Abstract
Development of type 1 diabetes has been attributed to T-cell-mediated autoimmunity, which is regulated by antigen-presenting cells. To study the role of liver-derived B220+ regulatory dendritic cells (DCs) in the development of diabetes in non-obese diabetic (NOD) mice, we found that liver 220+ DCs could easily be propagated from young NOD mice, but that such propagation was extremely difficult from mice older than 11 weeks, when insulitis began. This was not simply an age-related phenomenon, because liver B220+ DCs were readily propagated from both young and old congenic non-obese diabetic-resistant (NOR) and normal BALB/c mice. It was therefore speculated that the development of diabetes might be associated with a lack of precursors of B220+ DC in the liver in this animal model. Unfortunately, the specific marker for precursors of liver B220+ DC has not been identified. An alternative approach to supplement liver B220+ DCs by intravenous administration significantly inhibited the development of diabetes by inducing T-cell hyporesponsiveness via enhancement of their apoptotic death. Liver B220+ DCs were capable of effectively presenting antigens but, unlike plasmacytoid DCs, did not express CD11c and were not interferon-α producers. These observations may throw new light on the aetiopathology of type 1 diabetes.
Keywords: apoptosis, dendritic cells, diabetes, liver immunology, T cells
Introduction
Type 1 diabetes is an autoimmune disease in which the destruction of islet β-cells is mediated by the cellular immune system, including the actions of β-cell-specific cytotoxic T cells.1 The nature of immune dysregulation leading to β-cell destruction remains poorly understood but it is clearly influenced by multiple genetic and environmental factors, in particular by antigen-presenting cells (APCs), which have a significant impact on T-cell activation and differentiation. Dendritic cells (DCs) are the primary APCs that can initiate immune responses. Recent accumulating data demonstrate that DCs can also suppress cellular immunity. The diverse functions of DCs in immune regulation are partly the result of the diversity of subsets, lineages and associated maturation stages of DCs.2 Subpopulations of DCs have been described based on their anatomical location and phenotypic or functional characteristics. We have recently identified a DC population, propagated from the mouse liver non-parenchymal cell fraction in response to interleukin-3 (IL-3) and anti-CD40 monoclonal antibody (mAb), which express the B220 antigen. These B220+ DCs, although phenotypically mature, exhibit strong immune regulatory properties by their induction of activated T-cell apoptotic death and significant prolongation of allograft survival in an antigen-specific manner.3 Identification of two counterpart DC populations in the liver has been linked to its immune regulatory property, which was initially recognized by the spontaneous acceptance of liver allografts in many species as well as by tolerance to antigens delivered through the mouth or portal vein (oral tolerance).4
The role of liver B220+ DCs in the development of type 1 diabetes is unclear. To study this, we attempted, in this study, to generate liver B220+ from non-obese diabetic (NOD) mice, and surprisingly found that they were readily propagated from the livers of young NOD mice, but were extremely difficult to obtain from old mice, in which insulitis was manifest. This appeared not to be related to the age, because liver B220+ DCs could easily be generated in adult non-obese diabetes-resistant (NOR) mice that are congenic to NOD mice, indicating that the development of diabetes in adult NOD mice is associated with a lack of precursors of B220+ DC in the liver. Because the cell surface marker for precursors of B220+ DC is unknown, as an alternative we administered liver B220+ DC propagated from young NOD mice, which markedly prevented diabetes development in adult NOD mice, suggesting a link between the deletion of liver B220+ DC precursors in adult NOD mice and the development of diabetes.
Materials and methods
Animals
Female NOD (H2g7) mice, NOR H2g7 mice, BALB/c (H2d) mice, and DO10.11 (H2d) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The T-cell receptor (TCR) on DO11.10 CD4+ T cells is encoded by a transgene that recognizes a chicken ovalbumin-derived peptide OVA323−339 presented by I-Ad. This TCR can be identified by the anti-clonotypic mAb, KJ1-26. Animals were maintained in the specific pathogen-free facility of the University of Pittsburgh Medical Center, and used in accordance with institutional and National Institutes of Health guidelines.
DC culture
Liver B220+ DCs were propagated as previously described.3 Briefly, mouse livers were perfused in situ with collagenase solution followed by further ex vivo digestion. The non-parenchymal cells were then isolated by centrifugation over a Percoll gradient (Sigma Chemical Co., St Louis, MO). Liver non-parenchymal cells were depleted of T cells, B cells, granular cells and macrophages by complement-dependent lysis using a mAb cocktail comprising anti-CD3, anti-CD19, anti-CD14 and anti-Gr-1 (all from BD PharMingen, San Diego, CA) and low toxicity rabbit complement (Accurate Chemical & Scientific Co., Westbury, NY). Thereafter, 2 × 106 lineage-negative cells were cultured in 2 ml RPMI-1640 (Life Technologies, Gaithersburg, MD) supplemented with antibiotics and 10% (v/v) fetal calf serum (referred to subsequently as complete medium), and mouse recombinant IL-3 (10 ng/ml, BioSource, Camarillo, CA) plus anti-CD40 mAb (2 ng/ml, BD PharMingen) in flat-bottom, 24-well culture plates for 5–7 days. Non-adherent cells released from clusters were harvested for further characterization. For comparative purposes, conventional myeloid DCs (MDCs) and plasmacytoid DCs (PDCs) were propagated from the bone marrow of age-matched mice in the presence of granulocyte–macrophage colony-stimulating factor (4 ng/ml) plus IL-4 (1000 U/ml) (both from Schering Plough, Kenilworth, NJ) for 5–7 days, or in the presence of Flt3 ligand (100 ng/ml, Immunex, Seattle, WA) for 10 days, respectively.5,6 All DCs were purified using magnetic beads (Miltenyi Biotec, Aubum, CA). The purity determined by flow analysis was > 95% (CD11c+ for MDCs, B220+ CD11c– for liver B220+ DCs, B220+ CD11c+ for PDCs). Because propagation of liver B220+ DCs from old NOD mice was very difficult, all the DCs used in this study were propagated from young (6-week-old) female NOD mice.
