Abstract
Natural killer (NK) cells have the ability to control dendritic cell (DC)-mediated T cell responses. However, the precise mechanisms by which NK receptor-mediated regulation of NK cells determines the magnitude and direction of DC-mediated T cell responses remain unclear. In the present study, we applied an in vitro co-culture system to examine the impact of NK cells cultured with hepatic cells on DC induction of regulatory T cells. We found that interaction of NK cells and non-transformed hepatocytes (which express HLA-E) via the NKG2A inhibitory receptor resulted in priming of DCs to induce CD4+ CD25+ T cells with regulatory properties. NKG2A triggering led to characteristic changes of the cytokine milieu of co-cultured cells; an increase in the transforming growth factor (TGF)-β involved in the generation of this specific type of DC, and a decrease in the tumour necrosis factor-α capable of antagonizing the effect of TGF-β. The regulatory cells induced by NK cell-primed DCs exert their suppressive actions through a negative costimulator programmed death-1 (PD-1) mediated pathway, which differs from freshly isolated CD4+ CD25+ T cells. These findings provide new insight into the role of NK receptor signals in the DC-mediated induction of regulatory T cells.
Keywords: NK receptor, regulatory T cell, HLA-E, liver, HCV
Introduction
CD4+ CD25+ regulatory T (Treg) cells have been identified as the main suppressors of immune responses.1–5 Although the mechanisms by which CD4+ CD25+ Treg cells exert their suppressive actions have not been fully elucidated, negative costimulatory signals via cytotoxic T lymphocyte antigen-4 (CTLA-4) or inducible costimulator (ICOS)-mediated signals, have been suggested to play a key role in the activation of CD4+ CD25+ Treg cells.6,7 Programmed death-1 (PD-1), another molecule identified as a negative costimulatory receptor, has also serves as a negative regulator for effector immune responses.8 Recent reports have demonstrated that PD-1 is expressed in CD4+ CD25+ Treg cells, suggesting its potential roles in the regulation of T cell tolerance.9 However, the precise roles of PD-1 in CD4+ CD25+ Treg cell functions remain elusive.
The mechanisms by which CD4+ CD25+ Treg cells are generated have been extensively investigated. Dendritic cells (DCs), the sentinels between innate and adaptive immunity, have recently emerged as candidate cells involved in the differentiation and/or activation of CD4+ CD25+ Treg cells.10 Various kinds of factors have been identified as involved in DC induction of CD4+ CD25+ Treg cells. Mouse immature DC promotes the differentiation of CD4+ CD25+ Treg cells through the DEC 205-mediated targeting of self-antigen in the steady state.10,11 The immune regulatory cytokines interleukin (IL)-10/transforming growth factor (TGF)-β have also been reported to play important roles in DC generation and activation of CD4+ CD25+ Treg cells.12–14
Several lines of evidence have revealed that natural killer (NK) cell-mediated innate immunity regulates DC functions to determine the direction and magnitude of adaptive T cell immunity.15–18 It has also been established that NK cell function is regulated by positive and negative signals through their receptor and ligand interactions.19 We previously reported that, upon exposure to non-transformed hepatocytes (NHs), IL-2-primed NK cells negatively regulated DC functions, which appeared to be dependent on NKG2A inhibitory signals during co-culture of NK cells and NHs. Immunosuppressive cytokines such as IL-10 and TGF-β, but not direct NK−DC contact, were responsible for this action.20 However, it remains unclear whether these NK/hepatocyte co-cultures can also influence the induction as well as activation of CD4+ CD25+ Treg cells.
In the present study, we investigated whether DCs stimulated with the co-culture supernatant of IL-2-prestimulated NK cells and NHs can modulate Treg cell functions. We found that TGF-β produced from NK cell/hepatocyte co-culture via NKG2A activation is responsible for modulating DCs to induce and maintain regulatory phenotypes and functions of CD4+ CD25+ Treg cells. Furthermore, the generated CD4+ CD25+ Treg cells suppressed T cell activation via interaction between PD-1 and programmed death ligand 1 (PDL-1). These findings represent new evidence that NK receptor-mediated modulation of NK cells may dictate DC-induced adaptive immunity toward an immunogenic or tolerogenic status via induction of Treg cells.
Materials and methods
Antibodies
Anti-NKG2A monoclonal antibody (mAb) (Z199), PC5-labelled CD25 mAb or isotype-matched control IgG1 and IgG2a mAb were purchased from Beckmann-Coulter (Fullerton, CA). Anti-IL-10, anti-TGF-β, anti-CTLA-4, anti-GITR (glucocorticoid-induced TNF receptor) and anti-PD-1 polyclonal Abs were purchased from R & D Systems (Minneapolis, MN) and phycoerythrin (PE)-labelled mAb CTLA-4 from BD Biosciences (San Jose, CA). Anti-HLA-E mAb 3D12 was kindly provided by Dr E. Geraghty (Fred Hutchinson Cancer Research Institute, Seattle, WA) and used as reported previously.21 Anti-MIC mAb 6D4, anti-ULBP1 mAb 3F1 and anti-ULBP2 mAb DH1 were kindly provided by Drs T. Spies and V. Groh (Fred Hutchinson Cancer Research Institute) and used as reported previously.22
Human hepatic cells
Human non-transformed hepatocytes (NHs) derived from mixed heterogeneous donors were purchased from the Applied Cell Biology Research Institute (Kirkland, WA) and cultured in CS-C complete medium according to the manufacturer's instructions.
