Abstract
The number and function of human T cells in the periphery are regulated by homeostatic signals received from antigen-presenting cells (APCs) and the common gamma chain (γc) cytokines interleukin (IL)-7 and IL-15. We found that, in the absence of introduced antigen, blood monocytes or myeloid dendritic cells (MDCs) in the presence of IL-7 and IL-15 (IL-7/IL-15) can regulate CD4+ T memory (Tm) cell numbers by polyclonal cell proliferation. The dynamics of CD4+ Tm cell proliferation, in the presence of IL-7/IL-15, was dependent on contact with MDCs and to a lesser extent on contact with monocytes. IL-7/IL-15 either alone or combined with monocytes or MDCs enhanced the proportion of CD4+ Tm cells with activated and effector phenotype and diminished the helper function of CD4+ Tm cells. These CD4+ Tm cells, preconditioned with IL-7/IL-15 alone or with monocytes or MDCs and IL-7/IL-15, reduced T cell-dependent immunoglobulin M (IgM) and IgG responses. This appeared to be a contact-dependent effect involving a reduction in antibody-producing CD27+ B memory cells, but contact-independent suppression by soluble factors also contributed to the antibody-producing capacity of CD27+ B memory cells. These results indicate that blood monocytes, MDCs and the cytokines IL-7/IL-15 contribute to homeostasis of CD4+ Tm cells by regulating their number, activation state and helper/suppressor (regulatory) function. In healthy individuals, this mode of regulating CD4+ Tm cell homeostasis may provide a basis for the control of autoimmune responses.
Keywords: CD4+ T memory cell homeostasis, helper/suppressor function, T-cell dependent antibody
Introduction
When naïve CD4+ T cells encounter foreign peptide complexed with major histocompatibility complex (MHC) class II molecules on antigen-presenting cells (APCs), a robust immune response is generated. This results in proliferation and differentiation of naïve CD4+ T cells into short-lived cytokine-secreting effector cells, but some cells survive to become long-lived CD4+ T memory (Tm) cells.1 CD45RA down-regulation and CD45RO isoform up-regulation mark the transition of human CD4+ T cells from a naïve to a memory state. Although the majority of CD4+ Tm cells result from immune responses to foreign peptides, some may arise in response to self-peptides (e.g. those derived from apoptotic cells).2
In the absence of disease, human CD4+ Tm cell numbers are maintained at a near constant level, presumably by homeostatic signals received from APCs and common gamma chain (γc) cytokines, such as interleukin (IL)-7 and IL-15. This concept derives from the finding that maintenance of mouse CD4+ Tm cells is dependent on MHC class II recognition, IL-7 and, to a lesser extent, IL-15.3,4 Studies on the maintenance of human CD4+ Tm cells are limited to in vitro models, and the data to date suggest that together IL-7 and IL-15 (hereafter referred to as IL-7/IL-15), or cytokines secreted by monocyte-derived dendritic cells (MoDCs), can maintain CD4+ Tm cell numbers by cell proliferation.5 This in vitro‘homeostatic’ proliferation of human CD4+ Tm cells occurs in the absence of introduced antigen and does not appear to be dependent on MHC class II recognition.5 Whether human CD4+ Tm cells maintained in the presence of IL-7/IL-15 and APCs still retain their resting phenotype and helper function has not been studied.
We considered that homeostatic signals received from naturally occurring APCs such as blood monocytes or myeloid dendritic cells (MDCs) and the cytokines IL-7/IL-15 might regulate CD4+ Tm cell homeostasis in the periphery, and by carrying out in vitro studies we sought insights into these processes.
Methods
Blood samples
Blood was obtained from healthy donors, with appropriate informed consent according to the Mater Adult Hospital Ethics Committee Guidelines. Human pooled AB serum was prepared from AB donors and provided by the Australian Red Cross Blood Service.
Antibodies and reagents
Unconjugated monoclonal antibodies (mAbs) specific for CD3, CD8, CD11c, CD14, CD19, CD20, CD34, CD45RA, CD56, CCR7, glycophorin-A and CD16 were obtained from Coulter Immunotech (Gladesville, NSW, Australia). Unconjugated mAbs specific for CD3 (OKT3, IgG2a), CD8 (OKT8, IgG2a) and human leucocyte antigen (HLA)-DR (L243, IgG2a) prepared in our laboratory from hybridomas were obtained from the American Culture Collection. Fluorescein isothiocyanate (FITC)-conjugated mAbs for CD4, CD5 and CD15, isotype control IgG1, phycoerythrin (PE)-conjugated mAbs for CD4, CD14, CD45RO, CD62L, CD40 and CD25, isotype control mAbs (IgG1, IgG2a and IgG2b), peridinin-chlorophyll-protein (PerCp)-conjugated mAb for CD4, allophycocyanin-conjugated mAbs for CD3, CD11c and CD19, blocking mAbs for HLA-DR, DP and DQ and isotype control mAb were all obtained from BD Biosciences (Sydney, NSW, Australia). FITC-conjugated goat anti-mouse was obtained from Silenus (Melbourne, VIC, Australia). Blocking mAb for CD40 was obtained from Bio Scientific (Gymea, NSW, Australia). The T-cell receptor (TCR) Vβ Repertoire Kit was from Beckman Coulter (Gladesville, NSW, Australia). PE-conjugated mAbs for CD69, CD27, CD70, IL-4, IL-10, interferon-γ (IFN-γ) and IL-2 were all obtained from BD Pharmigen (Sydney, NSW, Australia). N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and fetal calf serum (FCS) were purchased from Invitrogen (Mount Waverly, VIC, Australia). Human IL-7 was obtained from Sigma (St Louis, MO), and granulocyte–macrophage colony-stimulating factor (GM-CSF) from Schering-Plough (Sydney, NSW, Australia). IL-4 and IL-15 were donated by Novartis Pharmaceuticals (North Ryde, NSW, Australia) and by Amgen (Seattle, WA), respectively. Carboxyfluorescein diacetate succinimidyl ester (CFSE) was obtained from Molecular Probes (Eugene, OR).
