Abstract
Human infection with Mycobacterium leprae, an intracellular bacterium, presents as a clinical and immunological spectrum; thus leprosy provides an opportunity to investigate mechanisms of T-cell responsiveness to a microbial pathogen. Analysis of the T-cell receptor (TCR) repertoire in leprosy lesions revealed that TCR BV6+ T cells containing a conserved CDR3 motif are over-represented in lesions from patients with the localized form of the disease. Here, we derived a T-cell clone from a leprosy lesion that expressed TCR BV6 and the conserved CDR3 sequence L-S-G. This T-cell clone produced a T helper type 1 cytokine pattern, directly lysed M. leprae-pulsed antigen-presenting cells by the granule exocytosis pathway, and expressed the antimicrobial protein granulysin. BV6+ T cells may therefore functionally contribute to the cell-mediated immune response against M. leprae.
Keywords: bacteria, major histocompatibility complex, T cell, T-cell receptor BV genes
Introduction
Mycobacterium leprae is the causative agent of leprosy, a potentially debilitating disease that is still prevalent worldwide.1 Moreover, the clinical manifestations of the disease correlate with the type of immune response to the pathogen M. leprae, providing an extraordinary model to understand human host defence to microbial infection. At one end of a spectrum, tuberculoid patients (T-Lep) mount a cell-mediated immune response and limit the infection. They present with few lesions containing rare bacilli and T helper type 1 (Th1) cytokine-producing CD4 T cells in organized granulomas.2,3 At the other end of the spectrum, lepromatous patients (L-Lep) present with numerous disseminated lesions containing high numbers of bacilli and Th2 cytokine-producing T cells, which are ineffective at containing the infection. Importantly, approximately one in 50 T cells from T-Lep lesions proliferate in response to M. leprae, whereas few T cells could be derived from L-Lep lesions that proliferated in response to M. leprae.4
A powerful immunological approach to understand the breadth of the T-cell response in disease is the assessment of the T-cell receptor (TCR) repertoire according to variable (V) gene diversity. Using this strategy for the study of human leprosy, we determined that T cells bearing the BV6 TCR were over-represented in the skin lesions of the resistant T-Lep form as compared to the more disseminated form of leprosy.5 In addition, T cells bearing the BV6 TCR were over-represented in lesions of reversal reaction,6 a reactional state of leprosy in which patients are actively upgrading from the L-Lep to the T-Lep form.7 Furthermore, TCR transcripts from these BV6+ T cells encoded a conserved motif, the V-J junction (CDR3 region), indicating recognition of an immunodominant antigen.5 As a first step towards understanding the generation of TCR diversity in microbial infection, we studied a T-cell clone derived from a reversal reaction lesion that expressed the BV6 TCR.
Materials and methods
Patient and donor specimens
The leprosy patient (male, Asian-Pacific Islander) from whom the biopsy was obtained was diagnosed and classified by one of us (THR) using the criteria of Ridley and Jopling8 at the Hansen's Disease Clinic of the Los Angeles County-University of Southern California Medical Center. The patient had been referred to this clinic for management of a reversal reaction, occurring after multiple antibiotic treatments for borderline lepromatous leprosy. The reversal reaction was controlled by prednisone; however the reversal reaction recurred 13 months after termination of prednisone, at which time the biopsy, the source of the T-cell clone described, was obtained. Blood and lesion samples were acquired after informed consent using protocols approved by the institution's internal review board. Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by density gradient centrifugation using Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) and irradiated (3000 rads) for use as antigen-presenting cells (APCs). Human leucocyte antigen (HLA) typing was performed at the UCLA Tissue Typing facility. T cells were isolated from lesions by physical separation of the tissue9,10 and cultured in RPMI-1640 medium enriched with sodium pyruvate, penicillin, streptomycin and l-glutamine (all obtained from Invitrogen, San Diego, CA) in the presence of human serum (2%) and fetal calf serum (8% FCS; Hyclone, Logan, UT). T cells were cultured with M. leprae sonicate (3 μg/ml) with either autologous or heterologous HLA-DR-matched healthy donor PBMCs followed by a total of three feedings of interleukin-2 (IL-2; 1 nm, Chiron Diagnostics, Norwood, MA) 3 days apart. The T-cell clone was derived by limiting dilution.10
Antigens and antibodies
Bacterial lysates of M. leprae and M. tuberculosis, strain H37Rv were prepared by probe sonication and French press, respectively.11,12 The following antibodies were used for flow cytometry: CD4 (Caltag, Burlingame, CA); CD8 (Caltag); CD3 (Becton-Dickinson/Pharmingen, San Diego, CA); immunoglobulin G controls (Caltag). The following antibodies were used in blocking experiments: OKT6 (anti-CD1a13); BCD1b3.1 (anti-CD1b14); F10/21A3 (anti-CD1c15); OKT3 [anti-CD3; American Type Culture Collection (ATCC), Manassas, VA]; OKT4 (anti-CD4; ATCC); OKT8 (anti-CD8; ATCC); immunoglobulin G controls (Sigma Chemical Corp., St Louis, MO). Antigen-presenting PBMCs were incubated with antigen and blocking antibodies for at least 60 min before the addition of T cells. The following antibodies were used in immunofluorescent cell labelling: DH4 (anti-granulysin16); dG9 (anti-perforin; Pharmingen, San Diego, CA). Immunofluorescence studies were carried out as previously described.17 Anti-Fas antibody was used in the cytotoxicity assays (Immunotech, Westbrook, IL).
