Abstract
It has recently been shown that immature dendritic cells (DCs) stimulated by a danger signal undergo transient maturation followed by exhaustion. However, the exact mechanism for this has not been elucidated. In this study, we show that interleukin-10 (IL-10) secreted from transiently matured DCs stimulated by danger signals is responsible for this rapid DC exhaustion. Blocking of the autocrine IL-10 enabled transient mature DCs to maintain the mature phenotype for several days. However, these DCs remained phenotypically unstable because the addition of IL-10 altered the transient mature DCs to exhausted DCs. More importantly, stimulation of DCs by CD40 protected transient mature DCs from IL-10-dependent exhaustion, with the result that mature DCs remained stable in the presence of IL-10. Furthermore, in vivo administration of stable mature DCs pulsed with ovalbumin protein induced antigen-specific cytotoxic T lymphocytes (CTLs) effectively, whereas neither exhausted DCs nor transient mature DCs were able to prime a strong antigen-specific CTL response. These results indicate that DC−T cell engagement via CD40−CD154 is required for stable DC maturation leading to effective CTL induction. Otherwise, DCs stimulated solely by a danger signal are temporarily activated, but then rapidly lose their immune-activating capacity under the influence of autocrine IL-10.
Keywords: dendritic cells, interleukin-10, cytotoxic T cells
Introduction
Activation of the immune system is always initiated by danger signals, which include microbial products termed pathogen-associated molecular patterns (PAMPs) and extracts from dying cells (endogenous adjuvant), accompanied by certain cells that provide innate immunity.1–3 Among these cells, dendritic cells (DCs) are particularly significant as mediators between innate and adaptive immunity. DCs are normally present in peripheral tissue, in an immature form with a low ability to stimulate T cells, sometimes defined as a tolerogenic form.4–6 When DCs are exposed to danger signals, they immediately transform to active mode − in a process termed ‘maturation’− where they express increased amounts of major histocompatibility complex (MHC) antigens and costimulatory molecules on the cell surface and release cytokines.3,7 Activated DCs then migrate to the lymph nodes, where they make close contact with antigen-specific CD4+ T cells to initiate the primary T cell response.8 During DC−T cell contact, the maturation of DCs is strengthened by CD40 ligation provided by CD154 on activated T cells.9,10 By contrast, it has been demonstrated that cytokine production of bone marrow (BM) DCs is upregulated immediately after stimulation by danger signals, but gradually downregulated without simultaneous stimulation via CD40.11,12 However, the mechanism of the phenomenon previously described as ‘exhaustion’ has not been elucidated. In this study, we investigated the role of interleukin-10 (IL-10) within this mechanism.
Dendritic cell maturation is prevented by IL-10 secreted by DCs, macrophages, mast cells and some T cells. The addition of IL-10 to culture medium strongly inhibits human DC maturation induced by different danger signals.13,14 Maturation of murine DCs can also be downregulated by additional or autocrine IL-10.15,16 However, it has been reported that mature DCs are no longer susceptible to the suppressive effects of IL-10.17–19 Although the effect of IL-10 on DC maturation is undetermined, autocrine IL-10 diminishes T cell responses by inhibiting cytokine production14 and trafficking to lymph nodes.19
Although some cytotoxic T lymphocytes (CTLs) can be generated by CD40-independent mechanisms,20–22 the CD40–CD154 interaction between DCs and CD4+ T cells is considered critical to the induction of antigen-specific CTLs.23–25 Like the stimulation by danger signals, stimulation through CD40 is able to trigger the maturation of DCs.26–30 The differences between CD40-ligated DCs and DCs modulated by a microbial lipopolysaccharide (LPS) stimulus are not obvious in regard to class I MHC antigen expression, expression levels of costimulatory molecules and cytokine/chemokine production.30 Simultaneous stimulation by a CD40 agonist and a microbial stimulus enhances the production of IL-12p70 by DCs,31–33 which is supposed to help CTL induction. However, some reports suggest that IL-12 is not critical for priming and activation of CTLs,11,34 Therefore, the role of stimulation via CD40 for CTL activation is still obscure.
In this study we showed that autocrine IL-10 facilitated downregulation of DC function that had been matured transiently by danger signals. We also showed that a CD40 signal promoted stable maturation of transiently matured DCs, resulting in sufficient and effective CTLs in vivo. The roles of IL-10 and stimulation via CD40 in DC maturation are discussed.
Materials and methods
Mice
Female C57BL/6 (B6) mice, aged 5–6 weeks, were purchased from Japan SLC, Inc. (Hamamatsu, Japan). B6 background IL-10-deficient mice (IL-10–/–) were purchased from Jackson Laboratory (Bar Harbor, ME). These mice were housed under specific pathogen-free conditions in the Research Centre for Animal Life Science, Shiga University of Medical Science. As all experiments were performed under the auspices of the Shiga University of Medical Science Animal Experiment Committee, we usually used three mice in each group of experiments in order to limit the number of experimental animals.
