Abstract
Isolated lymphoid follicles (ILFs) are recently identified lymphoid structures in the small intestine with features similar to Peyer's patches (PPs). Using immunohistochemistry we characterized the composition of ILFs in the small intestines of immunocompetent mice and of mice that lacked PPs as a result of either genetic deficiency of lymphotoxin or temporary in utero lymphotoxin-β receptor-signalling blockade. We showed that although both immature and mature ILFs were present in the intestines of immunocompetent mice, PP-deficiency induced a significantly greater number of mature ILFs. We found that in addition to B-lymphocyte-containing germinal centres, mature ILFs also possessed large networks of follicular dendritic cells (FDCs). These features were not detected within immature ILFs. Indeed, the presence of FDCs could be used to reliably distinguish ILF maturity. Further analysis revealed that the area occupied by the FDCs within mature ILFs was substantial. The total area occupied by FDCs in all the mature ILFs in mice lacking PPs was equivalent to the total area occupied by FDCs in all the PPs and the few mature ILFs in immunocompetent mice. Based on these data we reasoned that in the absence of PPs, mature ILFs are important inductive sites for intestinal immune responses. Indeed, in mice that lacked PPs, ILF maturation coincided with a restoration of faecal immunoglobulin A levels to values that were comparable to those found in immunocompetent mice. Taken together, these data imply that the induction of germinal centres and FDC networks within mature ILFs in response to PP deficiency provides an important compensatory mechanism.
Keywords: follicular dendritic cells, intestine, isolated lymphoid follicles, lymphotoxin, Peyer's patches
Introduction
The gastrointestinal tract is a major interface between host and potential pathogenic micro-organisms. Highly organized lymphoid structures, such as Peyer's patches (PPs) and diffusely distributed effector cells within the epithelium and lamina propria, help to protect the mammalian gastrointestinal tract against infection by microbial pathogens. Secretory immunoglobulin A (IgA) is an important component of the intestinal immune response and is synthesized by plasma cells in the lamina propria.1 In the intestine, IgA isotype class switching occurs only in organized lymphoid structures such as PPs2 but antigen-specific IgA responses have been demonstrated in mice that are deficient in PPs.3 As a consequence, novel lymphoid clusters, termed isolated lymphoid follicles (ILFs), were identified along the antimesenteric wall of the mucosa of the small intestine.4 Isolated lymphoid follicles are much smaller than PPs and comprise a single B-lymphocyte follicle with a germinal centre (GC) and an overlying M-cell-containing epithelium similar to the follicle-associated epithelium of PPs.4 Isolated lymphoid follicles are distributed throughout the small and large intestines of mice4–6 and have also been demonstrated in the intestines of rabbits,7 rats,8 guinea-pigs9 and humans.10
Interactions between the membrane bound lymphotoxin (LT) α1β2 heterotrimer and the LTβ receptor (LTβR) during gestation are crucial for the formation of PPs and other secondary lymphoid tissues. Accordingly, mice that are deficient in LTα, LTβ or LTβR lack PPs and most other lymph nodes.11 Likewise, temporary in utero LTβR-blockade during the period of embryonic lymphoid tissue formation also blocks the development of PPs and certain lymph nodes.12 The factors required for ILF development share similarities with those required for PP development but there are key differences. For example, stimulation via LTβR is also important for the development of ILFs because they are absent in mice that are deficient in LT or LTβR.4,13 However, unlike PP formation, ILF formation occurs postnatally because in utero LTβR-signalling blockade does not inhibit ILF formation, and their development in adult LT-deficient mice can be restored by reconstitution with LT-expressing bone marrow.4,13,14
Follicular dendritic cells (FDCs) reside within B-lymphocyte follicles in GCs and are specialized to trap and retain antigen on their surfaces. Antigen trapped on the surface of FDCs is considered to promote immunoglobulin isotype class switching and affinity maturation of naive IgM+ B lymphocytes.15–19 Consistent with their role as important sites for the generation of IgA responses,2 PPs contain all the necessary cellular components required to generate IgA-committed B lymphocytes including B-lymphocyte follicles, with GCs, T cells and FDC networks. The ILFs appear structurally and functionally similar to PPs4 and their inductive nature implies that they are a complementary system for the generation of intestinal IgA responses.13 In this study we demonstrate that mature ILFs also contain large FDC networks. The presence of FDC networks within gut-associated lymphoid tissues is considered important for the induction of intestinal IgA responses.20 Here, the induction of FDC maturation within ILFs of mice lacking PPs and mesenteric lymph nodes (MLNs) coincided with a restoration of faecal IgA to levels comparable with those found in immunocompetent mice. Therefore, our data suggest that the FDC networks within ILFs provide the necessary microenvironment to promote efficient interaction between luminal derived antigens and B lymphocytes to stimulate the generation of effective IgA responses.