Isolation of peripheral blood lymphocyte populations
Resting NK cells (CD56+ CD3+), naive CD4+ T cells (CD45RA+ RO+) or CD8+ T cells were isolated from peripheral blood mononuclear cells (PBMCs) with a positive cell isolation kit according to the manufacturer's protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ CD25+ T cells were further separated from naive CD4+ T cells using anti-CD25 microbeads (Miltenyi Biotech). Their purity was > 90% by flow cytometry. Informed consent was obtained from all blood donors.
Generation of monocyte-derived DC
Monocytes were isolated by plastic adherence from PBMCs and cultured in RPMI-1640 supplemented with granulocyte−macrophage colony stimulating factor (GM-CSF) (PeproTech, London, UK) and IL-4 (PeproTech). At day 6, they were stimulated with or without the co-culture supernatant of NK cells and hepatic cells. At day 7, non-adherent cells were harvested and used as described below.
Stimulation of DCs by co-culture supernatants of NK cells and hepatic cells
Freshly isolated NK cells were cultured with or without IL-2 for 24 hr. IL-2-prestimulated or non-stimulated NK cells were seeded in 24-well plates and then co-cultured for 24 hr with NHs (1 × 105 cells/well), respectively. Monocyte-derived DCs were cultured for 24 hr with 1 ml of the co-culture supernatant of IL-2-prestimulated NK cells and NHs (NH/IL-2 NK-primed DC). In some experiments, anti-NKG2A mAb (Z199) or isotype-matched control Ab was added during the co-cultures of NK cells and hepatic cells. Z199 mAb was previously confirmed to block the NKG2A-mediated signal.23 In some experiments, the supernatant of NK/hepatic cell co-cultures was also treated with anti-IL-10 or anti-TGF-β neutralizing Ab and used for DC stimulation for 24 hr. In some experiments, tumour necrosis factor (TNF)-α, TGF-β or both were used for DC stimulation for 24 hr.
Isolation of CD4+ CD25+ T cells
DCs (1 × 105) were stimulated for 24 hr with the supernatant obtained from the co-cultured medium. After washing three times, DCs were cultured with allogeneic CD4+ T cells for 48 hr; CD4+ CD25+ fractions were isolated from DC and CD4+ co-culture and subjected to further analysis. CD4+ CD25+ fractions were also isolated from PBMCs and cultured with 1 µg/ml plate-bound anti-CD3 mAb (UCHT1; Beckmann-Coulter) for 24 hr to efficiently induce their suppressive properties as described previously.3 These cells are referred to as natural CD4+ CD25+ T cells.
Flow cytometry
The expression of NK inhibitory ligands (human leucocyte antigen, HLA, class I, HLA-E) was examined on NHs by using w6/32 or 3D12, respectively. MIC, ULBP1 or ULBP2 expression on hepatocytes was also evaluated by mAb 6D4, 3F1 or DH1, respectively. For CD4+ CD25+ T cell staining, the cells were costained with PC5-labelled CD25 mAb with PE-labelled mAb of CTLA-4, GITR or PD-1 polyclonal Ab. The cells were analysed by flow cytometry using a fluorescence-activated cell sorter (FACScan) system, and data analysis was performed using cellquest software.
Measurements of cytokine production in culture supernatant
The culture supernatants of interferon (IFN)-γ, TNF-α, IL-10 and TGF-β were examined using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers' instructions (IFN-γ, TNF-α and IL-10, Endogen, Tokyo, Japan; TGF-β, R & D Systems).
Analysis of Foxp3 mRNA expression
Polymerase chain reaction (PCR) analysis was performed to determine Foxp3 mRNA expression of CD4+ T cells using a commercial PCR panel according to the manufacturer's instructions (Gibco BRL, Rockville, MD). The following primers were used: 5′-CCCACTTACAGGCACTCCTC-3′ (forward) and 5′-CTTCTCCTTCTCCAGCACCA-3′ (reverse).24 Amplification was carried out for 35 cycles of 20 seconds at 95°, 20 seconds at 58 ° and 30 seconds at 72°. As a control for the integrity of mRNA, primers specific for GAPDH (glyceraldehyde 3-phosphate dehydrogenase) were used as follows: 5′-GCCACCCAGAAGACTGTGGATGGC-3′ (forward) and 5′-CATGTAGGCCATGAGGTCCACCAC-3′ (reverse). The PCR products were analysed by ethidium bromide-stained 1·5% agarose gel electrophoresis.