Monoclonal antibodies and flow cytometry
Cell surface antigen expression was analysed by cytofluorography using an EPICS ELITE flow cytometer (Coulter Corporation, Hialeah, FL). The mAbs against mouse H2Kd, CD19 [both mouse immunoglobulin G2a (IgG2a)], IAd (clone AMS-32.1 mouse IgG2b, cross-reacted with H2g7 according to the manufacturer's data sheet), B220, CD40, CD80, CD86, LFA (all rat IgG2a), CD11b, CD45 (both rat IgG2b), CD3, CD11c and intracellular adhesion molecule 1 (ICAM-1) (all hamster IgG) were all purchased from BD PharMingen. Anti-CD-205 mAb was generously provided by Dr R.M. Steinman (The Rockefeller University, New York, NY). Appropriate isotype and species-matched irrelevant mAbs were used as controls.
Detection of apoptosis
For single cell analysis, T cells were stained accordingly with phycoerythrin-conjugated anti-CD3, anti-CD4, anti-CD8 or anti-KJ1.26 mAb. DNA strand breaks were identified by fluorescein isothiocyanate-conjugated or tetramethylrhodamine (TMR)-conjugated terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL). Following cell surface marker staining, cells were fixed in 4% paraformaldehyde, and permeabilized with 0·1% Triton X-100 and 0·1% sodium citrate. The TUNEL reaction mixture from the Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) was then added according to the manufacturer's instructions. Cells incubated with label solution in the absence of terminal transferase were used as negative controls. Quantitative analysis was performed by flow cytometry, with 5000 events acquired from each sample. For identification of apoptotic cells in tissue cryostat sections, an incorporated biotin-dUTP by peroxidase-labelled avidin method was used, followed by an enzyme reaction using avidin–biotin–alkaline phosphatase complex as the substrate.
RNase protection assay
Total RNA was extracted from cells by the guanidinium isothiocyanate–phenol–chloroform method using total RNA isolation (TRI) reagent (Sigma) as described elsewhere.3 Cytokine mRNA expression was determined using the RiboQuant multiprobe RNase protection assay system (PharMingen) following the manufacturer's instructions. Briefly, 5 μg total RNA was hybridized to 32P-labelled RNA probes overnight at 56°, followed by treatment with RNase for 45 min at 30°. The murine L32 and GADPH riboprobes were used as controls. Protected fragments were submitted to electrophoresis through a 7·0 m urea/5% polyacrylamide gel and then exposed to Kodak X-omat film for 72 hr.
T-cell proliferation assay
T-cell proliferation was determined in a one-way mixed lymphocyte reaction assay. For examining DC allostimulatory activity, the graded numbers of γ-irradiated (20 Gy) DCs propagated from NOD mice were used as stimulators, while T cells (2 × 105) obtained from C3H spleens purified by a nylon wool column were used as responders. To determine DC islet-related antigen stimulatory activity, the graded number of γ-irradiated DCs from NOD mice that were incubated with insulin (10 μg/ml) for the last 4 culture days, and used as stimulators. Cultures were established in 200 μl/well in triplicate in 96-well round-bottom microculture plates, and were maintained in complete medium for 3–4 days in 5% CO2 in air at 37°. [3H]TdR (1 μCi/well) was added for the final 18 hr of culture, and incorporation of [3H]TdR into DNA was assessed by liquid scintillation counting on an automated counter. Results are expressed as mean counts per minute (c.p.m.) ± 1 SD.
DC administration and assessment of diabetes
Female NOD mice were injected intravenously with 2 × 106 DCs between 7 and 9 weeks of age. Mice were monitored for the development of diabetes by measurement of blood glucose levels every week. The first day of two consecutive readings of blood glucose > 350 mg/dl was defined as the date of diabetes onset. To score islet inflammatory response, pancreata were fixed in 4% paraformaldehyde and embedded in paraffin. Multiple 4-mm sections were prepared, and stained with haematoxylin and eosin. The insulitis was scored according to mononuclear cell infiltration and destruction of islet architecture: 0, intact islet; 1, early insulitis, accumulation of mononuclear cells at the periphery of the islet; 2, intermediate insulitis, infiltration of mononuclear cells at the periphery and ∼ 50% of the islet centre; 3, late insulitis, massive infiltration of mononuclear cells throughout the islet; 4, end-stage insulitis, small, retracted islets with or without residual infiltration as described elsewhere.7
Labelling of cells with CFSE
T cells (107/ml) were labelled with 5 μm 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) from a 10 mm stock solution (in dimethylsulphoxide) for 15 min at 37°. Cells were washed before culture. The intensity of CFSE was examined by flow cytometry.
Statistical analyses
Statistical significance was assessed using Student's t-test or Kaplan–Meier log-rank test; P < 0·05 was considered statistically significant.