Materials and methods
Mice
Both LTα–/– mice21 and LTβ–/– mice22 were obtained from B & K Universal Ltd (Hull, UK) and were maintained on a C57BL/6 background. Age-matched and sex-matched C57BL/6 mice were used as immunocompetent wild-type (WT) controls in the studies using LTα–/– mice and LTβ–/– mice. Severe combined immunodeficiency (SCID) mice were maintained on a 129/Ola background.23
γ-irradiation and bone marrow reconstitution
Bone marrow from the femurs and tibias of immunocompetent C57BL/6 WT mice was prepared as a single-cell suspension (3 × 107 to 4 × 107 viable cells/ml) in Hank's balanced salt solution (Life Technologies, Paisley, UK). Recipient adult (6–8 weeks old) LTα–/– mice, LTβ–/– mice and C57BL/6 mice were γ-irradiated (950 rads) and 24 hr later were reconstituted with 0·1 ml bone marrow by injection into the tail vein.
PP-deficient mice
To create progeny mice that were deficient in PPs, timed pregnant C57BL/Dk mice were given a single intravenous injection of 100 μg of a fusion protein containing the soluble LTβR domain linked to the Fc portion of human IgG1 (LTβR-Ig24) on day 11·5 of gestation.
Immunohistochemical and immunofluorescent analysis
Spleens were snap-frozen at the temperature of liquid nitrogen. Small intestine from each mouse was divided into three roughly equal parts, gently squeezed to remove the gut contents, coiled, embedded in Tissue-Tek® OCT Compound™ (Bayer Plc., Newbury, UK) and snap frozen at the temperature of liquid nitrogen. Serial frozen sections (10 μm thickness) were cut on a cryostat.
Follicular dendritic cells were visualized by staining with 8C12 monoclonal antiserum to detect CR1 (CD35; BD Biosciences PharMingen, Oxford, UK) or 7G6 monoclonal antiserum to detect CR2/CR1 (CD21/CD35; BD Biosciences PharMingen). Complement components C3 and C4 were detected using RMC7H8 (Connex, Martinsreid, Germany) and FDC-M2 (AMS Biotechnology, Oxon, UK) monoclonal antisera, respectively. B lymphocytes were detected using B220 monoclonal antiserum to detect CD45R (Caltag, Towcester, UK), or biotin-conjugated peanut agglutinin (Sigma, Poole, UK) to detect GC B lymphocytes. T lymphocytes were detected using CD3ε-specific monoclonal antiserum 2C11 (BD Biosciences Pharmingen).
For light microscopy, following the addition of primary antibody, biotin-conjugated species-specific secondary antibodies (Stratech, Soham, UK) were applied followed by alkaline phosphatase coupled to the avidin/biotin complex (Vector Laboratories, Peterborough, UK). Vector Red (Vector Laboratories) was used as a substrate, and sections were counterstained with haematoxylin to distinguish the cell nuclei. For fluorescent microscopy, following the addition of primary antibody, species-specific secondary antibodies coupled to Alexa dyes (Invitrogen, Paisley, UK) 488 (green) or 594 (red) were used. Sections were mounted in fluorescent mounting medium (Dako, Ely, UK) and examined using a Zeiss LSM5 confocal microscope (Zeiss, Welwyn Garden City, UK).
ILF enumeration and morphometric analysis of FDC networks
Entire small intestines were divided into three equal parts, coiled, fixed in paraformaldehyde and embedded in paraffin wax. Serial sections (10 μm thickness) were deparafinnized and pretreated with Target Retrieval Solution (DAKO Ltd, Ely, UK). Sections were immunostained with antisera specific for CD45R or CR2/CR1 as described above and examined using a confocal microscope. The total number of B-lymphocyte-containing ILFs in the entire small intestine of each mouse analysed was counted microscopically using CD45R expression for identification. The maturity of each ILF was determined according to size and the location and density of component cells as previously described.13 An image of each mature ILF was captured and the area covered by FDCs (CR2/CR1+ CD45R– cells) was measured using image pro plus software (Media Cybernetics, Wokingham, UK).
Measurement of faecal IgA
Faecal pellets were collected from individual mice and faecal IgA levels were compared by enzyme-linked immunosorbent assay. Briefly, 10% faecal homogenates were prepared in carbonate–bicarbonate coating buffer (Sigma) and centrifuged for 10 min at 12 000 g. Supernatant was removed and absorbed to flat-bottomed, high-binding microwell plates (Costar®, High Wycombe, UK) for 18–24 hr at 4°. Plates were then blocked with 0·01 m phosphate-buffered saline (pH 7·5) containing 5% bovine serum albumin. Bound IgA was detected using alkaline phosphatase-conjugated goat anti-mouse IgA (Southern Biotechnology, Birmingham, AL). Bound alkaline phosphatase activity was measured by incubation with p-nitrophenyl phosphate liquid substrate (Sigma). Optical density was measured at 450 nm using a V-max kinetic microplate reader (Molecular Devices, Sunnyvale, CA). Faecal pellets from C57BL/6 WT mice, C57BL/Dk control mice and SCID mice were used as reference controls.
Statistical analysis
Data are presented as mean ± standard error (SE). Significant differences between samples in different groups were sought by one-way analysis of variance (anova).
Results
Temporary in utero LTβR-signalling blockade induces the maturation of ILFs in progeny mice
To temporarily blockade the LTβR-signalling pathway in utero, pregnant C57BL/Dk mice were given a single intravenous injection of 100 μg LTβR-Ig on day 11·5 of gestation. Consistent with previous reports,12 the intestines from age-matched, untreated, immunocompetent C57BL/Dk (control) mice contained approximately six PPs per mouse (n = 11), whereas no PPs were detected in any of the small intestines taken from progeny mice treated in utero with LTβR-Ig (n = 12). Microscopic analysis revealed the presence of both PPs and ILFs in the intestines of untreated C57BL/Dk (control) mice (Fig. 1), whereas only ILFs were found in the intestines of mice treated in utero with LTβR-Ig (termed PP-deficient C57BL/Dk mice; Fig. 1).
