Abstract
We have previously shown that the major dog allergen Can f 1 contains seven T cell epitope regions, none of which was preferentially recognized. To identify the immune characteristics of Can f 1 epitopes and to verify their suitability for peptide-based allergen immunotherapy, short-term T cell lines were generated with epitope-containing peptides from peripheral blood mononuclear cells of Can f 1 skinprick test-positive allergic and healthy control subjects. The lines were examined for their proliferative capacity and cytokine production upon stimulation with the allergen peptide, a homologous peptide from human tear lipocalin (TL) and Can f 1 and TL proteins. Can f 1 peptides induced proliferation of T cells and gave rise to T cell lines with comparable efficiencies. In particular, the T cell lines of allergic subjects induced with p33–48 and p107–122 favoured the production of interferon-γ and interleukin-10, respectively. A greater number of Can f 1-specific T cell lines were generated from allergic than from healthy individuals. Two p107–122-induced Can f 1-specific T cell lines also reacted to a homologous peptide of human TL. Our results suggest that several T cell epitope-containing peptides should be used in combination for specific immunotherapy in Can f 1 allergy.
Keywords: T cells, allergens, peptide immunotherapy, proliferation, cytokines
Introduction
The major dog allergen Can f 1 is one of the allergenic members of the lipocalin protein family.1 To date, the T cell epitopes of three lipocalin allergens, cow Bos d 2, rat Rat n 1 and dog Can f 1, have been determined.2–4 In Can f 1, the epitopes recognized by allergic and control subjects were localized in the same regions of the allergen, a finding previously observed with non-lipocalin allergens.5–8 When the sequences of the three lipocalin allergens with known T cell epitopes were aligned, the locations of the antigenic determinants showed similarities.4 However, whereas Bos d 2 contained one immunodominant T cell epitope,2 the T cell reactivity to Can f 1 was more evenly distributed in the molecule.4
In the treatment of allergies, specific immunotherapy (SIT) is currently the only method directed against allergic sensitization.9,10 By injecting increasing doses of allergen preparation subcutaneously, alleviation or even abrogation of symptoms for several years has been attained.11 The treatment's lengthy duration and the need for more efficient and safer allergen preparations have prompted the development of new modalities for desensitization.12
An alternative to conventional immunotherapy which has been observed to diminish the risk for severe IgE-mediated reactions is peptide-based allergen immunotherapy. The short peptides of an allergen lack the 3-dimensional structure needed for cross-linking IgE on the surface of mast cells and basophils.13 The ability of a peptide to induce a T cell response in persons with different human leucocyte antigen (HLA) backgrounds is desirable in specific immunotherapy.
The results obtained in clinical trials with peptides of cat14–16 or bee venom17,18 allergens are encouraging. For example, therapy with Fel d 1 peptides enhanced the pulmonary function of cat-allergic patients and improved tolerance to cats,14 whereas therapy with bee venom peptides improved tolerance to bee stings.17 The favourable results in bee venom allergy were associated with the increased secretion of interleukin (IL-)10 and interferon (IFN-)γ by peptide-specific T cells.18 Enhanced secretion of IL-10 has also been observed in the peripheral blood mononuclear cells (PBMCs) of cat-allergic persons upon stimulation with whole cat dander after treatment with Fel d 1 peptides.19 As differences in the proportions of allergen-specific subsets of T helper (Th)1, Th2 and regulatory T cells may lead to different outcomes in healthy and allergic subjects upon allergen exposure,20 modulating the activity of these T cell populations by immunotherapy is an attractive concept.
Although the use of a single peptide for immunotherapy would be desirable in dog allergy, the epitope mapping of Can f 1 revealed several epitopes that were equally recognized by the T cells of 15 individuals.4 To study epitope-specific T cell responses in more detail, short-term T cell lines were induced from the PBMCs of Can f 1 skinprick test-positive (SPT+) and healthy control subjects with Can f 1 peptides. The most prominent finding was that p33–48 induced elevated production of IFN-γ, whereas p107–122 promoted the production of IL-10, especially in the T cell lines of allergic subjects. Interestingly, two Can f 1-specific T cell lines from an allergic subject responded, with low functional avidity, to the peptide of human tear lipocalin (TL), a protein homologous to Can f 1. We infer from our results that a feasible approach to modify the immune response of dog-allergic subjects to Can f 1 by peptide-specific immunotherapy is to use several peptides of the molecule in combination.
Materials and methods
Subjects
Seven dog-allergic patients and five randomly selected healthy non-atopic dog-owners were recruited to the study as dog-allergic and control subjects, respectively. Allergy to dog was confirmed at the Pulmonary Clinic, Kuopio University Hospital and has been described in detail elsewhere.21 For a person to be classified as dog-allergic, the following criteria had to be fulfilled: the dog UniCAP fluoroenzyme-immunometric assay (FEIA) (Pharmacia, Uppsala, Sweden) of > 0·7 kU/l and an SPT with dog allergen (epithelial extract; ALK Abelló, Hørsholm, Denmark) of ≥ 3 mm. In one subject, who had allergic symptoms upon exposure to dog dust and a positive SPT reaction with the commercial dog epithelial extract, no specific antibody was found in the UniCAP FEIA. The SPTs with recombinant (r) Can f 1 revealed that four of the dog-allergic subjects were sensitized to Can f 1, whereas the other three subjects were non-sensitized. The control subjects were negative in the test. All subjects provided signed informed consent to participation and the study was approved by the Ethics Committee, Kuopio University Hospital.