Cell preparation
MDCs and plasmacytoid dendritic cells (PDCs) were prepared by labelling peripheral blood mononuclear cells (PBMC) with mAbs for lineage markers CD3, CD14, CD19, CD20, CD56, CD34 and glycophorin-A, followed by incubation with goat anti-mouse beads (Miltenyi Biotech, Sydney, NSW, Australia) and magnetic depletion of lineage+ cells by AutoMACS (Miltenyi Biotech). MDCs and PDCs were sorted from the lineage– cell fraction as CD11c+ CD4– and CD11c– CD4+ events (FACSVantage; BD Biosciences; > 98% purity). B cells and monocytes were prepared by labelling PBMCs with mAb for CD19, CD20 or CD14, respectively, followed by incubation with goat anti-mouse beads and AutoMACS positive selection (> 90% and > 95% purity for B cells and monocytes, respectively). CD4+ Tm cells (CD4+ CD45RO+ cells) were prepared by labelling PBMCs with mAbs for CD19, CD20, CD11c, CD14, CD34, CD56, HLA-DR, glycophorin-A, CD8 and CD45RA, followed by incubation with goat anti-mouse beads and AutoMACS negative selection (> 90% purity). MoDCs were produced by culturing monocytes with GM-CSF (800 U/ml) and IL-4 (1000 U/ml) for 7 days. Lipopolysaccharide (LPS)-treated MoDCs (MoDCs/LPS) were produced by the addition of LPS (1 μg/ml; Sigma) at day five of MoDC culture.6
Co-culture, transwell assays and TruCOUNTTMassay (BD Biosciences)
CFSE-unlabelled or CFSE-labelled CD4+ Tm cells (1 × 105) were cultured without or with autologous APCs (e.g. monocytes, MDCs, PDCs, MoDCs or MoDCs/LPS) (1 × 104 cells), with IL-7/IL-15 (25 ng/ml) alone or with autologous APCs and IL-7/IL-15 for 5–7 days. In transwell assays, CFSE-unlabelled or CFSE-labelled CD4+ Tm cells were seeded in 96-well round-bottomed culture plates and autologous APCs were seeded in a transwell insert. [3H]thymidine (1 µCi/well) was added for the last 16 hr of a 7-day culture. Proliferation was measured by [3H]thymidine uptake or by CFSE division-tracking assay7 and flow cytometry (CellQuest acquisition/analysis and Modfit LT software, BD Biosciences). Absolute CD4+ Tm cell numbers recovered in co-culture or transwell assays at day 5–7 of culture were quantified using TruCOUNTTM bead assay (BD Biosciences).8
Cytokine detection assay
Intracellular cytokine expression was assessed in CD4+ Tm cells at day 7 of culture. CD4+ Tm cells were expanded with phorbol myristate acetate (PMA; 10 ng/ml) and ionomycin (1 ng/ml; both from Sigma) for the last 6 hr of culture; labelled with anti-CD3-FITC mAb; fixed/permeabilized; labelled with IL-4-PE, IL-10-PE, IFN-γ-PE or IL-2-PE mAb, and analysed for intracellular cytokine expression by flow cytometry.6
B-cell culture
CFSE-unlabelled or CFSE-labelled B cells were cultured in the presence of pokeweed mitogen (PWM; 2 µg/ml; Sigma) with various numbers (1–10 × 104/well) of either fresh, control preconditioned CD4+ Tm cells (preconditioned in RPMI) or preconditioned CD4+ Tm cells (preconditioned with IL-7/IL-15 alone or with monocytes or MDC and IL-7/IL-15) in both co-culture and transwell assay (in RPMI/10% FCS with 25 mm HEPES). The proportion of total B cells and CD27+ B memory cells and the division of CFSE-labelled B cells were analysed at day 4–7 of culture by flow cytometry.
Quantification of IgM and IgG production
IgM and IgG in culture supernatants, collected from PWM-stimulated B-cell cultures or from PWM-stimulated B-cell/CD4+ Tm cell co-culture and transwell assay at day 7, were quantified by enzyme-linked immunosorbent assay (ELISA). Briefly, plates were coated overnight at 4° with goat anti-human IgM and IgG (H + L chains) capturing Ab (Jackson ImmunoResearch, West Grove, PA). Human IgM and IgG standards (Sigma) or culture supernatant samples were added to the coated plate and allowed to bind for 2 hr. Bound IgM and IgG were revealed with peroxidase-conjugated goat mAb specific for human IgG-Fc or IgM-Fc fragment (Jackson ImmunoResearch) using O-phenylenediamine dihydrochloride (Sigma) as substrate.
Cytotoxicity assay
From PWM-stimulated B-cell/CD4+ Tm co-cultures, B cells were sorted as CD19+/– CD5– and CD4+ Tm cells were sorted as CD5+ CD19– events at day 3 of culture. Following sorting, B cells were labelled with 200 µCi of 51Cr (GE-Healthcare, Castle Hill, NSW, Australia) and re-cultured with sorted CD4+ Tm cells originating from the same culture. Subsequent 4- or 13-hr 51Cr-release assays were performed at effector:target ratios of 0·5, 1, 2 and 5 with 3 × 104 51Cr-labelled target B cells/well. The percentage of specific lysis was calculated as 100 × (experimental release – spontaneous release/total – spontaneous release).
Statistical analysis
Data are expressed as the mean ± the standard error of the mean (SEM). The statistical significance of differences was determined by the paired two-tailed Student t-test using the GraphPad Prism software (GraphPad Software Inc., San Diego, CA). Significance was defined as P < 0·05.
Results
The inclusion of IL-7/IL-15 with myeloid APCs regulated CD4+ Tm cell number by cell proliferation
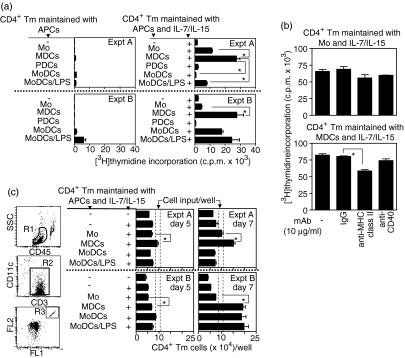
Evidence that in vitro generated MoDCs synergize with IL-7/IL-15 to enhance CD4+ Tm cell proliferation5 prompted us to test whether blood APCs have a similar capacity. CD4+ Tm cell proliferation was analysed after culture with (i) autologous APCs either isolated from blood (monocytes, MDCs or PDCs) or generated in vitro (immature MoDCs or mature MoDCs/LPS), (ii) IL-7/IL-15 and (iii) autologous APCs and IL7/IL-15, by measuring [3H]thymidine uptake at day 5–7 of culture. CD4+ Tm cell proliferation after culture with monocytes, MDCs or MoDCs was low [< 1·5 × 103 counts per minute (c.p.m.)] and after culture with MoDCs/LPS was somewhat higher but inconsistent (1–6 × 103 c.p.m.; Fig. 1a, experiments A and B; left-hand graphs). CD4+ Tm cell proliferation after culture with PDCs was negligible. CD4+ Tm cell proliferation after culture with IL-7/IL-15 was higher compared to that after culture with autologous APCs, but was still low (< 2·5 × 103 c.p.m.; Fig. 1a, experiments A and B; right-hand graphs). IL-7 or IL-15 alone induced at most 62 or 26%, respectively, of the CD4+ Tm cell proliferation induced by their combination (data not shown).
Figure 1.
