Abstract
Unmethylated CpG motifs in bacterial DNA, but not in vertebrate DNA, are known to trigger an inflammatory response of antigen-presenting cells (APC). In this study, we investigated the cytokine release from murine dendritic cells (DC) by the addition of various types of DNA in the free or complexed form with cationic lipids. Naked plasmid DNA and Escherichia coli DNA with immunostimulatory unmethylated CpG motifs induced pro-inflammatory cytokine secretion from granulocyte–macrophage colony-stimulating factor (GM-CSF)-cultured bone marrow-derived DC and the DC cell-line, DC2.4 cells, though vertebrate calf thymus DNA (CT DNA) with less CpG motifs did not. These characteristics differed from mouse peritoneal resident macrophages that do not respond to any naked DNA. The amount of cytokines released from the DC was significantly increased by complex formation with cationic lipids when CpG-motif positive DNAs were used. Unlike murine macrophages or Flt-3 L cultured DC, GM-CSF DC did not release inflammatory cytokines in response to the addition of CT DNA/cationic lipid complex, suggesting that the activation is completely dependent on CpG motifs. Taken together, the results of the present study demonstrate that murine DC produce pro-inflammatory cytokines upon stimulation with CpG-containing DNAs and the responses are enhanced by cationic lipids. These results also suggest that DC are the major cells that respond to naked CpG DNA in vivo, although both DC and macrophages will release inflammatory cytokines after the administration of a DNA/cationic lipid complex.
Keywords: CpG motifs, dendritic cells, TLR9, DNA and DNA uptake
Introduction
It is well known that unmethylated CpG sequences (CpG motifs) in bacterial DNA, but not in vertebrate DNA, are recognized by the immune system as a danger signal.1 Cytokines such as tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-12 and interferon-α (IFN-α) are secreted from antigen presenting cells, especially macrophages or dendritic cells (DC), upon stimulation with CpG DNA and synthetic oligodeoxynucleotides (ODN) containing CpG motifs. These cytokines significantly modify the therapeutic effects of DNA-based therapies in different ways.2 For example, in gene therapy, cytokine production generally seems inappropriate because these inflammatory cytokines significantly reduce transgene expression in target cells through their direct cytotoxicity and promoter attenuation.3–5 On the other hand, it is essential for DNA vaccination because these cytokines can enhance the immune responses and profoundly affect the balance of these cytokines and the nature of the immune responses.6–9
DC are one of the most important cell populations as far as both innate and acquired immunity are concerned. They influence a variety of immunological responses associated with the therapeutic use of CpG DNA.10,11 In addition to cytokine secretion, the expression of surface major histocompatibility complex (MHC) class I and II molecules as well as costimulatory molecules increases, and the maturation of DC is induced upon stimulation with CpG motifs.12 The initial important step for all these processes associated with CpG DNA is cellular uptake because the receptor of CpG DNA, Toll-like receptor-9 (TLR9), is expressed within cells.13,14 Our previous in vitro study using a DC cell line, DC2.4 cells, in mice demonstrated that DC take up pDNA via a mechanism specific to some defined polyanions15 similar to cultured mouse peritoneal macrophages.16,17
There is a rapidly growing body of information about the mechanism of antigen-presenting cell (APC) activation by CpG DNA. This activation requires endosomal acidification and recognition by TLR9.18–20 CpG DNA appears to use a TLR9 signaling pathway for NF-κB and c-Jun NH2-terminal kinase (JNK) and IRF-7 through MyD88.19,21 However, these proposed mechanisms are mainly based on studies using synthetic phosphorothioate CpG ODN, and there is little information about the activation induced by native DNA. Our previous study has demonstrated that, in contrast to macrophage cell lines, primary cultured mouse peritoneal macrophages secrete almost no inflammatory cytokines upon stimulation with pDNA, in spite of extensive uptake of the CpG DNA22. However, DNA/cationic lipid complex can activate the murine macrophages to induce inflammatory cytokines, whether they have replete CpG motifs or not.23 Flt-3-ligand cultured bone-marrow DC (Flt-3 L DC) exhibit a different type of activation.24,25 Upon stimulation with naked DNA, bacterial pDNA and CpG ODN stimulate Flt-3 L DC to induce cytokines IFN-α or IL-6 although vertebrate CT DNA does not. However, TLR9 in Flt-3 L DC can react when CT DNA is combined with cationic lipid N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP).24 Methylated CpG motifs or non-canonical CpG motifs complexed with DOTAP induce the activation of TLR9 in Flt-3 L DC. Further experiments have proved that the other sequences also induce the activation of TLR9 when ODNs are translocated to endosomes by DOTAP.25 While receptor-mediated endocytosis restricts the uptake of DNA, adsorptive endocytosis by cationic lipids does not. Thus, enhancement of DNA uptake seems to control the activation of TLR9 by vertebrate DNA. In the present study, we used a different type of DC and showed that the cells could respond to only DNA with CpG motifs even if the DNA was translocated to endosomes by cationic lipids.