Antigens
Recombinant Can f 1 was produced in Pichia pastoris, as described previously.21 The recombinant human TL (von Ebner's gland protein, VEGP) was produced in a similar way. Six 16mer peptides of Can f 1 were chosen from the epitope regions of the allergen, defined previously as A−E.4 Can f 1 peptides and the corresponding TL peptides (see below) were synthesized and purified as described previously.4
The synthesized Can f 1 peptides were p15–30 (GKWYLKAMTADQEVPE), p33–48 (DSVTPMILKAQKGGNL), p49–64 (EAKITMLTNGQCQNIT), p73–88 (PGKYTAYEGQRVVFIQ), p81–96 (GQRVVFIQPSPVRDHY) and p107–122 (RQIRMAKLLGRDPEQS). The Can f 1 and TL sequences were aligned (SIB BLAST network service at the Swiss Institute of Bioinformatics, January 20, 2004) and the TL peptides (ESVTPMTLTTLEGGNL and KPVRGVKLVGRDPKNN) corresponding to the Can f 1 peptides p33–48 and p107–122, respectively, were synthesized.
Generation of T cell lines
Peripheral-blood mononuclear cells were isolated by Ficoll−Paque density gradient centrifugation according to the manufacturer's protocol (Amersham Pharmacia Biotech, Uppsala, Sweden). For inducing specific T cell lines, the split-well culture method, as described by Monsonego et al.,22 was adopted, with some modifications. In brief, PBMCs were cultured in 30 wells (2 × 105 cells/well) on round-bottomed 96-well microtitre plates (Corning, NY) in the presence of peptides (10 µm). The culture medium was RPMI 1640 (BioWhittaker, Verviers, Belgium) supplemented with 2 mm l-glutamine, 20 µm 2-mercaptoethanol, sodium pyruvate (BioWhittaker), non-essential amino acids (BioWhittaker), 100 IU/ml penicillin and 100 µg/ml streptomycin, 10 mm HEPES (BioWhittaker) and 5% non-autologous male AB serum (Finnish Red Cross, Helsinki, Finland). At day 5, half the medium was replaced, adjusting the concentration of recombinant human IL-2 (Strathmann Biotech, Hanover, Germany) to 10 U/ml. At day 7, half the medium was replaced with medium without IL-2. At day 10, the cultures were split in two and specificity to the peptides was examined by radionuclide uptake (see below). At day 14, positive cultures with a stimulation index (SI; ratio between the mean counts per minute, cpm, in cultures with antigen-presenting cells plus antigen and the mean cpm in cultures without antigen) ≥ 2 were restimulated with the peptides on 24-well plates in 1-ml volumes using γ-irradiated (3000 rad) autologous PBMCs as antigen-presenting cells. Three days later, 0·5 ml of fresh medium containing rIL-2 (30 U/ml) was added. Thereafter, half the volume was replaced with fresh medium supplemented with 20 U/ml IL-2 every 2–3 days.
Lymphocyte proliferation assays
The proliferative responses of PBMCs were tested in 12 replicates (3 × 105 cells/well) on round-bottomed 96-well microtitre plates in synthetic AIM-V medium (Gibco™; Invitrogen Corp., Paisley, UK) containing the peptides (10 µm). Wells with purified protein derivative (PPD) (10 µg/ml; Statens Serum Institut, Copenhagen, Denmark) and those without a stimulant were included as positive and negative controls, respectively. Reactivity to the Can f 1 protein was studied in six replicate wells at 100 µg/ml. After a 5-day culture, the cells were pulsed for 16 hr with 1·0 µCi [3H]thymidine per well (specific activity 2·0 Ci/mmol; Amersham Pharmacia Biotech, Little Chalfont, UK). Radionuclide uptake was measured by scintillation counting (Wallac MicroBeta®; Trilux, Turku, Finland) and the number of positive cultures per peptide was determined using the mean cpm + 3 SD of 12 negative control cultures as a cut-off.
The proliferative responses of T cell lines (5 × 104 cells/well) were tested in three to five replicate wells on round-bottomed 96-well microtitre plates, the irradiated autologous PBMCs (1 × 105 cells/well) serving as antigen-presenting cells. The cells were stimulated with the Can f 1 peptide used in the induction of the line and with the corresponding TL peptide (at 0·05, 0·5, 5 and 50 µm) as well as with Can f 1 and TL proteins (5 µm). Wells containing phytohaemagglutinin (PHA; 10 µg/ml) and those without a stimulant were included as positive and negative controls, respectively. The results were indicated as stimulation indices and responses with SI ≥ 2 were regarded as specific. The functional T cell receptor (TCR) avidity of the lines was studied by determining the concentration needed to obtain the half-maximal response (EC50) values from four-parameter dose–response curves, as described previously.23,24 A high EC50 value refers to low functional TCR avidity: i.e. a high Ag concentration is needed to obtain the half-maximal response, and vice versa. The HLA restriction of T cell responses to Can f 1 was examined by inhibiting the response to Can f 1 peptides (5 µm) with monoclonal antibodies (mAbs) to HLA-DR, -DP and -DQ at several dilutions of 0−250 µg/ml (BD Biosciences, San Jose, CA).