Immunological assays
T-cell proliferation and cytokine release assays were carried out as follows: T cells (104) were cultured with heterologous HLA DR-matched irradiated PBMCs (105) with antigen, and culture medium (RPMI-1640 with 10% human serum). The assay was carried out in triplicate wells for 72 hr at 37°; [3H]thymidine (1 μCi/well; ICN, Costa Mesa, CA) was added (4 hr) followed by harvest onto scintillation filters (Ready Filter; Beckman, Fullerton, CA) and [3H]thymidine incorporation was measured on the Beckman LS 6000IC scintillation counter (Beckman-Coulter, Brea, CA). Cytokine release was measured by enzyme-linked immunosorbent assay for interferon-γ (IFN-γ; Pharmingen, San Diego, CA) and interleukin-4 (IL-4; Biosource, Camarillo, CA). Cytotoxicity assays measuring 51Cr-release were performed as previously described18 with a pretreatment (18 hr) of T cells with 30 mm strontium (Sr2+ Aldrich, Milwaukee, WI) or the addition of anti-Fas antibody (1 μg/ml, Immunotech).
Reverse transcription-polymerase chain reaction (RT-PCR) and sequencing of the TCR
Total RNA was isolated from T cells and cDNA synthesized using a previously described protocol.6 CD3 analysis was employed for normalization using the following oligonucleotide primer sequences: 5′-CTGGACCTGGGAAAACGCATC; 3′-GTACTGAGCATCATCTCGATC. Twenty-eight primers amplifying all 24 BV family genes were synthesized (Gibco BRL Life Technologies, Gaithersburg, MD) as previously described5 with the addition of the BV6 common primer sequence: TGTATCTCTGTGCCAGCAGC. The RT-PCR was carried out as previously described19 using the following 35 cycle programme: 94° for 1 min; 62° for 1 min; 72° for 1·5 min; 72° for 10 min; 4° indefinitely. The PCR product was visualized on a 2% agarose gel. Direct DNA sequencing was performed by first purifying the PCR product using the QIAquick PCR purification kit (QIAGEN, Chatsworth, CA); sequencing the PCR product using the Sequenase Version 2·0 DNA sequencing kit (USB-Amersham Life Science, Cleveland, OH); and analysis was carried out using an automated sequencer at the UCLA core sequencing facility.
Results
Derivation of a BV6+ T-cell clone from a T-Lep lesion
T cells isolated from leprosy lesions provide useful tools to dissect the host response to the pathogen. Previous studies indicated that the frequency of CD4+ T cells that recognize M. leprae is greater in tuberculoid lesions compared to the blood of the same donors, as well to lesions of lepromatous patients.20–22Mycobacterium leprae-reactive T cells from tuberculoid lesions can be expanded as T-cell clones, providing useful tools with which to evaluate functional T-cell responses in cell-mediated immunity to the pathogen. Several CD4+ T-cell clones were derived from a leprosy patient undergoing a reversal reaction that recognized M. leprae (data not shown).