Materials and methods
Chemicals
RPMI-1640 medium was obtained from Nissui Pharmaceutical (Tokyo, Japan). Escherichia coli DNA (EC DNA) and calf thymus DNA (CT DNA) were purchased from Sigma (St Louis, MO). Lipofectin reagent and Opti-MEM were purchased from Invitrogen (Rockville, MD). Mouse recombinant GM-CSF (rGM-CSF) and Triton-X-114 were purchased from Nacalai Tesque (Kyoto, Japan). [α-32P]dCTP (3000 Ci/mmol) was obtained from Amersham (Amersham, UK). Fetal Bovine Serum (FBS) was purchased from Thermo Trace (Melbourne, Australia).
Cell culture
Male ICR mice (5 weeks) were purchased from Shizuoka Agricultural Cooperative Association for Laboratory Animals (Shizuoka, Japan). After bone marrow was flushed out of the bones of the hind legs of the mice, the cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1000 U/ml rGM-CSF. After a 4–5 day incubation at 37° in 5% CO2-95% air, cells were collected and centrifuged at 200 g for 10 min. After removal of the supernatant, the cells were resuspended in 400 µl phosphate-buffered saline (PBS) containing 0·5% bovine serum albumin (BSA) per 108 total cells. The cell suspension was mixed thoroughly with 100 µl magnetic-activated cell sorting (MACS) CD11c MicroBeads (Miltenyi Biotec, Germany), and incubated for 15 min at 4°. After incubation, the cells were washed, centrifuged at 200 g for 10 min, and resuspended in 500 µl PBS containing 0·5% BSA. Then, magnetic separation with MACS was carried out to isolate the DC by selecting CD11c-positive cells from the cultured cells. These isolated cells were washed and then plated on 24-well culture plates (Falcon, Becton Dickinson, Lincoln Park, NJ) at a density of 5 × 105 cells/well and cultured for 24 hr. The murine DC2.4 cells were a gift from Dr Kenneth Rock (Department of Pathology, University of Massachusetts Medical School, MA). DC2.4 cells display dendritic morphology, express dendritic cell-specific markers, MHC molecules, and costimulatory molecules, and exhibit phagocytic activity and an antigen-presenting capacity.26 DC2.4 cells were cultured with RPMI-1640 medium supplemented with 10% FBS, 2 mm l-glutamine, 100 µm non-essential amino acids, 50 µm 2-mercaptoethanol, and antibiotics. They were then plated on a 24-well culture plate at a density of 5 × 105 cells/well and cultured for 24 hr.
