Abstract
Allergen-specific immunoglobulin E (IgE) mediates immediate-type hypersensitivity reactions and plays a central role in allergic diseases. Although antigen-driven B-cell maturation and isotype switching occur within germinal centres (GCs), the role of GCs in IgE production is poorly understood. In view of this, we investigated the development of IgE-expressing cells within GCs in response to an extensively characterized antigen, 2-phenyloxazolone (phOx). The phOx-specific IgE-expressing cells localized within GCs 7 days after immunization, and peaked in number on day 11. Surprisingly, very few IgE-positive cells were found in the T-cell areas of the lymph node. Flow cytometric studies confirmed that IgE was expressed by B cells and was not the result of trapping by follicular dendritic cells. The specificity of the antibody response was confirmed by microdissection and reverse transcription-polymerase chain reaction using phOx-specific IgE primers. IgE-positive cells were primarily found within GCs while, in contrast, many IgG1-positive cells could also be detected outside GCs in the T-cell areas. Taken together, these data highlight the importance of GCs in the production of antigen-specific IgE antibody.
Keywords: B cells, germinal centre, immunoglobulin E, isotypes/isotype switching, microenvironment
Introduction
The origin of immunoglobulin E (IgE)-producing B cells and the development of memory are of fundamental importance in understanding the generation of antigen-driven allergic responses. In response to protein antigens, germinal centres (GCs) form within secondary lymphoid tissue and are sites of antigen-driven B-cell proliferation and maturation.1 In addition, memory B-cell formation is associated with the development of GCs.2 Small numbers of follicular dendritic cells (FDCs) are also present in GCs as well as antigen-specific CD4+ T cells that produce interleukin-4 (IL-4), a cytokine critical for isotype switching and IgE production.3 Isotype switching can occur both within GCs and outside GCs in T-cell areas of lymphoid tissue.4 Yet, the maturational outcome of antigen-stimulated B cells appears to differ depending on their location within lymphoid tissue. For instance, precursor cells binding low levels of the monoclonal antibody J11D give rise to GCs and the development of memory B cells.5–7 In contrast, precursor cells binding high levels of J11D give rise to antibody-forming cells and do not produce GCs or memory B cells.5–7 It is possible that the microenvironment at sites within the lymphoid tissue where B cells reside provide distinct signals that regulate B-cell development and antibody production. This aspect of B-cell regulation is important and should be considered when devising strategies to effectively block antigen-driven IgE responses.
Only a few studies to date have examined the in vivo origin of IgE-producing B cells. Lymphocytes expressing IgE were first observed in Peyer's patches 8 days after immunization, suggesting that IgE-producing cells originate in gut-associated secondary lymphoid tissue.8 Wang et al. demonstrated that the lamina propria of the intestine produced the greatest number of IgE+ cells following an oral challenge with Trichinella spiralis.9 A peak in IgE+ cells was first seen in Peyer's patch GCs, followed by a peak in the lamina propria of the intestine.9 Using ovalbumin and a tracheal challenge model, inflammatory infiltrates containing ovalbumin-binding plasma cells and GCs were found in the lung after intratracheal challenge.10 Taken together, these studies suggest that IgE-secreting plasma cells might arise from GCs within mucosal tissues after antigen stimulation. Other studies have identified IgE+ cells within GCs, but failed to differentiate de novo IgE production from IgE trapped on the surface of GCs cells via Fc receptors that are defined by CD23 expression.11 Because of the potential for IgE trapping by CD23-expressing cells such as FDCs12 there is limited evidence that IgE-producing cells arise within GCs.
In this study we monitored in vivo development of antigen-induced IgE-producing cells in mice in response to the well-characterized hapten 2-phenyloxazolone (phOx). By using a number of techniques, we found that phOx-specific IgE+ B cells are generated primarily within GCs. Thus, therapies targeting isotype switching within GCs may be a viable approach for controlling IgE-dominated immune reactions such as allergies.
Materials and methods
Mice
Six- to 8-week-old female BALB/c mice were purchased from Harlan-Sprague Dawley (Indianapolis, IN) and housed in an Association for the Assessment and Accreditation of Laboratory Animal Care-approved facility. The experimental protocol was approved by the institutional animal-use review committee. Animals were given access to food and water as desired and housed as groups of five mice per cage.
Antigen preparation and immunization
The phOx was conjugated to bovine serum albumin (BSA) as previously described.13 Briefly, 150 mg of 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one and 2 g BSA (Sigma, St Louis, MO) were added to 40 ml 5% NaHCO3, and stirred for 24 hr at 4°. A spontaneous reaction occurs with loss of the ethoxymethylene group during the coupling of phOx to BSA. The solution was then centrifuged at 30 000 g for 30 min and extensively dialysed against 0·15 m sodium chloride. The conjugate contained 32 molecules of phOx per molecule of BSA based on the ability of phOx to absorb light at 352 nm. The succinic ester of 4-hydroxy-3-nitrophenyl acetyl (NP) (Cambridge Research Biochemical, Cambridge, UK) was used as a negative control hapten and was conjugated to BSA as described.4 The conjugate contained 24 molecules of NP per molecule of BSA, based on spectrophotometric analysis. The hapten conjugates were precipitated with alum (Sigma)14 and the concentration of alum-precipitated antigens was adjusted to 400 μg/ml in sterile saline. Mice were injected intradermally in both front footpads with 10 μg of either phOx-BSA-alum or NP-BSA-alum in a 25-μl volume.