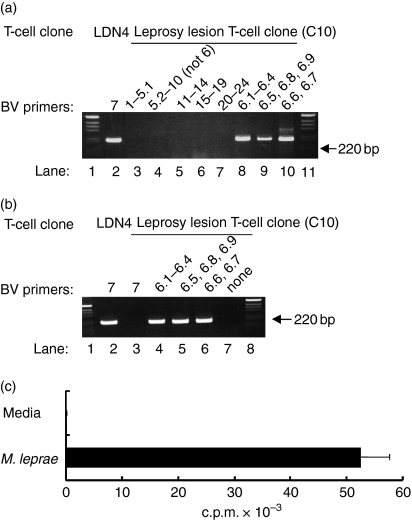
To characterize the TCR of these T-cell clones, RT-PCR was performed. Since multiple family members exist for BVs 5, 6 and 13, 28 primers were designed to amplify the genes of 24 BV families. All primers, except for the BV6 primers, were initially pooled and each pool contained five primers at equimolar concentrations. One clone was found to be exclusively BV6+ and product was amplified using all three BV6 primers. In Fig. 1(a), lanes 3–7 show that cDNA from this T-cell clone was not amplified for any other BV gene while lanes 8–10 show the BV6 gene products utilizing all three BV6 primers. Another T-cell clone, LDN4,23 expressing a BV7 TCR was included as a positive control for the RT-PCR (Fig. 1a, lane 2). To confirm BV6 expression, negative controls were carried out. The BV7 primer, which amplifies LDN4 cDNA (Fig. 1b, lane 2), does not amplify any product from the BV6+ T-cell clone (lane 3), while all the BV6 primers do (Fig. 1b, lanes 4–6). A further control confirms that the BV6 amplification is not a false-positive because in the absence of BV6 primers, no amplification of product occurs (Fig. 1b, lane 7). Based on these results and previous studies indicating that the TCR BV6 gene was over-represented in the lesions of patients with T-Lep5 we selected this T-cell clone for more detailed analysis. Moreover, the BV6+ T-cell clone exhibited a robust proliferative response to an extract of M. leprae (Fig. 1c).
Figure 1.
PCR analysis of a BV6+ T-cell clone. (a) Pools of BV primers at equal concentrations were used to assess the BV chain gene usage of a leprosy-lesion-derived T-cell clone (C10); lanes 1 and 11 contain the 1-kb ladder marker; lane 2 contains BV7-amplified cDNA from a BV7-expressing T-cell clone, LDN4; pools of primers are as follows, lane 3, BVs 1, 2, 3, 4, 5.1; lane 4, BVs 5.23, 7, 8, 9, 10; lane 5, BVs 11, 12, 13.1, 13.2, 14; lane 6, BVs 15, 16, 17, 18, 19; lane 7, BVs 20, 21, 22, 23, 24; lane 8, BV 6.1/2/3/4; lane 9, BV 6.5/8/9; lane 10, BV 6.6/7. (b) Lane 2, BV7+ T-cell clone; lane 3, leprosy lesion clone (C10) plus BV7 primer (as a negative control); lanes 4, 5 and 6, leprosy lesion clone (C10) plus BV 6.1/2/3/4, 6.5/8/9, and 6.6/7 primers; lane 7, leprosy lesion clone with no BV primers (negative control lanes). (c) Proliferative response of a leprosy lesion T-cell clone to M. leprae antigen. The BV6+ T-cell clone was stimulated with M. leprae bacterial lysates in a [3H]thymidine incorporation assay. The data represent one of more than 30 experiments. Values are expressed as the mean ± SEM of triplicate cultures.
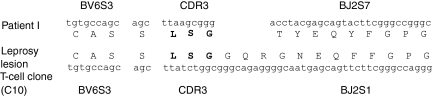
We previously demonstrated that BV6+ T cells in T-Lep lesions contained a specific amino acid motif in the CDR3.5,6 To determine if the BV6+ T-cell clone also contained a similar motif, the sequence of the BV6+ T-cell clone was determined. The TCR sequence of T cells previously isolated from the lesion of a T-Lep patient,5 patient I, is similar to that of the BV6+ T-cell clone (Fig. 2). The BV chain from both patient I and the BV6+ T-cell clone specifically utilizes the same BV6S3A1N1T gene, previously designated as Vβ6.4 and interchangeably designated as BV6S3.24 Importantly, although the BV6+ T-cell clone utilizes a different BJ chain than patient I,5 the T-cell clone does contain the conserved L-S-G motif in the CDR3 (Fig. 2).
Figure 2.
Sequence analysis of the BV6+ T-cell clone and comparison to a previously identified T-Lep patient BV6+ TCR. The T-cell clone shares the same BV chain as a patient TCR from a previous study.5 The T-cell clone also expresses the exact CDR3 ‘L-S-G’ motif, but uses a different BJ gene.