Analysis of CD4+ CD25+ T cell suppressor functions
DCs (5 × 104/well) were cultured with allogeneic CD4+ T cells (5 × 105/well) for 48 hr, after which CD4+ CD25+ T cells were isolated from the co-cultured cells. CD4+ CD25– T cells were freshly isolated from the same donors and activated with 1 µg/ml plate-bound anti-CD3 mAb in the presence or absence of autologous CD4+ CD25+ T cells for 48 hr. The ability of CD4+ CD25+ T cells to suppress proliferation and IFN-γ production of activated CD4+ CD25– T cells was determined by [3H]thymidine incorporation and ELISA assay, respectively. To further examine the mechanisms of CD4+ CD25+ T cell suppressive actions, neutralizing Ab of IL-10 or TGF-β, anti-CTLA-4, anti-GITR or anti-PD-1 was added at the beginning of CD4+ CD25+ T cell and CD4+ CD25– T cell co-cultures.
Statistical analysis
Comparisons between groups were analysed by t-test with Welch's correction or anova for experiments with more than two subgroups. Differences were considered significant when the P-value was < 0·05.
Results
IL-2-primed NK cells upon exposure to NH-modulated DCs on the induction of regulatory CD4+ CD25+ T cells
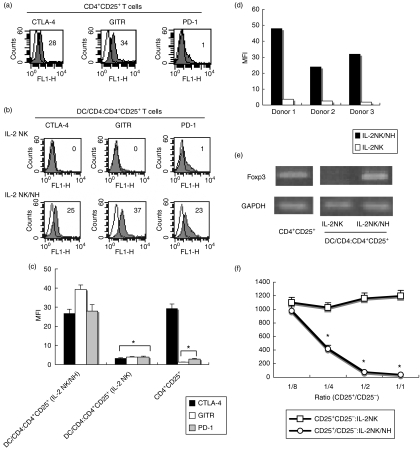
Natural CD4+ CD25+ T cells from human peripheral blood lymphocytes (PBLs) expressed CTLA-4 and GITR, both of which have been identified as regulatory markers,6,25 but did not express PD-1 (Fig. 1a). To examine whether DCs can modulate the expression of these regulatory markers on CD4+ CD25+ T cells, we stimulated monocyte-derived DCs for 24 hr, either by the culture supernatant of IL-2-stimulated NK cells (IL-2 NK) or by the co-culture supernatant of NH/IL-2 NK. After washing, the resulting DCs were cultured for 48 hr with CD4+ T cells isolated from allogeneic donors. CD4+ CD25+ T cells were isolated from the DC and CD4+ T cell co-culture and subjected to analysis for regulatory markers. The expression levels of CTLA-4 and GITR decreased on CD4+ CD25+ T cells after stimulation of IL-2 NK-primed DCs (Fig. 1b). By contrast, CD4+ CD25+ T cells stimulated with NH/IL-2 NK-primed DCs remained positive for CTLA-4 and GITR on their surface. Of note is the finding that PD-1 was induced on these cells, showing their phenotypic properties to differ from natural CD4+ CD25+ T cells (Fig. 1b, c). The induction of PD-1 on CD4+ CD25+ T cells was further confirmed when IL-2NK/NH-primed DCs from different donors were used as stimulators (Fig. 1d). The supernatant of NH without NK cells had little effect on phenotypic changes of CD4+ CD25+ T cells by DCs (data not shown).
Figure 1.
Human non-transformed hepatocyte (NH) modulation of activated natural killer (NK) cells endows dendritic cells (DCs) with the ability to induce CD4+ CD25+ regulatory T cells. (a) Freshly isolated CD4+ CD25+ T cells were cultured in the presence of plate-bound anti-CD3 antibody (Ab) for 24 hr, and then subjected to flow cytometry to examine their expression of cytotoxic T lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced TNF receptor (GITR) and programmed death-1 (PD-1) (closed histograms). Open histograms represent the staining of control Ab. Numbers on the upper right indicate the mean fluorescence intensity (MFI) of each type of stained cells. (b) NK cells were preactivated with 50 ng/ml interleukin (IL)-2, and co-cultured in the absence (IL-2 NK) or presence (IL-2 NK/NH) of NHs at a ratio of 1 : 1 for 24 hr. DCs (1 × 105) were stimulated for 24 hr with the supernatant obtained from the co-cultured medium. After washing three times, DCs were cultured with allogeneic CD4+ T cells for 48 hr. CD4+ CD25+ fractions were isolated from the DC/CD4+ T co-culture and subjected to flow cytometry for expression of CTLA-4, GITR or PD-1 (closed histograms). Open histograms show isotype control staining. Numbers on the upper right indicate the MFI of each type of stained cell. (c) All experiments in (a) and (b) were performed three times and the composite results with statistical analysis are shown as the MFI of the staining cells. *P < 0·05 vs. responses of IL-2 NK/NH group. The experiment was performed with a different set of donors and similar results were obtained. (d) PD-1 expression on CD4+ CD25+ T cells stimulated with allogeneic DCs from three different donors, shown as the MFI.(e)CD4+ CD25+ T cells were prepared as described above. The mRNA expression of Foxp3 and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was examined by reverse transcription-polymerase chain reaction (RT-PCR). (f) CD4+ CD25+ fractions were isolated from DC/CD4+ T cell co-cultures. Different numbers of these CD4+ CD25+ T cells were co-cultured with freshly isolated autologous CD4+ CD25– T cells (1 × 105/well) in the presence of plate-bound anti-CD3 Ab (CD4+ CD25+/CD4+ CD25–). The anti-CD3 Ab-activated CD4+ CD25– T cells alone were used as a positive control (CD4+ CD25–). IFN-γ was measured for each supernatant obtained after 48 hr of co-culture by enzyme-linked immunosorbent assay. *P < 0·05.