Quantification of serum IgE
Blood was collected before and 11 days after immunization with phOx. The serum was separated and stored at − 20° until analysis. Immunlon 2 microtitre plates (Dynatech, Chantilly, VA) were coated with 10 μg/ml of rat anti-mouse IgE antibody (clone 02111D; BD Biosciences, San Jose, CA) overnight at room temperature. Wells were then washed three times with phosphate-buffered saline containing 0·5% Tween-20. Serial dilutions of mouse sera were added to wells in duplicate and incubated for 3 hr at room temperature. After washing, 2 μg/ml of biotin-conjugated rat anti-mouse IgE antibody (clone 02122D; BD Biosciences) was added to all wells and incubated for 1 hr at room temperature. Plates were then washed and streptavidin–horseradish peroxidase (HRP) (Pierce; Rockford, IL) was added. After washing, the substrate 3-amino-9-ethylcarbazole (Sigma) was added to each well for 45 min and optical densities were determined at 405 nm. Serum IgE concentrations were determined by comparison to a standard curve obtained using purified mouse IgE antibody (clone 03121D; BD Biosciences).
Immunohistochemistry
Brachial lymph nodes were snap frozen in OCT embedding medium (Fisher Scientific, Pittsburgh, PA) and stored at − 70°.14 Tissue sections (5 μm thickness) were fixed in cold acetone, washed in phosphate-buffered saline and placed in methanol : H2O2 for 30 min to quench the endogenous peroxidase activity. Tissue biotin sites were blocked by the addition of avidin followed by biotin. After blocking with 10% goat serum, tissue sections were incubated with rat antibodies against mouse IgE (clone R35-118) or IgG1 (clone A85-3) from BD Biosciences for 45 min at room temperature in a humidified chamber. A purified preparation of IgG1 rat myeloma protein (IR863) was used as a negative control.14,15 Sections were then incubated with a biotin-conjugated goat anti-rat antibody at 14 μg/ml (BioSource International, Camarillo, CA), followed by streptavidin-conjugated alkaline phosphatase (Zymed, San Francisco, CA). 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate (Roche Molecular Biochemicals, Indianapolis, ID) was used for blue colour development. GCs were visualized using a peanut agglutinin (PNA)-HRP conjugate (Sigma) and developed with Fast Red RT salt (Pierce) to visualize pink-staining PNA+ GC cells. Only cells with a cytoplasmic rim of stain surrounding the entire cell were considered positive. Positive cells were counted in a blinded fashion throughout the entire GC section and were expressed as positive cells per mm2. Photographs were generated using a video camera (Sony Electronics, Inc., San Jose, CA) and Pax-it! software (Midwest Information Systems, Inc., Franklin Park, IL).
Flow cytometry
Single-cell suspensions of brachial lymph nodes were prepared and stained using a previously described microplate method.16 Briefly, cell suspensions (4 × 105 cells) were treated with anti-CD16/CD32 (BD Biosciences) to minimize Fc receptor binding. Cell suspensions were then incubated with the following rat anti-mouse antibodies from BD Biosciences: CD45R-peridinin chlorophyll (B220, clone RA3-6B2); GL7 conjugated to fluorescein isothiocyanate (clone GL7/Ly77); and either IgE conjugated to phycoerythrin (clone R35-72) or CD23-phycoerythrin (clone B3B4). Rat IgG1-fluorescein isothiocyanate and IgG2a-phycoerythrin served as isotype-matched negative controls. Analysis was performed using a FACScan flow cytometer (BD Biosciences) equipped with a 488-nm argon laser and Lysys II software. The instrument was calibrated with CaliBRITE beads using AutoComp software and the same settings were used throughout the study. Dead cells were excluded on the basis of forward angle and 90° light scatter and 10 000 live cells were analysed.
Isolation of GC cells from tissue sections
Frozen sections of brachial lymph nodes (18 μm in thickness) were placed on RNase-free slides coated with poly l-lysine. The slides were sequentially numbered with three to five slides being selected that were representative of the entire area of tissue that was sectioned. Representative slides were incubated with 10% normal goat serum to reduce non-specific binding, followed by PNA-HRP (Sigma). The GCs were identified after the addition of a metal-enhanced diaminobenzidine substrate (Pierce). The location of GCs in unstained slides was determined based on the location of GCs in adjacent sections stained with PNA and by the slightly larger size of the GC cells, compared to cells in other areas of the lymph node. The sections were covered with 30 μl of a 25% solution of glycerol containing 250 units RNase inhibitor (Promega Corp., Madison, WI). Cells were removed from GCs using a heat-drawn micropipette controlled by a micromanipulator (World Precision Instruments, Sarasota, FL). Microdissected cells from six GCs (same GC from two or three slides) at days 7 and 9 were pooled and placed in microcentrifuge tubes containing 0·5 ml of denaturing solution (see below) and stored at − 20°. Approximately 50 cells were routinely obtained from each GC using this technique. After microdissection, the remaining lymph node section was stained with PNA-HRP to confirm that only cells within GCs had been isolated.