The BV6+ T-cell clone from a leprosy lesion is CD4+ and major histocompatibility complex (MHC) class II-restricted
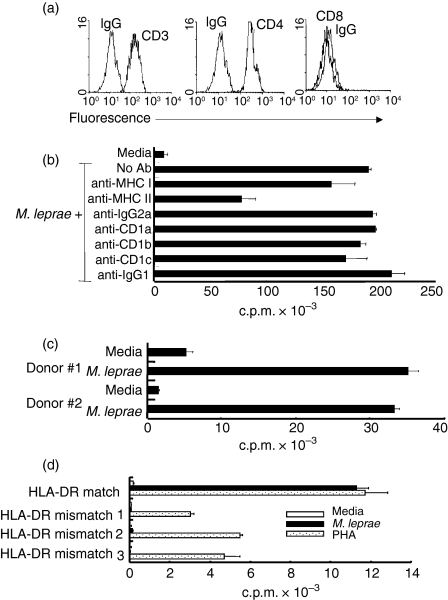
The host response to mycobacterial infection requires both CD8+ MHC class I and CD4+ class II-restricted T cells.25,26 Moreover, both TCR-αβ-positive and TCR-γδ-positive cells play a role in the host response to mycobacterial infection.25,27,28 To identify the TCR expression and MHC restriction of the BV6+ T-cell clone derived from a tuberculoid leprosy lesion, we first evaluated TCR and T-cell co-receptor expression. We found that the BV6+ T-cell clone expresses a TCR-αβ and the CD4 co-receptor, and does not express TCR-γδ or CD8 co-receptor (Fig. 3a). To identify MHC restriction, neutralizing antibodies against MHC class I and class II, and CD1 antigen-presenting molecules were used in a T-cell proliferation assay. MHC class II antibodies blocked the proliferative response of the BV6+ T-cell clone by 60%, whereas anti-MHC class I, anti-CD1a, anti-CD1b, or anti-CD1c antibodies did not show appreciable neutralizing activity (20% or less) on the BV6+ T-cell clone (Fig. 3b). The phenotype analysis and the antibody blocking experiments indicate that the BV6+ T-cell clone is a CD4+ MHC class II-restricted T-cell clone.
Figure 3.
Co-receptor expression and MHC restriction of BV6+ T-cell clone. (a) Phenotypic analysis of the BV6+ T-cell clone was performed by flow cytometry. Isotype control and specific antibodies are labelled on the histograms. (b) APCs were cultured with neutralizing antibodies to MHC and MHC-like antigen-presenting molecules (60 min) before T cells were added. Data shown are representative of three experiments. Values are expressed as the mean ± SEM of triplicate cultures. (c) APCs were from two HLA-DR-matched individuals. Data shown are representative of three experiments. (d) T-cell clone does not respond to antigen presented by HLA-DR mismatched APCs. Values are expressed as the mean ± SEM of triplicate cultures. Data shown are representative of multiple experiments.
Characterization of the precise HLA restriction element for the BV6+ T-cell clone was pursued by tissue typing the donor of the T-cell clone. Tissue typing of blood from the leprosy patient from which the BV6+ T-cell clone was derived indicated a genotype of HLA-DRB1*1501 and HLA-DRB5*0101, DR15 and DR51a, respectively.29 Tissue typing of several healthy donors identified two DR-matched individuals (Table 1). The PBMCs from the two DR-matched donors were cultured with the BV6+ T-cell clone to evaluate their ability to present antigen to the BV6+ T-cell clone. The BV6+ T-cell clone responded effectively to antigen presented by the DR-matched donors (Fig. 3c). However, because DR15 and DR51a are linked30 we were not able to determine with which of these two class II molecules the BV6+ TCR sees as the antigen restriction element. Using HLA mismatched donors, T-cell proliferation to M. leprae was undetectable, although the ability to support T-cell responses to mitogen was intact with the mismatched donors (Fig. 3d). Together, these data confirm that the BV6+ T-cell clone is a MHC class II HLA-DR 15, 51a-restricted CD4+ T-cell clone.
Table 1.
