Abstract
γδ T-cell receptor+ T lymphocytes are an important element of the innate immune system. Early production of interferon (IFN)-γ by γδ T cells may have a role in linking innate and adaptive immune responses and contribute to T helper-1 bias. We investigated the role of cytokines in the activation and induction of IFN-γ secretion by bovine workshop cluster 1+ (WC1+) γδ T cells. The effects of culture with interleukin (IL)-12, IL-18, IL-15 and IL-2 were investigated; these cytokines are known to influence murine and human γδ T cells. We report that bovine WC1+γδ T cells are synergistically stimulated by IL-12 and IL-18 to secrete large quantities of IFN-γ. Neonatal calves were shown to have significantly higher numbers of circulating WC1+γδ T cells than adult animals. In addition, the response of peripheral blood WC1+γδ T cells was significantly higher in neonatal calves compared with adult animals. However, in adult animals the response of lymph node WC1+γδ T cells to IL-12/IL-18 was more pronounced than that of peripheral blood WC1+γδ T cells. We hypothesize that the induction of IFN-γ secretion from WC1+γδ T cells by IL-12 and IL-18 is likely to be an important element of the innate response to pathogens such as Mycobacterium bovis. The high numbers of WC1+γδ T cells in neonatal calves, and their inherent ability to respond to inflammatory cytokines, could be a key factor in the enhanced responses seen in calves to BCG vaccination.
Keywords: neonatal, bovine, workshop cluster 1+γδ T cells, interferon-γ, interleukin-12
Introduction
γδ T-cell receptor+ T cells are thought to constitute a first line of defence and, as such, are an important element of the innate immune system. This is inferred from their localization in skin and in the epithelial layers of the intestine and respiratory tract. Studies of knockout mice also suggest that these cells are important early mediators of immune responses against intracellular bacteria, including Mycobacterium tuberculosis.1–3 However, although these cells have been associated with immunity to many infectious and inflammatory diseases, their activation requirements and their mechanisms for antigen recognition are not well understood. It is known that they have a wide antigenic recognition4,5 and suggested ligands for γδ T cells include heat shock proteins, phosphoantigens such as isopentyl phosphate from mycobacteria, and other non-peptide antigens. In addition, γδ T cells can respond to stimulation via the T-cell receptor (TCR) and through CD3 stimulation,6–8 leading to the induction of interferon (IFN)-γ expression in these cells. Some studies suggest that γδ T cells express CD28 and cytotoxic T-lymphocyte antigen (CTLA)-4 Ig,9 and that CD86 is involved in their costimulation.10 Dendritic cells (DCs) have been shown to be more efficient stimulators of γδ T cells compared with macrophages,10,11 which may suggest that CD80, CD86 or other molecules expressed at high levels on DCs are involved in this interaction. As an apparent paradox, other studies have found that bovine γδ cells do not express CD28,11,12 indicating that some γδ T cells may use alternative pathways for costimulation.
Many studies assessing γδ T cell activation and costimulatory requirements report that the responses of these cells are dependent upon the presence of exogenous interleukin (IL)-2; this is associated with expression of IL-2R by γδ T cells. However, γδ T cells are also influenced by other cytokines, including IL-12, IL-15, IL-18 and tumour necrosis factor (TNF)-α which may act alone, or in synergy to promote γδ T cell activation and IFN-γ secretion.13–18
γδ T cells exist in relatively small numbers in mice and humans. However, in cattle large numbers of these cells are evident, particularly in young calves where γδ T cells may comprise up to 50% of circulating peripheral blood mononuclear cells (PBMCs).19,20 In cattle γδ T cells exist as subpopulations which are defined by surface phenotype and which have different tissue distributions and functions. One differentiation antigen that is expressed on ruminant γδ T cells is the workshop cluster 1 (WC1) molecule, which is present on the majority of peripheral γδ T cells in cattle.21–23 This molecule is likely to be involved in control of proliferation of γδ T cells and may also be involved in homing and cytokine production by γδ T cells.24,25 WC1+γδ T cells have been shown to express IL-2, IL-4, IL-10 and TNF-α, and to respond to DCs present in afferent lymph preparations.11,12,26
Subsets exist of WC1+γδ T cells that may have different functions. We demonstrated previously that WC1+γδ T cells present in lymph nodes, but not in blood, produced IFN-γ in response to mitogen activation. This may indicate the presence of activated or alternative subsets of WC1+γδ T cells present within lymph nodes.27 The WC1 family of molecules are encoded by a multigene family and at least three isoforms of WC1 have been described. Differential expression of WC1 isoforms on subpopulations of cells has been associated with distinct functions of these cells.28 Expression of the WC1·1 isoform indicates the capacity to proliferate and secrete IFN-γ upon antigenic stimulation, and to secrete IFN-γ without proliferation in the presence of IL-12. Expression of WC1·1 and WC1·2 is largely mutually exclusive; cells bearing WC1·1 decline with age, whereas those expressing WC1·2 are maintained.
In addition to the secretion of IFN-γ, WC1+ cells have been shown capable of IL-12 and TNF-α secretion,12,26 which would contribute to early defence and T helper 1 (Th1) bias against intracellular pathogens. Early production of IFN-γ by WC1+γδ T cells postinfection may have a role in linking the innate and adaptive immune responses.