The forkhead transcription factor Foxp3 has been recently identified as a master gene for defining Treg cells.26 We therefore performed reverse transcription-PCR (RT-PCR) analysis of CD4+ T cells to evaluate the mRNA expression of Foxp3. Foxp3 expression was detected in natural CD4+ CD25+ T cells. When CD4+ T cells were stimulated with IL-2 NK-primed DCs for 24 hr, Foxp3 was not expressed on CD4+ CD25+ T cells. By contrast, they dominantly transcribed Foxp3 at levels comparable with those of natural CD4+ CD25+ T cells when stimulated with NH/IL-2 NK-primed DCs (Fig. 1e). Taken together, CD4+ CD25+ T cells, when stimulated by NH/IL-2 NK-primed DCs, maintained regulatory phenotypes such as CTLA-4, GITR and Foxp3, and properties distinct from those of natural CD4+ CD25+ Treg cells in terms of PD-1 expression.
CD4+ CD25+ T cells on stimulation of NH/IL-2 NK-primed DC suppressed effector cell functions
We next analysed the functions of CD4+ CD25+ T cells stimulated by NH/IL-2 NK-primed DC. CD4+ CD25+ T cells were co-cultured for 72 hr with CD4+ CD25– T cells freshly isolated from the same donors. During the co-cultures, CD4+ CD25– T cells were stimulated with plate-bound anti-CD3 Ab. The CD4+ CD25+ T cells induced by NH/IL-2 NK-primed DCs dose-dependently suppressed the proliferation of co-cultured cells, whereas those induced by IL-2 NK-primed DC did not (data not shown). CD4+ CD25+ T cells induced by NH/IL-2 NK-primed DCs also dose-dependently inhibited IFN-γ production of the co-cultured cells, by contrast with those induced by IL-2 NK-primed DCs (Fig. 1f). The suppressive activities of these CD4+ CD25+ Treg cells were similar to those of natural CD4+ CD25+ Treg cells (data not shown). These results demonstrate that CD4+ CD25+ T cells induced by NH/IL-2 NK-primed DCs exert suppressive actions to effector cell functions, consistent with their expression of regulatory markers. Taken together, these results indicated that NK cell modulation of DCs leads to the CD4+ CD25+ Treg cell-mediated suppression of effector cell responses when NK cells encounter hepatocytes.
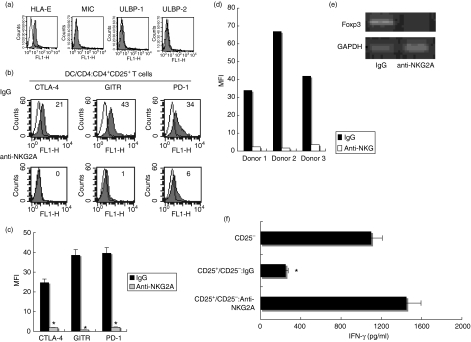
NKG2A signal of NK cells is responsible for the modulation of DCs to activate CD4+ CD25+ Treg cells
We examined the expression of various ligands for NK cell receptors on NHs. NHs expressed HLA-E, the ligand of NKG2A, but did not express NKG2D receptor ligands, MIC and ULBP1-2 (Fig. 2a). Given our previous findings that NHs negatively regulated IL-2 NK-mediated modulation of DC functions through the interaction of the NKG2A inhibitory receptor and its ligand HLA-E,20 we evaluated the role of these receptor signals in the induction of CD4+ CD25+ Treg cells by DCs. When anti-NKG2A Ab was added during the co-culture of NH and IL-2 NK and DCs were stimulated with the resultant supernatant, the expression of CTLA-4, GITR and PD-1 was diminished on CD4+ CD25+ T cells (Fig. 2b, c). NKG2A blockade also suppressed PD-1 expression on CD4+ CD25+ T cells stimulated with IL-2NK/NH-primed DCs from three different donors (Fig. 2d). The anti-NKG2A neutralizing Ab treatment also abrogated Foxp3 expression in CD4+ CD25+ Treg cells (Fig. 2e). Moreover, the blockade of NKG2A signals during NH and IL-2 NK co-cultures resulted in inhibition of the DC ability to induce CD4+ CD25+ T cells with regulatory functions; these CD4+ CD25+ T cells did not suppress proliferation or IFN-γ production (Fig. 2f and data not shown) of CD4+ CD25– T cells. Altogether, the activation of NKG2A inhibitory signals during NK cell and hepatocyte interaction was required for the DC induction of CD4+ CD25+ T cells with regulatory phenotypes and functions.
Figure 2.