DNA
pCMV-Luc encoding firefly luciferase gene was constructed, as described previously.27 pDNA was purified using an Endo-free plasmid Giga kit (Qiagen, Valencia, CA). For the cellular association experiments, pDNA was radiolabelled with [α-32P]dCTP by nick translation.28 For the activation experiments, all DNA samples were extensively purified with Triton-X-114, a non-ionic detergent, to minimize the activation by contaminated lipopolysaccharide (LPS). Extraction of endotoxin from pDNA, EC DNA, and CT DNA samples was performed according to reviously published methods29,30 with slight modifications. DNA samples were purified by extraction with phenol : chloroform : isoamyl alcohol (25 : 24 : 1) and ethanol precipitation. Then, 10 mg DNA was diluted with 20 ml pyrogen-free water, followed by the addition of 200 µl Triton-X-114 and mixing. The solution was placed on ice for 15 min and incubated for 15 min at 55°. Subsequently, the solution was centrifuged for 20 min at 25°, 600 g. The upper phase was transferred to a new tube, 200 µl Triton-X-114 was added, and the previous steps were repeated at least three times. The activity of LPS was measured by Limulus amoebocyte lysate (LAL) assay using the Limulus F Single Test kit (Wako, Tokyo, Japan). After purification using the Endo-free plasmid Giga kit, 1 µg/ml pDNA contained 0·01–0·05 EU/ml endotoxin. After Triton-X-114 extraction, the endotoxin levels of the DNA samples could no longer be determined by LAL assay, i.e. 1 µg/ml DNA contained less than 0·001 EU/ml. Without extraction of endotoxin by Triton-X-114, 100 µg/ml naked pDNA, which contains 1–5 EU/ml endotoxin, could release 521 ± 73 pg/ml TNF-α at 24 hr from peritoneal macrophages.
Cationic liposome formation
Lipofectin complexes were prepared according to the manufacturer's instructions. In brief, DNA was diluted in 100 µl Opti-MEM per 1 µg DNA (solution A) and 5 µl Lipofectin reagent was diluted in another 100 µl Opti-MEM (solution B). Then solutions A and B were combined and mixed gently. After a 15 min incubation at room temperature, complex was added to the cells.
Cellular association experiments
DC2.4 cells cultured in 24-well plates were washed three times with 0·5 ml Hanks' balanced salt solution (HBSS) without phenol red and 0·5 ml HBSS containing 0·1 µg/ml naked [32P]pDNA or 0·1 µg/ml [32P]pDNA/Lipofectin complex was added. After incubation at 37 or 4° for a specified time, the HBSS was removed and the cells were washed five times with ice-cold HBSS and then solubilized with 1·0 ml 0·3 N NaOH with 0·1% Triton-X-100. Aliquots of the cell lysate were taken for the determination of 32P radioactivity using an LSA-500 scintillation counter (Beckman, Tokyo, Japan) and the protein content was measured using the modified Lowry method with BSA as a standard.
Confocal microscopy
DC2.4 cells were washed three times with 1·0 ml HBSS and incubated with HBSS containing fluorescein-labelled pDNA (FL-pDNA) or FL-pDNA/Lipofectin complex. After a 3 hr incubation, the cells were washed five times and fixed with 4% paraformaldehyde for 10 min.
Cytokine secretion
BMDC or DC2.4 cells cultured in 24-well plates were washed three times with 0·5 ml RPMI-1640 before use. Naked DNA was diluted in 0·5 ml Opti-MEM. The cells were incubated with the naked DNA solution continuously for 8 hr. In the case of DNA/Lipofectin complexes, cells were incubated for 2 hr with 0·5 ml of the solutions containing the complexes. Then, the cells were washed with RPMI-1640 and incubated with RPMI-1640 with 10% FBS. After a 6 hr incubation, the supernatant was collected for ELISA and kept at −80°. The levels of TNF-α, IL-6, and IL-12p70 in the supernatants were determined by the OptEIA Set (BD Biosciences, San Diego, CA).
Results
Uptake of DNA with cationic lipid complexes is not saturated, although normal uptake is saturated in GM-CSF DC
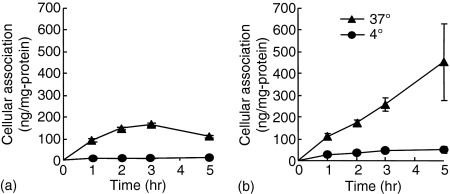
TLR9 exists in the endosomal-lysosomal compartment.13,14 The amount of naked DNA in the compartment can be limited because naked DNA is supposed to be taken up by DC via receptor-mediated endocytosis.15 However, DNA/cationic lipid complexes are supposed to be taken up by DC via a non-specific mechanism based on electrostatic interaction, so-called adsorptive endocytosis. Therefore, cationic lipid Lipofectin was used to deliver DNA efficiently to the compartment. To examine the binding and uptake of naked pDNA and pDNA/cationic lipid complexes in DC, we carried out cellular uptake experiments using naked [32P]pDNA and [32P]pDNA/Lipofectin complexes. As expected, the uptake of naked [32P]pDNA by DC2.4 cells at 37° was increased up to 2 hr (Fig. 1a). Following an incubation of 2–5 hr, the amount of DNA remained unchanged, probably due to continued uptake and degradation.15 On the other hand, complexation with cationic lipids enhanced the DNA uptake. Cationic lipids enhanced [32P]pDNA binding and uptake in DC2.4 cells and the amount of [32P]pDNA increased in a time-dependent manner (Fig. 1b).