We investigated the role of cytokines in the activation and induction of IFN-γ secretion by bovine WC1+γδ T cells. The effects of culture with IL-12, IL-18, IL-15 and IL-2 were investigated. We report that bovine WC1+γδ T cells can be synergistically stimulated by IL-12 and IL-18 to secrete large quantities of IFN-γ. In addition, we compare the functions of adult and neonatal WC1+γδ T cells and show that the capacity of WC1+ cells to secrete IFN-γ is affected by both age and tissue location. These differences may contribute to the different responses to BCG vaccination observed in adult animals and neonatal calves.
Materials and methods
Experimental animals
Conventionally reared British Holstein-Friesians (Bos taurus) bred at the Institute for Animal Health were used for these investigations. Animals under 6 weeks of age are considered as neonates and those over 2 years as adults. The experiments were performed in accordance with UK Home Office guidelines and were approved by the local ethics committee.
Cytokines
Recombinant human (rhu) IL-18 was purchased from R & D Systems Ltd. (Oxford, UK) and rhu IL-15 from Peprotech EC Ltd. (London, UK). Recombinant bovine (rbo) IL-12 and IL-2 were expressed in COS cells as previously described.29 Dilutions of these cytokines were initially selected based on previous experience within this laboratory.29 The concentrations selected for initial experiments were IL-12 10 U/ml, IL-18 10 ng/ml, IL-2 10 U/ml, and IL-15 0·2 ng/ml.
Isolation of mononuclear cells and purification of lymphocytes
PBMCs were isolated from heparinized blood as previously described.30 WC1+ cells were isolated from PBMCs following staining with mononuclear antibodies (mAbs) CC3922 or CC1531 (both anti-WC1, IgG1 or IgG2a, respectively) and anti-mouse IgG super-paramagnetic particles (Miltenyi-Biotech, Bergish-Gladbach, Germany). The purity of the cells, as evaluated by flow cytometry, was > 98%.
Prescapular lymph nodes (PSLNs) were taken from cattle post-mortem and forced through a fine steel mesh. Cell clumps were removed by passage through a 40-µm filter and the mononuclear cells were collected after density gradient centrifugation.27
Cells were cultured at a density of 2 × 105 per well in a total volume of 200 µl of tissue culture medium (TCM; RPMI containing 10% fetal calf serum, FCS, 5 × 10−5m 2-Me, 50 µg/ml gentamicin) in 96-well tissue culture plates. Cytokines were added to give the indicated final concentrations. TCM was used as a negative control.
Detection of intracytoplasmic IFN-γ expression and flow cytometric analyses
PBMCs or purified WC1+ cells were cultured in the presence or absence of cytokines for approximately 46 hr. Brefeldin-A (10 µg/ml final) was added for the last 16 hr of culture. The cells were washed extensively, then fixed with 1% paraformaldehyde and permeabilized (FACS permeabilization solution; Becton Dickinson, Oxford, UK). Expression of WC1 was detected following staining with mAbs CC39 or CC15 and IFN-γ expression was detected with mAbs 6H532 or CC302 (Serotec) (both antibovine IFN-γ; IgG2a and IgG1, respectively). Bound antibody was detected with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) labelled anti-mouse isotype specific reagents (Southern Biotechnology Associates, Birmingham, AL). Immunofluorescent staining was analysed using fcs express (De Novo Software, Thornhill, Canada) and the double positive cells expressed as a percentage of the total WC1+ population:
A minimum of 10 000 events were collected.
Assessing proliferation of WC1+ cells
PBMC were labelled with carboxy-fluorescein diacetate, succinimidyl ester (CFDA-SE) (Vybrant CFDA-SE Cell Tracer Kit; Molecular Probes, Eugene, OR) according to the kit instructions. Briefly, 5 µl of a 10 mm stock of CFDA-SE was diluted in 10 ml warm phosphate-buffered saline (PBS) to give a 5 µm stock. Cells were diluted to 1 × 107 ml−1 in warm PBS, and an equal volume of 5 µm CFDA-SE added to give 2·5 µm CFDA-SE. The cells were incubated for 15 min at 37 °, washed once in warm PBS and then the probe was modified by incubation of the cells in warm TCM containing FCS for 30 min, at 5 × 106 cells ml−1. The cells were then cultured and stimulated in the same way as previously described, with different combinations of IL-2, IL-15, IL-18 and IL-12 added. WC1 expression was detected using CC39 conjugated to Alexa Fluor 647 (Alexa Fluor 647 Protein Labelling Kit; Invitrogen, Paisley, UK) and IFN-γ was detected using CC302 conjugated to PE (mouse-anti-bovine IFN-γ-phycoerythrin (PE), Serotec, UK). Immunofluorescent staining was analysed using fcs express.
Measurement of IFN-γ by enzyme-linked immunosorbent assay
Purified WC1+ cells were cultured in the presence of cytokines or TCM alone for approximately 46 hr. Supernatants were removed and assessed for IFN-γ by enzyme-linked immunosorbent assay (ELISA) as previously described.33 Results are expressed as pg per ml.