NKG2A signals of natural killer (NK) cells are required for the dendritic cell (DC) induction of CD4+ CD25+ T cells with the regulatory phenotype. (a) Surface expression of the ligands of NKG2A (HLA-E) as well as NKG2D (MIC, ULBP1 and ULBP2) in human non-transformed hepatocytes (NHs) were assessed by flow cytometry (closed histograms). Open histograms show isotype control staining. (b, c) Interleukin (IL)-2-preactivated NK cells were co-cultured with NHs in the presence of 30 µg/ml of anti-NKG2A neutralizing antibody (Ab) (anti-NKG2A) or control IgG. DCs (1 × 105) were then stimulated with the supernatant obtained from the co-cultured medium for 24 hr. After washing three times, DCs were cultured with allogeneic CD4+ T cells for 48 hr. CD4+ CD25+ cells isolated from the co-culture were subjected to FCM for their surface expression of cytotoxic T lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced TNF receptor (GITR) and programmed death-1 (PD-1) (closed histograms). Open histograms show isotype control staining. Numbers on the upper right indicate the mean fluorescence intensity (MFI) of each type of stained cell. All experiments were performed three times. Representative data (b) and composite results with statistical analysis (c) are shown as the MFI of the staining cells. *P < 0·05 vs. responses of IgG group. The experiment was performed in different set of donors and similar results were obtained. (d) The inhibitory effect of anti-NKG2A Ab on PD-1 expression of CD4+ CD25+ T cells stimulated with allogeneic DCs from three different donors. Data are shown as MFI.(e)CD4+ CD25+ T cells were stimulated and purified as described above. The mRNA expression of Foxp3 and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) was examined by reverse transcription-polymerase chain reaction (RT-PCR. (f) CD4+ CD25+ T cells (1 × 105/well) isolated from DC and CD4+ T cell co-cultures were cultured with freshly isolated autologous CD4+ CD25– T cells at a ratio of 1 : 1 in the presence of plate-bound anti-CD3 Ab (CD25+/CD25–). The anti-CD3 Ab-activated CD4+ CD25– T cells alone were used as a positive control (CD25–). Interferon (IFN)-γ was measured for each supernatant obtained after 48 hr of co-culture by enzyme-linked immunosorbent assay (ELISA). *P < 0·05. All experiments were performed three times; representative results are shown.
Change of cytokine milieu, triggered by NKG2A signals, plays a critical role in DC-mediated induction of CD4+ CD25+ Treg cells
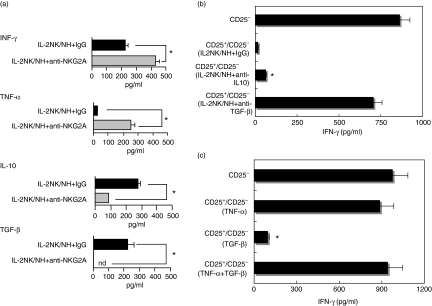
TNF-α has been well known as a critical factor for NK cell-mediated maturation of DCs.27 By contrast, IL-10 and TGF-β are known to act as suppressive factors of effector immune responses, and their roles in modulating DCs for Treg cell induction has recently been validated.12–14 These findings led us to evaluate the change in cytokine production patterns in NH and IL-2 NK co-cultures in the presence or absence of anti-NKG2A Ab. ELISA data showed that the production of IFN-γ and TNF-α from NH and IL-2 NK co-cultures were substantially increased in the presence of anti-NKG2A Ab. By contrast, the addition of NKG2A masking Ab during the co-culture resulted in the marked reduction of IL-10 and TGF-β from co-cultured cells (Fig. 3a).
Figure 3.
Change of cytokine production pattern of natural killer (NK) cells through NKG2A signals is responsible for the dendritic cell (DC) induction of CD4+ CD25+ Treg cells. (a) NK cells prestimulated with interleukin (IL)-2 were cultured with human non-transformed hepatocytes (NHs) in the presence of masking antibodies (Abs) of NKG2A (IL-2 NK/NH + anti-NKG2A) or isotype control IgG (IL-2 NK/NH + IgG) for 24 hr. *P < 0·05. (b) IL-2 activated NK cells were co-cultured with NHs (IL-2 NK/NH). DCs (1 × 105) were stimulated with the culture supernatant in the presence of anti-IL-10, anti-transforming growth factor (TGF)-β neutralizing Ab or control IgG for 24 hr. DCs were washed thoroughly and co-cultured with allogeneic CD4+ T cells for 48 hr. Next, the isolated CD4+ CD25+ T cells (1 × 105/well) were co-cultured with autologous CD4+ CD25– T cells in the presence of plate-bound anti-CD3 Ab at a ratio of 1 : 1. Interferon (IFN)-γ production from the culture supernatant was examined by enzyme-linked immunosorbent assay. *P < 0·05 vs. responses of anti-CD3 Ab-stimulated CD4+ CD25– T cells. (c) DCs (1 × 105) were stimulated with 50 ng/ml TNF-α, 100 ng/ml TGF-β or both for 24 hr. After thorough washing, they were co-cultured with allogeneic CD4+ T cells for 48 hr. CD4+ CD25+ T cells (1 × 105/well) were isolated from the DC and CD4+ co-cultures and cultured with freshly isolated autologous CD4+ CD25– T cells at a ratio of 1 : 1 in the presence of plate-bound anti-CD3 Ab. IFN-γ production was examined as described above. *P < 0·05 vs. responses of anti-CD3 Ab-stimulated CD4+ CD25– T cells.