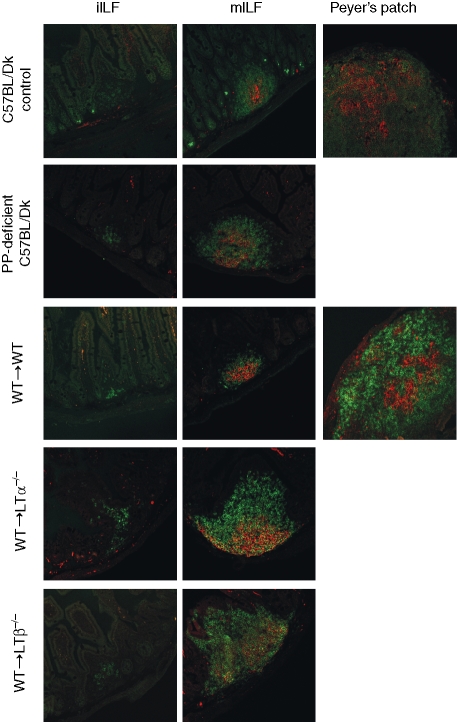
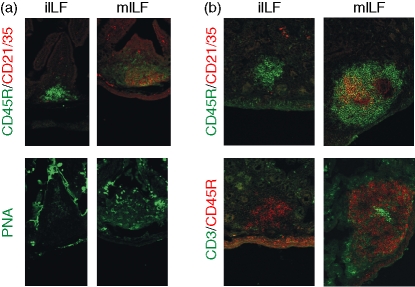
Figure 1.
Detection of B lymphocytes and FDCs within ILFs and PPs in the small intestine. Entire small intestines were examined from each mouse group and representative images are shown. Sections were stained with the CD45R-specific monoclonal antiserum B220 to detect B lymphocytes (green) and 7G6 monoclonal antiserum to detect CR2/CR1 (CD21/35) expressing FDCs (red). In each mouse group analysed, FDCs were absent within the B-lymphocyte aggregations of immature isolated lymphoid follicles (iILFs; left column), whereas both B lymphocytes and FDCs were detected within all mature ILFs (mILFs; middle column). Original magnification ×200.
The maturity of ILFs has previously been defined according to size and the location and density of the component cells.13 Immature ILFs (iILFs) comprise loosely clustered CD45R+ (B220) cells located at the base of a villus. Mature ILFs (mILFs), in contrast, are well-organized nodular structures, of similar width to or greater than the width of one villus, occurring singly or in groups of two, with a GC and an overlying dome typical of the follicle-associated epithelium of a PP. Using these criteria, double immunostaining for B lymphocytes (CD45R+ cells) and FDCs (CR2/CR1+ cells) on the same tissue section revealed that the presence of FDCs (CR2/CR1+ CD45R– cells) could also be reliably used to distinguish ILF maturation status. Our analysis showed that all mILFs contained FDCs, whereas iILFs did not (Fig. 1). The FDCs characteristically trap and retain antigens in the form of immune complexes composed of antigen, antibody and/or complement components, storing them in native form on the cell surface for long periods.25 Immune complex retention on FDCs is highly dependent on complements and the expression of CR1 (CD35) and CR2 (CD21). Complement components C3 and C4, in association with FDCs expressing CR1 and CR2, were detected within mILFs (Fig. 2) but not within iILFs (not shown). Taken together, these data clearly demonstrate that mILFs in the intestines of PP-deficient C57BL/Dk mice contain immune complex-trapping FDCs.
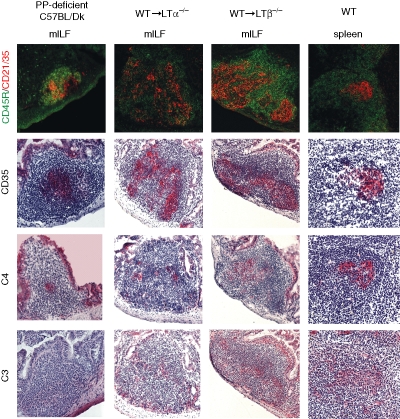
Figure 2.
Immunohistochemical characterization of FDCs within mILFs. Sections of small intestine or spleen (far right column) were stained with CD45R-specific monoclonal antiserum B220 to detect B lymphocytes (top row, green), CR2/CR1 (CD21/35)-specific (top row, red) or CR1 (CD35)-specific monoclonal antisera to detect FDCs (second row, red) and monoclonal antisera specific for complement components C4 (third row, red) and C3 (bottom row, red). Complement components in association with CR1- and CR2-expressing FDCs were detected in both mILFs and the spleen. Original magnification ×200.