Figure 1.
Cellular association time courses of naked [32P]pDNA (a) or [32P]pDNA/Lipofectin complex (b) in DC2.4 cells. Cells were incubated at 37° (closed triangle) or 4° (closed circle). Each point represents the mean ± SD (n = 3).
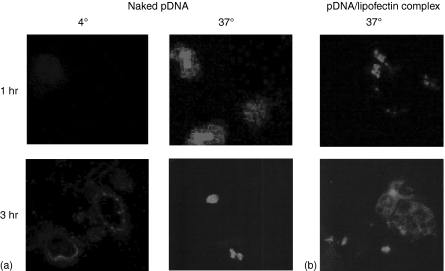
Next, we examined the localization of fluorescence-labelled DNA (FL-pDNA). In the confocal microscopy experiments, the fluorescence derived from naked FL-pDNA is bound to the cellular membrane at 4°(Fig. 2a). At 37°, FL-pDNA was observed inside the cells after 1 hr and it appeared to accumulate in the nucleus after a 3 hr incubation. On the other hand cationic lipids completely changed the localization of DNA. The fluorescence of the FL-pDNA/Lipofectin complex was observed in a punctuated pattern at 1 hr, then diffused into the cells after a 3 hr incubation (Fig. 2b).
Figure 2.
Uptake of naked FL-pDNA (a) or FL-pDNA/Lipofectin complex (b) by DC2.4 cells. The cells were incubated with 5·0 µg/ml naked FL-pDNA or 30 µg/ml FL-pDNA/Lipofectin complex.
The activation of GM-CSF DC by DNA
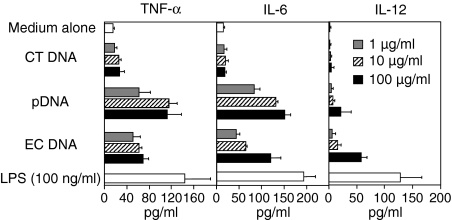
Next, cytokine production from DC by naked DNA was examined. Plasmid DNA and E. coli DNA were used as models of bacterial CpG DNA, and calf thymus DNA was used as a model of vertebrate DNA. As shown in Fig. 3, naked bacterial plasmid DNA and E. coli DNA with replete immunostimulatory CpG motifs induced TNF-α, IL-6 and IL-12 secretions from bone marrow-derived DC. The results are consistent with previous studies demonstrating that plasmid DNA stimulates GM-CSF DC to induce TNF-α and IL-12.18 Vertebrate calf thymus DNA (CT DNA) containing less CpG motifs did not. LPS induced small amounts of cytokines, probably because of relatively short-term incubation (8 hr). Similar results were observed in the experiment using DC2.4 cells, although the cells released a higher amount of cytokines (Fig. 4). These results demonstrate that the cytokine secretion from the DC corresponds to the difference between endogenous DNA and exogenous DNA.
Figure 3.
Cytokine secretion induced by naked DNA from GM-CSF DC. The cells were incubated with EC DNA, pDNA, or CT DNA for 8 hr. The supernatants were collected and the amount of TNF-α, IL-6, and IL-12 secreted from the cells was determined by ELISA. Each result represents the mean ± SD (n = 3).
Figure 4.
Cytokine secretion induced by naked DNA from DC2.4 cells. The cells were incubated with EC DNA, pDNA, or CT DNA for 8 hr. The supernatants were collected and the amount of TNF-α and IL-6 secreted from the cells was determined by ELISA. Each result represents the mean ± SD (n = 3).