Generation of myeloid DCs
Dendritic cells were generated from mouse BM as previously described.35 In brief, BM cells of B6 mice were depleted of T cells, B cells, macrophages and granulocytes by killing with lineage-specific antibodies and complement. Subsequently, the cells were cultured in RPMI-1640 (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal calf serum (FCS), 4 ng/ml recombinant mouse granulocyte/macrophage colony-stimulating factor (rmGM-CSF; Kirin Brewery Co. Ltd, Tokyo, Japan) and 50 μm b-mercaptoethanol for 6 days in 24-well culture plates. The culture medium was exchanged for fresh medium every 2 days. We usually acquired 1 × 107 immature DCs from each mouse.
DC stimulation
Maturation of DCs was induced by adding 5 µg/ml anti-CD40 mAb (HM40-3), 1 µg/ml LPS from Escherichia coli (Nacalai Tesque), 1 µg/ml peptidoglycan type III from Staphylococcus aureus (Wako Pure Chemicals, Osaka, Japan), 0·1 µm phosphothioate-protected cytosine-phosphorothioate-guanine rich oligodeoxynuleotides (CpG) 5002 (5′-TCCATGACGTTCTTGATGTT-3′; Hokkaido System Science, Sapporo, Japan) or derivatives from frozen and thawed 1 × 107 splenocytes (as necrotic cells) into each culture well. In some experiments, we added anti-IL-10 antibody (10 µg/ml) (JES5–2A5; BD PharMingen, San Diego, CA) or anti-IL-10 receptor antibody (10 µg/ml) (1B1·3a; BD PharMingen) in the culture of DC stimulation for 24 hr.
Flow cytometry
Dendritic cells were treated with anti-FcγRII/III mAb (2·4G2) and then stained with fluoresce in (fluorescein isothiocyanate, FITC)-conjugated anti-CD11c, phycoerythrin (PE)-conjugated anti-CD80, anti-CD86 or I-Ab, and biotin-conjugated antihamster IgG, I-Kb (BD PharMingen), followed by streptavidin-PE. Stained cells were acquired with a fluorescence-activated cell sorter (FACS) (BD Immunocytometry System, San Jose, CA).
Cytokine measurement with enzyme-linked immunosorbent assay
Variously matured subsets of DCs sorted by flow cytometry using anti-CD86 (5 × 106/well) were cultured in a 24-well plate for 6 hr and cytokines in supernatants were measured with enzyme-linked immunosorbent assay (ELISA). Briefly, IL-12p40 or IL-12p70 in supernatants was measured by a sandwich ELISA that used clone C15·6 (Caltag Laboratories, Burlingame, CA) or 9A5 (Pierce Biotechnology, Rockford, IL), respectively, for capture and biotinylated clone C17·8 for detection (Caltag Laboratories) and followed by peroxidase conjugate ExtrAvidin (Sigma-Aldrich Co., St. Louis, MO). IL-10 was detected using JES5–2A5 (BD PharMingen) for capture and biotinylated SXC-1 (BD PharMingen) for detection. Peroxidase activity was assessed with 3,3′,5,5′-tetramethylbenzidine substrate (Sigma-Aldrich Co.) for detection of IL-12p40 or Super Signal® ELISA Femto Maximum Sensitivity substrate (Pierce Biotechnology Inc.) for detection of IL-10.
In vivo cytotoxicity assay
Splenocytes of B6 mice were labelled with either 0·5 µm or 5 µm carboxyfluorescein diacetate succinimidyl ester (CFSE) (Sigma-Aldrich Co.) for 15 min at room temperature and washed twice. CFSE bright cells were subsequently pulsed with 0·5 µg/ml ovalbumin (OVA)257−264 (SIINFEKL) peptide for 90 min at 37 °. CFSE dull cells were pulsed with irrelevant nucleoprotein (NP)366−374 (ASNENMDAM) peptide for 90 min at 37 ° as a control. Cells were mixed at a 1 : 1 ratio, and then 5 × 106 total cells were injected intravenously into mice previously inoculated with OVA-ingested exhausted, transient mature or stable mature DCs. Eight to 10 hr later, splenocytes from each mouse were analysed by flow cytometry. The reduction rate of OVA257-264-pulsed CFSE bright cells was calculated as an indication of antigen-specific cytotoxicity induction efficiency.
Statistical analyses
Statistical analyses were carried out using Student's t-test. P-values < 0·05 were considered significant.