RNA isolation, cDNA synthesis and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from microdissected cells by the method of Chomczynski et al.17 Denaturing solution consisted of 4 m guanidinium thiocyanate, 25 mm sodium citrate (pH 7·0), 0·1 m 2-mercaptoethanol and 0·5%N-laurylsarcosine (Sigma). For RNA extraction, water-saturated phenol (Boehringer Mannheim Biochemicals, Indianapolis, IN), chloroform and chloroform : isoamyl alcohol (49 : 1) were added to each tube in a sequential fashion. The recovered RNA from the aqueous phase was precipitated in isopropyl alcohol, followed by absolute ethanol. After vacuum drying, the RNA was resuspended in diethylpyrocarbonate-treated deionized water. The cDNA synthesis and PCR using 2·5 μl cDNA were performed exactly as described.3 A 10× dilution of the first PCR product was subjected to a second round of PCR, called nested PCR, using oligonucleotides internal to those used in the first PCR.
Heavy-chain mRNAs produced in response to phOx exhibit restricted heterogeneity allowing PCR primers to be designed that detect the majority of phOx-specific mRNAs.18 Oligonucleotides were synthesized to recognize variable regions of heavy-chain mRNA specific for phOx (sense primers) and constant regions of the heavy chain specific for IgE (antisense primers). Oligolucleotide sequences used in the first PCR were sense 5′-TCACAGAGCCTGTCCATCACTTGCACT-3′ and antisense 5′-GTCCATTAGCCAGCTGAC-3′. Oligolucleotide sequences used in the second PCR were sense 5′-CTGCAAACTGATGACACAGCCATGTAC-3′ and antisense 5′-CAGCTGGTCACTTGGCTGGTGGTGAC-3′. PCR reaction conditions were 1·25 min at 94°, 1·5 min at 55° and 2 min at 72°, for a total of 40 cycles. The PCR products were separated in 3% NuSieve GTG agarose (FMC BioProducts, Rockland, ME) and visualized under ultraviolet illumination after ethidium bromide staining. The specificity of the PCR was confirmed by the size of the product (284 base pairs) and by demonstrating that three different restriction enzyme digests yielded fragments of expected size. In addition, the nested IgE PCR product was sequenced by the UCLA sequencing core facility using an ABI automated sequencer (Applied Biosystems, Foster City, CA).
Statistical analysis
Assessment of statistical differences between control and experimental groups were determined using the unpaired t-test or Mann–Whitney rank sum test. Statistical tests were performed on SigmaStat software (Jandel Scientific, San Rafael, CA). P < 0·05 was considered statistically significant.
Results
Serum IgE concentrations in response to phOx
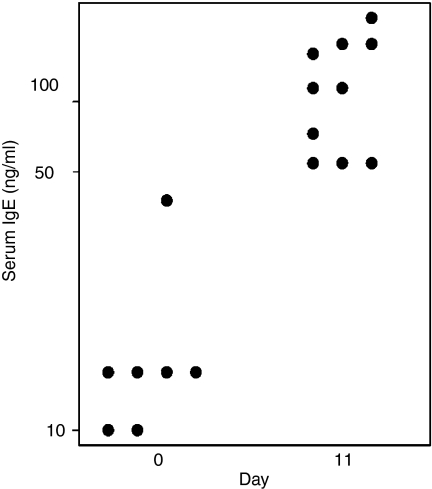
The hapten phOx has been extensively used to study somatic mutations within B cells.19 During a primary immune response to phOx, the majority of anti-phOx antibody generated by BALB/c mice utilizes the same heavy-chain and light-chain germline genes.18 The mRNA sequences from these genes show limited heterogeneity with only minor differences in the D region and joining boundaries.19 To elicit an IgE response, BALB/c mice were injected in each front footpad with 10 μg alum-absorbed phOx-BSA in a 25-μl volume. This concentration of antigen was selected because previous studies have shown that optimal IgE responses are elicited upon immunization with small antigen doses.20,21 In initial studies, we monitored serum IgE concentrations and found a significant increase in IgE at day 11 following immunization (Fig. 1). The serum IgE concentrations that were detected are comparable to those observed in other antigen systems,22 verifying that our immunization protocol induced an IgE response.
Figure 1.
Serum IgE concentrations following immunization with phOx. Total serum IgE concentrations were determined by a sandwich enzyme immunoassay before (day 0) and 11 days after immunization. Dots represent serum IgE levels for individual mice. There was a statistically difference between groups (P < 0·001; Mann–Whitney Rank Sum test).