Statistical analysis
Analysis was performed using minitab software. Differences between WC1+ responses between animals of different ages or between WC1+ cells isolated from different tissues were assessed by Students' t-test. P-values < 0·05 were considered significant.
Results
Determination of the optimal conditions of IL-12 and IL-18 stimulation required for IFN-γ induction
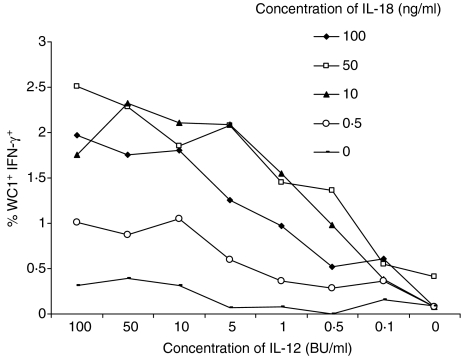
The effective dose of IL-12 and IL-18 was determined by stimulating PBMCs with a range of concentrations (Fig. 1) of these cytokines and assessment of IFN-γ expression by WC1+ cells. The optimal concentration of IL-12 was determined as 10 biological units (BU) per ml and IL-18 as 10 ng per ml. Optimal concentrations of IL-2 and IL-15 had been previously determined in this laboratory.29 It was determined in preliminary experiments that a culture period of 46 hr was optimal for the induction of IFN-γ, with the addition of Brefeldin A at 30 hr (data not shown).
Figure 1.
Determination of optimum concentrations of interleukin (IL)-12 and IL-18 for stimulation of workshop cluster 1+ (WC1+) γδ T cells. Various concentrations of IL-12 (biological units [BU]/ml; x axis) and IL-18 (ng/ml) were titrated to determine the optimum dilutions for induction of interferon (IFN)-γ production by WC1+γδ T cells. Cells were cultured for 46 hr; Brefeldin A was added for the final 16 hr of culture. Cells were fixed in paraformaldehyde, permeabilized and analysed by flow cytometry for the expression of intracellular IFN-γ (monoclonal antibody, mAb, 6H5)) and the WC1 antigen (mAb CC39). Results shown are from one animal; repeats in two other animals yielded similar results.
Effects of cytokines on the capacity of WC1+ cells to produce IFN-γ
Bovine PBMC were stimulated with a combination of IL-18, IL-15, IL-12 and IL-2 (Fig. 2a), IL-12 and IL-18 (Fig. 2b) or TCM alone for 46 hr (Fig. 2c). Brefeldin A was added for the final 16 hr and IFN-γ expression by WC1+ cells was assessed by flow cytometry. A percentage of WC1+ cells expressed high levels of intracellular IFN-γ in response to cytokine stimulation (Fig. 2a, b) but not in response to TCM alone (Fig. 2c). WC1− cells also expressed IFN-γ when PBMCs were stimulated by cytokine combinations. Additional analysis showed that IFN-γ was expressed by NK, CD4+ and CD8+ T lymphocytes (data not shown).
Figure 2.
Stimulation of workshop cluster 1+ (WC1+) γδ T cells by cytokines. Peripheral blood mononuclear cells (PBMCs) were stimulated for 46 hr with cytokines (a) interleukin (IL)-15, IL-12, IL-18 and IL-2; (b) IL-12 and IL-18, or (c) medium alone. Brefeldin A was added for the final 16 hr of culture. Cells were fixed in paraformaldehyde, permeabilized and analysed by flow cytometry for the expression of intracellular interferon (IFN)-γ (monoclonal antibody, mAb, 6H5) and the WC1 antigen (mAb CC39).
Determination of cytokines required for IFN-γ expression and proliferation by WC1+ cells
To define the required cytokines for IFN-γ secretion by WC1+ cells more precisely, PBMCs were exposed to various combinations of IL-12, IL-15, IL-2 and IL-18 and each of the cytokines alone (Table 1). Significant IFN-γ expression within the WC1+ population was induced by combinations containing IL-12 and IL-18, although IFN-γ was produced in response to other cytokines. Thus, IL-12 and IL-18 were considered to be the most critical cytokines for induction of IFN-γ (Fig. 2b). Assessment of IFN-γ production by dividing cells was carried out following CFDA-SE labelling of PBMCs (Fig. 3). IL-12 and IL-18 together (Fig. 3d) induced division of WC1+ cells, which could be enhanced by adding IL-15 (Fig. 3c), IL-2 (not shown) or IL-2 + IL-15 (Fig. 3b). The increased secretion of IFN-γ in cultures containing IL-2/IL-15 (Table 1) is due to the ability of IL-2 and IL-15 to induce IFN-γ production in higher numbers of WC1+ cells overall (Fig. 3b, c). Interestingly in each case, although the majority of WC1+ cells were undivided (Table 2 and Fig. 3), the highest proportion of WC1+ cells expressing IFN-γ was in the first division (Table 2 and Fig. 3c).
Table 1.