Results
Prolonged activation of LPS-stimulated BM-DCs induced by CD40 ligation
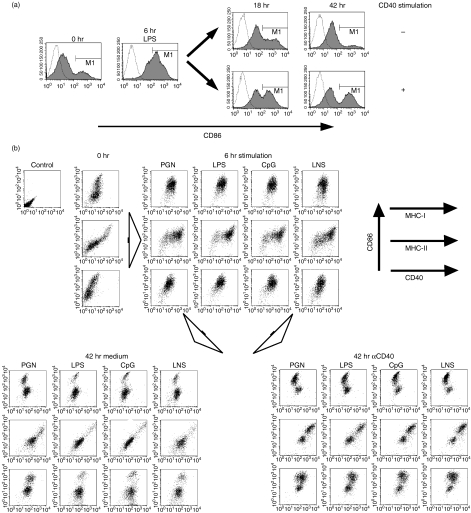
In the previous study, we compared BM-DC maturation by simulation of LPS with that by stimulation of anti-CD40 mAb (αCD40). Here we examined how sequential stimulation of αCD40 followed LPS-affected BM-DC maturation. We stimulated BM-DC with LPS for 6 hr, then washed and stimulated the cells with αCD40 for another 42 hr. CD86 costimulatory molecules, one of the maturation markers of DCs, were upregulated on BM-DCs at 6 hr after LPS stimulation, then downregulated gradually for another 42 hr without αCD40. By contrast, a certain number of DCs with a high concentration of CD86 (CD86high DCs) persisted for 42 hr after addition of αCD40 (Fig. 1a).
Figure 1.
Lipopolysaccharide (LPS)-induced transient activation and successive inactivation.(a) Immature dendritic cells (DCs) derived from B6 bone marrow (BM) cells were stimulated by LPS for 6 hr. The cells were then cultured with or without αCD40 for an additional 18 hr or 42 hr. Activation of DCs was analysed by fluorescence-activated cell sorter staining of anti-CD11c-FITC and anti-CD86-PE with propidium iodide live gating. Open lines indicate isotype control. (b) Immature BM-DCs of B6 mice were stimulated for 6 hr by 1 µg/ml peptidoglycan (PGN) as a toll-like receptor (TLR) 2 agonist, 1 µg/ml LPS as a TLR4 agonist, 0·1 µm cytosine-phosphorothioate-guanine (CpG) 5002 as a TLR9 agonist and lysate of necrotic B6 splenocytes (LNS), respectively. The cells were cultured for a further 42 hr with or without 5 µg/ml αCD40. The expression of Kb (MHC-I), Ab (MHC-II) and CD40 together with CD86 on BM-DCs was analysed by flow cytometry at the time-points indicated.
Certain surface molecules on DCs are known as maturation markers: i.e. MHC class I (MHC-I) and class II (MHC-II) antigens and CD40. We monitored the expression kinetics of these molecules on BM-DCs (Fig. 1b). Another costimulatory molecule, CD80, was expressed in parallel with CD86 (data not shown). The expression levels of MHC-I, MHC-II and CD40 increased all at once in the transient mature stage (6-hr LPS stimulation). Increased expression of these three molecules persisted during a further 42-hr incubation with αCD40, with the exception of MHC-I expression on CD86high DCs. However, expression of MHC-II, CD40 and CD86 decreased during a further 42-hr culture without stimulation. Therefore, the expression level of MHC-I molecules on BM-DCs was LPS stimulation-dependent in the primary stimulation but CD40 stimulation-independent in the secondary stimulation. By contrast, MHC-II and CD40 expression was not only dependent on an initial LPS stimulation but also on a secondary CD40 stimulation, similarly to CD86 expression kinetics. Furthermore, other PAMPs, peptidoglycan (PGN), CpG and lysate of necrotic splenocytes (LNS) functioned as LPS.
In a previous study, we demonstrated that CD86low DCs at 48 hr after LPS stimulation, termed expired DCs, were refractory to further stimulation via CD40 or LPS. This phenomenon is identical to that previously termed ‘exhaustion’. From these results, we surmise that stimulation by PAMPs is required for the transient maturation of DCs that subsequently change to exhausted DCs, and that the CD40 signal prevents the shift to exhausted DCs and keeps the maturation phase of DCs stable.
Cytokine production of BM-DC phenotypes
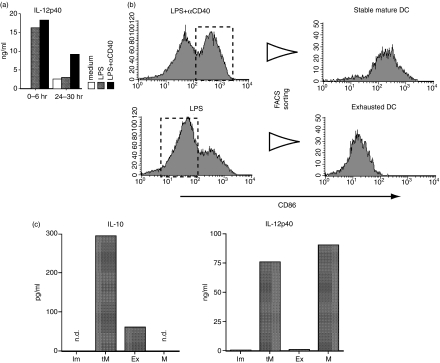
We quantified IL-12 p40 production by ELISA after 6-hr cultures of LPS-stimulated BM-DCs with or without CD40 stimulation. High amounts of IL-12p40 were produced within 6 hr of the beginning of LPS stimulation, regardless of simultaneous CD40 stimulation. However, the level of IL-12 production from BM-DCs stimulated with LPS alone decreased considerably at 24–30 hr compared with that from BM-DCs stimulated with LPS and αCD40 (Fig. 2a). These results supposedly reflect the percentage of CD86high DCs in the total cell population.