Two DR-matched individuals identified by tissue typing of several healthy donors
| Donor | HLA-DR type |
|---|---|
| Leprosy patient from whom | DRB11501, DRB50101 |
| BV6+ T-cell clone was derived | |
| APC donor 1 | DRB11501, DRB50101 |
| APC donor 2 | DRB11501, DRB50101 |
Functional characterization of a TCR BV6+ T-cell clone
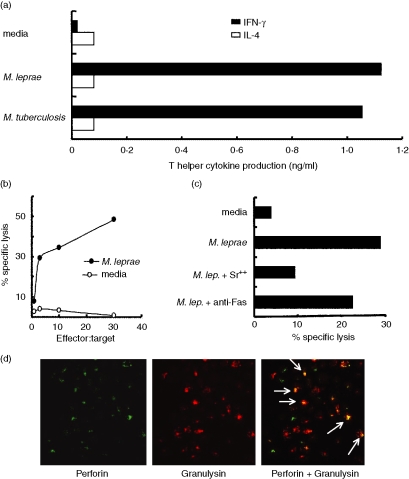
T-Lep and reversal reaction lesions have been characterized as containing inflammatory Th1 cytokine secreting CD4+ T cells.3,31 T cells that were previously isolated from T-Lep and reversal reaction leprosy lesions and shown to predominantly express the BV6+ TCR5,6 were not tested for their cytokine secretion profiles. Hence, we were interested to know the Th designation of the BV6+ clone. T-cell supernatants were used in enzyme-linked immunosorbent assays to test for the production of IFN-γ and IL-4. The BV6+ T cells produce IFN-γ, a definitive Th1 cytokine, in response to both M. leprae and M. tuberculosis(Fig. 4a); but does not produce any IL-4, the classical Th2 cytokine (Fig. 4a).
Figure 4.
T-cell effector functions of the BV6+ T-cell clone. (a) Cytokines (IFN-γ and IL-4) were measured from supernatants of T cells stimulated with antigen (M. leprae or M. tuberculosis) or media. The data are presented as the mean of triplicate wells and are representative of three experiments. (b) Cytolytic analysis of the BV6+ T-cell clone. Leprosy lesion T-cell clones can affect cytolytic activity against M. leprae-pulsed APCs as measured in a 51Cr-release assay. The data are representative of four separate experiments. (c) T cells were incubated with strontium (18 hr) to degranulate cytolytic mediators or with anti-Fas antibody to prevent Fas–FasL interactions. Monocyte-derived dendritic cells were then incubated with T cells. Cytolytic activity of the T-cell clones measured by a 51Cr-release assay. The data are representative of three experiments. (d) Two-colour immunofluorescent cell labelling of the BV6+ T-cell clone reveals that both granulysin and perforin co-localize in individual cells (white arrows).
There are reports of cytolytic CD4 T cells,32–35 therefore the BV6+ T-cell clone was studied for possible cytolytic capability. APCs were pulsed with M. leprae lysate and mixed with different ratios of BV6+ T cells in a 51Cr-release cytotoxicity assay. The BV6+ T-cell clone was able to kill up to 50% of antigen-pulsed targets at an effector to target ratio of 30 : 1 (Fig. 4b). Even at an effector to target ratio of 10 : 1, the BV6+ T-cell clone was able to lyse approximately 35% of antigen-pulsed targets. To investigate the mechanism of killing, cytotoxicity assays were performed with the addition of anti-Fas antibodies to block the Fas–Fas ligand mode of killing, or with the addition of strontium to degranulate intracellular cytotoxic molecules. Figure 4(c) shows that the BV6+ T-cell clone's ability to lyse antigen-pulsed targets is inhibited by the addition of the degranulating strontium, but the cytolytic activity is not significantly inhibited by the addition of anti-Fas antibodies. The data indicate that the BV6+ T-cell clone utilizes a granule-based method for killing.
The secretory granules of CD8 lymphocytes contain perforin, a protein that polymerizes to form pores in the membranes of target cells. Perforin is thought to allow proteins, such as granzymes, to enter target cells and to induce apoptosis. Granulysin is another cytolytic granule protein that is released into target cells. Granulysin is known to kill a variety of microbial pathogens including bacteria, fungi and parasites.15 We therefore wanted to know if the BV6+ T-cell clone contained either perforin or granulysin to identify its mechanism of cytolysis. Two-colour immunofluorescent staining was carried out on the BV6+ T cells as shown in Fig. 4(d). The BV6+ T cells contained both perforin and granulysin, co-localizing in the same cells (white arrows). Collectively, the data indicate that the BV6+ T-cell clone utilizes the granule exocytosis pathway to lyse target cells and contains the antimicrobial protein, granulysin.