Cytokine requirements for interferon (IFN)-γ production by workshop cluster 1+ (WC1+) γδ T cells. Peripheral blood mononuclear cells (PBMCs) were cultured for 46 hr in the presence of different combinations of cytokines with Brefeldin A added for the final 16 hr. Cells were fixed in paraformaldehyde, permeabilized and stained for intracellular IFN-γ (monoclonal antibody, mAb, 6Η5) and the WC1 antigen (mAb CC39). Results are means of three animals and are expressed as the percentage of the total WC1+ population
| Cytokine combination | Percentage of WC1+ cells that are IFN-γ+ |
|---|---|
| IL-12 + IL-18 + IL-15 + IL-2 | 7·9 ± 3·91 |
| IL-12 + IL-18 + IL-15 | 6·2 ± 2·61 |
| IL-12 + IL-18 | 3·9 ± 2·21 |
| IL-12 + IL-15 | 2·7 ± 1·01 |
| IL-12 + IL-2 | 1·2 ± 0·41 |
| IL-2 + IL-15 | 0·9 ± 0·21 |
| IL-18 + IL-15 | 0·4 ± 0·3 |
| IL-18 + IL-2 | 0·3 ± 0·1 |
| IL-12 | 1·3 ± 0·31 |
| IL-18 | 0·2 ± 0·1 |
| IL-2 | 0·8 ± 0·3 |
| IL-15 | 0·2 ± 0·2 |
| No cytokine added | 0·3 ± 0·3 |
Differs significantly (P ≤ 0·05) from medium control.
Figure 3.
Proliferation of workshop cluster 1+ (WC1+) γδ T cells in response to cytokine stimulation. Peripheral blood mononuclear cells (PBMCs) were isolated and labelled with carboxy-fluorescein diacetatesuccinimidyl ester (CFDA-SE) according to the kit instructions. The cells were cultured in the same way as for Figure 1 with different combinations of interleukin (IL)-2, IL-15, IL-18 and IL-12 added, and were then fixed in 1% paraformaldehyde, permeabilized and stained for intracellular interferon (IFN)-γ (CC302-PE conjugate) and the WC1 antigen (CC39-Alexa Fluor 647 conjugate). Cytokine combinations used were (a) medium; (b) IL-2, IL-12, IL-15, IL-18; (c) IL-12, IL-15, IL-18, and (d) IL-12, IL-18. The PBMCs were gated on WC1+ cells. The quadrants were set according to the medium control staining. (e) Three populations of CFDA-SE-labelled WC1+ cells were identified by applying markers to a histogram of the WC1+ cells shown in (b).
Table 2.
Expression of interferon (IFN)-γ by dividing workshop cluster 1+ (WC1+) cells, showing the percentage of the total WC1+ population in each division based on the markers (M) set in Fig. 3(e), and the percentage of WC1+ cells secreting IFN-γ within each population
| Cytokine | Division | % WC1+ cells | % IFN-γ+ |
|---|---|---|---|
| IL-2, 12, 15, 18 | Undivided | 86·8 | 7·7 |
| 1 | 8·1 | 44·4 | |
| 2 | 5·1 | 21·9 |
| IL-12, 15, 18 | Undivided | 89·7 | 5·8 |
| 1 | 6·9 | 43·9 | |
| 2 | 3·5 | 21·4 |
| IL-12, 18 | Undivided | 86·7 | 3·8 |
| 1 | 8·8 | 21·5 | |
| 2 | 4·4 | 9·4 |
| Medium | Undivided | 95·8 | 0 |
| 1 | 4·0 | 0 | |
| 2 | 0·1 | 0 |
IFN-γ production by WC1+ cells from cattle of differing ages
To assess whether the capacity of WC1+ cells to secrete IFN-γ is related to age or maturation of the immune system, PBMCs from six calves and six adult animals were stimulated by IL-12 and IL-18 as above and IFN-γ expression by WC1+ cells was assessed (Table 3). Young calves had up to 33-fold more WC1+ IFN-γ+ cells, expressed as a percentage of the total WC1+ cells, in response to IL-12 and IL-18 compared with the adults (Table 3; P = 0·00015). There were also significantly (P = 0·00046) more circulating WC1+ cells present in the calves than in the adult animals (means 57·1% and 20·3%, respectively; data not shown).
Table 3.
Interferon (IFN)-γ production by workshop cluster 1+ (WC1+) γδ T cells in young and adult animals. Peripheral blood mononuclear cells (PBMCs) from calves and adult animals were cultured for 46 hr in the presence of interleukin (IL)-12 and IL-18 at optimized dilutions, with Brefeldin A added for the final 16 hr. Cells were fixed in paraformaldehyde, permeabilized and stained for intracellular IFN-γ (monoclonal antibody, mAb, 6Η5) and WC1 antigen (mAb CC39)
| Calf no. | % WC1+/IFN-γ+ | Adult no. | % WC1+/IFN-γ+ |
|---|---|---|---|
| 103 | 24·3 | 320 | 0·7 |
| 106 | 16·0 | 349 | 0·5 |
| 108 | 11·4 | 172 | 3·3 |
| 128 | 16·8 | 775 | 3·8 |
| 135 | 27·3 | 009 | 1·3 |
| 134 | 11·5 | 010 | 0·6 |
| Mean/SD | 17·9 ± 6·61 | 1·7 ± 1·4 |
P = 0·00015.