Figure 2.
Cytokine production of bone marrow dendritic cells (BM-DCs) during maturation and phenotype change.(a) Immature BM-DCs of B6 mice were stimulated by lipopolysaccharide (LPS) (1 µg/ml) or LPS (1 µg/ml) with αCD40 (5 µg/ml) to induce exhausted or stable mature phenotypes. During the culture, the culture media were collected and exchanged with fresh medium at 6 hr, 24 hr and 30 hr, and the amount of IL-12p40 secreted by the DCs in the cultures was measured by enzyme-linked immunosorbent assay (ELISA) for the periods 0–6 hr and 24–30 hr. (b) BM-DCs stimulated by LPS or LPS with αCD40 for 24 hr were sorted using a cell sorter. CD86high stable mature DCs and CD86low exhausted DCs were purified. (c) IL-10 and IL-12p40 production by each BM-DC phenotype was determined by ELISA: supernatants of 6-hr cultures of fluorescence-activated cell sorter-purified stable mature DCs (M), exhausted DCs (Ex), immature DCs (Im) and transient mature DCs (tM), respectively, were analysed (n.d., not detected).
Next, using flow cytometry we purified stable mature CD86high DCs as stable mature DCs after simultaneous stimulation with LPS and αCD40 and CD86low DCs as exhausted DCs after stimulation with LPS alone (Fig. 2b). Following LPS stimulation, IL-10 was secreted by transient mature DCs (tM); production decreased subsequently in exhausted DCs (Ex) and was almost undetectable in the culture medium of stable mature DCs (tM) (Fig. 2c). By contrast, both transient and stable mature DCs produced comparable levels of IL-12p40, but exhausted DCs produced very low amounts (Fig. 2c). Similar IL-10 and IL-12 production patterns were seen in each BM-DC phenotype after using CpG as the initial stimulator (data not shown). Altogether, it appeared that transient mature DCs produced IL-10 and IL-12p40, and they altered to stable mature DCs mainly producing IL-12p40 in the presence of CD40 stimulation or exhausted DCs mainly producing IL-10 in the absence of CD40 stimulation.
IL-10 partial responsibility for the inactivation of transient mature DCs
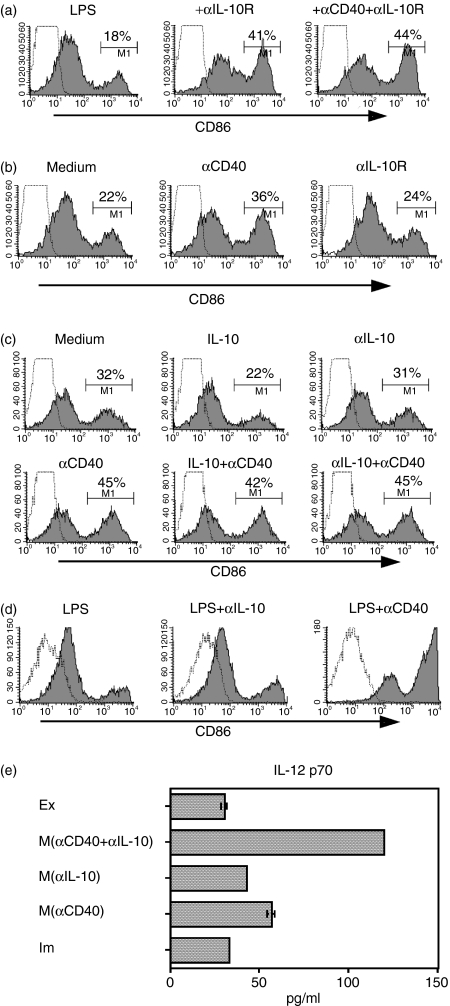
Having shown that BM-DCs produce a certain amount of IL-10 immediately after LPS stimulation, we then evaluated the involvement of IL-10 in DC maturation in the BM-DC culture system. When we blocked IL-10 ligation to BM-DCs by adding anti-IL-10 receptor antibody (αIL-10R) at the beginning of the culture maturing BM-DCs, the number of CD86high DCs was increased at 30 hr (Fig. 3a). Subsequently, after removal of IL-10 by washing transient mature DCs, we protected BM-DCs from the IL-10 effect by αIL-10R or anti-IL-10 neutralizing antibody (αIL-10). Unexpectedly, no significant rescue from downregulation of CD86 was observed by IL-10 blocking during the late (exhaustion) phase (Fig. 3b, c). Overall, during the early (transient maturation) phase, autocrine IL-10 seems to facilitate the downregulation of CD86 on transient mature DCs, which eventually leads to an increase in exhausted DCs. By contrast, adding IL-10 (10 ng/ml, which is an excessive amount compared with that of autocrine IL-10) to the culture medium after removal of autocrine IL-10 accelerated the downregulation of CD86 on BM-DCs (Fig. 3c), suggesting that a high dose of IL-10 still has some effect during the late phase of DCs.