Results
Propagation of liver B220+ DCs from NOD mice
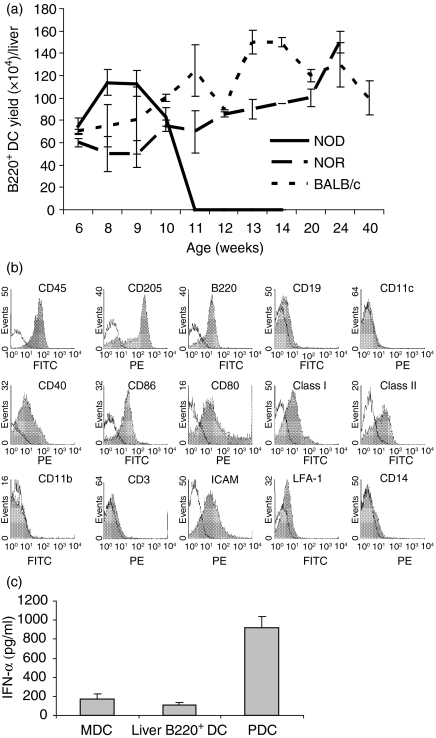
The study was initiated with propagation of liver B220+ DCs from female NOD (H2g7) mice. It turned out to be extremely difficult to generate them in female mice older than 11 weeks when severe insulitis began. By contrast, these cells could be easily propagated from the liver of young NOD mice. This appeared to occur specifically in NOD mice because liver B220+ DCs were easily propagated from old congenic female non-diabetic NOR (H2g7) mice that shared the same major histocompatibility complex (MHC) background with NOD mice, but had no diabetic tendency,8 as well as old normal BALB/c mice (Fig. 1a). Interestingly, we could also successfully propagate liver B220+ DCs from adult (15-week-old) male NOD mice (yield number from 1 × 106 to 2 × 106/liver). Male NOD mice are known to be resistant to the development of diabetes. Taken together, these results strongly suggest that development of diabetes in adult female NOD mice is associated with a lack of precursors of liver B220+ DCs. We were not able to obtain direct evidence for this because the surface marker for precursors of liver B220+ DC remained unknown. As an alternative, we performed the following experiments to determine whether supplementation with liver B220+ DCs propagated from non-diabetic young NOD mice (6 weeks old) prevented diabetes development in adult female NOD mice. The phenotype and function of liver B220+ DCs used in this study were closely monitored.
Figure 1.
Propagation of liver B220+ DCs. (a) Yield of B220+ DCs per liver from female NOD, NOR and BALB/c mice at different ages. In animals younger than 11 weeks, the number of B220+ DCs propagated from NOD mice was not statistically different from that of age-matched NOR and BALB/c mice (P > 0·05). However, it is extremely difficult to propagate these DCs from NOD mice older than 11 weeks (P < 0·05 compare with age-matched NOR and BALB/c). (b) Surface antigen expression on liver B220+ DCs that were propagated from 6-week-old NOD mice. Cell surface antigen expression was determined by staining with fluorescein isothiocyanate-conjugated mAbs, and analysed by flow cytometry (filled histograms). Appropriate species-matched isotype immunoglobulins were used as controls (open histograms). (c) Liver B220+ DCs do not produce IFN-α. MDCs, liver B220+ DCs, or PDCs generated from NOD mice (2 × 106/ml) were cultured in completed medium in the presence of CpG (10 μm, InvivoGen, San Diego, CA) for 2 days. The supernatant IFN-α levels were determined by enzyme-linked immunosorbent assay. In contrast to PDCs that secreted large amount of IFN-α, MDCs and liver B220+ DCs produced very low IFN-α (P < 0·05 compared with PDCs; P > 0·05 compared with MDCs).
Phenotype of liver B220+ DCs from young NOD mice
Liver B220+ DCs propagated from young (6 weeks) female NOD mice expressed high levels of CD45, MHC class I, MHC class II, costimulatory molecules (CD40, CD80 and CD86), ICAM-1 and CD205. The molecules associated with MDCs (CD13, CD11b, CD11c or CD14), and T cells (CD3, CD4 or CD8) were absent. These cells expressed B220 but lacked B-cell-restricted molecule CD19 (Fig. 1b). The results were very similar to those previously observed in other mouse strains.3 Liver B220+ DCs were unlikely to be PDCs because they were CD205+ CD11c–, while PDCs expressed high CD11c, low CD205. In addition, a striking feature of PDCs was the secretion of large amounts of interferon-α (IFN-α) following an appropriate stimulation.9,10 We compared the IFN-α levels in the culture supernatant of MDCs, liver B220+ DCs and PDCs following stimulation by CpG 1826 oligonucleotide (5′-TCCATGACGTTCCTGACGTT, InvivoGen, San Diego, CA). In contrast to PDCs, which produced large amounts of IFN-α, very low levels of IFN-α were detected in liver B220+ DC culture, similar to MDCs (Fig. 1c), although mouse liver B220+ DCs expressed toll-like receptor 9 (data not shown). Liver B220+ DCs did not produce IFN-α following stimulation by lipopolysaccharide (data not shown). These results suggest that liver B220+ DCs are not PDCs.
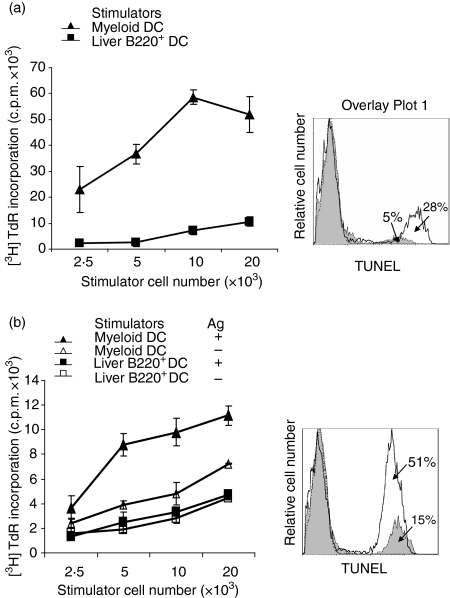
Liver B220+ DCs stimulate low T-cell thymidine uptake and induce T-cell apoptosis
In contrast to NOD MDCs, which stimulated vigorous proliferative responses in C3H (allogeneic) T cells, or NOD (syngeneic) T cells in the presence of insulin (Sigma), liver B220+ DCs induced very low T-cell thymidine uptake in response to either alloantigen or insulin. (Fig. 2a,b, left panels). However, a direct inspection of the T cells over the course of the culture revealed similar levels of T-blast development in response to MDCs or liver B220+ DCs. We therefore speculated that T cells stimulated by liver B220+ DC might rapidly die of apoptosis. To address this, cells cultured for 2 days were double stained with anti-CD3 mAb and TUNEL, and were analysed by flow cytometry. Liver B220+ DCs induced much higher T-cell apoptosis than MDCs in either alloantigen or insulin stimulation (Fig. 2a,b, right panels).