Cytokine production by T cell lines
Supernatants (0·1 ml/well) were collected from the proliferation assay plates 72 hr after the beginning of T cell stimulation. The samples were stored at − 70° until analysed. The concentrations of IL-4, IL-10 and IFN-γ were measured in duplicate using commercial enzyme-linked immunosorbent assay (ELISA) kits (DuoSet; R & D Systems, Minneapolis, MN) with AMDEX amplification (Amersham Pharmacia Biotech) according to the manufacturers' protocols.
Flow cytometric analyses
The phenotype of the T cell lines was examined by a fluorescence-activated cell sorter (FACScan) flow cytometer (Becton Dickinson, Mountain View, CA) and CellQuest™ 3·1 software (Becton Dickinson) using fluorochrome-labelled antibodies to CD4, CD8, CD3 (Becton Dickinson) and 24 TCR Vβ chains (IOTest Beta Mark kit; Immunotech, Marseille, France), as described previously.25
Statistical analyses
Statistical differences between groups were assessed using the Kruskall−Wallis test, and post hoc comparisons were made using the Mann–Whitney U-test. The difference in the frequency of cases classified as positive or negative was analysed with Fisher's exact test. P-values < 0·05 were considered significant.
Results
PBMC responses to the T cell epitope containing Can f 1 peptides
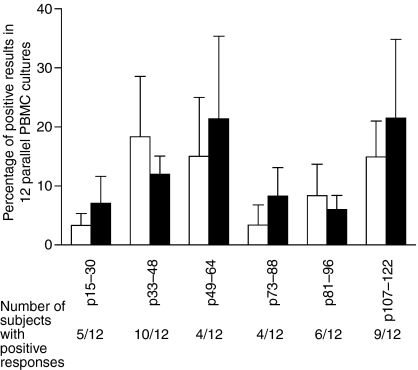
To examine the stimulatory capacity of peptides from the immunodominant epitope regions A−E of Can f 1,4 PBMCs of seven dog-allergic and five non-allergic subjects were stimulated with the peptides (10 µm). As lipocalin allergens have been shown to have a poor stimulatory capacity for human PBMCs,2,25 the cells were tested in 12 replicate wells on 96-well plates. The frequency of cultures with positive proliferative responses between the peptides or between dog-allergic and non-allergic subjects did not show statistically significant differences (P > 0·05; Fig. 1). However, peptides p33–48, p49–64 and p107–122 tended to induce a greater number of cultures with positive responses than did p15–30, p73–88 and p81–90 (Fig. 1). Surprisingly, only four of 12 subjects showed reactivity to p49–64, whereas the PBMCs of 10 and nine of 12 subjects responded to p33–48 and p107–122, respectively (Fig. 1). Although the differences were not statistically significant, p33–48 and p81–96 tended to induce more frequent responses in the PBMCs of control subjects than in those of dog-allergic subjects (Fig. 1), whereas the reverse was true of p15–30, p49–64, p73–88 and p107–122 (Fig. 1); p33–48 was selected from the first group and p107–122 from the latter group for more detailed examination.
Figure 1.
Proportion of positive results as percentages in 12 parallel peripheral blood mononuclear cell (PBMC) cultures stimulated with Can f 1 peptides. Specific proliferation of PBMCs of dog-allergic and non-allergic subjects was determined using the mean counts per minute (cpm) + 3 SD of 12 negative control cultures as a cut-off.24 Thereafter, a percentage of positive cultures per peptide was calculated for each subject (e.g. 1 positive culture in 12 = 8·3%). Results are presented as the mean of individual percentages ± SEM per peptide.
The reactivity of PBMCs to Can f 1 protein was determined in six replicate wells on 96-well plates at a concentration of 100 µg/ml (5 µm). The PBMCs of three of seven dog-allergic subjects and three of four non-allergic subjects showed positive responses. Although a greater proportion of non-allergic subjects reacted to Can f 1, the frequency of wells with positive responses tended to be higher in the cultures of the three dog-allergic subjects (50·0% ± 0; mean ± standard error of the mean, SEM) than in those of the three non-allergic subjects (27·7% ± 5·3). All subjects showed a vigorous proliferative response to PPD (data not shown).
Capacity of p33–48 and p107–122 to induce specific T cell lines
To induce specific T cell lines, PBMCs of four Can f 1 SPT+ and three control subjects were stimulated in 30 wells on 96-well plates with p33–48 or p107–122. The specificity of the cultures was determined 14 days later, as described in Materials and methods. p33–48 induced three to five (mean 3·9) specific cell cultures per person, and p107–122 one to 11 (mean 5·9). In total, 27 specific T cell cultures were established with p33–48 and 41 with p107–122. p33–48 induced slightly higher SIs (4·15 ± 1·09) than did p107–122 (3·73 ± 0·30). None of the differences were statistically significant (P > 0·05).