WC1+ cells from lymph nodes express IFN-γ in response to IL-12 and IL-18 stimulation
We have previously shown that WC1+ cells from different anatomical compartments (blood and lymph nodes) respond differently to stimulation by mitogen.27 We therefore compared WC1+ cells from blood with those present in PSLN from animals aged 6−12 months (Table 4). WC1+ cells from the PSLN responded to IL-12 and IL-18 by producing IFN-γ to a greater extent than blood-derived WC1+ cells, although the difference between the two anatomical populations was not significant (P = 0·06) due to large animal-to-animal variation. However, in individual animals, the lymph node-derived cells were consistently more responsive than the blood-derived cells. These differences may reflect the presence of distinct functional subsets of WC1+γδ T cells in different anatomical areas.
Table 4.
Interferon (IFN)-γ production by blood and lymph node-derived workshop cluster 1+ (WC1+) γδ T cells. WC1+γδ T cells from peripheral blood mononuclear cells (PBMCs) and prescapular lymph nodes (PSLNs) in 1-month-old animals were cultured for 46 hr in the presence of interleukin (IL)-12 and IL-18 at optimized dilutions, with Brefeldin A added for the final 16 hr. Cells were fixed in paraformaldehyde, permeabilized and stained for intracellular IFN-γ (monoclonal antibody, mAb, 6Η5) and WC1 antigen (mAb CC39)
| Animal | % WC1+/IFN-γ+ (PSLN) | % WC1+/IFN-γ+ (PBMC) |
|---|---|---|
| 476 | 4·1 | 0·5 |
| 918 | 1·6 | 0·4 |
| 919 | 2·5 | 1·6 |
| 921 | 2·1 | 0·3 |
| 915 | 13·6 | 2·2 |
| 917 | 5·7 | 1·3 |
| Mean/SD | 4·9 ± 4·5 | 1·0 ± 0·7 |
P = 0·06.
IFN-γ production by purified populations of WC1+ cells
Each of the experiments described above utilized mixed populations of PBMCs in which bystander effects could influence the WC1+ cell response. To define the effect of cytokines more precisely, the WC1+ cells were highly purified (> 98%) and stimulated by IL-12 and IL-18. Purified WC1+ cells were assessed for both intracellular IFN-γ by flow cytometry (Fig. 4a), and secretion of IFN-γ by ELISA (Fig. 4b). In populations of purified WC1+ cells, intracellular IFN-γ expression was detected (Fig. 4a) and the cells appeared to respond equally as well as observed in whole PBMC cultures. High concentrations of secreted IFN-γ were detected by ELISA following culture of WC1+ cells with IL-12 and IL-18 (Fig. 4b).
Figure 4.
Production of interferon (IFN)-γ by purified workshop cluster 1+ (WC1+) γδ T cells. (a) Intracellular IFN-γ expression was measured using purified WC1+ cells. WC1+ cells were cultured and stained as in Figure 1. One representative dot plot is shown. (b) WC1+γδ T cells were stimulated with interleukin (IL)-12 and IL-18 or medium and cultured for 46 hr. IFN-γ production was measured by enzyme-linked immunosorbent assay. Stimulations were set up as four biological repeats per animal per condition and each was assayed in triplicate. Mean values ± standard error are indicated.
Discussion
Early production of IFN-γ by γδ T cells may have a role in linking innate and adaptive immune responses. These cells are influenced by a number of costimulatory molecules including cytokines such as IL-12 and IL-18, which are also implicated as important mediators of innate immune mechanisms. We report here that bovine WC1+γδ T cells produce high levels of IFN-γ in response to IL-12 and IL-18. These cytokines acted synergistically to induce IFN-γ expression in a proportion of WC1+ cells. Roles for IL-2 and IL-15 in this response were also implicated, although these cytokines induced only low levels of IFN-γ expression by the WC1+ cells in the absence of IL-12/IL-18. Stimulation through IL-2R by IL-2 or IL-15 may be more important for inducing proliferative responses of WC1+ cells than for the induction of IFN-γ by these cells. By labelling cells with CFDA-SE and assessing cell division following stimulation with cytokine combinations it was evident that IL-2 and IL-15 induced IFN-γ production in higher numbers of WC1+ cells overall. It is likely that IL-2 and IL-15 become more important during the transition from an early innate response to an adaptive response, involving CD4 and CD8 T cells, as they clearly boost the production of IFN-γ by T cells. However, IL-12 and IL-18 may be important in the production of IFN-γ in the early stages of an immune response, prior to the significant expansion of the WC1+ population.
The response to IL-12 and IL-18 was observed in WC1+ cells isolated from animals of different ages. However, in young calves a significantly higher percentage of the WC1+ cells expressed IFN-γ. Previously, Baldwin et al.7 showed that γδ T cells isolated from calves immediately after birth were unresponsive to IL-12. This implies that IL-18 is a critical factor in the response of these cells. It appeared from this study that by limiting concentrations of IL-18, the ability of IL-12 to induce high levels of IFN-γ was reduced (data not shown).