Discussion
Although T cells are essential to the host defence against microbial infection, the mechanism of T-cell responsiveness to the pathogen is not well understood. Previously, we analysed the TCR repertoire in the human disease leprosy and found that a particular TCR, BV6, was over-represented in the self-limited form of the disease compared to the progressive form.5,6 Here, we have derived a T-cell clone from a tuberculoid leprosy lesion that expressed the BV6 TCR. The BV6+ T-cell clone was found to be CD4+ and to produce the Th1 cytokine IFN-γ. It also lysed M. leprae-pulsed APCs via the granule exocytosis pathway and contained the antimicrobial protein, granulysin. In this manner, BV6+ T cells representing the dominant TCR in T-Lep lesions probably contribute to cell-mediated immunity against M. leprae.
An effective immune response against infection by intracellular bacteria requires the generation and local accumulation of antigen-specific CD4+ T cells. In human leprosy, for example (a) CD4+ T cells are more prominent in the lesions of patients with the localized form T-Lep as compared to the disseminated form L-Lep of the disease;4 (b) the precursor frequency of M. leprae-reactive CD4+ T cells is five-fold greater in the T-Lep lesions compared to the blood of the same donors,4 whereas few M. leprae-reactive CD4+ T cells could be detected in lepromatous lesions;4 and (c) the TCR repertoire is skewed, with an over-representation of the BV6 TCR in T-Lep lesions.5,6 We analysed the TCR of a BV6+ T cell clone, derived from a T-Lep lesion and found it to exclusively express the BV6S3 chain, previously designated as Vβ6.4, the same TCRV region that is over-represented in T-Lep patient lesions.5,6 Furthermore, because specific amino acids in the CDR3 can be selected for by antigenic epitopes,36–40 we analysed the BV6+ T-cell clone and found it to contain L-S-G, one of the conserved CDR3 motifs previously identified in BV6+ T cells in leprosy lesions (L-X-G; L-T-S-G; L-X-G-X-G5). The data here indicate that BV6+ T cells in leprosy lesions can recognize M. leprae antigens. That this M. leprae-reactive T-cell clone expressed the BV6 TCR, a TCR that is over-represented in lesions from tuberculoid leprosy patients,5,6 and shared aspects of the conserved VDJ junction found in BV6 TCR transcripts from tuberculoid leprosy lesions is consistent with the recognition of a nominal antigen as opposed to a superantigen. Clearly, this is a single T-cell clone; further characterization of the antigen recognized should provide additional insights into the shaping of the TCR repertoire.
In conjunction with the surrounding cytokine environment, the TCR is activated by the specific and exclusive recognition of an epitope in the context of an antigen-presenting molecule. In the case of the T-Lep and reversal reaction lesions, inflammatory cytokines such as IFN-γ3 and IL-1241 are produced while IL-4 and IL-10 cytokines are not. We investigated cytokine production of the BV6+ T-cell clone and determined that it is a Th1 T-cell clone, producing IFN-γ but not IL-4 in response to M. leprae. Interferon-γ is part of the local cytokine pattern in T-Lep lesions and can contribute to cell-mediated immunity by its ability to stimulate cells of the monocyte/macrophage lineage.
Another capacity in which the BV6+ T-cell clone can contribute to host defence is through its cytolytic capability. Our data indicate that the BV6+ T-cell clone can lyse M. leprae-pulsed APCs, with as much as 50% specific lysis at a 30 : 1 effector to target ratio. This cytolytic activity can be inhibited by the addition of the degranulator, strontium, but not by the addition of anti-Fas antibodies. Hence, the BV6+ T-cell clone does not kill by the traditional Fas-Fas ligand method, but instead uses a granule-based mechanism of cytolysis. The BV6+ T-cell clone expressed both perforin and granulysin, which co-localized in the same granules. Perforin is known for creating pores in the membranes of target cells, allowing apoptosis-inducing granzymes access to the cytosplasm of those targets. Granulysin, a member of the SAPLIP family, is produced by cytotoxic T lymphocytes and has been shown to express antimicrobial activity.42 Granulysin was shown to directly kill extracellular M. tuberculosis17 but it killed intracellular M. tuberculosis only in the presence of purified perforin.43 Furthermore, L-Lep lesions do not produce granulysin as do T-Lep lesions, although both lesions show equal amounts of perforin.44
The T-cell clone used in this study produced a Th1 cytokine profile, expressed the cytolytic granule proteins perforin and granulysin, and lysed antigen-pulsed monocytes in a granule-dependent manner. These characteristics are part of the cell-mediated immune programme that is known to restrict the growth of intracellular pathogens such as mycobacteria. This is consistent with the origin of the T-cell clone, it having been derived from the lesion of a patient undergoing a reversal reaction, i.e. upgrading clinically from the lepromatous to tuberculoid form of the disease. Evaluation of additional BV6 T cells derived from lesions will strengthen the correlation between the TCR over-represented in tuberculoid lesions with T cells exhibiting functions that are consistent with the promotion of protective immunity against leprosy infection. Given that the T cells expressing the dominant TCR contribute to cell-mediated immunity, delineation of the antigen recognized by the TCR BV6+ T cells should provide additional insight into the mechanism of immune responsiveness in human host defence against infection.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (AR 40312, AI47868) to R.L.M. We thank Drs John Belisle and Patrick Brennan for their insightful discussions. We thank the UCLA tissue-typing laboratory for HLA typing of patients, the UCLA flow cytometry core laboratory, and individuals for this study.