Maintenance of CD4+ T memory (Tm) cell numbers by cell proliferation. CD4+ Tm cells were cultured (i) without or with different types of autologous antigen-presenting cell (APC) (as indicated), (ii) in the presence of interleukin (IL)-7/IL-15 alone, (iii) with autologous APCs and IL-7/IL-15 (as indicated) or (iv) with either monocytes or myeloid dendritic cells (MDCs) with IL-7/IL-15 in the presence of either isotype control monoclonal antibody (mAb), blocking anti-major histocompatibility (MHC) class II or anti-CD40 mAb. (a) Proliferation of CD4+ Tm cells was measured by [3H]thymidine incorporation at day 7 of culture. The data are displayed as mean counts per minute (c.p.m.) values ± standard error of the mean (SEM) for triplicate measurements of two representative experiments of 20 (Expts A and B). (b) The effect of blocking anti-MHC class II or anti-CD40 mAb (10 µg/ml) on CD4+ Tm cell proliferation induced by monocytes with IL-7/IL-15 (upper panel) or by MDCs with IL-7/IL-15 (lower panel) is shown. The data are displayed as mean c.p.m. values ± SEM for triplicate measurements of one representative experiment of four. (c) Absolute numbers of CD4+ Tm cells recovered either in the absence or in the presence of IL-7/IL-15 alone or with autologous APCs with IL-7/IL-15 at day 5–7 of culture. Cultured cells were labelled with anti-CD3-phycoerythrin (PE), anti-CD11c-allophycocyanin and anti-CD45-peridinin-chorophyll-protein (PerCp) mAbs and analysed by flow cytometry TruCOUNTTM assay. Regions R1, R2 and R3 were created to define live CD45+ cells, CD3+ T cells and TruCOUNT™ beads, respectively. Data are displayed as the total number of CD3+ T cells/well (corresponding to CD4+ Tm cells/well; mean ± SEM of triplicate measurements) for two representative experiments of seven. IgG, immunoglobulin G; Mo, monocytes; MoDCs, monocyte-derived dendritic cells; LPS, lipopolysaccharide; PDCs, plasmacytoid dendritic cells.
The inclusion of IL-7/IL-15 with either monocytes, MDCs, MoDCs or MoDCs/LPS enhanced CD4+ Tm cell proliferation, albeit with considerable variability (Fig. 1a, right-hand graphs). In the presence of IL-7/IL-15, MDCs induced higher CD4+ Tm cell proliferation than monocytes in 85% of experiments and similar proliferation to monocytes in 15% of experiments (Fig. 1a, experiments A and B; n = 20). The CD4+ Tm cell proliferation induced by MoDCs, or MoDCs/LPS with IL-7/IL-15, was similar to that induced by monocytes in 75% of experiments and similar to that induced by MDCs in 25% of experiments (Fig. 1a, experiments A and B; n = 8). Compared with immature MoDCs, MoDCs/LPS with IL-7/IL-15 always induced slightly higher CD4+ Tm cell proliferation, a feature that might be attributed to their advanced maturation status. In contrast to myeloid APCs, the inclusion of IL-7/IL-15 with PDCs failed to enhance CD4+ Tm cell proliferation and therefore this option was excluded from subsequent investigation.
The addition of anti-MHC class II mAb (10–20 µg/ml) did not significantly change the CD4+ Tm cell proliferation induced by monocytes with IL-7/IL-15 (Fig. 1b, upper panel, and data not shown). However, the addition of anti-MHC class II mAb significantly decreased the CD4+ Tm cell proliferation induced by MDCs with IL-7/IL-15, as measured by [3H]thymidine uptake at day 7 of culture (Fig. 1b, lower panel, and data not shown). In contrast, the addition of anti-CD40 mAb (10–20 µg/ml) resulted in no significant changes in CD4+ Tm cell proliferation induced by either monocytes or MDCs with IL-7/IL-15 (Fig. 1b, upper and lower panels, and data not shown).
In parallel with CD4+ Tm cell proliferation, we assessed the absolute number of CD4+ Tm cells recovered at day 5–7 of culture by flow cytometry TruCOUNTTM assay.8 MDCs with IL-7/IL-15 consistently maintained the highest CD4+ Tm cell numbers, with the major increase occurring during the last 2 days of culture (Fig. 1c, experiments A and B). The number of CD4+ Tm cells recovered after culture with MoDCs or MoDCs/LPS with IL-7/IL-15 was more variable (Fig. 1c, experiments A and B) and, to obtain consistent data, CD4+ Tm cells maintained with IL-7/IL-15 alone, or combined with monocytes or MDCs, were used in subsequent experiments.
The dynamics of CD4+ Tm cell proliferation depended on the manner of stimulation
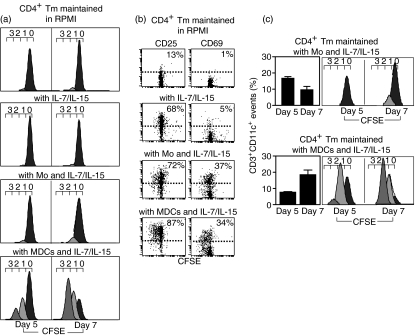
We analysed the dynamics of CD4+ Tm cell proliferation induced by IL-7/IL-15 alone or combined with monocytes or MDCs using the CFSE division-tracking assay.7 In this assay, as cells divide, their CFSE fluorescence halves sequentially and each successive cell generation is visualized as a distinct peak. The vast majority of CD4+ Tm cells remained undivided over a 5–7-day culture, in the presence of IL-7/IL-15 alone (Fig. 2a). In the presence of IL-7/IL-15, CFSE peaks of divided CD4+ Tm cells were generally hard to visualize and therefore the dynamics of their proliferation was not analysed further. In co-culture with monocytes and IL-7/IL-15, the proportion of CD4+ Tm cells that had divided once was 4–7% (Fig. 2a). This was associated with a fourfold increase in the number of proliferative precursors (calculated by dividing the number of cells at n divisions by 2n)9 during the last 2 days of a 7-day culture (Table 1). In this co-culture assay, mitotic events (calculated by subtracting the number of proliferative precursors from the number of cells generated by each division)9 slowly accumulated during the last 2 days of a 7-day culture, representing a fourfold increase over the first 5 days and giving rise to 1·1 mitotic events per average CD4+ Tm cell (Table 1). In co-culture with MDCs and IL-7/IL-15, the proportion of CD4+ Tm cells that had divided once or twice ranged from 24 to 64% and the number of proliferative precursors was increased sixfold during the last 2 days of a 7-day culture (Fig. 2a and Table 1). Furthermore, in this co-culture assay, mitotic events represented a ninefold increase over the first 5 days of culture and gave rise to 2·1 mitotic events per average CD4+ Tm cell (Table 1). Despite the different dynamics of CD4+ Tm cell proliferation, similar proportions of activated CD4+ Tm cells expressing the early activation markers CD25 and CD69 were found in co-culture with monocytes or MDCs with IL-7/IL-15 (Fig. 2b).
Figure 2.