FDCs in mILFs compensate for PP deficiency
We next assessed the number of ILFs in the intestines of PP-deficient mice and control mice, and also compared the size of the FDC networks within them. Entire small intestines were double immunostained with monoclonal antisera specific for B lymphocytes (CD45R+ cells) and CR2/CR1-expressing FDCs. Sections were examined microscopically, the ILFs were counted, their maturation status was recorded and the area within them occupied by FDCs was measured. The total number of ILFs in the small intestine of PP-deficient mice was found to be significantly greater than the number in C57BL/Dk (control) mice (P = 0·001; Fig. 3a). Approximately four-fold more ILFs were detected in the intestines of PP-deficient mice than in those of control mice. Although the small intestines of both groups of mice were found to contain both mILFs and iILFs (Fig. 1), a significantly greater proportion of the ILFs in PP-deficient mice were mature when compared to those in control mice (46% and 15%, respectively; P = 0·001; Fig. 3b).
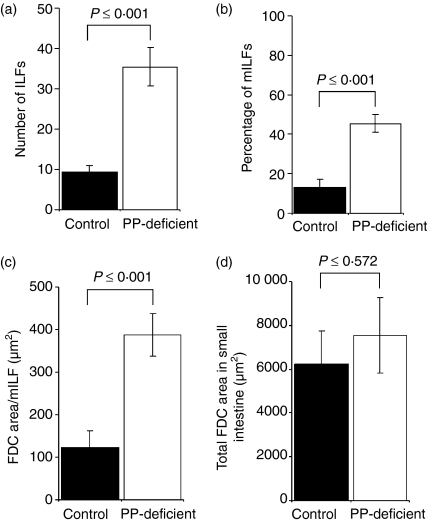
Figure 3.
Enumeration of ILFs and FDC area in the small intestines of PP-deficient C57BL/Dk mice and immunocompetent C57BL/Dk (control) mice. For each mouse group, sections were stained with B-lymphocyte- (CD45R) and FDC- (CD21/35) specific antisera and the entire small intestine was examined microscopically. The number of ILFs was counted and the area occupied by FDCs was measured. (a) Intestines from PP-deficient mice contained significantly more (approximately four-fold more) ILFs than control mice. (b) Intestines from PP-deficient mice were found to have a significantly higher percentage of mILFs (46%) when compared to control mice (13%). (c) The area occupied by FDCs in individual mILFs from PP-deficient mice was significantly larger than those in mILFs from control mice. (d) The total area covered by FDCs in the mILFs in PP-deficient C57BL/Dk mice was equivalent to the total area covered by FDCs from all the PPs and mILFs in control mice. Each bar represents the mean ± SE for each group of mice (n = 12 for PP-deficient mice, n = 11 for control mice).
Next, we measured the area of the FDC networks within the mILFs in PP-deficient mice and those within both the PPs and mILFs in control mice. We found that the area occupied by FDCs within individual mILFs from PP-deficient mice was significantly larger than the area they occupied in mILFs from control mice (P = 0·001; Fig. 3c). Furthermore, the total area occupied by FDCs in all the mILFs in PP-deficient mice was equivalent to the total area occupied by FDCs in all the PPs and the few mILFs found in control mice (P = 0·572; Fig. 3d).
LT-expressing bone marrow-derived cells induce the development of ILFs in LT-deficient mice
Both PPs and ILFs are absent in the intestines of LT-deficient mice because signalling via the LTβR is required for their formation.11,13,26 However, reconstitution of adult LTα–/– mice13 or LTβ–/– mice with LT-expressing WT bone marrow (termed WT→LTα–/– mice, and WT→ LTβ–/– mice, respectively) restored the formation of ILFs (Figs 1 and 4), but not the development of PPs (data not shown). When assessed 105 days after WT bone marrow reconstitution, the total number of ILFs in the intestines of WT→LTα–/– mice and WT→LTβ–/– mice was equivalent to the number detected in WT→WT mice (Fig. 4a).
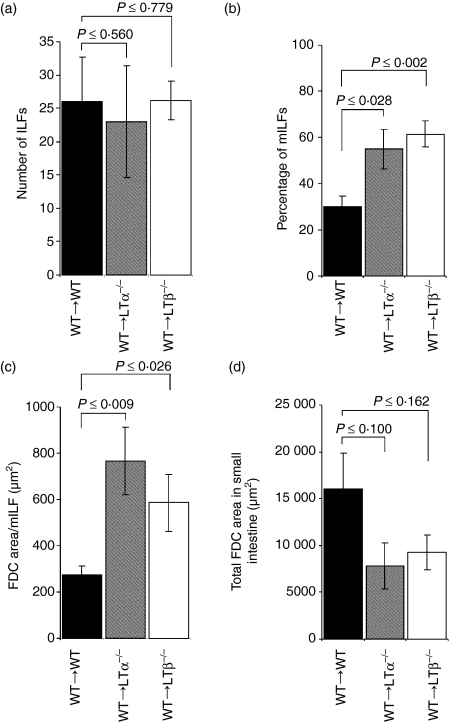
Figure 4.