Figure 2.
Liver B220+ DCs induce T-cell low [3H]TdR uptake. (a) In a one-way mixed lymphocyte reaction culture, C3H (H2k) splenic T cells (2 × 105) were cultured with graded numbers of γ-irradiated NOD (H2g7) MDCs or liver B220+ DCs. Left panel: cultured for 3 days; liver B220+ DCs induced significantly lower T-cell proliferative responses (P < 0·05). Right panel: culture at DC : T ratio of 1 : 10 for 2 days; cells were double-stained with anti-CD3 and TUNEL, and analysed by flow cytometry gated in CD3+ cell population. T-cell apoptotic activity in liver B220+ DC group (open histogram) was markedly higher than MDC group (filled histogram). (b) NOD spleen T cells (2 × 105) were cultured with graded numbers of γ-irradiated NOD MDCs or liver B220+ DCs in the presence or absence of antigen (insulin 10 μg/ml). Left panel: cultured for 4 days; MDCs stimulated strong T-cell proliferative responses in the presence of antigen, while liver B220+ DCs stimulated low T-cell proliferative responses in the presence of antigen (P < 0·05, compared with MDCs plus antigen group), which was not statistically different from either the MDC no-antigen group or the liver B220+ DC no-antigen group (both P > 0·05). Right panel: culture at DC : T ratio of 1 : 10 for 2 days in the presence of insulin. Cells were double-stained with anti-CD3 and TUNEL, and analysed by flow cytometry gated in a CD3+ cell population. T-cell apoptotic activity in liver B220+ DC group (open histogram) was markedly higher than MDC group (filled histogram). The data are representative of three separate experiments.
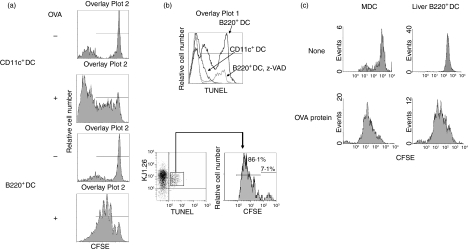
Liver B220+ DCs elicit T-cell division in vitro
To exclude the possibility that extensive apoptosis in T cells stimulated by liver B220+ DC was simply the result of a lack of antigen presentation capacity, we directly examined T-cell division using TCR transgenic (Tg) DO11.10 (H2d) mice. In this strain, the CD4+ T cells express an OVA-specific transgene-encoded TCR that can be identified by specific mAb KJ1-26. T cells from DO11.10 mice were labelled with CFSE and cultured with liver B220+ DCs from NOD mice (H2g7 containing IAdα-chain) in the presence of IAd-restricted OVA323−339 peptide (IAd-restricted peptide) for 3 days. Cells were double-stained with anti-KJ1.26 mAb and TUNEL. OVA-specific CD4+ T-cell division and apoptotic activity were determined in KJ1.26+ cell populations. Liver B220+ DCs efficiently presented OVA peptide to CD4+ KJ1.26+, resulting in active division of KJ1.26+ cells (Fig. 3a). Eight-six per cent of TUNEL-positive cells underwent cell division, clearly indicating that liver B220+ DCs are capable of eliciting T-cell proliferation. They, however, rapidly died of apoptosis (Fig. 3b, lower panel). The data demonstrated in Fig. 3(b; upper panel) clearly showed that more KJ1.26+ T cells underwent apoptosis in response to liver B220+ DCs than in response to MDCs. The T-cell apoptosis induced by liver B220+ DCs were markedly suppressed by the addition of z-benzyloxycarbonyl-valyl-alartyl-aspartyl (z-VAD) an inhibitory peptide of common caspase pathway. To further ascertain that liver B220+ DCs were capable of processing and presenting antigens, NOD liver B220+ DCs were pulsed with whole-length OVA protein, and then the presentation of OVA antigen to specific CD4+ T cells from DO11.10 mice was examined by CFSE dilution assay. Bone-marrow-derived MDCs were used to determine the saturation concentration of OVA protein (data not shown), and as positive controls. Both MDCs and liver B220+ DCs induced comparable strong division of OVA-specific CD4+ T cells with saturation OVA protein concentrations of 200 μg/ml (Fig. 3c), indicating a professional antigen presentation capacity of liver B220+ DCs.
Figure 3.
Liver B220+ DCs stimulate T-cell division and apoptotic death. CFSE-labeled spleen and LN T cells (2 × 105) isolated from DO11.10 (H2d) mice were cultured with irradiated NOD (H2g7) MDCs or liver B220+ DCs in the presence of OVA323−339 peptide (0·3 μm) at DC : T ratio of 1 : 10 for 3 days. Cells were stained with Cy-Chrome-conjugated KJ1.26 mAb and TMR-conjugated TUNEL. (a) CFSE dilution was analysed in a KJ1.26-positive cell population. MDCs and liver B220+ DCs stimulated OVA-specific T-cell division in the presence of OVA peptide. (b) Lower panel: CFSE dilution was analysed in TUNEL+ KJ1.26+ cell population. Majority of apoptotic OVA-specific T cells induced by liver B220+ DCs were dividing cells. Upper panel: in a separate experiment, T cells (2 × 105) from DO11.10 mice were cultured with irradiated NOD MDCs or liver B220+ DCs in the presence of OVA323−339 peptide (0·3 μm) at DC : T ratio of 1 : 10 for 3 days. To inhibit T-cell apoptosis, z-VAD (50 μm/ml, a common caspase inhibitory peptide) was added at the beginning of the culture. Cells were stained with phycoerythrin-conjugated KJ1.26 and fluorescein isothiocyanate-conjugated TUNEL and analysed by flow cytometry. The histogram showed the intensity of TUNEL in KJ1.26 cells. (c) To ascertain the protein processing and antigen presentation capacity, CFSE-labelled T cells (2 × 105) isolated from DO11.10 mice were cultured with irradiated NOD MDCs or liver B220+ DCs in the presence of OVA protein (200 μg) at a DC : T ratio of 1 : 10 for 3 days. Cells were stained with phycoerythrin-conjugated KJ1.26 mAb. CFSE dilution was analysed in KJ1.26-positive cell population. Liver B220+ DCs could comparably process OVA protein and present antigen to specific CD4+ T cells. The data are representative of three separate experiments.