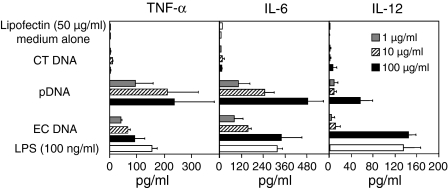
Next, cellular activation in DC by DNA/cationic lipid complexes was examined. The E. coli DNA/Lipofectin complexes stimulated GM-CSF cultured DC to produce cytokines, TNF-α, IL-6 and IL-12 in a dose-dependent manner (Fig. 5). Similar results were observed with pDNA/Lipofectin complex. The amounts of cytokines released from the DC were significantly increased by complex formation with cationic lipids compared with naked DNA (Fig. 3). The DC were unable to produce a significant amount of pro-inflammatory cytokines following stimulation with vertebrate calf thymus DNA (CT DNA) containing less CpG motifs when DNA is complexed to Lipofectin. Lipids alone were unable to stimulate the DC sufficiently to release pro-inflammatory cytokines. Similar results were obtained in DC2.4 cells (Fig. 6). These results demonstrate that GM-CSF DC discriminate between bacterial DNA and mammalian DNA.
Figure 5.
Cytokine secretion induced by DNA/Lipofectin complex from GM-CSF DC. The cells were incubated with EC DNA, pDNA, or CT DNA/Lipofectin complex (5 µl Lipofectin per 1 µg DNA). After a 2 hr incubation, liposomes were removed and fresh growth medium was added to the cells. The supernatants were collected 8 hr after the incubation with liposomes. The amount of TNF-α, IL-6, and IL-12 secreted from the cells was determined by ELISA. Each result represents the mean ± SD (n = 3).
Figure 6.
Cytokine secretion induced by DNA/Lipofectin complex from DC2.4 cells. The cells were incubated with EC DNA, pDNA, or CT DNA/Lipofectin complex (5 µl Lipofectin per 1 µg DNA). After a 2 hr incubation, liposomes were removed and fresh growth medium was added to the cells. The supernatants were collected 8 h after the incubation with liposomes. The amount of TNF-α and IL-6 secreted from the cells was determined by ELISA. Each result represents the mean ± SD (n = 3).
Discussion
The most important role of immune system is to distinguish between ‘self’ and ‘non-self’. Although the TLR9 subfamily (TLR7, 8 and 9) recognizes non-self nucleic acids31 under special conditions, such as systemic lupus erythematosus, these TLRs are stimulated in response to self nucleic acids. For example chromatin–immunoglobulin complexes trigger DC activation in a TLR9-dependent and TLR9-independent manner.32 Recently, Barton et al. have demonstrated that the fusion protein of TLR4/9, which is delivered to cellular membranes, is activated by vertebrate DNA.33 One proposed hypothesis is that compartmentalization of TLR9 prevents the response induced by endogenous DNA.
In the present study, we have demonstrated that GM-CSF-derived DC activation is triggered by exogenous naked DNA. Bacterial DNA induces cytokine secretion from DC, although vertebrate DNA does not. Flt-3 L cultured murine DC (Flt-3 L DC) also induce activation of TLR9 in response to naked bacterial DNA, but not naked vertebrate DNA.24 Therefore, these studies imply that both GMCSF-DC and Flt-3 L DC can discriminate between bacterial non-self DNA and vertebrate self DNA.
On the other hand, these characteristics are different from murine macrophages.22 Primary macrophages do not respond to naked DNA in spite of TLR9 expression, although the macrophage-like cell line RAW264.7 cells do. Both primary macrophages and DC take up DNA via a similar mechanism.15–17 The mechanism of unresponsiveness of macrophages to DNA has not been elucidated, although TLR9 is present in the cells. Macrophages have deoxyribonuclease II (DNase II) in the lysosomal compartment, and they are responsible for apoptotic cell engulfment, DNA digestion and erythroid cell differentiation.34 In erythropoiesis, macrophages take up nuclei and digest DNA. In DNase II-deficient mice, undigested DNA in macrophages causes IFN-β production via unknown receptors.35 The cytokine production is mediated by the TLR9/MyD88 pathway and novel pathways that have been identified recently.36,37 Therefore, the mechanism of the unresponsiveness of macrophages to naked DNA may involve the limited uptake and degradation by DNase II. However, further investigation is required.