The enhanced responsiveness of WC1+ cells to IL-12 and IL-18 in young calves, along with their increased number compared with adults, suggests that this is an important element of innate immunity that may be particularly relevant in immunologically naive, neonatal animals. Herein we assessed WC1+ responses in animals that were very different in terms of age. As studies have demonstrated that neonatal calves respond more effectively to BCG vaccination,34,35 it is potentially important to determine at what age the immune responses demonstrated in this study wane, as this enhanced response could contribute significantly to the efficacy of vaccination strategies in neonatal animals. Whether or not this response is affected by changes in immune status following environmental exposure, infection or vaccination is also of interest. Preliminary data from this laboratory indicated that the response of young calves had decreased significantly by 6–8 weeks of age and that, whereas BCG vaccination induced a transient boost in WC1+ responsiveness to IL-12 and IL-18, this was not maintained for more than 2 weeks. However, these responses need to be confirmed in larger groups of animals and additional functions of WC1+ cells also need to be assessed in parallel.
Previous data from this laboratory showed that WC1+ cells from different anatomical compartments showed differential IFN-γ responses following stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin.27 Thus, WC1+ cells from lymph nodes but not blood expressed IFN-γ in response to mitogen stimulation. This observation suggested that different, possibly activated, subsets of WC1+ cells are resident in lymph nodes and that the circulating WC1+ cells are resting or naive cells. In accord with this, IFN-γ transcripts were not detected in resting WC1+ cells isolated from bovine PBMCs.12 In adult animals assessed in the studies described herein, lymph node WC1+ cells were more responsive to IL-12 and IL-18 stimulation than were peripheral blood WC1+ cells from the same animal. However, we have shown here that WC1+ cells present in peripheral blood can be stimulated to produce high levels of IFN-γ by culture with IL-12 and IL-18, but that this response is associated with age. This suggests that production of IFN-γ by peripheral WC1+ cells is not only dependent upon appropriate stimulation. It seems likely that with increasing age and exposure to increasing numbers of antigens present in the environment, there are changes in immune status that could stimulate changes in responsiveness and changes in distribution of WC1+ cells, with recruitment of subsets of WC1+ cells from blood to lymphoid organs.
It is clear that not all the WC1+ cells expressed IFN-γ. In most of the young calves, approximately 5% (up to 10% in some animals) of these cells expressed high levels of IFN-γ as determined by flow cytometry and showed high secretion levels assessed by ELISA. This may reflect the existence of distinct subsets of WC1+ cells within the peripheral pool or the presence of subsets of WC1+ cells that have divergent roles in the immune response. It has been reported that at least two isoforms of WC1 exist, which identify largely non-overlapping subpopulations of γδ T cells, with differing functional properties. The WC1·1+ population, the major contributor to IFN-γ production in response to antigenic stimulation, is seen to decline from the periphery with increasing age. A comparison of 4-week-old calves with 4-month-old calves showed that there were significantly (P = 0·003) more WC1·2+γδ T cells in the peripheral blood of calves of both age groups compared with WC1·1+ cells. Significantly more WC1·1 expressing cells were induced to produce IFN-γ by IL-12 and IL-18 than WC1·2 positive cells (P = 0·04) within an individual (data not shown). Whether the WC1+ cells responding to IL-12 and IL-18 are a subpopulation of WC1·1+ cells with the capacity to become memory or effector cells that can be further defined on the basis of phenotype or function remains to be determined. We have shown here that the WC1+ populations in the lymph node and peripheral blood differ in their ability to respond to cytokine stimulation, which may reflect functionally distinct populations.
We have recently shown that bovine DCs infected with Mycobacterium bovis and M. bovis BCG can express IL-12 and IL-18 that could influence γδ T cells.36,37 Substantial increases in IL-12 and IL-18 secretion would allow the rapid stimulation of γδ T cells early in the response that might act to enhance Th1 bias and aid early resolution of infection. In cattle, γδ T cells are among the first cells to accumulate at sites of infection38–40 and they may play an important role in the early response to M. bovis through secretion of IFN-γ.41 The capacity for M. bovis-infected DCs to activate γδ T cells and the role of IL-12 and IL-18 is under current investigation.
In summary, we have demonstrated that bovine γδ T cells respond to stimulation with IL-12 and IL-18 by secretion of large quantities of IFN-γ, particularly in neonatal animals. This is likely to be an important element of the initiation of immune responses to pathogens, including M. bovis.
Acknowledgments
We gratefully acknowledge the staff of the animal facilities for care of the cattle. This work was funded by the Biotechnology and Biological Sciences Research Council, UK.