Thirty-seven of the peptide-specific T cell cultures were obtained from the PBMCs of Can f 1 SPT+ subjects, and 31 from the PBMCs of control subjects. Individually, the frequency of specific cultures from allergic subjects ranged from two to eight (mean 4·6), whereas that from non-allergic subjects ranged from one to 11 (mean 5·2). The cells of allergic subjects tended to show higher SIs (5·53 ± 2·23) upon stimulation with p33–48 than the cells of non-allergic subjects (2·87 ± 0·21). The difference was even smaller with p107–122, as the mean SI of T cell lines from allergic subjects was 3·99 ± 0·42 and that of non-allergic subjects 3·36 ± 0·43. The differences were not statistically significant (P > 0·05).
Phenotype of peptide-specific T cell lines
Thirty-six peptide-specific T cell cultures with a sustained expansion capacity were analysed in more detail. T cell lines isolated from Can f 1 SPT+ subjects were dominated by CD4+ T cells (83% ± 5·2). The majority of the lines from non-allergic control subjects were also of CD4+ phenotype (67% ± 7·6) but three of the cell lines induced with p107–122 from these persons contained mainly CD8+ T cells (78% ± 5·9). The responses of the peptide-specific T cell lines were mostly restricted by HLA-DR, as 22 of the 36 T cell lines showed at least a 50% inhibition with the specific anti-HLA-DR mAb. In five T cell lines, none of the antibodies inhibited the response notably. Regardless of the peptide used in the induction or the allergic status of the donors, T cell lines showed variability in their TCR Vβ usage with no predilection for any Vβ subtypes (data not shown).
Proliferative responses of peptide-induced T cell lines
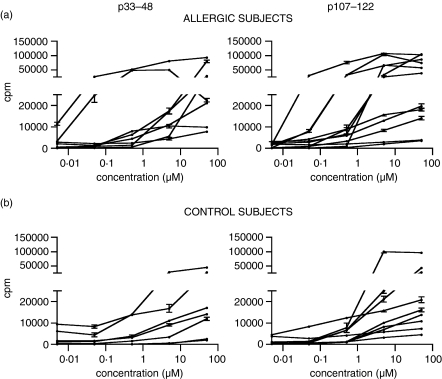
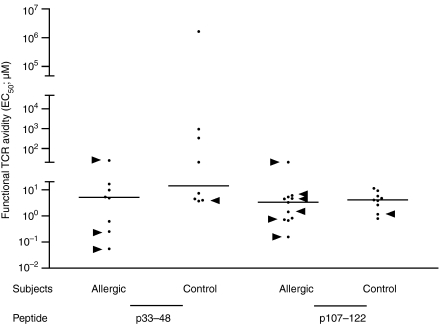
Sixteen T cell lines induced with p33–48 and 20 induced with p107–122 were stimulated with the peptide used in the induction, the corresponding peptide of human TL, and Can f 1 and TL proteins. The T cell lines from allergic and non-allergic subjects reacted to p33–48 and p107–122 in a dose-dependent manner but showed no obvious differences in their proliferative capacities (Fig. 2). When the functional avidity of the T cell lines was studied by determining EC50 values,23,24 the T cell lines of allergic subjects showed slightly higher avidity (lower EC50 values) than those of non-allergic control subjects, but the difference was not statistically significant (P > 0·05; Fig. 3).
Figure 2.
Proliferative responses of the peptide-specific T cell lines of Can f 1 skinprick test-positive allergic (a) and non-allergic control (b) subjects upon stimulation with p33–48 and p107–122. Peripheral blood mononuclear cells were isolated from heparinized blood samples and stimulated with p33–48 or with p107–122. After the first stimulation cycle, specificity of the cultures was determined using the thymidine incorporation test. Lines with stimulation indices ≥ 2 were re-stimulated for further study. The reactivity of these T cell lines to the peptide used in the induction was examined at 0–50 µm. Results are presented as mean counts per minute ± SEM.
Figure 3.
Peptide concentrations needed for inducing half-maximal proliferative responses (EC50) in the T cell lines of Can f 1 skinprick test-positive allergic and healthy control subjects. Can f 1 peptide-specific T cell lines were stimulated at 0–50 µm with the peptide used in the induction of the lines. Functional T cell receptor (TCR) avidity of the lines was studied by determining EC50 values from four-parameter dose–response curves using non-linear curve fitting.23,24 A high EC50 value refers to low functional TCR avidity (i.e. a greater Ag concentration is needed to obtain the half maximal response) and vice versa. Horizontal lines represent medians and arrowheads indicate the T cell lines responsive to Can f 1 protein.
The T cell lines isolated from allergic subjects showed reactivity to Can f 1 protein significantly more often than the lines from the non-allergic subjects (P < 0·05; Table 1). Six of nine protein-reactive T cell lines isolated from allergic subjects were induced with p107–122, whereas the rest of the lines were induced with p33–48 (Fig. 3). Both peptides induced only one protein-reactive T cell line from the non-allergic persons (Fig. 3).
Table 1.