Enumeration of ILFs and FDC area in the small intestines of WT→WT mice, WT→LTα–/– mice and WT→LTβ–/– mice 105 days after reconstitution with WT bone marrow. For each mouse group, sections were stained with B-lymphocyte- (CD45R) and FDC- (CD21/35) specific antisera and the entire small intestine was examined microscopically. The number of ILFs was counted and the area occupied by FDCs was measured. (a) Similar numbers of ILFs were found in WT→WT mice, WT→LTa–/– mice and WT→ LTβ–/– mice. (b) However, WT→LTα–/– and WT→LTβ–/– mice were found to have a significantly higher percentage of mILFs when compared to WT→WT mice. (c) The area occupied by FDCs in individual mILFs from WT→LTα–/– mice and WT→LTβ–/– mice was significantly larger that those in WT→LTα–/– mice. (d) The total area covered by FDCs in all the mILFs in WT→LTα–/– mice or WT→LTβ–/– mice was equivalent to the total area covered by FDCs in all the PPs and mILFs in WT→WT mice. Each bar represents the mean ± SE for each group of mice (n = 4 for each group).
Mature ILFs in WT bone marrow reconstituted LT-deficient mice contain FDCs
We next examined the ILFs in intestines from WT→WT mice, WT→LTα–/– mice and WT→LTβ–/– mice in greater detail. Entire small intestines were double immunostained with monoclonal antisera specific for B lymphocytes (CD45R+ cells) and CR2/CR1-expressing FDCs. Sections were examined microscopically, the ILFs were counted, their maturation status was recorded and the area within them occupied by FDCs was measured. Although both iILFs and mILFs were detected in the intestines of each group of mice (Fig. 1), a significantly greater proportion of the ILFs in WT→LTα–/– mice and WT→LTβ–/– mice were mature when compared to those in WT→WT mice (P = 0·028, n = 6, and P = 0·002, n = 5, respectively, when compared to WT→WT mice; Fig. 4b).
Consistent with data from the analysis of PP-deficient mice, all the mILFs in the intestines of WT→LTα–/– mice, WT→LTβ–/– mice and WT→WT mice contained functional FDC networks (Fig. 2). When the area of these FDC networks was measured, those within the mILFs of WT→LTα–/– mice and WT→LTβ–/– mice were found to be significantly larger than those of WT→WT mice (P = 0·009, n = 6, and P = 0·026, n = 5, respectively, when compared to WT→WT mice, n = 6; Fig. 4c). In accordance with data from PP-deficient C57BL/Dk mice, the total area occupied by FDCs in all the mILFs in WT→LTα–/– mice or WT→LTβ–/– mice was equivalent to the total area occupied by FDCs in all the PPs and the mILFs found in WT→WT mice (P = 0·099, n = 6, and P = 0 ·162, n = 5, respectively, when compared to WT→WT mice; Fig. 4d).
Mature ILFs contain T lymphocytes and GC B lymphocytes
Further immunohistochemical analysis revealed that GC B lymphocytes (peanut agglutinin-positive cells) were readily detected in the mILFs of all five groups of mice and were rarely detected in iILFs (Fig. 5a). Although ILFs lack the interfollicular T-lymphocyte regions of PPs, CD3-positive T lymphocytes were detected within both iILF and mILFs, and appeared to be more frequent in mILFs (Fig. 5b).
Figure 5.
Immunohistochemical detection of (a) GC B lymphocytes and (b) T lymphocytes within ILFs. Entire small intestines were examined from PP-deficient C57BL/Dk mice and representative images are shown. Sections were stained with B lymphocyte- (CD45R, green) and FDC- (CD21/35, red) specific antisera to distinguish between iILFs and mILFs (upper row). (a) To detect GC B lymphocytes, adjacent sections were stained with peanut agglutinin (green) (lower row). Peanut agglutinin-binding GC B lymphocytes were detected only in mILFs. (b) To detect T lymphocytes, adjacent sections were stained with CD3-specific monoclonal antiserum (green) (lower row). T lymphocytes were found in mILFs, whereas few or none were found in iILFs. Original magnification ×200.
Enhanced intestinal IgA synthesis in the presence of mILFs
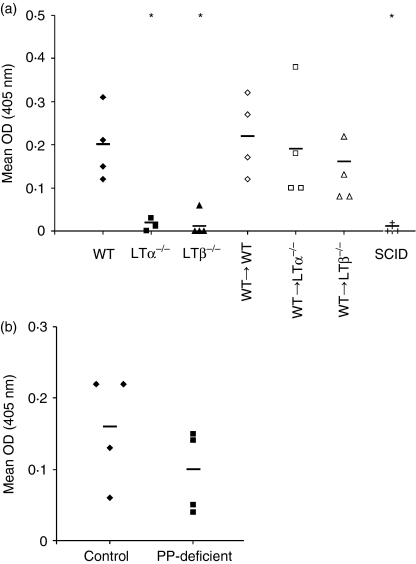
The finding that mILFs contain GC B lymphocytes and large FDC networks suggests that mILFs have the potential to function as inductive sites for mucosal immune responses. To assess this potential, we next compared the levels of IgA secreted into the intestines of mice lacking PPs and/or ILFs. Coincident with the absence of both PPs and mILFs, IgA levels in the faeces of LTα–/– mice and LTβ–/– mice were substantially lower than those in the faeces of immunocompetent WT mice (Fig. 6a). Similarly, IgA was undetectable in the faeces of highly immunodeficient SCID mice. In contrast, the induction of ILF maturation in WT→LTα–/– mice and WT→LTβ–/– mice (Fig. 4) coincided with a significant increase in intestinal IgA synthesis (P = 0·05, n = 4, and P = 0·020, n = 4, respectively, when compared to unreconstituted LTα–/– mice and LTβ–/– mice) to levels equivalent to those in both WT mice and WT→WT mice (Fig. 6a). Similarly, in PP-deficient C57BL/Dk mice which had no PPs but which had significantly increased numbers of mILFs (Fig. 3), faecal IgA levels were equivalent to those found in C57BL/Dk (control) mice (P = 0·245, n = 4; Fig. 6b). The absence of the MLNs in WT→LTα–/– mice did not adversely affect faecal IgA levels because they were equivalent to those of WT mice, WT→WT mice and WT→LTβ–/– mice, which possess them. Taken together these results suggest that mILFs are important inducible components of the mucosal immune system.