Quantification of IgE- and IgG1-expressing B cells during GC development
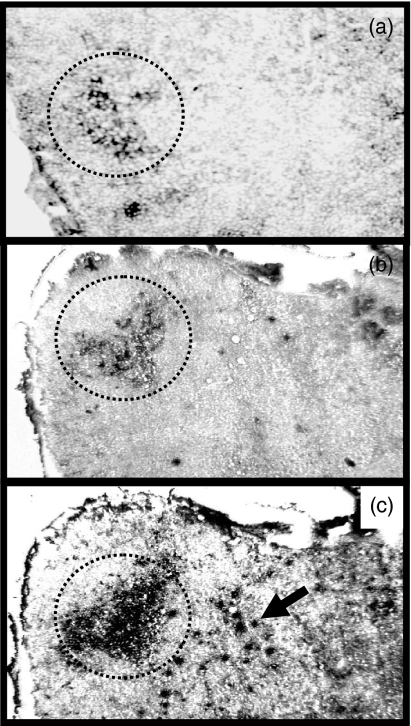
To detect and quantify IgE- and IgG1-expressing B cells within GCs we employed a dual immunohistochemical staining technique, using PNA to identify GCs, together with antibodies against either IgE or IgG1. Small numbers of IgE+ cells (dark cells) could easily be identified in GCs (dotted outline) 7 days after immunization (Fig. 2a). The amount of IgE staining was increased at day 9 (Fig. 2b) and could still be detected at day 18 (data not shown). The IgE-staining pattern was primarily localized to the light zones of GCs at day 7 and appeared in this site during early GC development (Fig. 2a,b). Interestingly, very few IgE+ cells were found in areas of the lymph node other than GCs at days 7 and 9 (Fig. 2a,b). Since IgG1 production is usually observed in mice producing IgE, we examined its expression and found that IgG1-producing cells were present within GCs but were also found in other areas (arrow) of the lymph node (Fig. 2c).
Figure 2.
IgE and IgG1 expression within GCs in response to phOx. Brachial lymph node sections were stained with PNA to identify GCs and either anti-IgE or anti-IgG1 antibodies by immunohistochemistry. IgE+ cells can be observed within GCs at day 7 (a) and day 9 (b) following immunization. In contrast, IgG1+ cells were found both inside and outside (arrow) GCs at day 9 (c). GCs (PNA+ area) are outlined by a dotted line. Original magnification is 40 ×.
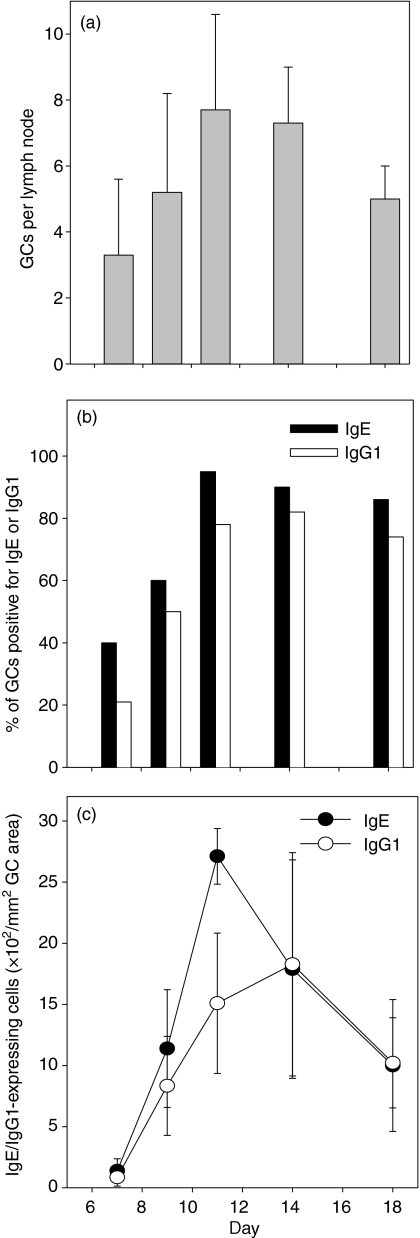
Quantification of GC numbers at various times following phOx-BSA injection showed that an average of three PNA+ GCs could be detected 7 days after immunization with phOx-BSA (Fig. 3a). Germinal centre formation peaked by day 11 and began to wane at day 18 (Fig. 3a). These data indicate that a low dose of phOx is capable of inducing GC formation with kinetics similar to those observed in other experimental systems using higher antigen doses.1,14,15 At day 7 when GCs were first detectable only 40% contained IgE+ cells (Fig. 3b). The number of IgE+ cells within GCs continued to increase over time and reached a peak at day 11, with the majority of the GC area in 22 of 23 GCs containing IgE (Fig. 3b). The majority of GCs also contained IgE at days 14 and 18. When IgG1 was examined, a similar kinetic pattern was observed within GCs (Fig. 3b). Although the absolute number of IgG1+ cells increased at a slower rate compared to IgE at days 7–11, equivalent numbers of IgE+ and IgG1+ cells were observed within GCs at days 14 and 18 (Fig. 3c). Taken together, these data indicate that phOx favours IgE production inside GCs, whereas IgG1 production takes place both inside and outside GCs.
Figure 3.
Kinetics of GC formation and IgE/IgG1 expression following immunization with phOx. Brachial lymph node frozen tissue sections were stained with PNA to identify GCs, and antibodies against either IgE or IgG1 to determine: (a) mean number of GCs per lymph node, (b) percentage of GCs containing IgE+ and IgG1+ cells, and (c) numbers of IgE+ and IgG1+ cells within each GC. Four lymph node sections were examined from each mouse and three to five mice were used at each time-point. The GC area was determined using an ocular grid. Standard deviation bars are shown.