References
- 1.Leprosy. Global situation. Wkly Epidemiol Rec. 2002;77:1–8. [PubMed] [Google Scholar]
- 2.Salgame P, Abrams JS, Clayberger C, Goldstein H, Convit J, Modlin RL, Bloom BR. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 3.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 4.Modlin RL, Melancon-Kaplan J, Young SMM, Pirmez C, Kino H, Convit J, Rea TH, Bloom BR. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci USA. 1988;85:1213–17. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Golkar L, Uyemura K, et al. T cells bearing Vβ6 T-cell receptors in the cell-mediated immune response to Mycobacterium leprae. J Immunol. 1993;151:7105–16. [PubMed] [Google Scholar]
- 6.Wang X-H, Ohmen JD, Uyemura K, Rea TH, Kronenberg M, Modlin RL. Selection of T lymphocytes bearing limited T-cell receptor beta chains in the response to a human pathogen. Proc Natl Acad Sci USA. 1993;90:188–92. doi: 10.1073/pnas.90.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamura M, Wang X-H, Ohmen JD, Uyemura K, Rea TH, Bloom BR, Modlin RL. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–5. [PubMed] [Google Scholar]
- 8.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 9.Modlin RL, Mehra V, Wong L, et al. Suppressor T lymphocytes from lepromatous leprosy skin lesions. J Immunol. 1986;137:2831–4. [PubMed] [Google Scholar]
- 10.Mehra V, Bloom BR, Bajardi AC, et al. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J Exp Med. 1992;175:275–84. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckman EM, Melian A, Behar SM, et al. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J Immunol. 1996;157:2795–803. [PubMed] [Google Scholar]
- 12.Hirschfield GR, McNeil M, Brennan PJ. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis. J Bacteriol. 1990;172:1005–13. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF. Discrete stages of human intrathymic differentiation. Analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980;77:1588–92. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behar SM, Porcelli SA, Beckman EM, Brenner MB. A pathway of costimulation that prevents anergy in CD28– T cells. B7-independent costimulation of CD1-restricted T cells. J Exp Med. 1995;182:2007–18. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melian A, Geng YJ, Sukhova GK, Libby P, Porcelli SA. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol. 1999;155:775–86. doi: 10.1016/S0002-9440(10)65176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson DA, Kaspar AA, Poulain FR, Krensky AM. Biosynthesis of granulysin, a novel cytolytic molecule. Mol Immunol. 1999;36:413–22. doi: 10.1016/s0161-5890(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 17.Stenger S, Hanson DA, Teitlebaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–5. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 18.Stenger S, Mazzaccaro RJ, Uyemura K, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–7. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 19.Uyemura K, Deans RJ, Band H, Ohmen J, Panchamoorthy G, Morita CT, Rea TH, Modlin RL. Evidence for clonal selection of gamma/delta T-cells in response to a human pathogen. J Exp Med. 1991;174:683–92. doi: 10.1084/jem.174.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan G, Weinstein DE, Steinman RM, Levis WR, Elvers U, Patarroyo ME, Cohn ZA. An analysis of in vitro T cell responsiveness in lepromatous leprosy. J Exp Med. 1985;162:917–29. doi: 10.1084/jem.162.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom BR, Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 22.Godal T, Myklestad B, Samuel DR, Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971;9:821–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Sieling PA, Chatterjee D, Porcelli SA, et al. CD1-restricted T cell recognition of microbial lipoglycans. Science. 1995;269:227–30. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 24.Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogenetics. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- 25.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–17. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladel CH, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann SH. Contribution of alpha/beta and gamma/delta T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette–Guérin: studies with T cell receptor-deficient mutant mice. Eur J Immunol. 