, MacDonald HR, Dronenberg M, Smyth MJ, Kaer LV. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 42.Mathiot ND, Krueger R, French MA, Price P. Percentage of CD3+CD4–CD8–γδTCR– T cells is increased in HIV disease. AIDS Res Hum Retroviruses. 2001;17:977–80. doi: 10.1089/088922201750290096. [DOI] [PubMed] [Google Scholar]
- 43.Bonnet F, Dequeae-Merchadou L, Taupin J, et al. Increase in CD3+CD4– T lymphocytes in patients with AIDS and disseminated Mycobacterium avium–intracellulare complex infection: a prospective study. Microbes Infect. 1999;1:771–6. doi: 10.1016/s1286-4579(99)80079-3. [DOI] [PubMed] [Google Scholar]
- 44.Moody DB, Porcelli SA. Intracellular pathways of CD1 antigen presentation. Nat Rev Immunol. 2003;3:11–22. doi: 10.1038/nri979. [DOI] [PubMed] [Google Scholar]
- 45.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 47.Belz GT, Stevenson PG, Castrucci MR, Altman JD, Doherty PC. Postexposure vaccination massively increases the prevalence of γ-herpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc Natl Acad Sci. 2000;97:2725–30. doi: 10.1073/pnas.040575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Malherve L, Zhang D, Zingler K, Glaichenhaus N, Killeen N. CD4 promotes breadth in the TCR repertoire. J Immunol. 2001;167:4311–20. doi: 10.4049/jimmunol.167.8.4311. [DOI] [PubMed] [Google Scholar]