The dynamics of CD4+ T memory (Tm) cell proliferation defined by the carboxyfluorescein diacetate succinimidyl ester (CFSE) division-tracking assay. CFSE-labelled CD4+ Tm cells were cultured either in the absence or in the presence of interleukin (IL)-7/IL-15 alone, or combined with monocytes or myeloid dendritic cells (MDCs), for 5–7 days. (a) Cultured cells were labelled with anti-CD3-phycoerythrin (PE), anti-CD11c-allophycocyanin and anti-CD45-peridinin-chorophyll-protein (PerCp) monoclonal antibodies (mAbs) and analysed by flow cytometry. Regions R1 and R2 were created to define live CD45+ cells and CD3+ T cells (see Fig. 1). Histograms show the fluorescence profile of CFSE-labelled CD3+ T cells (corresponding to CD4+ Tm cells). The numbers (0–3) above the histograms denote division number, with undivided CFSE-labelled CD4+ Tm cells residing under peak ‘0’ and proliferating CD4+ Tm cells exhibiting serial halving of CFSE fluorescence residing under peaks ‘1–3’. Division peaks were determined using standard CellQuest acquisition/analysis and ModFitLT software. (b) Cultured cells were labelled with anti-CD11c-allophycocyanin and anti-CD45-PerCp mAbs combined with either anti-CD25-PE or anti-CD69-PE mAb and analysed by flow cytometry. Dot plots display CD25 or CD69 expression as a function of division of CFSE-labelled CD4+ Tm cells, with the percentages of CD4+ CD25+ Tm or CD4+ CD69+ Tm cells indicated in each dot plot (events above dashed line; dashed line shows staining with control mAb). (c) Cultured cells were labelled with anti-CD3-PE, anti-CD11c-allophycocyanin and anti-CD45-PerCp mAbs and analysed by flow cytometry. Aggregates between monocytes or MDCs and CFSE-labelled CD4+ Tm cells (determined as CD3+ CD11c+ events) were expressed as a proportion of total CD3+ Tm cells [bar; mean ± standard error of the mean (SEM) for three separate experiments]. Histograms show the CFSE fluorescence profiles of CD4+ Tm cell/monocyte or CD4+ Tm cell/MDC aggregates. Mo, monocytes.
Table 1.
Quantitative measure of the dynamics of CD4+ T memory (Tm) cell proliferation
| CD4+ Tm cells maintained with | ||||
|---|---|---|---|---|
| Monocytes and IL-7/IL-15 [day 5 (day 7)] | MDCs and IL-7/IL-15 [day 5 (day 7)] | |||
| Proliferative precursors | Mitotic events | Proliferative precursors | Mitotic events | |
| Co-culture | 553 ± 187 | 675 ± 170 | 7050 ± 282 | 9672 ± 108 |
| (n = 3) | (2340 ± 786) | (2742 ± 761) | (40491 ± 2925) | (84772 ± 2478) |
| Transwell | 619 ± 137 | 656 ± 131 | 665 ± 96* | 778 ± 86* |
| (n = 3) | (1506 ± 279) | (1914 ± 441) | (1582 ± 284*) | (1926 ± 323*) |
CD4+ Tm cells were maintained with monocytes or myeloid dendritic cells (MDCs) and interleukin (IL)-7/IL-15 in co-culture or transwell assay. The numbers of proliferative precursors and mitotic events9 recovered at day 5 or day 7 of culture were obtained by gating on live CD45+ CD3+ gate and quantified using the TruCOUNTTM bead assay (see Fig. 1). Data are expressed as the mean value ± standard error of the mean.
Values from co-culture and transwell assays differed significantly.
When co-cultured with either monocytes or MDCs and IL-7/IL-15, CD4+ Tm cells formed aggregates with the monocytes and MDCs, respectively (defined as CD3+ CD11c+ events; Fig. 2c). However, these aggregates included CD4+ Tm cells with different proliferation histories, such that CD4+ Tm/monocyte aggregates always contained fewer dividing CD4+ Tm cells than CD4+ Tm/MDC aggregates (Fig. 2c). On the basis of this evidence, we presumed that the dynamics of CD4+ Tm cell proliferation might depend on contact with MDCs.
The dynamics of CD4+ Tm cell proliferation was dependent on contact with MDCs and to a lesser extent on contact with monocytes
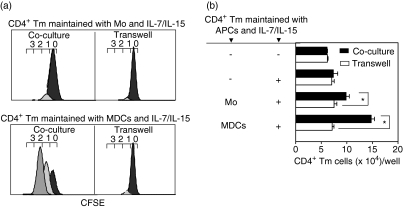
We analysed the dynamics of CD4+ Tm cell proliferation induced by monocytes or MDCs with IL-7/IL-15 using a co-culture or transwell assay, to either allow (co-culture) or prevent (transwell) contact between monocytes or MDCs and CD4+ Tm cells. Prevention of contact between monocytes and CD4+ Tm cells reduced the proportion of dividing cells by less then 10%, without significant changes in the number of proliferative precursors and mitotic events, compared with the co-culture assay (Fig. 3a and Table 1). Prevention of contact between monocytes and CD4+ Tm cells significantly decreased the absolute number of CD4+ Tm cells recovered at the end of culture in 71% of experiments (Fig. 3b; n = 7), suggesting that contact with monocytes may contribute to survival of the CD4+ Tm cells. In contrast, preventing contact between MDCs and CD4+ Tm cells markedly perturbed the dynamics of CD4+ Tm cell proliferation, decreasing the proportion of dividing cells by more than 70%and significantly reducing the numbers of proliferative precursors and mitotic events and the absolute number of CD4+ Tm cells, compared with the co-culture assay (Figs 3a and b; Table 1).
Figure 3.
The dynamics of CD4+ T memory (Tm) cell proliferation in co-culture and transwell assay. Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labelled CD4+ Tm cells were cultured in the presence of interleukin (IL)-7/IL-15 and in contact with monocytes or myeloid dendritic cells (MDCs) (co-culture assay) or separated from monocytes or MDCs (transwell assay) for 7 days. Cultured cells were labelled with anti-CD3-phycoerythrin (PE), CD11c-allophycocyanin and CD45-peridinin-chorophyll-protein (PerCp) monoclonal antibodies (mAbs) and analysed by flow cytometry. Regions R1, R2 and R3 were created to define live CD45+ cells, CD3+ T cells or TruCOUNTTM beads, respectively (see Fig. 1). (a) Histograms show the fluorescence profile of CFSE-labelled CD3+ T cells (corresponding to CD4+ Tm cells). The numbers (0–3) above the histograms denote division number (see Fig. 2). (b) The absolute number of CD4+ Tm cells recovered in co-culture and transwell assay were analysed by TruCOUNTTM assay (see Fig. 1). Data are displayed as the total number of CD3+ T cells/well [corresponding to CD4+ Tm cells/well; mean ± standard error of the mean (SEM) for triplicate measurements] of one representative experiment of four. APCs, antigen-presenting cells; Mo, monocytes.