Figure 6.
The presence of mILFs in mice lacking PPs and MLNs correlates with enhanced IgA synthesis. Faecal IgA levels were determined by enzyme-linked immunosorbent assay and are expressed as the mean optical density (OD) at 450 nm for triplicate samples from individual mice. The horizontal bar represents mean OD for each experimental group of mice (n = 4). (a) In contrast to WT mice, faecal IgA levels in both LTα–/– and LTβ–/– mice were extremely low, but increased significantly after reconstitution with WT bone marrow. (b) C57BL/Dk (control) mice and PP-deficient C57BL/Dk mice were found to have equivalent levels of faecal IgA. SCID mice (+) were used as negative controls. *Indicates significant difference from WT mice (P = 0·05).
Discussion
The composition of ILFs in the murine small intestine was investigated. We found that although both iILFs and mILFs were present in the intestines of immunocompetent mice, a significantly greater number of mILFs was induced in a LT-dependent manner in the absence of PPs. Immunohistochemical analysis revealed that the presence of functional FDCs could also be used to reliably distinguish ILF maturity: all mILFs contained large FDC networks whereas none were detected within iILFs. The total area occupied by FDCs in all the mILFs in mice lacking PPs was substantial and was equivalent to the total area occupied by FDCs in all the PPs and the few mILFs found in immunocompetent mice. These data imply that the induction of GC and FDC network formation within mILFs in response to PP deficiency provides a mechanism to compensate for the absence of PPs. Indeed, in mice that lacked PPs, ILF maturation coincided with a restoration of faecal IgA levels to those comparable to immunocompetent mice.
The formation of iILFs is dependent upon stimulation from LT-expressing lymphocytes which interact with LTβR-expressing stromal cells in the intestine and induce the formation of clusters of B220+ cells at the base of a villus. This initial stimulus can be provided by LT-expressing B lymphocytes, T lymphocytes or NK cells. However, in response to exogenous stimuli, LT-expressing B lymphocytes trigger the progression of iILFs into mILFs resembling lymphoid nodules containing a single B-lymphocyte follicle of predominantly B2-B lymphocytes with a GC and a follicle-associated epithelium containing M cells.2,13,14 In this study we also demonstrate that LT-expressing bone marrow-derived cells also induce the differentiation of large FDC networks within mILFs.
Follicular dendritic cells trap and retain antigens on their surfaces through interactions between complement components and cellular CR1 and CR2.27,28 In our morphometric analysis studies described above anti-CR2/CR1 antiserum was used to detect FDCs as they characteristically express these receptors at high levels.27 However, in the mouse CR1 and CR2 are also expressed on B lymphocytes, but at lower levels than on FDCs. Thus CR2/CR1+ B lymphocytes were excluded from our assessments of FDC size and our calculations were based only on CR2/CR1+ CD45R– cells. Parallel studies also demonstrated complement components C3 and C4, in association with large networks of CR1-expressing and CR2/CR1-expressing cells (Fig. 2). These data confirm that the CR2/CR1+ CD45R– cells identified within mILFs were functional FDCs capable of trapping and retaining C3-bearing and C4-bearing immune complexes.27,29
Several lines of evidence support the conclusion that antigen trapped on the surface of FDCs promotes immunoglobulin-isotype class switching and affinity maturation of naive IgM+ B lymphocytes.15–19 Follicular dendritic cells require important cytokine stimulation from lymphocytes, such as membrane LTα1β2 and tumour necrosis factor-α, to maintain their differentiated state.30 Hence, in the absence of LTβR-stimulation in LTα–/– mice, LTβ–/– mice and LTβR–/– mice, FDC networks and GCs do not develop and immunoglobulin-isotype class switching is dramatically impaired. However, reconstitution of LTα–/– mice and LTβ–/– mice with WT bone marrow induces the development of FDC networks and GCs in the spleen, supporting effective immunoglobulin-isotype class switching.15–18,31 Secretory IgA is a major component of the mucosal immune system and provides an important first line of defence against infection by microbial pathogens across mucosal surfaces.1 In this study, coincident with the lack of FDCs and organized gut-associated lymphoid tissues, IgA levels were undetectable in the faeces of LTα–/– mice and LTβ–/– mice. However, reconstitution of LTα–/– mice and LTβ–/– mice with WT bone marrow (WT→LTα–/– mice and WT→LTβ–/– mice, respectively), induced the formation of ILFs and the maturation of FDCs and GCs within them, and coincided with an increase in faecal IgA levels to those comparable of WT mice. IgA isotype class-switching has been shown to occur only within the GCs of organized gut-associated lymphoid tissues such as PPs and mILFs, and not in the diffuse lamina propria.2 In another study the presence of FDC networks within gut-associated lymphoid tissues was shown to be important for the induction of intestinal IgA responses.20 Taken together, our data imply that FDCs within the GCs of mILFs provide the necessary microenvironment to promote efficient interaction between luminal derived antigens and B lymphocytes to stimulate the generation of effective IgA responses.