Reactivity of p33–48 and p107–122-induced T cell lines upon stimulation with the peptides and the Can f 1 protein (P < 0·05, Fisher's exact test)
| Number of T cell lines from: | |||
|---|---|---|---|
| Reactivity to: | Allergic subjects | Control subjects | Total |
| Peptide | 10 | 15 | 25 |
| Peptide and protein | 9 | 2 | 11 |
| Total | 19 | 17 | 36 |
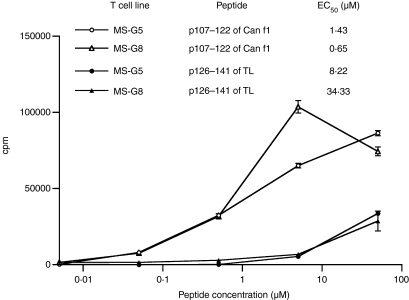
Two T cell lines (MS−G5 and MS−G8) induced with p107–122 from a Can f 1 SPT+ subject were observed to respond to the homologous peptide of human TL. The EC50 values of p107–122 of Can f 1 for the two T cell lines were 6- and 53-fold lower than the EC50 values of the TL peptide (Fig. 4). Both the lines also responded to Can f 1 protein but not to TL protein (data not shown).
Figure 4.
Proliferative responses of two p107–122-induced T cell lines upon stimulation with the Can f 1 peptide p107–122 and the corresponding tear lipocalin (TL) peptide. Can f 1 peptide-specific T cell lines were stimulated with the peptide used in the induction of the lines and with the corresponding human TL peptide at 0–50 µm. The functional T cell receptor (TCR) avidity of the lines was studied as for the data in Fig. 3. A high EC50 value refers to low functional TCR avidity and vice versa. The results are representative of two independent experiments and indicated as mean counts per minute ± SEM.
Cytokine production by the peptide-specific T cell lines
The production of IL-4 by the 19 T cell lines from allergic subjects induced and stimulated with either p33–48 or p107–122 tended to be greater than that by the 17 T cell lines from non-allergic subjects (Table 2). The p33–48-specific T cell lines from allergic subjects produced higher concentrations of IFN-γ than those specific to p107–122. This IFN-γ concentration was also higher than that obtained with either of the peptides from the T cell lines of control subjects (Table 2). Interestingly, the production of IL-10 tended to dominate in the p107–122-specific cell lines of allergic subjects, whereas the production of IL-10 in the p33–48-specific T cell lines was at a similar level to that in the lines of control subjects (Table 2).
Table 2.
Cytokine production of the peptide-induced T cell lines upon stimulation with p33–48 or p107–122 at a concentration of 50 µm. Results are shown as mean concentrations (pg/ml) ± SEM
| p33–48 | p107–122 | |||
|---|---|---|---|---|
| Allergic subjects | Control subjects | Allergic subjects | Control subjects | |
| Interleukin-4 | 269·9 ± 127·7 | 60·61 ± 19·92 | 420·2 ± 163·4 | 63·85 ± 19·58 |
| Interferon-γ | 2344 ± 1034 | 1169 ± 476·7 | 1275 ± 372·4 | 1540 ± 378·6 |
| Interleukin-10 | 95·65 ± 50·91 | 71·02 ± 46·71 | 617·8 ± 326·3 | 73·05 ± 40·02 |
Discussion
To identify the characteristics of the T cell epitopes of Can f 1, the PBMCs of Can f 1-sensitized SPT+ dog-allergic and healthy control subjects were stimulated with peptides from the immunodominant regions of the allergen.4 p33–48, p49–64 and p107–122 tended to give rise to a positive response more frequently than the other three peptides but the differences were not significant (Fig. 1). The differences between individuals were more prominent, as only four of 12 subjects responded to p49–64, for example. This result is in accordance with those of previous reports indicating that individual reactivity to the epitope regions of an allergen can vary considerably.6,26–28
The Can f 1 peptides p33–48 and p107–122 induced positive proliferative responses in PBMCs most frequently (Fig. 1). They showed equal capacities to induce T cell lines from the PBMCs of allergic and non-allergic persons. This result is in line with several findings according to which PBMCs29,30 and T cells5–7 of non-allergic and allergic subjects show reactivity to the same peptides of an allergen. It also agrees with the present data on PBMC stimulations (Fig. 1). The tendency of p33–48 and p107–122 to induce slightly higher frequencies of positive responses with the PBMCs of allergic and non-allergic subjects, respectively, was not reflected in the induction of T cell lines.
Two p107–122-induced T cell lines from an allergic subject recognized the homologous sequence (KPVRGVKLVGRDPKNN) of human TL (Fig. 4). This is of particular interest because, to our knowledge, T cell cross-reactivity between endogenous and exogenous lipocalins has not been observed previously. Our present data show that the EC50 values, used for characterizing the functional avidity of T cells,24 were greater for the endogenous peptide than for the allergen peptide (Fig. 4). This is reasonable, as self-specific T cells that have escaped thymic negative selection are likely to have low-affinity TCRs.23,31 By contrast, a minor change in the peptide sequence can influence TCR-mediated signalling.32 Our previous study showed that the substitution of a single amino acid in the immunodominant peptide of the major cow allergen Bos d 2 resulted in an approximately 100-fold increase in the proliferative response of a specific T cell clone.33 Our present data suggest that T cells showing moderate avidity for an exogenous ligand can cross-react with low avidity to a closely related endogenous peptide. Recognition of an endogenous peptide with low avidity can be protective against autoimmunity, whereas somewhat stronger recognition may favour allergy. As discussed below, strong reactivity to an antigenic peptide seems to favour Th1-type responses.