We next examined whether these changes of cytokine profiles were responsible for the DC induction of the CD4+ CD25+ Treg cells. For this purpose, the NH and IL-2 NK co-culture supernatant was treated with neutralizing Ab of IL-10 or TGF-β before DC stimulation, and suppressive activity was evaluated by analysing CD4+ CD25+ T cells obtained from CD4+ and DC mixtures. The neutralization of IL-10 did not reverse the suppressive actions of CD4+ CD25+ Treg cells, but the blockade of TGF-β led to reversal of CD4+ CD25+ Treg cell activities (Fig. 3b).
We directly examined the effect of TGF-β on the modulation of DC ability to induce CD4+ CD25+ Treg cells. TGF-β endowed DCs with the ability to induce CD4+ CD25+ Treg cells. TNF-α inhibited TGF-β-mediated DC induction of CD4+ CD25+ Treg cells (Fig. 3c). By contrast, IFN-γ had little effect on the modulation of DC by TGF-β (data not shown). Taken together, these results strongly suggest that increased TGF-β and decreased TNF-α production, the change of cytokine profiles mediated by the NKG2A signals, are involved in DC-mediated CD4+ CD25+ Treg cell induction.
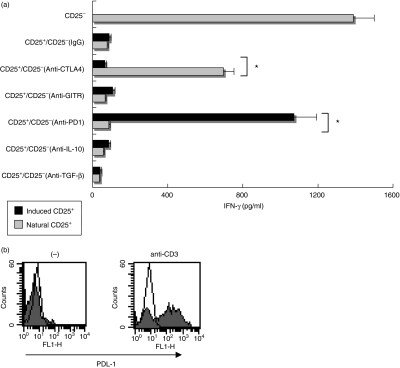
Suppressive actions of CD4+ CD25+ Treg cells, induced by NH/IL-2 NK-primed DCs, depends on PD-1-mediated negative costimulatory signals
The suppressive activities of CD4+ CD25+ Treg cells reportedly depend on various kinds of mediators, such as CTLA-4, IL-10 and/or TGF-β, but the exact mechanisms of the actions have not been fully elucidated.1,6,12–14 PD-1, recently identified as a negative costimulatory receptor of the B-7 family, is expressed in CD4+ CD25+ Treg cells, indicating that PD-1-mediated negative signals may be involved in the regulatory functions of CD4+ CD25+ Treg cells.9 Thus, we evaluated the involvement of these molecules in the suppressive activities of CD4+ CD25+ Treg cells. For this purpose, the blocking Ab of CTLA-4, GITR, PD-1, TGF-β or IL-10 was added during co-cultures of CD4+ CD25+/CD4+ CD25– T cells in the presence of anti-CD3 Ab. In case of natural CD4+ CD25+ T cells, their suppressive action was partially reversed on addition of anti-CTLA-4 Ab. By contrast, they preserved their suppressive capacity even in the presence of the blocking Ab of GITR, PD-1, TGF-β or IL-10 (Fig. 4a). When CD4+ CD25+ Treg cells induced by NH/IL-2 NK-primed DCs were used instead of natural CD4+ CD25+ T cells, their suppressive activity was markedly reduced on addition of the blocking Ab of PD-1 but not CTLA-4, IL-10, TGF-β or GITR (Fig. 4a). The regulatory functions of these Treg cells were required for direct cell-to-cell contact because separation of CD4+ CD25+ Treg cells and CD4+ CD25– T cells in transwell chambers virtually abolished their suppressive effects (data not shown). We also confirmed the presence of PDL-1 expression on CD4+ CD25– T cells when they were activated with anti-CD3 Ab (Fig. 4b), suggesting that effector cells themselves induce suppressive activities of CD4+ CD25+ Treg cells. Taken together, these results further reinforced the hypothesis that CD4+ CD25+ Treg cells induced by NH/IL-2 NK-primed DCs were different from natural CD4+ CD25+ Treg cells in their PD-1-dependent suppressive functions.
Figure 4.
CD4+ CD25+ Treg cells induced by interleukin (IL)-2 natural killer (NK)/human non-transformed hepatocytes (NH)-treated dendritic cell (DC) suppressed T cell activation through programmed death-1 (PD-1)/programmed death ligand-1 (PDL-1) interactions. (a) DCs (1 × 105) were stimulated with the IL-2 NK/NH supernatant for 24 hr, and then cultured with allogeneic CD4+ T cells for 48 hr. CD4+ CD25+ fractions were isolated from the DC/CD4+ T cell mixtures. Freshly isolated CD4+ CD25+ T cells (natural CD25+) or CD4+ CD25+ T cells induced by NK/NH-primed DCs (induced CD25+) were co-cultured with freshly isolated autologous CD4+ CD25– T cells at a ratio of 1 : 1 upon stimulation of plate-bound anti-CD3 antibody (Ab). Anti-CTLA-4 (cytotoxic T lymphocyte antigen-4) Ab, anti-GITR (glucocorticoid-induced TNF receptor) Ab, anti-PD-1 Ab, anti-IL-10 Ab, anti-TGF-β Ab or isotype control IgG (20 µg/ml for each) were incubated during CD4+ CD25+/CD4+ CD25– T cell co-cultures. Interferon (IFN)-γ was measured for each supernatant obtained after 72 hr of co-culture by enzyme-linked immunosorbent assay. *P < 0·05 vs. responses of anti-CD3 Ab-stimulated CD4+ CD25– T cells. (b) Freshly isolated CD4+ CD25– T cells were incubated with (anti-CD3) or without (–) plate-bound anti-CD3 Ab for 24 hr. PDL-1 expression was assessed by flow cytometry (closed histograms). Open histograms show isotype control staining.