Although PPs are absent in LTβ–/– mice, the MLNs are retained,32 raising the possibility that IgA isotype class-switching may also have occurred within GCs in the MLNs of WT→LTβ–/– mice. However, although LTα–/– mice lack both PPs and MLNs,33 their absence in WT→LTα–/– mice did not adversely affect faecal IgA levels, which were equivalent to those of WT mice, WT→WT mice and WT→LTβ–/– mice (which do possess them). These data demonstrate that IgA isotype class-switching in response to luminal antigens can occur in the absence of MLNs, but is critically dependent upon the presence of organized lymphoid tissues such as PPs and mILFs within the intestine.
One study has suggested that ILFs are not essential for the induction of intestinal IgA responses.20 In this study, mice which lacked PPs and MLNs, as a result of combined in utero TNFR1-signalling and LTβR-signalling blockade, failed to induce an IgA response after oral immunization. However, TNFR1-stimulation is essential for mILF formation but not iILF formation.13 Unfortunately, in the above study20 ILF maturation status in TNFR1- and LTβR-treated mice was not recorded, but it is plausible that ILF maturation was significantly impaired in these mice.
Consistent with other studies,13,14 we found mILFs to be highly infrequent in the intestines of unmanipulated immunocompetent control mice in our SPF colony. However, in mice that lacked PPs, stimuli from LT-expressing, BM-derived cells significantly expanded the numbers of mILFs. The stimuli that trigger ILF maturation and the role these structures play in intestinal immune responses are not fully understood. In the absence of immunoglobulin-isotype class switching and impaired IgA production in activation-induced cytidine deaminase (AID)-deficient mice, the number of ILFs in the intestine is increased.34 This implies that inflammatory responses precipitated by a relative deficiency in intestinal IgA stimulate the formation of mILFs, providing a mechanism to compensate for the absence of faecal IgA production. Indeed, our data show that ILF maturation in mice that lack PPs coincides with the restoration of faecal IgA levels to be comparable with those of immunocompetent mice. ILF formation has also been shown to be driven by the presence of luminal bacterial flora.13,34 Furthermore, following infection of mice with the parasitic helminth Trichuris muris,6 a significant increase in the numbers of ILFs in the large intestine has also been shown to occur.6
In summary, our study has demonstrated that PP-deficiency intensifies ILF maturation in the murine small intestine. We found that mILFs, unlike iILFs, possessed large FDC networks. The area occupied by the FDCs within the mILFs of mice lacking PPs was substantial, and was equivalent to the total area occupied by FDCs in all the PPs and the few mILFs in immunocompetent mice. ILF maturation coincided with a restoration of faecal IgA to levels comparable with those in immunocompetent mice, suggesting that mILFs are important inductive sites for intestinal immune responses. Taken together, these data imply that the inducible nature of ILFs and the components within them (e.g. GCs and FDC networks) provide the intestine with an additional capacity to respond to mucosal challenges.
Acknowledgments
This work was supported by funding from the Medical Research Council (Ref: G69/1867) and the Biotechnology and Biological Sciences Research Council. We thank Karen Brown, Irene McConnell, Rebecca Greenan and Simon Cumming (Institute for Animal Health, Edinburgh, UK) for excellent technical support. LTβR-Ig was kindly provided by Dr Jeffrey Browning (Biogen Inc., Cambridge, MA, USA) and requests for this reagent should be addressed to Jeff_Browning@biogen.com
References
- 1.Macpherson AJ, Hunzinker L, McKoy K, Lamarre A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect. 2001;3:1021–35. doi: 10.1016/s1286-4579(01)01460-5. [DOI] [PubMed] [Google Scholar]
- 2.Shikina T, Hiroi T, Iwatani K, et al. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria or airways and gut. J Immunol. 2004;172:6259–64. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M, Rennert P, McGhee JR, et al. Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J Immunol. 2000;164:5184–91. doi: 10.4049/jimmunol.164.10.5184. [DOI] [PubMed] [Google Scholar]
- 4.Hamada H, Hiroi T, Nishiyama Y, et al. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 5.Kweon MN, Yamamoto M, Rennert PD, et al. Prenatal blockage of lymphotoxin β receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol. 2005;174:4365–72. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]
- 6.Little MC, Bell LV, Cliffe LJ, Else KJ. The characterization of intraepithelial lymphocytes, lamina propria leukocytes, and isolated lymphoid follicles in the large intestine of mice infected with the intestinal nematode parasite Trichuris muris. J Immunol. 2005;175:6713–22. doi: 10.4049/jimmunol.175.10.6713. [DOI] [PubMed] [Google Scholar]
- 7.Keren DF, Holt PS, Collins HH, Gemski P, Formal SB. The role of Peyer's patches in the local immune response of rabbit ileum to live bacteria. J Immunol. 1978;120:1892–6. [PubMed] [Google Scholar]
- 8.Hitotsumatsu O, Hamada H, Naganuma M, Inoue N, Ishii H, Hibi T, Ishikawa H. Identification and characterisation of novel gut-associated lymphoid tissues in rat small intestine. J Gastroenterol. 2005;40:956–63. doi: 10.1007/s00535-005-1679-8. [DOI] [PubMed] [Google Scholar]