GC B cells express IgE
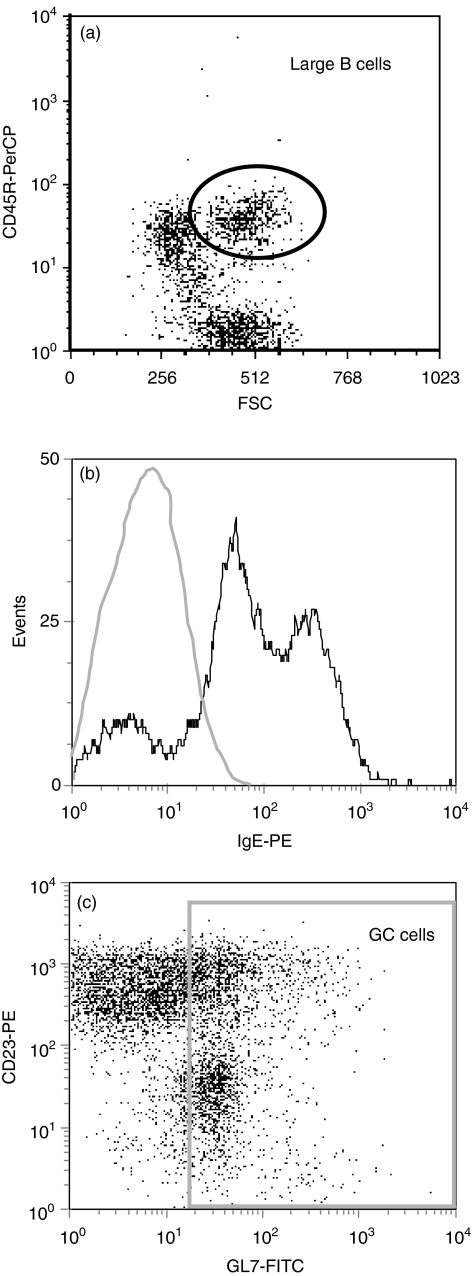
FDCs express low-affinity Fc receptors for IgE (CD23) and can trap and retain IgE on their surface in the form of immune complexes.12 In view of this, it was possible that the IgE we identified in GCs was produced at other sites and became trapped in GCs by FDCs. Although this was unlikely because IgE staining was in a typical ring-like B-cell pattern and did not resemble a reticular FDC pattern (Fig. 2a,b), we examined IgE expression by GC B cells by flow cytometry. Since GC B cells are a minor component of lymph nodes, GC B cells were enriched by gating on CD45R+ cells expressing high forward-angle light scatter (Fig. 4a). Not all large B cells are found within GCs therefore large B cells were only considered GC B cells if they were also GL7+.23–26 As shown in Fig. 4(b), approximately 80% of the large GL7+ GC B cells were IgE+ at day 11, a time when IgE expression was highest within GCs (Fig. 3c). This finding eliminates FDC trapping of IgE as the explanation for the appearance of IgE in GCs by immunohistochemistry. In addition, some large GL7– B cells were IgE+ and most likely represented IgE-expressing B cells outside GCs (data not shown). We also found two populations of IgE+ cells within GCs (GL7+); IgElo (70%) and IgEhi (30%) cells (Fig. 4b). Interestingly, the IgElo cells also expressed lower levels of GL7 compared to the IgEhi cells that expressed higher levels of GL7 (data not shown). This finding is consistent with a previous report demonstrating that GL7hi GC cells also express higher levels of a marker associated with memory B cells (J11d2) and produce larger amounts of antibody, compared with GL7lo GC cells.26
Figure 4.
Flow cytometry analysis of IgE expression on GC B cells. Brachial lymph nodes were harvested on day 11 after phOx-BSA injection and single cell suspensions were stained for flow cytometric analysis. (a) Large B cells were identified based on CD45R (B220) expression and high forward-angle light scatter (channel > 384). (b) Histogram showing IgE expression by GL7+ large B cells (CD45R+ cells with high forward-angle light scatter). For comparison, the staining profile to an irrelevant control antibody is indicated by the light grey line. (c) Dot plot of gated cells (large, CD45R+ B cells) showing CD23 expression on GC B cells based on GL7 (LY77) staining. Data are representative of two separate experiments.