Glossary
Abbreviations:
- BU
biological units
- CFDA-SE
carboxy-fluorescein diacetatesuccinimidyl ester
- PSLN
prescapular lymph node
- rbo
recombinant bovine
- WC
workshop cluster
References
- 1.Skeen MJ, Ziegler HK. Induction of murine peritoneal γ/δ T cells and their role in resistance to bacterial infection. J Exp Med. 1993;178:971–84. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 3.Ladel CH, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann SH. Contribution of α/β and γ/δ T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guerin: studies with T cell receptor-deficient mutant mice. Eur J Immunol. 1995;25:838–46. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 4.Hayday AC. γ/δ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann SH. γ/δand other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–9. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender A, Kabelitz D. CD4− CD8− human T cells: phenotypic heterogeneity and activation requirements of freshly isolated ‘double-negative’ T cells. Cell Immunol. 1990;128:542–54. doi: 10.1016/0008-8749(90)90047-u. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin CL, Sathiyaseelan T, Naiman B, White AM, Brown R, Blumerman S, Rogers A, Black SJ. Activation of bovine peripheral blood γδ T cells for cell division and IFN-γ production. Vet Immunol Immunopathol. 2002;87:251–9. doi: 10.1016/s0165-2427(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 8.Sathiyaseelan T, Rogers A, Baldwin CL. Response of bovine γδ T cells to activation through CD3. Vet Immunol Immunopathol. 2002;90:155–68. doi: 10.1016/s0165-2427(02)00244-1. [DOI] [PubMed] [Google Scholar]
- 9.Fikri Y, Nyabenda J, Denis M, Pastoret PP. Purification and characterization of bovine WC1+γδ T lymphocytes from peripheral blood. Vet Res. 2000;31:229–39. doi: 10.1051/vetres:2000118. [DOI] [PubMed] [Google Scholar]
- 10.Fikri Y, Pastoret PP, Nyabenda J. Costimulatory molecule requirement for bovine WC1+γδ T cells' proliferative response to bacterial superantigens. Scand J Immunol. 2002;55:373–81. doi: 10.1046/j.1365-3083.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- 11.Hanrahan CF, Kimpton WG, Howard CJ, Parsons KR, Brandon MR, Andrews AE, Nash AD. Cellular requirements for the activation and proliferation of ruminant γδ T cells. J Immunol. 1997;159:4287–94. [PubMed] [Google Scholar]
- 12.Collins RA, Sopp P, Gelder KI, Morrison WI, Howard CJ. Bovine γ/δ TCR+ T lymphocytes are stimulated to proliferate by autologous Theileria annulata-infected cells in the presence of interleukin-2. Scand J Immunol. 1996;44:444–52. doi: 10.1046/j.1365-3083.1996.d01-332.x. [DOI] [PubMed] [Google Scholar]
- 13.Davis WC, Brown WC, Hamilton MJ, Wyatt CR, Orden JA, Khalid AM, Naessens J. Analysis of monoclonal antibodies specific for the γδ TCR. Vet Immunol Immunopathol. 1996;52:275–83. doi: 10.1016/0165-2427(96)05578-x. [DOI] [PubMed] [Google Scholar]
- 14.Eberl M, Engel R, Beck E, Jomaa H. Differentiation of human γ−δ T cells towards distinct memory phenotypes. Cell Immunol. 2002;218:1–6. doi: 10.1016/s0008-8749(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 15.Garcia VE, Jullien D, Song M, Uyemura K, Shuai K, Morita CT, Modlin RL. IL-15 enhances the response of human γδ T cells to non-peptide [correction of non-petide] microbial antigens. J Immunol. 1998;160:4322–9. [PubMed] [Google Scholar]
- 16.Ueta C, Kawasumi H, Fujiwara H, Miyagawa T, Kida H, Ohmoto Y, Kishimoto S, Tsuyuguchi I. Interleukin-12 activates human γδ T cells: synergistic effect of tumour necrosis factor-α. Eur J Immunol. 1996;26:3066–73. doi: 10.1002/eji.1830261237. [DOI] [PubMed] [Google Scholar]
- 17.Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood γδ T cells toward Th1- or Th2-phenotype. Cell Immunol. 2001;212:110–7. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- 18.Skeen MJ, Ziegler HK. Activation of γδ T cells for production of IFN-γ is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J Immunol. 1995;154:5832–41. [PubMed] [Google Scholar]
- 19.Wilson RA, Zolnai A, Rudas P, Frenyo LV. T cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves and adult bovine. Vet Immunol Immunopathol. 1996;53:49–60. doi: 10.1016/0165-2427(95)05543-6. [DOI] [PubMed] [Google Scholar]
- 20.Wyatt CR, Madruga C, Cluff C, Parish S, Hamilton MJ, Goff W, Davis WC. Differential distribution of γδ T cell receptor lymphocyte subpopulations in blood and spleen of young and adult cattle. Vet Immunol Immunopathol. 1994;40:187–99. doi: 10.1016/0165-2427(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 21.Mackay CR, Maddox JF, Brandon MR. Three distinct subpopulations of sheep T lymphocytes. Eur J Immunol. 1986;16:19–25. doi: 10.1002/eji.1830160105. [DOI] [PubMed] [Google Scholar]
- 22.Morrison WI, Davis WC. Individual antigens of cattle. Differentiation antigens expressed predominantly on CD4− CD8− T lymphocytes (WC1, WC2) Vet Immunol Immunopathol. 1991;27:71–6. doi: 10.1016/0165-2427(91)90082-n. [DOI] [PubMed] [Google Scholar]