Figure 3.
Role of IL-10 in inactivation of transient mature dendritic cells (DCs).(a) Bone marrow DCs (BM-DCs) were cultured in the presence of anti-IL-10 receptor (αIL-10R, 10 µg/ml) with or without αCD40 (5 µg/ml), after which CD86 expression at 30 hr after stimulation was analysed by flow cytometry. (b) A portion of lipopolysaccharide (LPS)-stimulated DCs were washed at 6 hr, then incubated for a further 24 hr in the presence of αCD40 or αIL-10R. (c) Immature BM-DCs stimulated by LPS (1 µg/ml) for 6 hr were washed with phosphate-buffered saline and then cultured for a further 24 hr in the presence of IL-10 (10 ng/ml), anti-IL-10 (αIL-10, 10 µg/ml) or αCD40 (5 µg/ml), respectively, or a combination of these stimuli. (d) Immature BM-DCs were stimulated by LPS in the presence of αIL-10 or αCD40 for 5 days. The cells were stained and gated as described in Figure 1. (e) IL-12p70 production by each BM-DC phenotype was determined by enzyme-linked immunosorbent assay: supernatants of 6-hr cultures of fluorescence-activated cell sorter-purified mature DCs (M), exhausted DCs (Ex), and immature DCs (Im), respectively, were analysed. Mature DCs (M) were produced by CpG + αIL-10, CpG + αCD40 or CpG + αCD40 + αIL-10.
Signalling via CD40 can compete with IL-10-induced inactivation of DCs
After removal of cytokines, including IL-10, from a 6-hr stimulated DC culture, αCD40 enhanced CD86 expression was compared with that by αIL-10R (Fig. 3b, c). Whereas the presence of a relatively high concentration of IL-10 in the culture medium of LPS-stimulated DCs after transient maturation accelerated exhaustion of DCs, the addition of αCD40 to the culture reversed the IL-10 effect (Fig. 3c).
We then questioned whether the CD86high DC population, produced by blocking IL-10 after LPS stimulation, belongs to the transient or stable mature phase. We cultured LPS-stimulated BM-DCs for 5 days by LPS with αIL-10 or αCD40 and then evaluated CD86 expression on the cell surface. Most of the LPS-stimulated DCs with αIL-10 lost CD86 expression, but αCD40-stimulated DCs retained the mature form (Fig. 3d). Therefore, IL-10 blocking during DC maturation simply prolongs the transient mature phenotype, whereas the CD40 signal alters transient mature DCs to stable mature DCs.
In order to see more accurate IL-12 production from the DCs, we measured IL-12p70 in the culture with DCs after various stimulations. The production of IL-12p70 in immature DCs did not differ from that in exhausted DCs (P = 0·1). By contrast, stable mature DCs produced more IL-12p70 compared with immature DCs (P < 0·001). Moreover, additional treatment with αIL-10 enhanced IL-12p70 production from stable mature DCs (Fig. 3e) (P < 0·001). Overall, IL-10 was profoundly involved in IL-12 production from DCs.
Confirmation of the role of IL-10 in DC maturation using IL-10-deficient mice
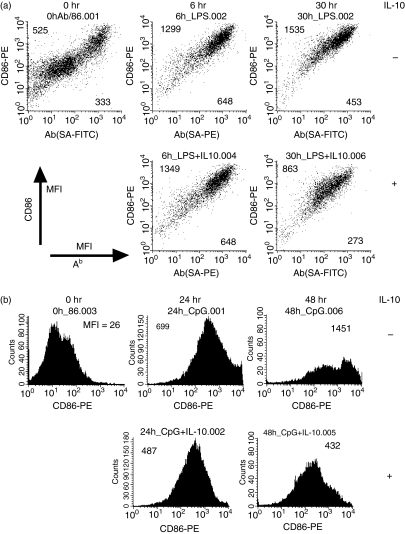
Further, we analysed maturation of BM-DCs derived from IL-10-deficient mice. Immature DCs derived from IL-10-deficient mice contained mainly low expressing cells of CD86 and MHC class II, like those of wild mice shown in Fig. 1(b). Expression of CD86 and MHC class II on BM-DCs derived from IL-10-deficient mice was not downregulated during the 30 hr after LPS stimulation (Fig. 4a). In splenic DCs, after 48 hr of CpG stimulation, the CD86low population slightly increased (Fig. 4b). This finding seems compatible with a finding that the transient mature DC phenotype was not maintained for long in the presence of αIL-10 (Fig. 3d). Furthermore, the expression of CD86 and MHC class II on BM-DCs derived from IL-10-deficient mice decreased at 30 hr after LPS stimulation in the presence of IL-10 (Fig. 4a). Similarly, CD86 expression on splenic DCs derived from IL-10-deficient mice decreased at 48 hr after CpG stimulation in the presence of IL-10 (Fig. 4b). These data show that IL-10 is a critical factor in changing transient mature DCs to exhausted DCs.