We then evaluated CD23 staining on GC B cells and found that not all GL7+ cells (both GL7hi and GL7lo) expressed CD23. In fact, a sizeable number of GC B cells (40%) were CD23– (Fig. 4c, lower population of cells in gate). These data emphasize that while some GC B cells are CD23+, there are a significant percentage of CD23– GC B cells that are IgE+, demonstrating that binding of IgE to surface CD23 does not account for all of the IgE expression that was observed within GCs by immunohistochemical staining. Furthermore, these date demonstrate that IgE+ B cells are present within GCs.
phOx-specific IgE mRNA is produced in GCs
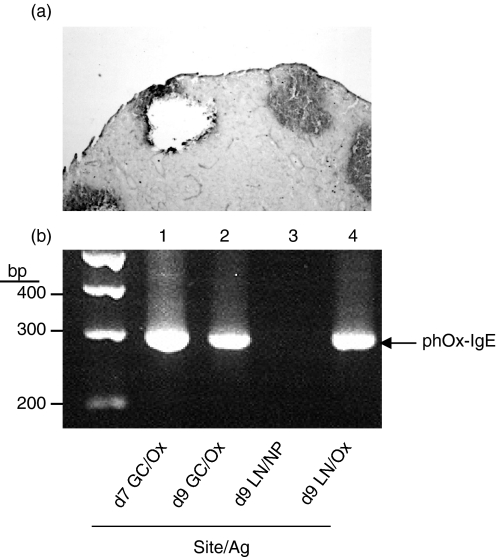
To verify that phOx-specific IgE was actually produced within GC cells from individual brachial lymph nodes, IgE mRNA was examined in individual GCs after microdissection. Germinal centres were isolated after morphological identification using a micromanipulator. Isolated cells from individual GCs were then examined for IgE gene expression by RT-PCR. Nested PCR primers were used to amplify a region spanning the variable region of the heavy-chain gene (phOx-specific) and the IgE constant heavy-chain gene. As shown in Fig. 5(a), GC cells could easily be isolated from frozen sections of brachial lymph nodes. When isolated GC cells were examined by RT-PCR, we found that phOx-specific IgE mRNA was detectable within GCs on days 7 and 9 and in whole lymph nodes of mice injected with phOx-BSA (Fig. 5b). Furthermore, phOx mRNA expression was found only in mice immunized with phOx and not in mice immunized with another antigen, NP (Fig. 5b, lane 3), confirming the specificity of the RT-PCR for phOx. Detection of mRNA for phOX occurred before IgE protein within GCs was maximal (Fig. 3c). Sequencing of the IgE expression mRNA PCR product confirmed that the sequences were specific for anti-phOx variable heavy region genes and the epsilon constant region gene (data not shown). These findings demonstrate that phOx-specific IgE mRNA is produced within GCs and confirms the protein data obtained by histological staining of tissue sections.
Figure 5.
GCs express Ox-specific mRNA for IgE. Cells were retrieved from individual GCs by microdissection and examined for Ox-specific IgE mRNA by nested RT-PCR. (a) A tissue section was stained with PNA after microdissection. (b) GC cells (day 7, lane 1; day 9, lane 2) and brachial lymph node cells (day 9, lanes 3 and 4) were examined for phOx-specific IgE mRNA following immunization with phOx (lanes 1, 2 and 4) or NP (lane 3). Molecular weight markers appear in the far left lane. The expected phOx-specific mRNA product is 284 base pairs (bp) in length.
Discussion
In this study, we characterized the kinetics and histological localization of IgE-producing cells within GCs of brachial lymph nodes during a primary immune response to phOx. We used phOx as an antigen because the antibody response to phOx has been extensively studied and the nucleotide sequence of antibodies generated to phOx have been sequenced and found to exhibit restricted heterogeneity.27 Immunoglobulin E-positive B cells were detectable within GCs 7–18 days after immunization. This was confirmed by flow cytometric studies and by demonstrating that isolated cells from individual GCs contained phOx-specific mRNA for IgE. Surprisingly, IgE+ cells were not present outside GCs on days 7 and 9, and very few IgE+ cells could be detected within T-cell regions at later times. In contrast, large numbers of IgG1+ cells were found outside GCs as early as day 9. Taken together, these data strongly suggest that IgE+ B cells develop within GCs and provide evidence for a role of the GC microenvironment in antigen-driven IgE responses.
IgE production by B cells normally requires T-cell help in the form of costimulatory signals mediated via CD40 and IL-4.28,29 GCs are specialized microenvironments that regulate B-cell maturation and contain all of the necessary factors needed for IgE production. For instance, GC T cells have been shown to express CD40 ligand (CD40L)30 and mutations in the CD40L gene abrogate isotype switching to IgE.31 In addition, CD40L knockout mice do not contain detectable levels of IgE.32 Likewise, IL-4-producing T cells have also been reported within GCs.3 These studies support our finding that IgE+ cells are localized to the light zones of GCs where T cells reside. In view of these data, it comes as no surprise that we found the majority of IgE+ B cells to be localized to GCs.
To our knowledge, this is the first report demonstrating that antigen-specific IgE mRNA and IgE-expressing B cells are detectable at early stages of GC development in lymph nodes draining the site of antigen injection. In support of our finding, Wang et al. found that IgE+ cells rapidly appeared within GCs of Peyer's patches following infection of rats with T. spiralis larvae.9 In contrast, Auci et al. found that immunization with benzylpenicilloyl-keyhole limpet haemocyanin resulted in the appearance of IgE+ cells within primary B-cell follicles, but not in GCs of Peyer's patches or mesenteric lymph nodes.8 The appearance of IgE in various sites may vary depending on the route and type of antigen used for immunization. Nevertheless, our findings and results from the study by Wang et al. suggest that isotype switching to IgE can occur within GCs.