- 23.Clevers H, MacHugh ND, Bensaid A, et al. Identification of a bovine surface antigen uniquely expressed on CD4− CD8− T cell receptor γδ+ T lymphocytes. Eur J Immunol. 1990;20:809–17. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- 24.Kirkham PA, Takamatsu HH, Parkhouse RM. Growth arrest of γδ T cells induced by monoclonal antibody against WC1 correlates with activation of multiple tyrosine phosphatases and dephosphorylation of MAP kinase erk2. Eur J Immunol. 1997;27:717–25. doi: 10.1002/eji.1830270321. [DOI] [PubMed] [Google Scholar]
- 25.Wijngaard PL, Metzelaar MJ, MacHugh ND, Morrison WI, Clevers HC. Molecular characterization of the WC1 antigen expressed specifically on bovine CD4− CD8−γδ T lymphocytes. J Immunol. 1992;149:3273–7. [PubMed] [Google Scholar]
- 26.Brown WC, Davis WC, Choi SH, Dobbelaere DA, Splitter GA. Functional and phenotypic characterization of WC1+γ/δ T cells isolated from Babesia bovis-stimulated T cell lines. Cell Immunol. 1994;153:9–27. doi: 10.1006/cimm.1994.1002. [DOI] [PubMed] [Google Scholar]
- 27.Sopp P, Howard CJ. IFN-γ and IL-4 production by CD4, CD8 and WC1 γδ TCR (+) T cells from cattle lymph nodes and blood. Vet Immunol Immunopathol. 2001;81:85–96. doi: 10.1016/s0165-2427(01)00334-8. [DOI] [PubMed] [Google Scholar]
- 28.Rogers AN, Vanburen DG, Hedblom EE, Tilahun ME, Telfer JC, Baldwin CL. γδ T cell function varies with the expressed WC1 coreceptor. J Immunol. 2005;174:3386–93. doi: 10.4049/jimmunol.174.6.3386. [DOI] [PubMed] [Google Scholar]
- 29.Hope JC, Sopp P, Collins RA, Howard CJ. Differences in the induction of CD8+ T cell responses by subpopulations of dendritic cells from afferent lymph are related to IL-1 α secretion. J Leukoc Biol. 2001;69:271–9. [PubMed] [Google Scholar]
- 30.Werling D, Hope JC, Chaplin P, Collins RA, Taylor G, Howard CJ. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–8. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 31.Howard CJ, Sopp P, Parsons KR, Finch J. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur J Immunol. 1989;19:757–64. doi: 10.1002/eji.1830190428. [DOI] [PubMed] [Google Scholar]
- 32.Weynants V, Gilson D, Furger A, et al. Production and characterization of monoclonal antibodies specific for bovine interleukin-4. Vet Immunol Immunopathol. 1998;66:99–112. doi: 10.1016/s0165-2427(98)00184-6. [DOI] [PubMed] [Google Scholar]
- 33.Collins RA, Camon EB, Chaplin PJ, Howard CJ. Influence of IL-12 on interferon-γ production by bovine leucocyte subsets in response to bovine respiratory syncytial virus. Vet Immunol Immunopathol. 1998;63:69–72. doi: 10.1016/s0165-2427(98)00083-x. [DOI] [PubMed] [Google Scholar]
- 34.Hope JC, Thom ML, Villarreal-Ramos B, Vordermeier HM, Hewinson RG, Howard CJ. Vaccination of neonatal calves with Mycobacterium bovis BCG induces protection against intranasal challenge with virulent M. Bovis. Clin Exp Immunol. 2005;139:48–56. doi: 10.1111/j.1365-2249.2005.02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buddle BM, Wedlock DN, Parlane NA, Corner LA, De Lisle GW, Skinner MA. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect Immun. 2003;71:6411–9. doi: 10.1128/IAI.71.11.6411-6419.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hope JC, Sopp P, Howard CJ. NK-like CD8 (+) cells in immunologically naive neonatal calves that respond to dendritic cells infected with Mycobacterium bovis BCG. J Leukoc Biol. 2002;71:184–94. [PubMed] [Google Scholar]
- 37.Hope JC, Thom ML, McCormick PA, Howard CJ. Interaction of antigen presenting cells with mycobacteria. Vet Immunol Immunopathol. 2004;100:187–95. doi: 10.1016/j.vetimm.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes SG, Hewinson RG, Vordermeier HM. Antigen recognition and immunomodulation by γδ T cells in bovine tuberculosis. J Immunol. 2001;166:5604–10. doi: 10.4049/jimmunol.166.9.5604. [DOI] [PubMed] [Google Scholar]
- 39.Smyth AJ, Welsh MD, Girvin RM, Pollock JM. In vitro responsiveness of γδ T cells from Mycobacterium bovis-infected cattle to mycobacterial antigens. predominant involvement of WC1 (+) cells. Infect Immun. 2001;69:89–96. doi: 10.1128/IAI.69.1.89-96.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollock JM, Welsh MD. The WC1 (+) γδ T cell population in cattle: a possible role in resistance to intracellular infection. Vet Immunol Immunopathol. 2002;89:105–14. doi: 10.1016/s0165-2427(02)00200-3. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy HE, Welsh MD, Bryson DG, Cassidy JP, Forster FI, Howard CJ, Collins RA, Pollock JM. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1 (+) γδ T cells. Infect Immunol. 2002;70:1488–500. doi: 10.1128/IAI.70.3.1488-1500.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-γ in host defence against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–51. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]