Figure 4.
Time−course analysis of lipopolysaccharide (LPS)-stimulated bone marrow dendritic cells (BM-DCs) from IL-10-deficient mice.(a) Immature BM-DCs derived from IL-10-deficient mice were stimulated by LPS (1 µg/ml) with or without IL-10, then analysed for CD86 and Ab expression by flow cytometry at 6 hr and 30 hr after stimulation. The numbers indicated in the upper left and lower right corners represent mean fluorescent intensity (MFI) of CD86 and Ab, respectively. (b) Time−course expression of CD86 on splenic DCs of IL-10-deficient mice was also analysed after cytosine-phosphorothioate-guanine stimulation with or without additional IL-10.
Efficiency of antigen cross-presentation by LPS-stimulated DC subsets
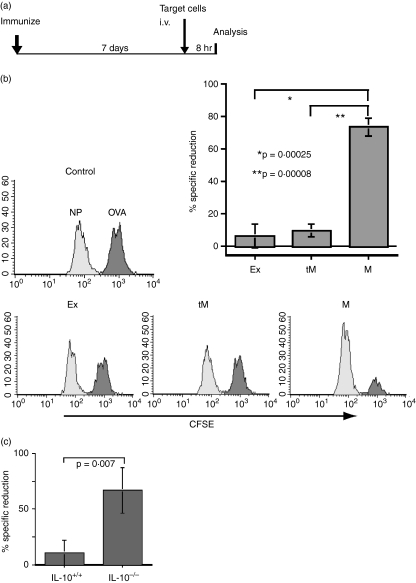
Finally, we checked the ability of LPS- and αCD40-modified BM-DCs to cross-prime antigen-specific CTLs. Immature BM-DCs were stimulated for 6 hr by LPS in OVA protein-containing medium to produce transient mature DCs. Then, a portion of the cells (5 × 104/mouse) was inoculated into a group of mice (three mice/group) subcutaneously. The rest of the transient mature DCs were washed to remove remaining OVA protein and further induced to stable mature DCs or exhausted DCs by incubation for another 24 hr with or without αCD40, respectively. Stable mature DCs and exhausted DCs were purified by cell sorter and then inoculated into two groups of mice (5 × 104/mouse), respectively. Six days later, OVA257-264-pulsed target cells were inoculated intravenously into the three groups of DC-immunized mice together with irrelevant peptide (NP366-374)-pulsed control cells. Eight hours later, remaining target cells and control cells in the spleens were analysed by flow cytometry (Fig. 5a). As we expected, only the mice immunized with stable mature DCs effectively removed OVA257-264-pulsed target cells (Fig. 5b). In the mice inoculated with transient mature DCs, some of the OVA peptide-pulsed target cells were removed.
Figure 5.
Efficacy of in vivo cytotoxic T lymphocyte (CTL) induction by several dendritic cell (DC) phenotypes.(a) Protocol for in vivo CTL induction by DC inoculation: 1 week after antigen-pulsed DC immunization, target cells were inoculated intravenously and residual cells in the spleen were analysed 8 hr later. (b) Histograms indicate ovalbumin (OVA)-specific killing in B6 mice immunized with OVA protein-pulsed exhausted DCs (Ex), transient mature DCs (tM) or stable mature DCs (M) (5 × 104/mouse). The bar graph shows the average reduction rate of OVA257-264-target cells (decreased number of OVA257-264-pulsed target cells/decreased number of nucleoprotein (NP)366-374-pulsed target cells) in the spleen from mice immunized with Ex, tM or M. (c) Bone marrow DCs derived from IL-10-deficient (IL-10–/–) or wild type (IL-10+/+) mice were stimulated with lipopolysaccharide in the presence of OVA protein (1 mg/ml) for 3·5 hr. B6 mice were immunized by those DCs, after which in vivo CTL activity was estimated.
We prepared transient mature DCs from IL-10-deficient mice in the presence of OVA protein, and then inoculated the DCs into B6 mice to determine the capacity for activating OVA-specific CTL in vivo. The transient mature DCs from IL-10-deficient mice were better able to induce OVA-specific CTL in vivo compared with transient mature DCs from wild mice (Fig. 5c). These data suggest that IL-10 is profoundly involved in CTL induction in vivo by its regulation of DC maturation.