The TLR4/9 fusion protein on the cell membrane is activated by vertebrate DNA.33 This research indicates that compartmentalization into cells avoids TLR9 responses to endogenous DNA. Therefore, we forced DNA to internalize into cells using cationic lipids. In fact, vertebrate DNA/cationic lipid complexes can induce cytokine secretion from murine macrophages and Flt-3 L DC.23,24 Following enhancement of DNA uptake by cationic lipids, these cells cannot distinguish between ‘self’ and ‘non-self’ DNA. In peritoneal macrophages, complexation of calf thymus DNA with cationic lipids elicited a similar level of inflammatory cytokine production to that obtained with bacterial E. coli DNA using cationic lipids.23 In addition, calf thymus DNA with cationic lipid DOTAP causes a high degree of IFN-α release from murine Flt-3 L cultured DC or human peripheral blood mononuclear cells.24 The amount of IFN-α induced by calf thymus DNA with DOTAP is similar to that induced by bacterial plasmid DNA. However, the result with GM-CSF DC is different from that in these cells. The cells only recognize bacterial DNA. Vertebrate DNA/cationic lipid complexes do not stimulate GM-CSF DC, although bacterial DNA does. There are two possibilities to explain these observations. One is the possibility that different types of cationic lipids lead to different forms of delivery of DNA, and result in different responses. For example, murine macrophages release inflammatory cytokines in response to the addition of vertebrate CT DNA/cationic lipid complexes.23 Lipofectamine was used for this research. Synthetic double-stranded DNA containing no CpG motif can stimulate macrophage cell lines when DNA is complexed with the cationic lipid Fugene 6.38 In addition, vertebrate CT DNA/cationic lipid Lipofectamine complexes induce macrophage activation via TLR9-dependent and -independent mechanisms.39 Flt-3 L cultured DC (Flt-3 L DC) also responds to vertebrate DNA/cationic lipid DOTAP complexes via TLR9-dependent and -independent pathways.24 TLR9-independent activation is also observed following transfection using Lipofectamine 2000.37 Honda et al. showed that different cellular distributions of DNA result in different cytokine responses.40 CpG-B ODN normally do not induce IFN-α release from plasmacytoid DC. However, following complexation with DOTAP, the same ODNs trigger IFN-α. Confocal microscopy reveals that DOTAP retains DNA in early endosomes, although ODNs without DOTAP are immediately transferred to lysosomal vesicles. Taken together, enhancement of the DNA uptake may not explain the response of TLR9 to vertebrate DNA and TLR9 may be present in specific compartments.
The other possibility is that GM-CSF DC, Flt-3 L DC and macrophages may contribute to the immune systems in different ways, by producing different types or degrees of induction. TLR9 is mainly expressed in B cells and plasmacytoid DC in humans.31 On the other hand, mouse TLR9 is also present in myeloid DC and macrophages. Although further studies are required to clarify the contribution of DC or macrophages to immune responses in vivo, the present study suggests that DC are the main cells that respond to naked bacterial DNA, although both DC and macrophages will release inflammatory cytokines after the administration of bacterial DNA/cationic lipid complexes.
Very recently Martin et al. have shown that GM-CSF DC release type I IFN upon stimulation of mammalian DNA complexed with Fugene, another kind of lipid for transfection.41 Interestingly, the cells do not produce TNF-α, IL-6 or IL-12. The activation is independent of TLR9 because GM-CSF DC from TLR9–/– deficient mice respond to mammalian DNA/Fugene complexes to secrete type I IFN. Another group has also demonstrated that non-CpG DNA/lipofectamine complexes stimulate GM-CSF DC to induce type I IFN.42 The activation is not dependent on the MyD88 or TRIF pathways. Based on these observations, one can hypothesize that, distinct from Flt-3 L DC, GM-CSF DC respond to only bacterial or viral DNA via TLR9-dependent pathway, and release cytokines, such as TNF-α IL-6 and IL-12. However when mammalian DNAs are translocated into cells, GM-CSF DC may not induce these cytokines. Instead, the cells may release IFN-α through a TLR9-independent pathway. Further studies are required for these TLR9-dependent and -independent mechanisms.