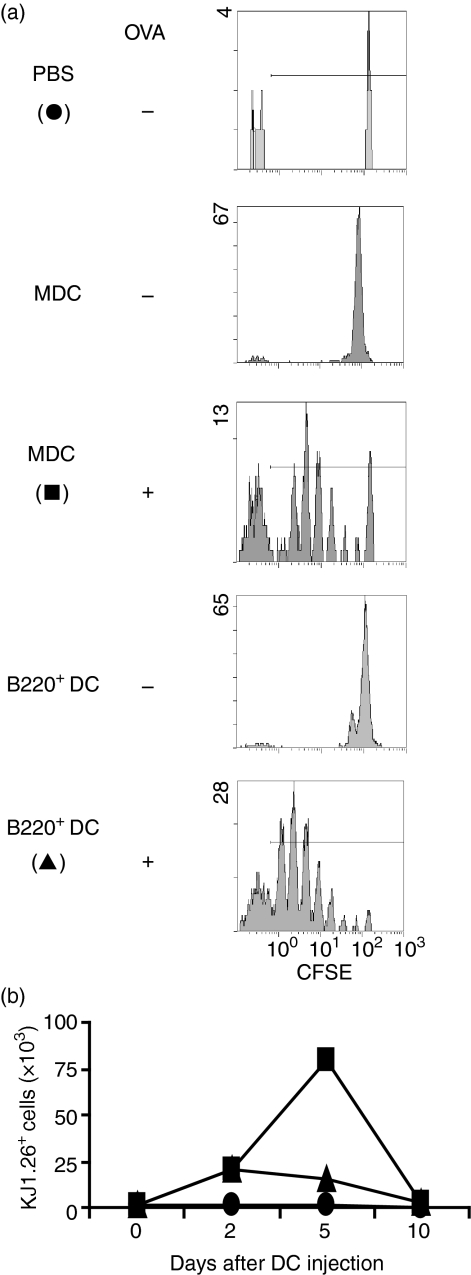
Liver B220+ DCs induces T-cell division and death in vivo
To examine the effect of liver B220+ DCs on T-cell responses in vivo, CFSE-labelled spleen T cells (eluted by a nylon wool column) from DO11.10 mice were adoptively transferred into syngeneic BALB/C mice followed by a footpad injection on the following day of BALB/c liver B220+ DCs that had been pulsed with OVA peptide. Lymphocytes were then isolated from popliteal lymph nodes on days 2 to10 after DC injection and examined for expansion and retention of KJ1.26+ T cells by flow cytometry. Injection of liver B220+ DCs, similar to MDCs, elicited strong OVA-specific CD4+ T-cell division by CFSE dilution analysis (Fig. 4a), indicating the antigen-presenting capacity of liver B220+ DCs. However, unlike injection of MDCs, which stimulated proliferation of KJ1.26 cells, the expansion of OVA-specific T cells in the liver B220+ DC injection group was limited (Fig. 4b). These results provide in vivo evidence of T-cell division followed by cell deletion, which is consistent with above in vitro observations.
Figure 4.
Liver B220+ DCs elicit antigen-specific T-cell dividing in vivo. CFSE-labelled spleen and lymph node T cells (2·5 × 106) isolated from DO11.10 mice (H2d) were adoptively transferred to BALB/c mice (H2d). On the following day, the mice received a footpad injection of 5 × 105 BALB/c MDCs or liver B220+ DCs that had, or had not, been pulsed with OVA323−339 peptide. (a) Three days after footpad injection priming, lymphocytes were isolated from DC injection side popliteal lymph nodes (pooled from three animals in each group), and stained with KJ1.26 mAb. The dividing of OVA-specific CD4+ T cells was analysed using CFSE dilution in KJ1.26+ cell population. (b) Lymphocytes were isolated from injection side popliteal lymph nodes at the indicating time post-DC injection and stained with KJ1.26 mAb. The data show the absolute numbers of KJ1.26+ T cells per lymph node, and representative of two separate experiments.