Discussion
Recent studies have revealed that activated NK cells positively regulate DC activation and maturation either through direct contact via NK cell receptors (NKp30, NKG2D, etc.) or in co-ordination with various kinds of cytokines (IFN-γ, TNF-α, etc.).15–18 However, the issue of whether NK cells are involved in DC-mediated Treg cell induction has not been resolved. In the present study, we report that the expression of regulatory markers and functions was markedly decreased on CD4+ CD25+ T cells upon exposure to IL-2 NK-primed DCs. By contrast, the interaction of activated NK cells and NH through the NKG2A inhibitory receptor led to DC induction of CD4+ CD25+ T cells with regulatory properties. Furthermore, NKG2A-mediated increase in TGF-β as well as decrease in TNF-α in an NH and NK cell mixture contributed to DC induction of CD4+ CD25+ Treg cells. This is consistent with previous reports showing that TGF-β plays a role in generating the specific DC that activates CD4+ CD25+ Treg cells.10,11 The findings that TNF-α suppressed TGF-β-mediated priming of DCs to induce Treg cells also extended the previously identified role of TNF-α as a positive regulator of DC activation. In line with our findings, previous reports showed that impairment of CD4+ CD25+ Treg cell activities restored their suppressive functions after blocking TNF-α signals in non-obese diabetic (NOD) mice or in patients with Crohn's disease.28,29 To our knowledge, the present study is the first description of modulation of NK cells and human hepatocytes through NKG2A-mediated inhibitory signals that profoundly affect DC functions towards CD4+ CD25+ Treg cells. Because NK cell functions are regulated by the balance between inhibitory and activating signals, any future clarification of the role of other NK inhibitory and activating receptors in DC modulation and Treg cell activation will be of great interest.
The cross-presentation of self-antigens by major histocompatibility complex (MHC) class II pathways constitutes an important step towards generating and/or expanding peripheral Treg cells.30 However, we initially settled our experimental design by using DCs and Treg cells from different donors, and DCs encountered CD4+ T cells in an ‘antigen-free’ condition. Therefore, Treg cells induced by NK/NH-primed DCs are generated independently of MHC class II-mediated self-antigen recognition. These results give rise to the possibility that the cross-talk of NK cells, DCs and hepatocytes represents an alternative pathway in the generation and expansion of peripheral Treg cells. However, it should be noted that these results may not apply to all donors because of the complexity of the allogeneic system and the relatively few donors tested.
PD-1-mediated suppressive activities were characteristic for CD4+ CD25+ Treg cells generated by NH/IL-2 NK-primed DCs. By contrast, natural CD4+ CD25+ Treg cells exerted their suppressive function, at least in part, in a CTLA-4-dependent fashion. Recent reports have clarified the existence of two subtypes of Treg cells: natural and inducible CD4+ CD25+ Treg cells. Inducible Treg cells exert suppressive activities by using molecular mechanisms distinct from those of natural regulatory cells.31 Our findings further identify the novel pathways by which inducible CD4+ CD25+ Treg cell activities triggered by NKG2A inhibitory signals are dependent on PD-1-mediated negative costimulation. A recent report identified the interaction of B7 on effector T cells with costimulatory molecules CD28/CTLA-4 on CD4+ CD25+ Treg cells as molecular mechanisms of their suppressor activity.32 Thus, it is possible that reverse signalling of PDL-1 on effector cells may also be crucial for the negative costimulator-mediated suppressive action of CD4+ CD25+ Treg cells. In the present study, we did not address the mechanisms by which NH/IL-2 NK-primed DCs induce CD4+ CD25+ Treg cells with PD-1-dependent suppressive functions. Further study will be needed to clarify this issue.
We previously showed that NKG2A is expressed at higher levels from NK cells isolated from peripheral blood in patients with chronic hepatitis C virus (HCV) infection than from those in healthy donors.20 HCV frequently persists in humans, at least in part, due to inefficient induction of NK activity as well as specific T cell responses.33–35 The small percentage of patients who spontaneously clear the virus and recover from chronic hepatitis C mount vigorous HCV-specific CD4+ and CD8+ T cell responses.36,37 Research has described an increased frequency of CD4+ CD25+ T cells in the blood of patients with persistent HCV infection compared with those who have spontaneously cleared HCV.38,39 Our current findings raise the interesting possibility that increased NKG2A expression on NK cells may lead to DC-mediated induction of Treg cells, leading to the inhibition of adaptive responses to HCV and failure to eliminate this virus. Indeed, CD4+ CD25+ T cells induced by HCV-NK/Hep3B hepatoma cell-primed DCs expressed and suppressed effector T cell functions at greater levels than those induced by N-NK/Hep3B-primed DCs (our unpublished data). Interestingly, a recent study identified PD-1-mediated signals as a critical pathway to induce anergic CD8+ T cells and impair antiviral CTL responses in chronic viral infection.40 In this regard, the therapeutic modification of the PD-1 pathway may synergistically augment antiviral immunity by suppressing Treg activity and recovering CTL responses. It is important to establish whether the PD-1 pathway in liver lymphocytes may be operable in vivo and play a critical role in suppression of virus-specific immunity in HCV infection.