In conclusion, the present study has demonstrated that murine GM-CSF DC or the DC cell line, DC2.4, produce pro-inflammatory cytokines following stimulation with CpG-containing DNAs and this production is increased when the DNAs are added to the cells in a complex form with cationic lipids. These findings form an important basis for future DNA-based therapies, including gene therapy and DNA vaccination.
Acknowledgments
This work was supported in part by 21st Century COE Program ‘Knowledge Information Infrastructure for Genome Science’, and also in part by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We would like to thank Dr Kenneth Rock (Department of Pathology, University of Massachusetts Medical School, MA, USA) for providing DC2.4 cells.
Glossary
Abbreviations
- APC
antigen-presenting cells
- DC
dendritic cells
- BMDC
bone-marrow derived dendritic cell
- CT DNA
calf thymus DNA
- TNF-α
tumour necrosis factor-α
- IL-6
interleukin-6
- IL-12
interleukin-12
- IFN-α
interferon-α
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- IRF
interferon regulatory factor
- LPS
lipopolysaccharide
- ODN
oligodeoxynucleotide
- MHC
major histocompatibility complex
- TLR
Toll-like receptor
- JNK
c-Jun NH2-terminal kinase
- Flt-3 L DC
Flt-3ligand cultured bone-marrow dendritic cells
- EC DNA
Escherichia coli DNA, pDNA, plasmid DNA
- FL-pDNA
fluorescein labelled plasmid DNA
- GM-CSF
DC
- granulocyte–macrophage
colony-stimulating factor cultured dendritic cells
- DNase II
deoxyribonuclease II
References
- 1.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 2.Yasuda K, Wagner H, Takakura Y. Role of immunostimulatory DNA and TLR9 in gene therapy. Crit Rev Ther Drug Carrier Syst. 2006;23:89–110. doi: 10.1615/critrevtherdrugcarriersyst.v23.i2.10. [DOI] [PubMed] [Google Scholar]
- 3.Haddad EB, Rousell J, Lindsay MA, Barnes PJ. Synergy between tumor necrosis factor alpha and interleukin 1beta in inducing transcriptional down-regulation of muscarinic M2 receptor gene expression. Involvement of protein kinase A and ceramide pathways. J Biol Chem. 1996;271:32586–92. doi: 10.1074/jbc.271.51.32586. [DOI] [PubMed] [Google Scholar]
- 4.Qin L, Ding Y, Pahud DR, Chang E, Imperiale MJ, Bromberg JS. Promoter attenuation in gene therapy. interferon-gamma and tumor necrosis factor-alpha inhibit transgene expression. Hum Gene Ther. 1997;8:2019–29. doi: 10.1089/hum.1997.8.17-2019. [DOI] [PubMed] [Google Scholar]
- 5.Sellins K, Fradkin L, Liggitt D, Dow S. Type I Interferons potently suppress gene expression following gene delivery using liposome (–) DNA complexes. Mol Ther. 2005;12:451–9. doi: 10.1016/j.ymthe.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Gurunathan S, Klinman DM, Seder RADNA. vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–74. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 7.Raz E, Tighe H, Sato Y, et al. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman M, Martin OE, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 9.Seder RA, Hill AV. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–8. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–73. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 11.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c (–) type 2 dendritic cell precursors and CD11c (+) dendritic cells to produce type I IFN. J Immunol. 2001;166:2291–5. doi: 10.4049/jimmunol.166.4.2291. [DOI] [PubMed] [Google Scholar]
- 12.Weiner GJ. The immunobiology and clinical potential of immunostimulatory CpG oligodeoxynucleotides. J Leukoc Biol. 2000;68:455–63. [PubMed] [Google Scholar]
- 13.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–68. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 15.Yoshinaga T, Yasuda K, Ogawa Y, Takakura Y. Efficient uptake and rapid degradation of plasmid DNA by murine dendritic cells via a specific mechanism. Biochem Biophys Res Commun. 2002;299:389–94. doi: 10.1016/s0006-291x(02)02648-7. [DOI] [PubMed] [Google Scholar]
- 16.Takagi T, Hashiguchi M, Mahato RI, Tokuda H, Takakura Y, Hashida M. Involvement of specific mechanism in plasmid DNA uptake by mouse peritoneal macrophages. Biochem Biophys Res Commun. 1998;245:729–33. doi: 10.1006/bbrc.1998.8521. [DOI] [PubMed] [Google Scholar]