1995;25:838–46. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 28.Modlin RL, Pirmez C, Hofman FM, Torigian V, Uyemura K, Rea TH, Bloom BR, Brenner MB. Lymphocytes bearing antigen-specific gamma/delta T-cell receptors in human infectious disease lesions. Nature. 1989;339:544–8. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 29.Schreuder GM, Hurley CK, Marsh SG, Lau M, Maiers M, Kollman C, Noreen H. The HLA dictionary 1999. a summary of HLA-A-B-C-DRB1/3/4/5-DQB1 alleles and their association with serologically defined HLA-A-B-C-DR, and -DQ antigens. Human Immunol. 1999;60:1157–81. doi: 10.1016/s0198-8859(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 30.Tiercy JM, Jeannet M, Mach B. Oligonucleotide typing analysis for the linkage disequilibrium between the polymorphic DRB1 and DRB5 loci in DR2 haplotypes. Tissue Antigens. 1991;37:161–4. doi: 10.1111/j.1399-0039.1991.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 31.Verhagen CE, Wierenga EA, Buffing AA, Chand MA, Faber WR, Das PK. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin: a follow-up study. J Immunol. 1997;159:4474–83. [PubMed] [Google Scholar]
- 32.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4(+) and CD8(+) T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand-independent mechanism. J Immunol. 2001;167:2734–42. doi: 10.4049/jimmunol.167.5.2734. [DOI] [PubMed] [Google Scholar]
- 33.Emoto M, Emoto Y, Kaufmann SH. TCR-mediated target cell lysis by CD4+NK1+ liver T lymphocytes. Int Immunol. 1997;9:563–71. doi: 10.1093/intimm/9.4.563. [DOI] [PubMed] [Google Scholar]
- 34.Lewinsohn DM, Bement TT, Xu J, Lynch DH, Grabstein KH, Reed SG, Alderson MR. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–9. [PubMed] [Google Scholar]
- 35.Williams NS, Engelhard VH. Perforin-dependent cytotoxic activity and lymphokine secretion by CD4+ T cells are regulated by CD8+ T cells. J Immunol. 1997;159:2091–9. [PubMed] [Google Scholar]
- 36.Hedrick SM, Engel I, McElligott DL, Fink PJ, Hsu ML, Hansburg D, Matis LA. Selection of amino acid sequences in the beta chains of the T-cell antigen receptor. Science. 1988;239:1541–4. doi: 10.1126/science.2832942. [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen JL, Esser U, Fazekas de St Groth B, Reay PA, Davis MM. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature. 1992;355:224–30. doi: 10.1038/355224a0. [DOI] [PubMed] [Google Scholar]
- 38.Lawson TM, Man S, Williams S, Boon AC, Zambon M, Borysiewicz LK. Influenza A antigen exposure selects dominant Vbeta17+ TCR in human CD8+ cytotoxic T cell responses. Int Immunol. 2001;13:1373–81. doi: 10.1093/intimm/13.11.1373. [DOI] [PubMed] [Google Scholar]
- 39.Lehner PJ, Wang EC, Moss PA, Williams S, Platt K, Friedman SM, Bell JI, Borysiewicz LK. Human HLA-A0201-restricted cytotoxic T lymphocyte recognition of influenza A is dominated by T cells bearing the V beta 17 gene segment. J Exp Med. 1995;181:79–91. doi: 10.1084/jem.181.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss PA, Moots RJ, Rosenberg WM, Rowland-Jones SJ, Bodmer HC, McMichael AJ, Bell JI. Extensive conservation of alpha and beta chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci USA. 1991;88:8987–90. doi: 10.1073/pnas.88.20.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sieling PA, Wang X-H, Gately MK, et al. IL-12 regulates T helper Type 1 cytokine responses in human infectious disease. J Immunol. 1994;153:3639–47. [PubMed] [Google Scholar]
- 42.Krensky AM. Granulysin. a novel antimicrobial peptide of cytolytic T lymphocytes and natural killer cells. Biochem Pharmacol. 2000;59:317–20. doi: 10.1016/s0006-2952(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 43.Thoma-Uszynski S, Stenger S, Modlin RL. CTL-mediated killing of intracellular Mycobacterium tuberculosis is independent of target cell nuclear apoptosis. J Immunol. 2000;165:5773–9. doi: 10.4049/jimmunol.165.10.5773. [DOI] [PubMed] [Google Scholar]
- 44.Ochoa MT, Stenger S, Sieling PA, et al. T-cell release of granulysin contributes to host defense in leprosy. Nat Med. 2001;7:174–9. doi: 10.1038/84620. [DOI] [PubMed] [Google Scholar]