In addition to GCs, isotype switching from IgM to IgG has been shown to occur outside GCs in T-cell areas.33 It is noteworthy that we found IgE+ B cells to be primarily restricted to GCs whereas IgG1+ cells were present in GCs and T-cell regions at early time-points. Perhaps some B cells within GCs directly switched from IgM to IgE at an early stage of GC development while IgG1+ B cells that were activated in T-cell areas entered GCs later and underwent further switching to IgE. Mouse hybridoma cell lines derived from GC B cells obtained 10 days after phOx immunization were predominately IgG+,34 suggesting that switching from IgM to IgG, then to IgE, would not be an early event during GC development. However, IgM to IgE has been reported in GC centrocytes (IgD–, CD38+, CD77–) and in Burkitt's lymphoma cell lines at the centrocyte stage, demonstrating that centrocytes express sterile transcripts for IgE35 and Sγ-Sμ/Sε-Sμ DNA switch recombination products.35,36 Our results are consistent with these findings because we found that IgE expression was localized to the light zone during GC development, suggesting that switching from IgM to IgE may have occurred during the centrocyte stage of GC development. Additional studies are needed to clarify this aspect of GC B-cell development during IgE-induced immune responses.
Follicular dendritic cells are specialized antigen-presenting cells found within GCs that can trap and retain antigens in the form of immune complexes for extended periods of time.37 Although FDCs express Fc receptors that can bind IgE (CD23)12 our studies demonstrated that B cells were expressing IgE within GCs using multiple techniques; immunohistochemistry, flow cytometry and mRNA expression by RT-PCR. Interestingly, it has been postulated that FDC-associated antigen plays a role in the long-term maintenance of IgE responses, most likely through the induction of IgE-specific memory T and B cells.38
A shift toward IgG2a and IgG2b antibody production would be desirable in certain IgE-mediated allergic immune responses. Recent studies have shown that treatment of mice with a soluble form of IL-13 receptor α1 resulted in increased production of IgG2a and IgG2b without alterations in IgG1.39 Interestingly, the IL-13 receptor α1 is restricted to B-cell follicles and is found on FDCs, but not T cells or interdigitating dendritic cells.39 These findings suggest that FDCs may play an important role in allergic responses and need to be considered when designing strategies to block antigen-driven IgE responses.
Acknowledgments
The authors thank Ms Qun Lu for her fine technical skills in microdissection. This work was supported by R01-AI26328 and by special funds from the Department of Pathology and Laboratory Medicine, Geffen School of Medicine at UCLA.
Glossary
Abbreviations
- BSA
bovine serum albumin
- FDC
follicular dendritic cell
- GC
germinal centre
- HRP
horseradish peroxidase
- IgE
immunoglobulin E
- IL-4
interleukin-4
- NP
4-hydroxy-3-nitrophenyl acetyl
- phOx
2-phenyloxazolone
- PNA
peanut agglutinin
- RT-PCR
reverse transcription polymerase chain reaction
References
- 1.Nieuwenhuis P, Opstelten D. Functional anatomy of germinal centers. Am J Anat. 1984;170:421–35. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- 2.Stedra J, Cerny J. Distinct pathways of B cell differentiation. I. Residual T cells in athymic mice support the development of splenic germinal centers and B cell memory without an induction of antibody. J Immunol. 1994;152:1718–26. [PubMed] [Google Scholar]
- 3.Butch AW, Chung GH, Hoffmann JW, Nahm MH. Cytokine expression by germinal center cells. J Immunol. 1993;150:39–47. [PubMed] [Google Scholar]
- 4.Jacob J, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. II. A common clonal origin for periarteriolar lymphoid sheath-associated foci and germinal centers. J Exp Med. 1992;176:679–87. doi: 10.1084/jem.176.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker DJ, Linton PJ, Zaharevitz S, Biery M, Gingeras TR, Klinman NR. Defining subsets of naive and memory B cells based on the ability of their progeny to somatically mutate in vitro. Immunity. 1995;2:195–203. doi: 10.1016/s1074-7613(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 6.Linton PJ, Lo D, Lai L, Thorbecke GJ, Klinman NR. Among naive precursor cell subpopulations only progenitors of memory B cells originate germinal centers. Eur J Immunol. 1992;22:1293–7. doi: 10.1002/eji.1830220526. [DOI] [PubMed] [Google Scholar]
- 7.Linton PL, Decker DJ, Klinman NR. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989;59:1049–59. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- 8.Auci DL, Chice SM, Heusser C, Athanassiades TJ, Durkin HG. Origin and fate of IgE-bearing lymphocytes. II. Gut-associated lymphoid tissue as sites of first appearance of IgE-bearing B lymphocytes and hapten-specific IgE antibody-forming cells in mice immunized with benzylpenicilloyl-keyhole limpet hemocyanin by various routes: relation to asialo GM1 ganglioside+ cells and IgE/CD23 immune complexes. J Immunol. 1992;149:2241–8. [PubMed] [Google Scholar]