- 17.Takakura Y, Takagi T, Hashiguchi M, et al. Characterization of plasmid DNA binding and uptake by peritoneal macrophages from class A scavenger receptor knockout mice. Pharm Res. 1999;16:503–8. doi: 10.1023/a:1018842210588. [DOI] [PubMed] [Google Scholar]
- 18.Hacker H, Mischak M, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–40. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–61. [PubMed] [Google Scholar]
- 20.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 21.Honda K, Yanai H, Mizutani T, et al. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci USA. 2004;101:15416–21. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasuda K, Kawano H, Yamane I, Ogawa Y, Yoshinaga T, Nishikawa M, Takakura Y. Restricted cytokine production from mouse peritoneal macrophages in culture in spite of extensive uptake of plasmid DNA. Immunology. 2004;111:282–90. doi: 10.1111/j.1365-2567.2004.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda K, Ogawa Y, Kishimoto M, Takagi T, Hashida M, Takakura Y. Plasmid DNA activates murine macrophages to induce inflammatory cytokines in a CpG motif-independent manner by complex formation with cationic liposomes. Biochem Biophys Res Commun. 2002;293:344–8. doi: 10.1016/S0006-291X(02)00210-3. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda KYuP, Kirschning CJ, et al. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J Immunol. 2005;174:6129–36. doi: 10.4049/jimmunol.174.10.6129. [DOI] [PubMed] [Google Scholar]
- 25.Yasuda K, Rutz M, Schlatter B, et al. CpG motif-independent activation of TLR9 upon endosomal translocation of ‘natural’ phosphodiester DNA. Eur J Immunol. 2006;36:431–6. doi: 10.1002/eji.200535210. [DOI] [PubMed] [Google Scholar]
- 26.Shen Z, Reznikoff G, Dranoff G, Rock KL. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol. 1997;158:2723–30. [PubMed] [Google Scholar]
- 27.Nomura T, Yasuda K, Yamada T, Okamoto S, Mahato RI, Watanabe Y, Takakura Y, Hashida M. Gene expression and antitumor effects following direct interferon (IFN)-gamma gene transfer with naked plasmid DNA and DC-chol liposome complexes in mice. Gene Ther. 1999;6:121–9. doi: 10.1038/sj.gt.3300792. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a Laboratory Manual. 2. Cold Spring Harbor, NY: Cold Spring. Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Cotten M, Baker A, Saltik M, Wagner E, Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994;1:239–46. [PubMed] [Google Scholar]
- 30.Hartmann G, Krieg AM. CpG DNA and LPS induce distinct patterns of activation in human monocytes. Gene Ther. 1999;6:893–903. doi: 10.1038/sj.gt.3300880. [DOI] [PubMed] [Google Scholar]
- 31.Wagner H. The immunobiology of the TLR9 subfamily. Trends Immunol. 2004;25:381–6. doi: 10.1016/j.it.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 34.Nagata S. DNA degradation in development and programmed cell death. Annu Rev Immunol. 2005;23:853–75. doi: 10.1146/annurev.immunol.23.021704.115811. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 36.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202:1333–9. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii KJ, Coban C, Kato H, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–8. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 38.Zhu FG, Reich CF, Pisetsky DS. Effect of cytofectins on the immune response of murine macrophages to mammalian DNA. Immunology. 2003;109:255–62. doi: 10.1046/j.1365-2567.2003.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasuda K, Ogawa Y, Yamane I, Nishikawa M, Takakura Y. Macrophage activation by a DNA/cationic liposome complex requires endosomal acidification and TLR9-dependent and -independent pathways. J Leukoc Biol. 2005;77:71–9. doi: 10.1189/jlb.0204089. [DOI] [PubMed] [Google Scholar]
- 40.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signaling for robust type-I interferon induction. Nature. 2005;434:1035–40. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 41.Martin DA, Elkon KB. Intracellular mammalian DNA stimulates myeloid dendritic cells to produce type I interferons predominantly through a toll-like receptor 9-independent pathway. Arthritis Rheum. 2006;54:951–60. doi: 10.1002/art.21677. [DOI] [PubMed] [Google Scholar]
- 42.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]