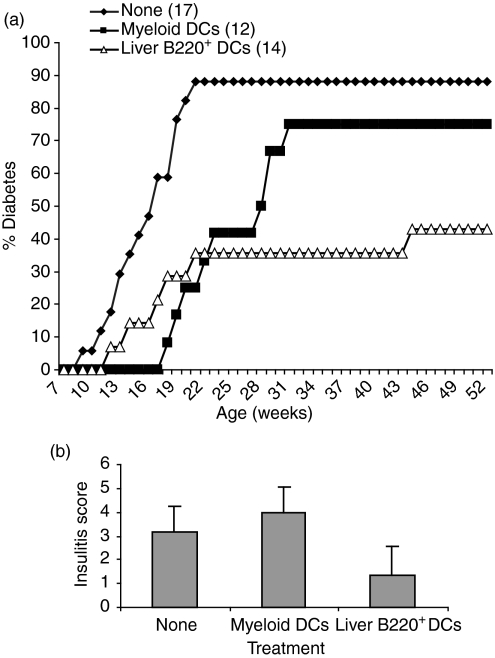
Administration of liver B220+ DCs inhibits development of diabetes
Our ultimate goal was directed towards assessing whether supplemental administration of liver B220+ DCs was able to prevent development of autoimmune diabetes in female NOD mice that spontaneously developed pathological insulitis at 8 weeks of age with peri-islet infiltration followed by a progressive destruction of pancreatic β-cells. Histological examination revealed that the insulitis score in untreated NOD mice was 3·14 ± 1·0 at 18 weeks of age with 88% of the mice developing destructive insulitis and diabetes by the age of 23 weeks (Fig. 5a,b). To determine the effect of liver B220+ DCs on the prevention of type 1 diabetes development, female NOD mice were treated at 7–9 weeks of age with a single intravenous injection of 2 × 106 liver B220+ DCs from NOD mice. Administration of bone marrow-derived MDCs was used for comparison. Administration of liver B220+ DCs protected 60% of NOD mice from the development of diabetes following up to the age of 53 weeks (Fig. 5a). Injection of MDCs appeared to slightly delay the onset of diabetes; however, 75% of mice ultimately developed diabetes by the age of 32 weeks (Fig. 5a). Histological examination of the islet grafts revealed that the severity of insulitis was markedly diminished with substantial reduction in peri- and intra-islet infiltration in mice treated with liver B220+ DCs. The pancreata from three mice in each group were scored for insulitis as described in the Materials and methods. The insulitis score in the liver B220+ DC administration group was significantly less than in non-treated and MDC-treated groups (Fig. 5b).
Figure 5.
Systemic administration of liver B220+ DCs prevents diabetes development in NOD mice. Female NOD mice were injected intravenously at 7–9 weeks of age with 2 × 106 NOD MDCs (n = 12) or liver B220+ DCs (n = 14). Mice without DC treatment (none, n = 17) were used as controls. Blood glucose levels were monitored and diabetes was diagnosed with blood glucose > 350 g/dl for the two consecutive testings. (a) Administration of liver B220+ DC markedly inhibited development of diabetes (P < 0·01, compared with either the untreated group or the MDC-treated group). (b) The pancreatic islets from female NOD mice at 18 weeks of age (10 weeks after DC treatment) were examined histologically. Three mice in each group were scored (50 islets per mouse) for insulitis as described in the Materials and methods. Insulitis was significantly less severe in mice treated with liver B220+ DCs than in those treated with MDC or without DC treatment (both P < 0·05).
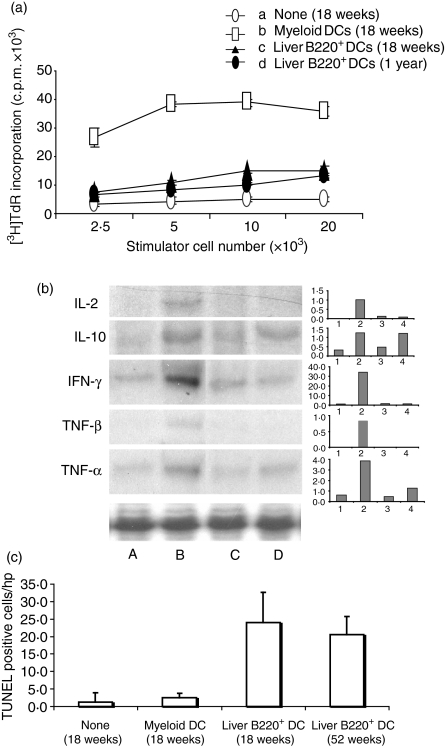
Administration of liver B220+ DCs induces T-cell hyporesponsiveness and apoptosis
T cells from pancreatic lymph nodes of NOD mice that had been previously treated with DCs were assessed for their proliferative responses in a mixed lymphocyte reaction assay. Unlike mice receiving MDCs, in which T cells demonstrated marked proliferative responses, T cells from liver B220+ DC-treated mice showed significantly lower proliferative responses to insulin stimulation, suggesting a T-cell hyporesponsiveness (Fig. 6a). MDC administration markedly enhanced IL-2, IFN-γ, IL-10 and tumour necrosis factor (TNF) gene transcripts in T cells compared with the untreated group. In the B220+ DC-treated group, IL-2 and TNF-γβ gene transcripts were complete suppressed, IFN-γ and TNF-α were markedly reduced, while. IL-10 and IFN-γ appeared to be the main cytokines detected; both of these cytokines have been shown to be produced by T regulatory 1 (Tr1) cells.11 Apoptotic activity in pancreatic lymph nodes of NOD mice following DC administration was examined by in situ TUNEL staining. Compared with the MDC-treatment group or the non-DC-treatment group, significantly higher levels of TUNEL-positive mononucleocytes were detected in the groups treated with liver B220+ DCs (Fig. 6c) Indicating that an enhanced apoptotic activity of peripheral lymphocytes is induced by administration of liver B220+ DCs.
Figure 6.