IL-7/IL-15 alone or combined with monocytes or MDCs induced CD4+ Tm cell activation
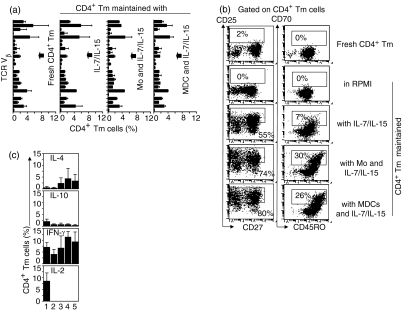
The surface phenotype and cytokine profile of CD4+ Tm cells were assessed before and after culture with IL-7/IL-15 alone or combined with either monocytes or MDCs. Regardless of the APC involvement (monocytes or MDCs), CD4+ Tm cells maintained polyclonal TCR Vβ gene usage similar to that of fresh CD4+ Tm cells (Fig. 4a). CD4+ Tm cells up-regulated CD25 and CD70 in the presence of IL-7/IL-15 alone, and this up-regulation was increased in the presence of IL-7/IL-15 combined with either monocytes or MDCs (Fig. 4b). Irrespective of the culture conditions, expression of CD45RO and CD62L was retained, CCR7 was down-regulated and CD154 was unchanged in CD4+ Tm cells (Fig. 4b, and data not shown). Thus, IL-7/IL-15 either alone or combined with APCs did not simply maintain the resting phenotype of CD4+ Tm cells but instead induced CD4+ Tm cell activation. In addition, IL-7/IL-15 either alone or combined with APCs appeared to change the cytokine profile of CD4+ Tm cells by enhancing (but not significantly) the number of IL-4- and IFN-γ-secreting cells and reducing the number of IL-2-secreting cells.
Figure 4.
T-cell receptor (TCR) Vβ usage, surface phenotype and cytokine expression in CD4+ T memory (Tm) cells maintained with interleukin (IL)-7/IL-15 alone or with monocytes or myeloid dendritic cells (MDCs) and IL-7/IL-15. Fresh CD4+ Tm cells or CD4+ Tm cells cultured in the presence of IL-7/IL-15 alone or combined with monocytes or MDCs for 7 days were analysed for TCR Vβ usage, surface phenotype and cytokine expression by flow cytometry. (a) Fresh or cultured cells were labelled with anti-CD3-allophycocyanin and anti-TCR Vβ Repertoire Kit monoclonal antibodies (mAbs). The proportion of CD3+ T cells (corresponding to CD4+ Tm cells) expressing specific TCR Vβ (y-axis, top to bottom: Vβ-1, -2, -3, -4, -5·1, -5·2, -5·3, -7·1, -7·2, -8, -9, -11, -12, -13·1, -13·2, -13·6, -14, -16, -17, -18, -20, -21·3, -22 and -23) is shown [bar; mean ± standard error of the mean (SEM) for two separate experiments]. (b) Fresh or cultured cells were labelled with anti-CD4-peridinin-chorophyll-protein (PerCp), anti-CD25-fluorescein isothiocyanate (FITC) and anti-CD27-phycoerythrin (PE) mAbs or alternatively with anti-CD70-FITC and anti-CD45RO-PE mAbs. CD4+ Tm cells are displayed in a dot plot of CD27 versus CD25 or in a dot plot of CD45RO versus CD70. The proportions of CD4+ CD25+ and CD4+ CD70+ CD45RO+ cells are indicated (numbers in boxes). Data are from one representative experiment of five. (c) Fresh or cultured cells were labelled with anti-CD3-FITC combined with anti-IL-4-PE, anti-IL-10-PE, anti-interferon (IFN)-γ-PE or anti-IL-2-PE mAb and analysed for intracellular cytokine expression by flow cytometry. The proportion of CD3+ Tm cells (corresponding to CD4+ Tm cells) expressing IL-4, IL-10, IFN-γ or IL-2 is shown (bar; mean ± SEM for three separate experiments). (1) Fresh CD4+ Tm cells; (2–5) CD4+ Tm cells maintained (2) in RPMI; (3) with IL-7/IL-15; (4) with monocytes and IL-7/IL-15; (5) with MDCs and IL-7/IL-15. Mo, monocytes.
CD4+ Tm cells preconditioned with IL-7/IL-15 alone or with monocytes or MDCs and IL-7/IL-15 reduced T cell-dependent antibody responses
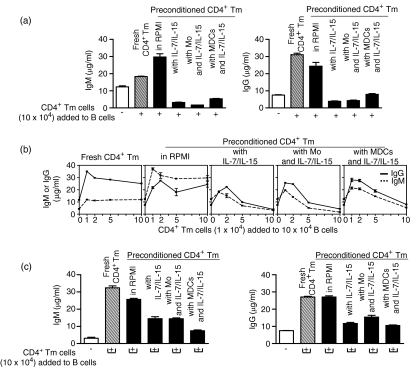
CD4+ Tm cells provide critical helper function for T cell-dependent B-cell responses through cell–cell contact and cytokine production.10 As IL-7/IL-15 either alone or combined with APCs changed the activation state and cytokine profile of CD4+ Tm cells, we presumed that these changes might affect the helper function of CD4+ Tm cells, such as that required for T cell-dependent B-cell responses. Therefore, we analysed fresh CD4+ Tm cells, CD4+ Tm cells preconditioned in RPMI (control preconditioned CD4+ Tm cells) and CD4+ Tm cells preconditioned with IL-7/IL-15 alone or with monocytes or MDCs and IL-7/IL-15 (preconditioned CD4+ Tm cells) for their capacity to help T cell-dependent PWM-driven B-cell responses. The addition of preconditioned CD4+ Tm cells (10 × 104), in lieu of fresh of control preconditioned CD4+ Tm cells, to PWM-driven B-cell culture reduced both IgM and IgG production to the basic PWM-induced levels (Fig. 5a). Suppression of IgM and IgG production was particularly evident when greater numbers of preconditioned CD4+ Tm cells (5–10 × 104) were added to the B-cell culture (Fig. 5b), whereas lower numbers of preconditioned CD4+ Tm cells (1–2·5 × 104) were still able to help, enhancing IgM and IgG production above PWM-induced levels (Fig. 5b). These results were in contrast to the consistent help and enhancement of IgM and IgG production found when increasing numbers of fresh CD4+ Tm cells or control preconditioned CD4+ Tm cells (1–10 × 104) were added to the PWM-driven B-cell culture (Figs 5a and b). Removal of contact between B cells and preconditioned CD4+ Tm cells (transwell assay) failed to restore IgM and IgG production to the levels obtained in the absence of contact with fresh or control preconditioned CD4+ Tm cells (Fig. 5c). These experiments revealed that the preconditioned CD4+ Tm cells develop suppressor/(regulatory) function along with the expected helper function. Our results also suggest that preconditioned CD4+ Tm cells exert their suppressor/(regulatory) function through contact with B cells but, albeit to a lesser extent, contact-independent suppression by soluble factors also makes a contribution.
Figure 5.