In conclusion, we have demonstrated that interaction of NK cells and hepatic cells via NKG2A leads to DC induction of CD4+ CD25+ T cells with PD-1-dependent regulatory activities. These findings also imply that NK receptor signals of NK cells may dictate DC-mediated adaptive immune responses towards tolerogenic or immunogenic status via induction of Treg cells.
Acknowledgments
This work was supported by a grant-in-aid from the Ministry of Culture, Sports, Science and Technology of Japan and a grant-in-aid for research on hepatitis and BSE from the Ministry of Health, Labour and Welfare of Japan. It was also partially supported by the 21st Century Centre of Excellence Programme of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Glossary
Abbreviations:
- CTLA-4
cytotoxic T lymphocyte antigen-4
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- GITR
glucocorticoid-induced TNF receptor
- HCV
hepatitis C virus
- HLA
human leucocyte antigen
- NH
human non-transformed hepatocyte
- NK
natural killer
- PD-1
programmed death-1
- PDL-1
programmed death ligand 1
- PBMC
peripheral blood mononuclear cell
- Treg
regulatory T
References
- 1.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 2.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification of functional characterization of human CD4+ CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+ CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkald Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 5.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumour-specific human CD4+ regulatory T cells and their ligands: implication for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 6.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD4+ CD25+ regulatory cells that control intestinal inflammation. J Exp Med. 2002;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+ CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–89. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–38. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- 9.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4 (+) CD25 (+) suppressor T cells in vivo. Nat Immunol. 2004;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Hawiger D, Nussenzweig D. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 11.Mahnke K, Quan Y, Knop J, Enk AH. Induction of CD4+ CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 12.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD4+ CD25+ T cells regulate the expansion of peripheral CD4+ T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 13.Yamagiwa S, Gray JD, Hashimoto H, Horwitz DA. A role of TGF-β in the generation and expansion of CD4+ CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–9. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-β regulates in vivo expansion of Foxp3-expressing CD4+ CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–7. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moretta A. The dialogue between human natural killer cells and dendritic cells. Curr Opin Immunol. 2005;17:306–11. doi: 10.1016/j.coi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural killer cells and dendritic cells: ‘L’union fait la force'. Blood. 2005;106:2252–8. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 17.Mocikat R, Braumuller H, Gumy A, et al. Natural killer cells activated by MHCLOW targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–9. doi: 10.1016/s1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 18.Van den Broeke LT, Daschbach E, Thomas EK, Andringa G, Berzofsky JA. Dendritic cell-induced activation of adaptive and innate antitumour immunity. J Immunol. 2003;171:5842–52. doi: 10.4049/jimmunol.171.11.5842. [DOI] [PubMed] [Google Scholar]
- 19.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–9. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 20.Jinushi M, Takehara T, Tatsumi T, et al. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to the altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–81. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 21.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. 1998;160:4951–60. [PubMed] [Google Scholar]
- 22.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 23.Pende D, Sivori S, Accame L, et al. HLA-G recognition by human natural killer cells. Involvement of CD94 both as inhibitory and as activating receptor complex. Eur J Immunol. 1997;27:1875–80. doi: 10.1002/eji.1830270809. [DOI] [PubMed] [Google Scholar]
- 24.Valerie V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4+ T cells by human mature autologous dendritic cells. Eur J Immunol. 2003;34:762–72. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD4+ CD25+ regulatory T cells through GITR break immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 26.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 27.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNF-α therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumour necrosis factor-α regulation of CD4+ CD25+ T cell levels in NOD mice. Proc Natl Acad Sci USA. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 31.Bluestone JA, Abbas AK. Natural and adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 32.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci USA. 2004;101:10398–403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad A, Alvarez F. Role of NK and NKT cells in the immunopathogenesis of HCV-induced hepatitis. J Leukoc Biol. 2004;76:743–59. doi: 10.1189/jlb.0304197. [DOI] [PubMed] [Google Scholar]
- 34.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies. Liver Transplant. 2006;12:363–72. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 35.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 36.Lauer GM, Barnes E, Lucas M, et al. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924–36. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Cox AL, Mosbruger T, Lauer GM, Pardoll D, Thomas DL, Ray S. Comprehensive analysis of CD8+ T cell responses during longitudinal study of acute human hepatitis C. Hepatology. 2005;42:104–12. doi: 10.1002/hep.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, Nelson DR. An immunomodulatory role for CD4+ CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 39.Rushbrook SM, Ward SM, Unitt E, Vowler M, Lucas M, Kleneman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–9. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]