Administration of liver B220+ DCs into NOD mice induces T-cell hyporesponsiveness to islet antigens. In a 3-day mixed lymphocyte reaction assay, T cells (2 × 105) purified from pancreatic lymph nodes of NOD mice at age of 18 weeks or 1 year that had received 2 × 106 NOD MDCs or liver B220+ DCs at age 7–9 weeks, and used as responders. T cells from NOD DC-untreated or MDC-treated (18 weeks) were used as controls. Graded number of γ-irradiated MDCs from NOD mice pulsed with insulin (10 μg/ml) was used as stimulator. T cells from liver B220+ DC-treated mice showed significantly suppressed proliferative responses (P < 0·05 compared with MDC-treated group). (b) Cytokine mRNA expression (RNase protection assay) in T cells purified from pancreatic lymph nodes of NOD mice that had been treated, at age of 7–9 weeks, with lane A, none; lane B, MDCs (killed at age 18 weeks); lanes C and D, liver B220+ DCs (killed at age of 18 weeks and 1 year, respectively). (c) Administration of liver B220+ DCs enhances apoptosis in pancreatic lymph nodes. Cryostat sections of pancreatic lymph nodes were stained with TUNEL staining in situ. Thirty high-power field of lymph nodes in each mouse were randomly selected and counted for TUNEL-staining-positive cells. The data were collected from three mice in each group, and are presented as positive cell numbers/high-power field ± 1SD. The results are representative of two separate experiments.
Discussion
A population of regulatory DCs propagated from mouse livers in response to IL-3 and anti-CD40 mAb (liver B220+ DCs) have the appearance of a mature DC phenotype but exhibit a distinct function in directing the suppression of naive T cells.3 The presence of these B220+ DCs in vivo has recently been demonstrated,12 implicating their biological significance. In the present study, interestingly, it was extremely difficult to propagate liver B220+ DCs from female NOD mice older than 11 weeks. This is unlikely to be an age-related phenomenon because sufficient liver B220+ DCs can be generated from BALB/c mice. A normal number of liver B220+ DCs can be propagated from the congenic NOR mice that share the same MHC background with NOD mice, but have no intention to be diabetic13 (Fig. 1a). The onset of autoimmunity in NOD mice begins at 6–8 weeks with infiltration of the pancreas by dendritic cells followed by macrophages and T cells, resulting in a marked decrease in pancreatic insulin content by about 12 weeks of age. The mice soon develop insulin-dependent diabetes.10 The observations described in this study suggest that the development of autoimmune diabetes in adult NOD mice may be associated with a deletion of precursors of B220+ DC in their livers, although we are not able to provide direct evidence because a method to identify these precursors is currently not available. However, we demonstrate that a supplemental administration of extra liver B220+ DCs propagated from non-diabetic NOD mice was able to prevent the development of diabetes in 60% of susceptible NOD mice. This prevention is robust and long-lasting because many survivors are older than 1 year, with abrogated insulitis (Fig. 5). This is in contrast to the injection of conventional MDCs, which resulted in minimal reduction in diabetes development or peri-islet inflammation. These results suggest that the lack of precursors of liver B220+ DCs may, at least partially, contribute to the development of type 1 diabetes in adult NOD mice, although there are other factors as to the cause of this autoimmunity.
The association between liver and the pathogenesis of diabetes has been limited to the function of hepatic glycogenolysis and gluconeogenesis, two key components of the glucose output. Thus, elevation of hepatic glucose secretion contributes significantly to hyperglycaemia in both type 1 and type 2 diabetes.14 Suppression of hepatic gluconeogenesis improves overall glycaemic control in both diabetic patients and animal models.15,16 An increase in gluconeogenesis in the liver is largely responsible for the enhanced hepatic glucose production and fasting hyperglycaemia in individuals with diabetes mellitus.17 Our study for the first time provides a clue that the liver may immunologically affect the development of autoimmune diabetes in NOD mice, and that liver-derived regulatory DCs may significantly influence pancreatic autoimmunity. This is not surprising because the close relationship between the liver and pancreas has been shown by their arising from adjacent areas in the anterior endoderm of the embryo.23 Signals from the cardiac mesoderm are responsible for specifying the two organs. The ventral foregut endoderm is to become pancreas, while a fibroblast growth fator (FGF)-like signal released from the cardiac mesoderm diverts the fate of some cells into liver, indicating that the liver and pancreas differ by a single developmental decision, presumably affecting the expression of a relatively small number of genes.18–20 In addition, the pancreatic lymph nodes are major sites for DCs to bring antigens processed from islets, liver-derived DCs may actively involve in pancreatic immune regulation.
The mechanism by which administration of liver B220+ DCs prevents diabetes is not fully understood. The uniqueness of liver B220+ DCs is their ability leading to T-cell initial expansion but ultimate programmed cell death. Thus, cultures of T cells with liver B220+ DCs in the presence of antigens demonstrated high apoptosis rates. In NOD mice rescued from diabetes with liver B220+ DCs injection, there is an increase in apoptotic activity of T cells. This high rate of T-cell apoptosis correlated strongly with diminished cellular infiltration and cytokine mRNA profile alteration in pancreatic lymph nodes. While IL-2 and IFN-γ mRNA were reduced, IL-10 mRNA was unaltered, consistent with published reports linking a T helper type 1 response to diabetogenesis as well as the potential of IL-10 in diabetes prevention (Figs 5 and 6). Apoptosis of activated T cells may result from a lack of the third signal not present on liver B220+ DCs, namely IL-12.3 While liver B220+ DCs express p35, the lack of p40 prevents the completion of the IL-12 dimer, and this lack of signal has been shown to prevent T-cell development.21 Prevention of diabetes in NOD mice by apoptosis appears to be a unique mechanism not yet described. In addition, B220+ DC, a population of IL-10-producing APC3 may also act on immune regulation through induction of T regulatory cell generation because IL-10 has been shown to be an import cytokine for promoting T regulatory cell differentiation.11
The liver B220+ DCs described in this study are also distinguished from PDCs because they express low CD11c (Fig. 1b) and produce very little IFN-α following stimulation (Fig. 1c). PDCs are known to be the major source of IFN-α following viral infection and in response to CpG oligodeoxynucleotides.10,22,23 Indeed, the liver contains a small population of DCs that produced IFN-α following viral stimulation.24 Although liver B220+ DCs are phenotypically mature, they induce low thymidine uptake of T cells in response to antigen stimulation. This is unlikely to be the result of a failure of liver B220+ DCs to activate T cells, because the results of the CFSE dilution assay show that liver B220+ DCs can process and present antigen to specific T cells inducing their division. This is associated with induction of activated T-cell apoptosis (Figs 3 and 4). The experiment of Fig. 3(a) demonstrates that NOD (H2g7) mouse liver B220+ DCs can present the IAd restricted OVA323−339 peptide to DO11.10 CD4+ T cells. There are several possible explantations: (1) MHC class II of H2g7 consists of an IAdα-chain and an IAg7 β-chain;25 (2) a crystal structure study on mouse class II MHC IAd in complex with OVA323−339 peptide reveals that either the α- or β-chain has an eight-residue (ssadlvpr) peptide bound to the C-terminus,26 suggesting that an IAdα- or β-chain alone may be sufficient to present OVA323−339 peptide. We have also examined NOD bone marrow-derived MDCs and PDCs. Both of them also effectively presented the OVA323−339 peptide to DO11.10 CD4 T cells (data not shown). Therefore, IAdα-chain with mutant β-chain (such as IAg7β-chain in NOD mice) appears to be able to present IAd-restricted peptide to specific T cells.