Preconditioned CD4+ T memory (Tm) cells suppress T-cell dependent antibody responses. Fresh or preconditioned CD4+ Tm [preconditioned in RPMI, with interleukin (IL)-7/IL-15 alone, or with monocytes or myeloid dendritic cells (MDCs) and IL-7/IL-15] were cultured with B cells in the presence of pokeweed mitogen (PWM) in (a, b) co-culture assay or (c) transwell assay. The culture supernatant was collected from co-culture or transwell assay at day 7 and assayed for human immunoglobulin M (IgM) and IgG by enzyme-linked immunosorbent assay (ELISA). The mean ± standard error of the mean (SEM) of triplicate ELISA measurements of human IgM and IgG is representative of five co-culture and three separate transwell experiments. Mo, monocytes.
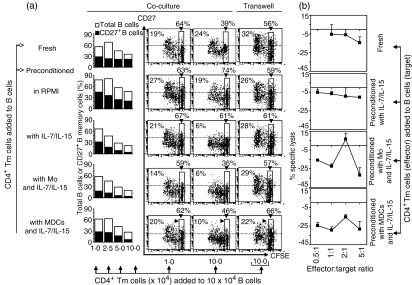
Reduced antibody production, mediated by preconditioned CD4+ Tm cells, was associated with a reduction in CD27+ B memory cells
It was possible that preconditioned CD4+ Tm cells reduced antibody production by affecting the persistence of antibody-producing CD27+ B memory cells.11 To address this possibility, we analysed the proportion of total B cells (sum of CD27+ and CD27– B cells) and CD27+ B memory cells after the addition of standardized numbers of fresh CD4+ Tm cells, control preconditioned CD4+ Tm cells or preconditioned CD4+ Tm cells (1–10 × 104 cells) to PWM-driven B-cell cultures (Fig. 6a). In co-culture with fresh CD4+ Tm cells or control preconditioned CD4+ Tm cells, the proportion of total B cells and CD27+ B memory cells was maintained (in relation to the number of CD4+ Tm cells added); however, the proportions were decreased in co-culture with preconditioned CD4+ Tm cells (Fig. 6a). This was particularly evident in co-cultures containing 5–10 × 104 preconditioned CD4+ Tm cells (Fig. 6a), consistent with reduced IgM and IgG production (Fig. 5b).
Figure 6.
Decrease in the proportion of CD27+ B memory cells in co-culture with preconditioned CD4+ T memory (Tm) cells. (a) Various numbers of fresh or preconditioned CD4+ Tm were cultured with carboxyfluorescein diacetate succinimidyl ester (CFSE)-unlabelled or CFSE-labelled B cells in the presence of pokeweed mitogen (PWM) for 4–7 days. Cells from culture containing CFSE-unlabelled B cells were harvested at day 7, stained with anti-CD4-fluorescein isothiocyanate (FITC), anti-CD27-phycoerythrin (PE) and anti-CD19-allophycocyanin monoclonal antibodies (mAbs) and analysed for the proportion of total B cells (defined as CD19+/– CD4– events) or for the proportion of CD27+ B cells (defined as CD19+/– CD27+ CD4– events). Data are from one representative experiment of three. Cells from co-culture and transwell assay containing CFSE-labelled B cells were harvested at day 4, stained with anti-CD27-PE mAb and analysed by flow cytometry. Dot plots display CD27 expression as a function of division of CFSE-labelled B cells with the percentages of CD27+ B cells indicated in each dot plot (dashed line shows staining with control mAb). Undivided CFSE-labelled B cells are indicated in each dot plot (numbers in boxes). Data are from events above dashed line; one representative experiment of four. (b) Fresh or preconditioned CD4+ Tm cells were cultured with 51Cr-labelled PWM-stimulated B cells (3 × 104) at effector:target ratios of 0·5 : 1 to 5 : 1 for 13 hr, at which time supernatants were collected and analysed for 51Cr release. The data are displayed as the mean ± standard error of the mean (SEM) for triplicate measurements and are representative of three separate experiments. Mo, monocytes.
Preconditioned CD4+ Tm cells, particularly those maintained with monocytes or MDCs and IL-7/IL-15, supported the division of PWM-stimulated B cells, similarly to fresh CD4+ Tm cells (Fig. 6a; dot plots). However, dividing B cells maintained CD27 expression in co-cultures with small numbers of preconditioned CD4+ Tm cells (e.g. 1 × 104) while in co-cultures with higher numbers of preconditioned CD4+ Tm cells (e.g. 10 × 104) CD27 expression was diminished or lost (Fig. 6a; dot plots). Loss of CD27 expression was dependent on contact with preconditioned CD4+ Tm cells (Fig. 6; co-culture versus transwell), occurred during the initial 4 days of co-culture and was typical for B cells, whereas CD4+ Tm cells originating from the same co-culture retained CD27 expression (Fig. 4b). We speculated that the apparent disappearance of CD27+ B memory cells might reflect their lysis by IFN-γ-producing T effector cells, as suggested in previous mouse studies.12,13 Therefore, fresh or preconditioned CD4+ Tm cells were tested for cytotoxic activity against PWM-stimulated B cells. No significant lysis of PWM-stimulated B cells was detected in either a 4-hr or extended 13-hr 51Cr-release assay at effector:target ratios of 0·5:1 to 5:1 (Fig. 6b and data not shown). Instead, in these experiments lower experimental than spontaneous 51Cr release (spontaneous 51Cr release was 30–50% of total 51Cr release) generated negative specific lysis values, suggesting that spontaneous B-cell death was occurring in the absence of help provided by the fresh or preconditioned CD4+ Tm cells. Thus, regulation of CD27+ B memory cells appeared to be an important functional feature of preconditioned CD4+ Tm cells; however, the contact-dependent mechanism(s) responsible for the disappearance of CD27+ B memory cells in co-culture with preconditioned CD4+ Tm cells requires further study.
Discussion
The maintenance of appropriate CD4+ Tm cell numbers and function is central to immunological memory. We show here that an environment involving myeloid APCs, such as monocytes and MDCs, and IL-7/IL-15, which maintains human CD4+ Tm cell numbers, also generates activated CD4+ Tm cells. Further, we make the new observation that such cells limited T cell-dependent antibody responses by contact-dependent mechanisms involving a reduction in antibody-producing CD27+ B memory cells, but contact-independent suppression by soluble factors also contributed to the antibody-producing capacity of CD27+ B memory cells.