The median EC50 value of the Can f 1 peptide-specific T cell lines (Fig. 3) resembled the distribution we have observed with Bos d 2. The EC50 values of Bos d 2-specific T cell clones ranged typically from 0·5 to 5·0 µm upon stimulation with the immunodominant allergen peptide.33 By contrast, the EC50 values of T cell clones specific to an influenza virus haemagglutinin, a non-self-antigen, have been shown to be ≤ 0·1 µm.34 The present findings support our previous result in that the Can f 1 peptide-specific T cells generally showed only moderate avidity for the allergen peptides.33
The T cell lines of allergic and non-allergic subjects did not show any significant differences in the EC50 values upon stimulation with Can f 1 peptides (Fig. 3). The lack of polarization in the EC50 values suggests that functional TCR avidity for the allergen peptides is probably not a decisive factor for the allergenic capacity of proteins. In our previous study, Bos d 2-specific T cell clones showed higher functional avidity for the heteroclitic analogues of the immunodominant epitope of Bos d 2 than for the natural ligand.35 These heteroclitic peptides skewed the cytokine response of the allergen-specific T cell clones from Th2/Th0 towards the Th1-type and increased susceptibility to hyporesponsiveness or even cell death. Although it seems that mechanisms other than functional TCR avidity for allergen peptides are involved in protecting healthy subjects from allergies, high-avidity peptides (altered peptide ligands, APLs) can, however, be therapeutically useful for allergic individuals.
When peptide-specific T cell lines were stimulated with Can f 1, we observed that a significantly greater number of protein-reactive T cell lines were induced from the PBMCs of Can f 1 SPT+ subjects than from those of healthy control subjects (Table 1). This suggests that allergic subjects have a higher number of allergen-specific T cells in circulation than do non-allergic persons. Our previous data with Can f 1-induced T cell lines support this idea.25 Parallel results were obtained in a study of timothy grass pollen allergy.36 Another explanation for the greater number of protein-responsive T cell lines inducible from the PBMCs of allergic subjects could be that the function of allergen-reactive T cells of healthy persons is downregulated in the periphery. Several studies have produced evidence that regulatory T cells play a role in preventing allergic diseases.37–39 Interestingly, the dominance of high-avidity T cells in autoimmune diseases has been interpreted as resulting from a defect in the function of regulatory T cells.40
Although the peptide-induced T cell lines of allergic subjects produced IL-4, they also produced concentrations of IFN-γ over twice as high upon stimulation with p33–48 and concentrations of IL-10 eight times as high upon stimulation with p107–122 as did the cell cultures of non-allergic subjects upon stimulation with the same peptides (Table 2). This is of interest as both cytokines have been associated with successful immunotherapy of allergies.18,41,42 As the T cell lines of allergic and non-allergic subjects showed similar EC50 values, functional TCR avidity is unlikely to explain the great difference in cytokine production. Moreover, the elevated production of IL-10 refers to the involvement of an additional cell population alongside the Th1- and Th2-type lymphocytes. We have previously suggested that a pool of seven epitope-containing Can f 1 peptides could be used in specific immunotherapy in dog allergy.4 Our present data support this view, as one epitope peptide induced the production of IL-10 and the other the production of IFN-γ.
In conclusion, the stimulation of PBMCs of Can f 1 SPT+ subjects with peptides containing Can f 1 epitopes resulted in a significantly greater frequency of allergen-specific T cell lines than did the stimulation of PBMCs of healthy control individuals. Additionally, two allergen-specific T cell lines from a Can f 1 SPT+ subject responded to an endogenous human lipocalin peptide. The most prominent difference between the Can f 1-derived peptides was that p33–48 and p107–122 induced elevated production of IFN-γ and IL-10, respectively. Importantly, the increase in the production of those cytokines was greater in the T cell lines of Can f 1 SPT+ subjects than in those of healthy subjects. Both cytokines have been associated with successful immunotherapy. The data support our previous suggestion that several Can f 1 peptides should be used concurrently in specific immunotherapy. However, before clinical trials, safety, including the capacity of the peptides to elicit immediate allergic reactions, must be verified in a representative group of Can f 1-allergic subjects.
Acknowledgments
The skilful technical assistance of Virpi Fisk, Pirjo Vänttinen and Raija Tukiainen is gratefully acknowledged. We thank Professor Rauno Mäntyjärvi MD, PhD for the critical review of the manuscript. This work was financially supported by Kuopio University Hospital (project #5021605), the Academy of Finland (contracts #48657 and #205871), the Ida Montin Foundation, the Finnish-Norwegian Medical Foundation, and the Finnish Allergy Research Foundation.