- 9.Wang CH, Richards EM, Block RD, Lezcano EM, Gutierrez R. Early induction and augmentation of parasitic antigen-specific antibody-producing B lymphocytes in the non-Peyer's patch region of the small intestine. Front Biosci. 1998;3:A58–65. doi: 10.2741/a253. [DOI] [PubMed] [Google Scholar]
- 10.Chvatchko Y, Kosco-Vilbois MH, Herren S, Lefort J, Bonnefoy JY. Germinal center formation and local immunoglobulin E (IgE) production in the lung after an airway antigenic challenge. J Exp Med. 1996;184:2353–60. doi: 10.1084/jem.184.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nahm MH, Takes PA, Bowen MB, Macke KA. Subpopulations of B lymphocytes in germinal centers, II. A germinal center B cell subpopulation expresses sIgD and CD23. Immunol Lett. 1989;21:201–8. doi: 10.1016/0165-2478(89)90105-3. [DOI] [PubMed] [Google Scholar]
- 12.Maeda K, Burton GF, Padgett DA, Conrad DH, Huff TF, Masuda A, Szakal AK, Tew JG. Murine follicular dendritic cells and low affinity Fc receptors for IgE (Fc epsilon RII) J Immunol. 1992;148:2340–7. [PubMed] [Google Scholar]
- 13.Makela O, Kaartinen M, Pelkonen JL, Karjalainen K. Inheritance of antibody specificity V. Anti-2-phenyloxazolone in the mouse. J Exp Med. 1978;148:1644–60. doi: 10.1084/jem.148.6.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller KA, Kanagawa O, Nahm MH. T cells within germinal centers are specific for the immunizing antigen. J Immunol. 1993;151:4505–12. [PubMed] [Google Scholar]
- 15.Kelly KA, Bucy RP, Nahm MH. Germinal center T cells exhibit properties of memory helper T cells. Cell Immunol. 1995;163:206–14. doi: 10.1006/cimm.1995.1118. [DOI] [PubMed] [Google Scholar]
- 16.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Kaartinen M, Griffiths GM, Markham AF, Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. Nature. 1983;304:320–4. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- 19.Berek C, Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 20.Dessein A, Germain RN, Dorf ME, Benacerraf B. IgE responses to synthetic polypeptide antigens. I. Simultaneous Ir gene and isotype-specific regulation of IgE responses to l-glutamic acid60-l-alanine30-l-tyrosine10 (GAT) J Immunol. 1979;123:463–70. [PubMed] [Google Scholar]
- 21.Hamaoka T, Newburger PE, Katz DH, Benacerraf B. Hapten-specific IgE antibody responses in mice. 3. Establishment of parameters for generation of helper T cell function regulating the primary and secondary responses of IgE and IgG B lymphocytes. J Immunol. 1974;113:958–73. [PubMed] [Google Scholar]
- 22.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 23.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–5. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 24.Laszlo G, Hathcock KS, Dickler HB, Hodes RJ. Characterization of a novel cell-surface molecule expressed on subpopulations of activated T and B cells. J Immunol. 1993;150:5252–62. [PubMed] [Google Scholar]
- 25.Han S, Zheng B, Takahashi Y, Kelsoe G. Distinctive characteristics of germinal center B cells. Semin Immunol. 1997;9:255–60. doi: 10.1006/smim.1997.0081. [DOI] [PubMed] [Google Scholar]
- 26.Cervenak L, Magyar A, Boja R, Laszlo G. Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol Lett. 2001;78:89–96. doi: 10.1016/s0165-2478(01)00239-5. [DOI] [PubMed] [Google Scholar]
- 27.Apel M, Berek C. Somatic mutations in antibodies expressed by germinal centre B cells early after primary immunization. Int Immunol. 1990;2:813–19. doi: 10.1093/intimm/2.9.813. [DOI] [PubMed] [Google Scholar]
- 28.Gascan H, Gauchat JF, Aversa G, Van Vlasselaer P, de Vries JE. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 29.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–8. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–31. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh N, Fuleihan R, Ramesh V, et al. Deletions in the ligand for CD40 in X-linked immunoglobulin deficiency with normal or elevated IgM (HIGMX-1) Int Immunol. 1993;5:769–73. doi: 10.1093/intimm/5.7.769. [DOI] [PubMed] [Google Scholar]
- 32.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–92. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 34.Finkelman FD, Katona IM, Urban JF, Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–41. [PubMed] [Google Scholar]
- 35.Liu YJ, Malisan F, de Bouteiller O, et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–50. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 36.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, Casali P. CD40 ligand and appropriate cytokines induce switching to IgG, IgA and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+IgD+ B cell line. J Immunol. 1998;160:2145–57. [PMC free article] [PubMed] [Google Scholar]
- 37.Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 38.Helm SL, Burton GF, Szakal AK, Tew JG. Follicular dendritic cells and the maintenance of IgE responses. Eur J Immunol. 1995;25:2362–9. doi: 10.1002/eji.1830250836. [DOI] [PubMed] [Google Scholar]
- 39.Poudrier J, Graber P, Herren S, Gretener D, Elson G, Berney C, Gauchat JF, Kosco-Vilbois MH. A soluble form of IL-13 receptor alpha 1 promotes IgG2a and IgG2b production by murine germinal center B cells. J Immunol. 1999;163:1153–61. [PubMed] [Google Scholar]