Our data reveal new attributes of the human CD4+ Tm cell responses to signals received from monocytes, MDCs and IL-7/IL-15. They suggest that the requirements for activation and proliferation of CD4+ Tm cells may be different. IL-7/IL-15 was sufficient to induce CD4+ Tm cell activation, as measured by the up-regulation of CD25 and CD69 (to a lesser extent in the latter case), but addition of either monocytes or MDCs (as APCs) further enhanced CD4+ Tm cell activation. IL-7/IL-15 alone induced measurable CD4+ Tm cell proliferation but the addition of monocytes or MDCs was required to induce substantial CD4+ Tm cell proliferation. Human blood PDCs were incapable of mediating this function. The CD4+ Tm cell proliferation induced either by IL-7/IL-15 alone or by monocytes or MDCs and IL-7/IL-15 maintained similar TCR Vβ repertoires to those in the fresh CD4+ Tm cells. Although either monocytes or MDCs with IL-7/IL-15 consistently induced significant CD4+ Tm cell proliferation, the levels of the proliferative responses varied somewhat amongst healthy individuals. This heterogeneity in humans is expected and points to potential variation in regulatory mechanisms to maintain CD4+ Tm cell numbers, which are likely to be dependent on background genes14 and to be particularly relevant to the pathogenesis of autoimmune diseases.
The availability of MHC class II promoted CD4+ Tm cell proliferation induced by MDCs and IL-7/IL-15, but was less relevant to the CD4+ Tm cell proliferation induced by monocytes and IL-7/IL-15. Similarly, MoDCs did not require MHC class II to induce CD4+ Tm cell proliferation in the presence of IL-7/IL-15,5 suggesting that monocytes and MoDCs may share a similar mechanism(s) for maintaining CD4+ Tm cell proliferation. Certainly, they induced similar levels of CD4+ Tm cell proliferation in 75% of our experiments. We also found that CD4+ Tm cell proliferation, in the absence of introduced antigen, did not appear to involve CD40 costimulation, distinguishing it from CD4+ T cell proliferation in response to foreign antigen. This is supported by mouse studies, which showed that homeostatic proliferation of T cells occurred in the absence of CD40 expression on the host APCs.15 Further studies are required to determine how signalling pathways triggered by MHC class II on MDCs and IL-7/IL-15 promote CD4+ Tm cell proliferation in the absence of introduced antigen.
Particularly striking is the observation that MDCs induced higher levels of CD4+ Tm cell proliferation than monocytes, in the presence of IL-7/IL-15. Using CFSE division-tracking and transwell experiments, we showed that MDCs facilitated CD4+ Tm cell proliferation, in a contact-dependent manner, by enhancing the proportion of dividing cells, the numbers of proliferative precursors and mitotic events and the number of CD4+ Tm cells recovered at the end of culture. In contrast, contact between monocytes and CD4+ Tm cells had a less pronounced effect on the dynamics of CD4+ Tm cell proliferation but probably contributed to CD4+ Tm cell survival.
CFSE division-tracking revealed asynchrony in the numbers of divisions reached by each CD4+ Tm cell in co-culture with either monocytes or MDCs and IL-7/IL-15, with some CD4+ Tm cells dividing twice, whereas others remained undivided. This may reflect heterogeneity in the ability of distinct CD4+ Tm subsets to be recruited into the dividing pool. However, our experiments showed that ‘memory’ markers, such as CD62L and CCR7, did not distinguish between the divided and undivided CD4+ Tm cells (data not shown). It is possible that human CD4+ Tm cells with greater TCR affinity for self-antigens may undergo more cell division, in the light of mouse experiments with transgenic T cells.16 Quantitative mathematical modelling17 may help our understanding of how the proliferation and differentiation of different subsets amongst the CD4+ Tm cell population are regulated by signals received from monocytes, MDCs and IL-7/IL-15 in healthy individuals and patients with autoimmune diseases.
The most important issue in the maintenance of human CD4+ Tm cells, apart from their number, is their functional capacity. There is evidence that the conditions permitting the maintenance of mouse CD4+ T cells can lead to impairment of their helper functions.18 We provide unequivocal evidence that preconditioned CD4+ Tm cells (maintained in the presence of IL-7/IL-15 alone or with monocytes or MDCs and IL-7/IL-15) have diminished helper function, i.e. they do not facilitate T cell-dependent antibody production in the same manner as control preconditioned CD4+ Tm cells (maintained in RPMI) or fresh blood CD4+ Tm cells. This was particularly evident when greater numbers of preconditioned CD4+ Tm cells (5–10 × 104) were added to the PWM-driven B-cell culture. Our interpretation of this observation is that the preconditioned CD4+ Tm cells evolved distinct helper and suppressor/regulatory functions, perhaps even attributable to the different phenotypic subsets that emerged amongst the preconditioned CD4+ Tm cells (e.g. CD69+, CD25+ or CD70+ cells). The immunoregulatory network is clearly complicated and may involve interactions amongst the preconditioned CD4+ Tm cells themselves as well as different interactions with B cells, which contribute to the fine tuning of B-cell antibody responses. In vivo, this could mean that T cell-dependent B-cell antibody responses might become flexible, depending on the availability of functionally distinct subsets of CD4+ Tm cells in the lymphoid tissues.
It is intriguing that the CD4+ Tm cells with putative suppressor/regulatory function emerged within the preconditioned CD4+ CD45RO+ Tm cell population, particularly, as human CD4+ T cells with antibody suppressive/regulatory capacity have been described in the CD4+ CD45RA+ population.19,20 Interaction between CD70 on preconditioned CD4+ Tm cells and its ligand CD27 on T and B memory cells is known to enhance both cell-mediated cytotoxicity and the formation of antibody-producing cells.21 Although activated CD25+ T cells within the preconditioned CD4+ Tm cells may include T regulatory cells (e.g. CD25+ CD27+) their suppressor/regulatory function is likely to be impaired by the presence of IL-7/IL-15.22 These findings are difficult to reconcile with the observed down-regulation of T cell-dependent antibody production in our experiments and point to another potential regulatory mechanism(s) being involved.
We predicted that the maintenance of CD27+ B memory cells, which are responsible for antibody production in the PWM-driven B-cell cultures,11 would directly influence antibody production following the addition of preconditioned CD4+ Tm cells. Indeed, we found that the addition of preconditioned CD4+ Tm cells sustained or even enhanced the division of PWM-stimulated B cells but did not maintained their CD27 expression. When greater numbers of preconditioned CD4+ Tm cells (5–10 × 104) were added to PWM-driven B-cell cultures, numbers of CD27+ B memory cells were particularly decreased in conjunction with reduced antibody responses. Contact between preconditioned CD4+ Tm cells and B cells, although not essential in the suppression of antibody responses, was involved in the reduction of numbers of CD27+ B memory cells. A possible explanation for this was that preconditioned CD4+ Tm cells dependent on contact with B cells down-regulated CD27 expression on B memory cells and/or induced CD27+ B memory cell death. The decrease in total B cells implies CD27+ B memory cell death; however, we cannot exclude the possibility that CD27+ B memory cells are able to down-regulate CD27 expression, substituting for naïve CD27– B cells which have died in culture (e.g. activation-induced B-cell death). The presence of IFN-γ-producing cells among preconditioned CD4+ Tm cells seems unlikely to be responsible for the elimination of CD27+ B memory cells by cytotoxic mechanisms.13 Thus, our data raise the new possibility that preconditioned CD4+ Tm cells down-regulate CD27 on B memory cells, or eliminate CD27+ B memory cells by a novel mechanism(s) distinct from IFN-γ-mediated cytotoxicity. The critical relevance of this phenomenon is emphasized by the data linking low CD27+ B memory cell numbers with profound hypoglobulinaemia in common variable immunodeficiency patients.23 However, antibody production remained very modest in the absence of contact between the higher numbers of preconditioned CD4+ Tm cells and B cells. Thus, the interactions mediated by soluble factors with suppressive properties (e.g. IL-424) also appear to contribute significantly to the suppressive effect of preconditioned CD4+ Tm cells on antibody production. Artificial suppression of antibody production by any contaminating Fc-expressing cells (which bind the Fc portion of immunoglobulin) is possible but seems unlikely given that such cells should also have been present in fresh or control preconditioned CD4+ Tm cells which provide consistent help and enhance antibody production.