Glossary
Abbreviations:
- cpm
counts per minute
- EC50 value
concentration needed to obtain the half-maximal response
- ELISA
enzyme-linked immunosorbent assay
- FEIA
fluoroenzyme-immunometric assay
- HLA
human leucocyte antigen
- IFN
interferon
- IL
interleukin
- mAb
monoclonal antibody
- PBMC
peripheral blood mononuclear cell
- PHA
phytohaemagglutinin
- PPD
purified protein derivative
- r
recombinant
- SI
stimulation index
- SIT
specific immunotherapy
- SPT
skinprick test
- TCR
T cell receptor
- Th cell
T helper cell
- TL
tear lipocalin
- VEGP
von Ebner's gland protein
References
- 1.Virtanen T, Mäntyjärvi R. Mammalian allergens. In: Lockey RF, Bukantz SC, Bousquet J, editors. Allergens and Allergen Immunotherapy. New York: Marcel Dekker; 2004. pp. 297–317. [Google Scholar]
- 2.Zeiler T, Mäntyjärvi R, Rautiainen J, Rytkönen-Nissinen M, Vilja P, Taivainen A, Kauppinen J, Virtanen T. T cell epitopes of a lipocalin allergen colocalize with the conserved regions of the molecule. J Immunol. 1999;162:1415–22. [PubMed] [Google Scholar]
- 3.Jeal H, Draper A, Harris J, Taylor AN, Cullinan P, Jones M. Determination of the T cell epitopes of the lipocalin allergen, Rat n. Clin Exp Allergy. 2004;34:1919–25. doi: 10.1111/j.1365-2222.2004.02126.x. [DOI] [PubMed] [Google Scholar]
- 4.Immonen A, Farci S, Taivainen A, et al. T cell epitope-containing peptides of the major dog allergen Can f 1 as candidates for allergen immunotherapy. J Immunol. 2005;175:3614–20. doi: 10.4049/jimmunol.175.6.3614. [DOI] [PubMed] [Google Scholar]
- 5.Ebner C, Schenk S, Najafian N, et al. Non-allergic individuals recognize the same T cell epitopes of bet v 1, the major birch pollen allergen, as atopic patients. J Immunol. 1995;154:1932–40. [PubMed] [Google Scholar]
- 6.Carballido JM, Carballido-Perrig N, Kagi MK, Meloen RH, Wuthrich B, Heusser CH, Blaser K. T cell epitope specificity in human allergic and non-allergic subjects to bee venom phospholipase A2. J Immunol. 1993;150:3582–91. [PubMed] [Google Scholar]
- 7.Mark PG, Segal DB, Dallaire ML, Garman RD. Human T and B cell immune responses to Fel d 1 in cat-allergic and non-cat-allergic subjects. Clin Exp Allergy. 1996;26:1316–28. [PubMed] [Google Scholar]
- 8.Cardaba B, Del Pozo V, Jurado A, et al. Olive pollen allergy: searching for immunodominant T cell epitopes on the Ole e 1 molecule. Clin Exp Allergy. 1998;28:413–22. doi: 10.1046/j.1365-2222.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–62. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- 10.Akdis CA, Blaser K. Role of IL-10 in allergen-specific immunotherapy and normal response to allergens. Microbes Infect. 2001;3:891–8. doi: 10.1016/s1286-4579(01)01449-6. [DOI] [PubMed] [Google Scholar]
- 11.Durham SR, Walker SM, Varga EM, et al. Longterm clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 12.Vrtala S, Focke-Tejkl M, Swoboda I, Kraft D, Valenta R. Strategies for converting allergens into hypoallergenic vaccine candidates. Methods. 2004;32:313–20. doi: 10.1016/j.ymeth.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 14.Maguire P, Nicodemus C, Robinson D, Aaronson D, Umetsu DT. The safety and efficacy of ALLERVAX CAT in cat allergic patients. Clin Immunol. 1999;93:222–31. doi: 10.1006/clim.1999.4795. [DOI] [PubMed] [Google Scholar]
- 15.Oldfield WL, Kay AB, Larche M. Allergen-derived T cell peptide-induced late asthmatic reactions precede the induction of antigen-specific hyporesponsiveness in atopic allergic asthmatic subjects. J Immunol. 2001;167:1734–9. doi: 10.4049/jimmunol.167.3.1734. [DOI] [PubMed] [Google Scholar]
- 16.Alexander C, Ying SB, Kay A, Larche M. Fel d 1-derived T cell peptide therapy induces recruitment of CD4+ CD25+ CD4+ interferon-γ+ T helper type 1 cells to sites of allergen-induced late-phase skin reactions in cat-allergic subjects. Clin Exp Allergy. 2005;35:52–8. doi: 10.1111/j.1365-2222.2005.02143.x. [DOI] [PubMed] [Google Scholar]
- 17.Muller U, Akdis CA, Fricker M, Akdis M, Blesken T, Bettens F, Blaser K. Successful immunotherapy with T cell epitope peptides of bee venom phospholipase A2 induces specific T cell anergy in patients allergic to bee venom. J Allergy Clin Immunol. 1998;101:747–54. doi: 10.1016/S0091-6749(98)70402-6. [DOI] [PubMed] [Google Scholar]
- 18.Fellrath JM, Kettner A, Dufour N, Frigerio C, Schneeberger D, Leimgruber A, Corradin G, Spertini F. Allergen-specific T cell tolerance induction with allergen-derived long synthetic peptides: results of a phase I trial. J Allergy Clin Immunol. 2003;111:854–61. doi: 10.1067/mai.2003.1337. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield WL, Larche M, Kay AB. Effect of T cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomized controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 20.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saarelainen S, Taivainen A, Rytkönen-Nissinen M, et al. Assessment of recombinant dog allergens Can f 1 and Can f 2 for the diagnosis of dog allergy. Clin Exp Allergy. 2004;34:1576–82. doi: 10.1111/j.1365-2222.2004.02071.x. [DOI] [PubMed] [Google Scholar]