Discussion
Although it is generally known that PAMPs or tumour necrosis factor (TNF) family molecules induce DC maturation, there is little information concerning the mechanisms of DC exhaustion. It has been demonstrated that immature DCs stimulated with PAMPs undergo transient maturation and successive exhaustion.11,12 In this study, IL-10 blocking experiments revealed that autocrine or exocrine IL-10 induced by PAMPs accelerated DC exhaustion. Furthermore, transient mature DCs from IL-10-deficient mice could activate antigen-specific CTLs, whereas those from wild mice could not. Thus, it appears that IL-10 plays a crucial role in DC exhaustion. These results may explain that some of argument that immune response in IL-10-deficient conditions is attributable to the suppression of the change to exhausted DCs, and in certain instances, IL-10 deficiency leads to autoimmune diseases.36,37
In addition, the stimulation of CD40 enabled DCs to avoid exhaustion by PAMPs-induced autocrine IL-10. The CD40 signal stabilizes the mature phenotype of DCs and produces ‘stable maturation’, which is hardly influenced by IL-10, as has been previously demonstrated.17–19 If transient mature DCs do not interact with T cells, DCs gradually lose their antigen-presenting capacity by IL-10-dependent DC exhaustion. Therefore, these results explain the synergistic effects of toll-like receptor (TLR) and CD40 agonists.
IL-12 is a critical cytokine in the immune system and is produced by DCs with innate stimuli.38 IL-10 is considered to suppress IL-12 production because autoimmune-like disease develops spontaneously in IL-10-deficient mice with increased IL-12 production.39,40 Although the mechanisms for IL-10-inhibition of IL-12 production are still unclear, our data may partly explain this: IL-10 combined with low production of IL-12 increased the rate of DC exhaustion; by contrast, IL-10 blocking increased the level of transient mature DCs, which were high IL-12 producers. Like IL-12, IL-6 is selectively produced by both transient and stable mature DCs, but is scarcely produced by exhausted DCs.12 Production of IL-6 supposedly assists T cell activation by stable mature DCs because IL-6 diminishes the effect of CD4+CD25+ regulatory T cells.41 Therefore, the polarization of cytokine production by DCs during maturation is functionally co-operative with stable mature or exhausted DC phenotypes.
Not all DC exhaustion is IL-10-dependent because we always observe a biphasic pattern of CD86 expression in BM-DCs stimulated by LPS + αCD40 or CpG + αCD40. The CD40 signal supposedly protects against only the IL-10-dependent exhaustion; no additive effect of αCD40 and αIL-10 treatment was observed compared with αCD40 or αIL-10 single use. IL-10-independent exhaustion of DCs may be dependent on IRAK-M42 or other unidentified molecules.
Cross-presentation of exogenous antigens by myeloid DCs is another important issue. Our data proved that myeloid DCs are able to cross-prime antigen-specific CTLs in vivo, if the DCs are matured in a stable fashion by CD40 stimulation. By contrast, transient mature DCs have much less capacity to cross-prime antigen-specific CTLs in vivo despite their expression of potent costimulatory molecules and cytokine production. The reason for this discrepancy seems to be that transient DCs easily lose antigen expression. However, peptide expression was detectable on DCs 24 hr after the initial stimulation (data not shown). Thus, transient mature DCs are likely to alter to exhausted DCs by autocrine IL-10, with the loss of expression of costimulatory molecules and cytokine production, before contact with CTLs in vivo, unless CD40L (CD154) on activated T cells stimulates transient DCs. Indeed, transient DCs induced from IL-10-deficient mice hardly lost their antigen-presenting capacity and activated CTLs even in the absence of CD40 stimulation.
Finally, it is important to modulate DCs for practical use, such as in antivirus vaccination, allergy or cancer immunity. Recent studies have proved that CD40 stimuli are essential for effective memory CTL induction, both directly and indirectly.43–45 However, activation of DCs by a single stimulation by αCD40 is not sufficient to induce functional CTLs because both CD40 and TLR triggering are needed to induce protective immunity in vivo.46 In this report, we also demonstrated that triggering of both CD40 and TLR induced stable mature DCs that produce considerable amounts of immunogenic cytokines and these DCs were able to activate functional antigen-specific CTLs in vivo. Conclusively, stable mature DCs induced by synergistic stimulation of CD40 and TLR will be applicable in the treatment of human diseases.