- 9.Rosner AJ, Keren DF. Demonstration of M cells in the specialized follicle-associated epithelium overlying isolated lymphoid follicles in the gut. J Leukoc Biol. 1984;35:397–404. doi: 10.1002/jlb.35.4.397. [DOI] [PubMed] [Google Scholar]
- 10.Moghaddami M, Cummins A, Mayrhofer G. Lymphocyte-filled villi: comparison with other lymphoid aggregations in the mucosa of the human small intestine. Gastroenterology. 1998;115:1414–25. doi: 10.1016/s0016-5085(98)70020-4. [DOI] [PubMed] [Google Scholar]
- 11.Chaplin DD, Fu Y-X. Cytokine regulation of secondary lymphoid organ development. Curr Opin Immunol. 1998;10:289–97. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- 12.Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor 1 function. J Immunol. 2003;170:5474–82. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 14.McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–8. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y-X, Molina H, Matsumoto M, Huang G, Min J, Chaplin DD. Lymphotoxin-α (LTα) supports development of splenic follicular structure that is required for IgG response. J Exp Med. 1997;185:2111–20. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y-X, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin α-dependent fashion. J Exp Med. 1998;187:1009–18. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endres R, Alimzhanov MB, Plitz T, et al. Mature follicular dendritic cell networks depend on expression of lymphotoxin β receptor by radioresistant stromal cells and of lymphotoxin β and tumour necrosis factor by B cells. J Exp Med. 1999;189:159–68. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y-X, Huang G, Wang Y, Chaplin DD. Lymphotoxin-α-dependent spleen microenvironment supports the generation of memory B cells and is required for their subsequent antigen-induced activation. J Immunol. 2000;164:2508–14. doi: 10.4049/jimmunol.164.5.2508. [DOI] [PubMed] [Google Scholar]
- 19.Aydar Y, Sukumar A, Szakal AK, Tew JG. The influence of immune complex-bearing follicular dendritic cells on the IgM response, Ig class switching, and production of high affinity IgG. J Immunol. 2005;174:5358–66. doi: 10.4049/jimmunol.174.9.5358. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Kweon MN, Rennert PD, Hiroi T, Fujihashi K, McGhee JR, Kiyono H. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J Immunol. 2004;173:762–9. doi: 10.4049/jimmunol.173.2.762. [DOI] [PubMed] [Google Scholar]
- 21.Riminton DS, Körner H, Strickland DH, Lemckert FA, Pollard JD, Sedgwick JD. Challenging cytokine redundancy: inflammatory cell movement and clinical course of experimental autoimmune encephalomyelitis are normal in lymphotoxin-deficient, but not tumour necrosis factor-deficient, mice. J Exp Med. 1998;187:1517–28. doi: 10.1084/jem.187.9.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngo VN, Korner H, Gunn MD, et al. Lymphotoxin α/β and tumour necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med. 1999;189:403–12. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown KL, Stewart K, Ritchie D, et al. Scrapie replication in lymphoid tissues depends on PrP-expressing follicular dendritic cells. Nat Med. 1999;5:1308–12. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 24.Force WR, Walter BN, Hession C, Tizard R, Kozak CA, Browning JL, Ware CF. Mouse lymphotoxin-beta receptor. J Immunol. 1995;155:5280–8. [PubMed] [Google Scholar]
- 25.Kosco-Vilbois MH. Are follicular dendritic cells really good for nothing? Nature Rev Immunol. 2003;3:764–9. doi: 10.1038/nri1179. [DOI] [PubMed] [Google Scholar]
- 26.Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins α and β revealed in lymphotoxin β-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida K, van den Berg TK, Dijkstra CD. Two functionally different follicular dendritic cells in secondary lymphoid follicles of mouse spleen, as revealed by CR1/2 and FcRγII-mediated immune-complex trapping. Immunology. 1993;80:34–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Nielsen CH, Fischer EM, Leslie RGQ. The role of complement in the acquired immune response. Immunology. 2000;100:4–12. doi: 10.1046/j.1365-2567.2000.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor PR, Pickering MC, Kosco-Vilbois MH, Walport MJ, Botto M, Gordon S, Martinez-Pomares L. The follicular dendritic cell restricted epitope, FDC-M2, is complement C4; localization of immune complexes in mouse tissues. Eur J Immunol. 2002;32:1883–96. doi: 10.1002/1521-4141(200207)32:7<1883::AID-IMMU1888>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Mackay F, Browning JL. Turning off follicular dendritic cells. Nature. 1998;395:26–7. doi: 10.1038/25630. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M, Mariathasan SV, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type 1 TNF receptor in the formation of germinal centers. Science. 1996;271:1289–91. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 32.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, et al. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc Natl Acad Sci USA. 1997;94:9302–7. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Togni P, Goellner J, Ruddle NH, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–7. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 34.Fagarasan S, Muramatsu M, Suzuki K, Nagaokam H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–7. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]