Acknowledgments
This work was supported by a Juvenile Diabetes Foundation International Program Project Grant P1893135 and by National Institutes of Health grant DK058316.
Abbreviations
- ABC
avidin–biotin–alkaline phosphatase complex
- Ag
antigen
- APC
antigen-presenting cell
- CFSE
5,6-carboxyfluorescein diacetate succinimidyl ester
- CpG
cytosine-guanine
- DC
dendritic cell
- IFN
interferon
- IL-3
interleukin-3
- mAb
monoclonal antibody
- MDC
myeloid dendritic cell
- MHC
major histocompatibility complex
- NOD
non-obese diabetic
- NOR
non-obese diabetic-resistant
- NPC
non-parenchymal cell
- OVA
ovalbumin
- PDC
plasmacytoid dendritic cell
- TCR
T-cell receptor
- TNF
tumour necrosis factor
References
- 1.Liblau RS, Wong FS, Mars LT, Santamaria P. Autoreactive CD8 T cells in organ-specific autoimmunity: emerging targets for therapeutic intervention. Immunity. 2002;17:1–6. doi: 10.1016/s1074-7613(02)00338-2. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineages, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, Bonham CA, Liang X, et al. Liver-derived DEC205+B220+CD19– dendritic cells regulate T cell responses. J Immunol. 2001;166:7042–52. doi: 10.4049/jimmunol.166.12.7042. [DOI] [PubMed] [Google Scholar]
- 4.Qian S, Thai NL, Lu L, Fung JJ, Thomson AW. Liver transplant tolerance: mechanistic insights from animal models, with particular reference to the mouse. Transplantation Rev. 1997;11:151–64. [Google Scholar]
- 5.Lu L, Qian S, Hershberger PA, et al. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol. 1997;158:5676–84. [PubMed] [Google Scholar]
- 6.Gilliet M, Boonstra A, Paturel C, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–8. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KS, Kang Y, Choi SE, et al. Modulation of glucocorticoid-induced GAD expression in pancreatic β-cells by transcriptional activation of the GAD67 promoter and its possible effect on the development of diabetes. Diabetes. 2002;51:2764–72. doi: 10.2337/diabetes.51.9.2764. [DOI] [PubMed] [Google Scholar]
- 8.Prochazka M, Serrez DV, Worthen SM, Leiter EH. Genetic control of diabetogenesis in NOD/Lt mice: development and analysis of congenic stocks. Diabetes. 1989;38:14–46. doi: 10.2337/diab.38.11.1446. [DOI] [PubMed] [Google Scholar]
- 9.Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003;111:675–97. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 10.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c– type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type 1 IFN. J Immunol. 2001;166:2291–5. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 11.Groux H, O'Garra A, Bigler M, Rouleau M, Antinenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;89:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 12.Burke F, Stagg AJ, Bedford PA, English N, Knight SC. IL-10-producing B220+CD11c– APC in mouse spleen. J Immunol. 2004;173:2362–72. doi: 10.4049/jimmunol.173.4.2362. [DOI] [PubMed] [Google Scholar]
- 13.Wicker LS, Miller BJ, Fischer PA, Pressey A, Peterson LB. Genetic control of diabetes and insulitis in the nonobese diabetic mouse. Pedigree analysis of a diabetic H-2nod/b heterozygote. J Immunol. 1989;142:781–4. [PubMed] [Google Scholar]
- 14.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 15.Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 16.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–7. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 17.Taylor SI. Deconstructing type 2 diabetes. Cell. 1999;97:9–12. doi: 10.1016/s0092-8674(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 18.Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 19.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–81. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 20.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Qian S, Liang X, et al. Prevention of diabetes in NOD mice by administration of dendritic cells deficient in NF-κB activity. Diabetes. 2003;52:1976–85. doi: 10.2337/diabetes.52.8.1976. [DOI] [PubMed] [Google Scholar]
- 22.Schnurr M, Toy T, Shin A, et al. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood. 2004;103:1391–7. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 23.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 24.Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, Reimann J. Hepatic dendritic cell subsets in the mouse. European J Immunol. 2004;34:355–65. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 25.Jackson Laboratory. Allelic Designation for H2 Congenic StrainJAX® Mice. Bar Harbor: The Jackson Laboratory; June 2005–May 2007. p. 656. Catalog. [Google Scholar]
- 26.Scott CA, Peterson PA, Teyton L, Wilson IA. Crystal structures of two I-Ad peptide complexes reveal that high affinity can be achieved without large anchor residues. Immunity. 1998;8:319–29. doi: 10.1016/s1074-7613(00)80537-3. [DOI] [PubMed] [Google Scholar]