Acknowledgments
We thank Drs R. Germain and H. Yagita for generous gifts of materials. This work was supported partly by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Glossary
Abbreviations:
- BM
bone marrow
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- CpG
cytosine-phosphorothioate-guanine rich oligodeoxynuleotides
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- NP
nucleoprotein
- OVA
ovalbumin
- PAMPs
pathogen-associated molecular patterns
- TLR
toll-like receptor
References
- 1.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 2.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nature Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Janway CA., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhodapkar MV, Steinman RM, Krasovsky J, et al. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–20. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 7.Bancherean J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell–dendritic cell interactions in lymph nodes. Science. 2002;296:1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima T, Caceres-Dittmar G, Tapia FJ, et al. T cell-mediated terminal maturation of dendritic cells: loss of adhesive and phagocytotic capacities. J Immunol. 1996;157:2340–7. [PubMed] [Google Scholar]
- 10.Josien R, Li HL, Ingulli E, et al. TRANCE, a tumour necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med. 2000;191:495–502. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of Th1, Th2 and non-polarized T cells. Nat Immunol. 2000;1:311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura I, Kajino K, Bamba H, et al. Phenotypic stability of mature dendritic cells tuned by TLR or CD40 to control efficiency to cytotoxic T cell priming. Microbiol Immunol. 2004;48:211–9. doi: 10.1111/j.1348-0421.2004.tb03508.x. [DOI] [PubMed] [Google Scholar]
- 13.Morel AS, Quaratino S, Douek DC, Londei M. Split activity of interleukin-10 on antigen capture and antigen presentation by human dendritic cells: definition of a maturative step. Eur J Immunol. 1997;27:26–34. doi: 10.1002/eji.1830270105. [DOI] [PubMed] [Google Scholar]
- 14.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–8. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 15.Redpath S, Angulo A, Gascoigne NRJ, Ghazal P. Murine cytomegalovirus infection downregulates MHC class II expression on macrophages by induction of IL-10. J Immunol. 1999;162:6701–7. [PubMed] [Google Scholar]
- 16.Commeren DL, van Soest PL, Karimi K, et al. Paradoxical effects of interleukin-10 on the maturation of murine myeloid dendritic cells. Immunology. 2003;110:188–96. doi: 10.1046/j.1365-2567.2003.01730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald KPA, Pettit AR, Quinn C, Thomas GJ, Thomas R. Resistance of rheumatoid synovial dendritic cells to the immunosuppressive effects of IL-10. J Immunol. 1999;163:5599–607. [PubMed] [Google Scholar]
- 18.Thurner B, Roder C, Dieckmann D, et al. Generation of large numbers of fully mature and stable dendritic cells from leukopheresis products for clinical application. J Immunol Meth. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 19.Demangel C, Bertolino P, Britton WJ. Autocrine IL-10 impairs dendritic cell (DC)-derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002;32:994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Ruedl C, Kopf M, Bachmann MF. CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo. J Exp Med. 1999;189:1875–84. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Z, Yuan L, Zhou X, et al. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J Exp Med. 2000;191:541–50. doi: 10.1084/jem.191.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuurhuis DH, Laban S, Toes REM, et al. Immature dendritic cells acquire CD8+ cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J Exp Med. 2000;192:145–50. doi: 10.1084/jem.192.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett SR, Carbone FR, Karamalis F, et al. Help for cytotoxic-T cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 24.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T helper and a T killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 25.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 26.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella M, Scheidegger D, Palmer-Lehmann K, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T−T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch F, Stanzl U, Jennewein P, et al. High level of IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinde S, Wu Y, Guo Y, et al. CD40L is important for induction of, but not response to, costimulatory activity: ICAM-1 as the second costimulatory molecule upregulated by CD40L. J Immunol. 1996;157:2764–8. [PubMed] [Google Scholar]
- 30.Kelleher M, Beverley PCL. Lipopolysaccharide modulation of dendritic cells is insufficient to mature dendritic cells to generate CTLs from naive polyclonal CD8+ T cells in vitro, whereas CD40 ligation is essential. J Immunol. 2001;167:6247–55. doi: 10.4049/jimmunol.167.11.6247. [DOI] [PubMed] [Google Scholar]
- 31.Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 32.Morelli AE, Zahorchak AF, Larregina AT, et al. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–23. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 33.Vaidyanathan H, Gentry JD, Weatherman A, Schwartzbach SD, Petro TM. Differential response of the murine IL-12 p35 gene to lipopolysaccharide compared with interferon-gamma and CD40 ligation. Cytokine. 2001;16:1–9. doi: 10.1006/cyto.2001.0938. [DOI] [PubMed] [Google Scholar]
- 34.Wan Y, Lu L, Bramson JL, et al. Dendritic cell-derived IL-12 is not required for the generation of cytotoxic, IFN-gamma-secreting, CD8 + CTL in vivo. J Immunol. 2001;167:5027–33. doi: 10.4049/jimmunol.167.9.5027. [DOI] [PubMed] [Google Scholar]
- 35.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bettelli E, Das MP, Howard ED, et al. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–306. [PubMed] [Google Scholar]
- 37.Toto P, Feliciani C, Amerio P, et al. Immune modulation in pemphigus vulgaris: role of CD28 and IL-10. J Immunol. 2000;164:522–9. doi: 10.4049/jimmunol.164.1.522. [DOI] [PubMed] [Google Scholar]
- 38.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 40.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10–/– mice: an overview. J Leukoc Biol. 1997;61:389–96. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 41.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 42.Janssen EM, Lemmens EE, Wolfe T, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi K, Hernandez LD, Galan JE, et al. IRAK-M is a negative regulator of toll-like receptor signalling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 44.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 45.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahonen CL, Doxsee CL, McGurran SM, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–84. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]