We suggest that, in steady-state conditions, MDCs with IL-7/IL-15 might be responsible for maintaining the majority of CD4+ Tm cells by facilitating their proliferation. However, we have demonstrated that maintenance of human CD4+ Tm cell numbers also generates mixed helper/suppressor (regulatory) function in CD4+ Tm cells. In healthy individuals, these CD4+ Tm cells may, through different interactions mediated by membrane-bound proteins or by soluble factors, suppress/regulate autoimmune B-cell responses whilst helping/enhancing protective immune responses in lymphoid tissues. According to this hypothesis, the mechanism regulating the number and/or function of CD4+ Tm cells might be altered in individuals with either a genetically or an environmentally induced predisposition to autoimmune disease, leading to induction or perturbation of otherwise prohibited autoimmune responses.
Acknowledgments
The authors thank Dr Ronald P. Gladue (Pfizer, Groton, USA) for his advice during the first stage of this project and Mary Sartor (Westmead Hospital, Sydney, NSW) for providing the TCR Vβ Repertoire Kit for this study. We also would like to thank the donors who provided blood, and Georgina Crosbie and Sonia Tepes for their help in donor blood collection.
Abbreviations
- APC
antigen-presenting cell
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- IFN-γ
interferon-γ
- Ig
immunoglobulin
- IL
interleukin
- mAb
monoclonal antibody
- MDC
myeloid dendritic cell
- MHC
major histocompatibility complex
- MoDC
monocyte-derived dendritic cell
- LPS
lipopolysaccharide
- PBMC
peripheral blood mononuclear cells
- PDC
plasmacytoid dendritic cell
- PE
phycoerythrin
- PerCp
peridinin-chorophyll-protein
- PMA
phorbol myristate acetate
- PWM
pokeweed mitogen
- SEM
standard error of the mean
- TCR
T-cell receptor
- Tm
T memory
- γc
gamma chain
References
- 1.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–62. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 2.Chernysheva AD, Kirou KA, Crow MK. T cell proliferation induced by autologous non-T cells is a response to apoptotic cells processed by dendritic cells. J Immunol. 2002;169:1241–50. doi: 10.4049/jimmunol.169.3.1241. [DOI] [PubMed] [Google Scholar]
- 3.Kondo T, Cortese I, Markovic-Plese S, Wandinger KP, Carter C, Brown M, Leitman S, Martin R. Dendritic cells signal T cells in the absence of exogenous antigen. Nat Immunol. 2001;2:932–8. doi: 10.1038/ni711. [DOI] [PubMed] [Google Scholar]
- 4.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–6. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 5.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–9. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osugi Y, Vuckovic S, Hart DN. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100:2858–66. doi: 10.1182/blood.V100.8.2858. [DOI] [PubMed] [Google Scholar]
- 7.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Meth. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 8.Vuckovic S, Gardiner D, Field K, et al. Monitoring dendritic cells in clinical practice using a new whole blood single-platform TruCOUNT assay. J Immunol Meth. 2004;284:73–87. doi: 10.1016/j.jim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion. Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–83. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHeyzer-Williams MG, McHeyzer-Williams LJ, Fanelli Panus J, Bikah G, Pogue-Caley RR, Driver DJ, Eisenbraun MD. Antigen-specific immunity. Th cell-dependent B cell responses. Immunol Res. 2000;22:223–36. doi: 10.1385/IR:22:2-3:223. [DOI] [PubMed] [Google Scholar]
- 11.Kindler V, Zubler RH. Memory, but not naive, peripheral blood B lymphocytes differentiate into Ig-secreting cells after CD40 ligation and costimulation with IL-4 and the differentiation factors IL-2, IL-10, and IL-3. J Immunol. 1997;159:2085–90. [PubMed] [Google Scholar]
- 12.Clayberger C, Dekruyff RH, Cantor H. Immunoregulatory activities of autoreactive T cells: an I-A-specific T cell clone mediates both help and suppression of antibody responses. J Immunol. 1984;132:2237–43. [PubMed] [Google Scholar]
- 13.Arens R, Tesselaar K, Baars PA, et al. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–12. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 14.Theofilopoulos AN, Dummer W, Kono DH. T cell homeostasis and systemic autoimmunity. J Clin Invest. 2001;108:335–40. doi: 10.1172/JCI12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol. 2001;167:5664–8. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 16.Ge Q, Rao VP, Cho BK, Eisen HN, Chen J. Dependence of lymphopenia-induced T cell proliferation on the abundance of peptide/MHC epitopes and strength of their interaction with T cell receptors. Proc Natl Acad Sci USA. 2001;98:1728–33. doi: 10.1073/pnas.98.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deenick EK, Gett AV, Hodgkin PD. Stochastic model of T cell proliferation: a calculus revealing IL-2 regulation of precursor frequencies, cell cycle time, and survival. J Immunol. 2003;170:4963–72. doi: 10.4049/jimmunol.170.10.4963. [DOI] [PubMed] [Google Scholar]
- 18.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–50. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 19.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159:2652–7. [PubMed] [Google Scholar]
- 20.Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114:1640–9. doi: 10.1172/JCI22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brugnoni D, Airo P, Marino R, Notarangelo LD, van Lier RA, Cattaneo R. CD70 expression on T-cell subpopulations: study of normal individuals and patients with chronic immune activation. Immunol Lett. 1997;55:99–104. doi: 10.1016/s0165-2478(96)02693-4. [DOI] [PubMed] [Google Scholar]
- 22.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquot S, Macon-Lemaitre L, Paris E, Kobata T, Tanaka Y, Morimoto C, Schlossman SF, Tron F. B cell co-receptors regulating T cell-dependent antibody production in common variable immunodeficiency: CD27 pathway defects identify subsets of severely immuno-compromised patients. Int Immunol. 2001;13:871–6. doi: 10.1093/intimm/13.7.871. [DOI] [PubMed] [Google Scholar]
- 24.Khaled AR, Durum SK. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat Rev Immunol. 2002;2:817–30. doi: 10.1038/nri931. [DOI] [PubMed] [Google Scholar]