- 22.Monsonego A, Zota V, Karni A, et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer's disease. J Clin Invest. 2003;112:415–22. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebe JA, Falk BA, Rock KA, Kochik SA, Heninger AK, Reijonen H, Kwok WW, Nepom GT. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur J Immunol. 2003;33:1409–17. doi: 10.1002/eji.200323871. [DOI] [PubMed] [Google Scholar]
- 24.Bielekova B, Sung MH, Kadom N, Simon R, McFarland H, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol. 2004;172:3893–904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- 25.Kinnunen T, Taivainen A, Partanen J, Immonen A, Saarelainen S, Rytkönen-Nissinen M, Rautiainen J, Virtanen T. The DR4−DQ8 haplotype and a specific T cell receptor vb T cell subset are associated with absence of allergy to Can f. Clin Exp Allergy. 2005;35:797–803. doi: 10.1111/j.1365-2222.2005.02247.x. [DOI] [PubMed] [Google Scholar]
- 26.van Neerven RJ, Ebner C, Yssel H, Kapsenberg ML, Lamb JR. T cell responses to allergens: epitope-specificity and clinical relevance. Immunol Today. 1996;17:526–32. doi: 10.1016/0167-5699(96)10058-x. [DOI] [PubMed] [Google Scholar]
- 27.Ebner C, Szepfalusi Z, Ferreira F, et al. Identification of multiple T cell epitopes on bet v 1, the major birch pollen allergen, using specific T cell clones and overlapping peptides. J Immunol. 1993;150:1047–54. [PubMed] [Google Scholar]
- 28.Counsell CM, Bond JF, Ohman JL, Jr, Greenstein JL, Garman RD. Definition of the human T cell epitopes of Fel d 1, the major allergen of the domestic cat. J Allergy Clin Immunol. 1996;98:884–94. doi: 10.1016/s0091-6749(96)80004-2. [DOI] [PubMed] [Google Scholar]
- 29.Raulf-Heimsoth M, Chen Z, Rihs HP, Kalbacher H, Liebers V, Baur X. Analysis of T cell reactive regions and HLA-DR4 binding motifs on the latex allergen hev b 1 (rubber elongation factor) Clin Exp Allergy. 1998;28:339–48. doi: 10.1046/j.1365-2222.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- 30.Haselden BM, Syrigou E, Jones M, Huston D, Ichikawa K, Chapman MD, Kay AB, Larche M. Proliferation and release of IL-5 and IFN-γ by peripheral blood mononuclear cells from cat-allergic asthmatics and rhinitics, non-cat-allergic asthmatics, and normal controls to peptides derived from Fel d 1 chain 1. J Allergy Clin Immunol. 2001;108:349–56. doi: 10.1067/mai.2001.117461. [DOI] [PubMed] [Google Scholar]
- 31.Reijonen H, Mallone R, Heninger AK, et al. GAD65-specific CD4+ T cells with high antigen avidity are prevalent in peripheral blood of patients with type 1 diabetes. Diabetes. 2004;53:1987–94. doi: 10.2337/diabetes.53.8.1987. [DOI] [PubMed] [Google Scholar]
- 32.de Visser KE, Cordaro TA, Kessels HW, Tirion FH, Schumacher TN, Kruisbeek AM. Low-avidity self-specific T cells display a pronounced expansion defect that can be overcome by altered peptide ligands. J Immunol. 2001;167:3818–28. doi: 10.4049/jimmunol.167.7.3818. [DOI] [PubMed] [Google Scholar]
- 33.Kinnunen T, Buhot C, Närvänen A, et al. The immunodominant epitope of lipocalin allergen Bos d 2 is suboptimal for human T cells. Eur J Immunol. 2003;33:1717–26. doi: 10.1002/eji.200322952. [DOI] [PubMed] [Google Scholar]
- 34.Korb LC, Mirshahidi S, Ramyar K, Sadighi Akha AA, Sadegh-Nasseri S. Induction of T cell anergy by low numbers of agonist ligands. J Immunol. 1999;162:6401–9. [PubMed] [Google Scholar]
- 35.Kinnunen T, Kwok WW, Närvänen A, Rytkönen-Nissinen M, Immonen A, Saarelainen S, Taivainen A, Virtanen T. Immunomodulatory potential of heteroclitic analogues of the dominant T cell epitope of lipocalin allergen Bos d 2 on specific T cells. Int Immunol. 2005;17:1573–81. doi: 10.1093/intimm/dxh332. [DOI] [PubMed] [Google Scholar]
- 36.Rimaniol AC, Garcia G, Till SJ, Capel F, Gras G, Balabanian K, Emilie D, Humbert M. Evaluation of CD4+ T cells proliferating to grass pollen in seasonal allergic subjects by flow cytometry. Clin Exp Immunol. 2003;132:76–80. doi: 10.1046/j.1365-2249.2003.02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34:1364–72. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 38.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T cell suppression of allergen-driven T cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 39.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 40.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J Immunol. 2004;172:5967–72. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 41.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116:961–8. doi: 10.1016/j.jaci.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–61. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]