Abstract
Lymphocyte homing to peripheral lymph nodes is governed by adhesion molecules, including lymphocyte function-associated antigen 1 (LFA-1). Statins are 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and exert anti-inflammatory effects, e.g. inhibition of LFA-1. It is still not known whether statin compounds are capable of inhibiting lymphocyte homing in vivo. We used a cervical lymph node preparation to study the effects of simvastatin on lymphocyte adhesion to high endothelial venules (HEVs) by means of intravital fluorescence microscopy (IVM). IVM revealed that firm adhesion of lymphocytes to HEV endothelium critically depends on the adhesive function of LFA-1. The number of firmly adherent lymphocytes was reduced by 58% in LFA-1-deficient mice (P < 0·05 versus wild-type controls). As in mutant mice, acute treatment with simvastatin (i.p. injection at 2 hr prior to IVM) inhibited the firm adhesion of lymphocytes to HEV endothelium of wild-type animals by 63% (P < 0·05 versus vehicle-treated wild-type controls). In addition, acute treatment with the synthetic statin-derivate LFA878 also reduced firm lymphocyte adhesion in HEVs by 63% (P > 0·05 versus placebo-treated controls). Histological analysis after a 10-day treatment with simvastatin showed reduced cellularity of cervical lymph nodes, as indicated by a reduction of the relative area of haematoxylin-stained cell nuclei in cervical lymph node cross-sections from 94 ± 0% in vehicle-treated controls to 77 ± 3% in simvastatin-treated mice (P < 0·05). We conclude that statin compounds are capable of inhibiting lymphocyte homing to murine peripheral lymph nodes in vivo. This may have novel implications for the treatment of adaptive immune responses, e.g. transplant rejection.
Keywords: adhesion molecules, homing, intravital microscopy, lymph node, lymphocyte function associated antigen 1
Introduction
The recirculation of lymphocytes constitutes a paramount feature of the adaptive immune system. Naive lymphocytes routinely travel through blood vessels, the lymphatic system, peripheral tissues and lymphoid organs to scrutinize the body for the appearance of foreign antigens.1 In peripheral lymph nodes, tissue-derived antigens are presented to blood-borne lymphocytes by antigen-presenting cells in conjunction with major histocompatibility complex class II proteins. Recognition of the cognate antigen that a lymphocyte is primed for, starts lymphocyte activation and clonal expansion to mount an effective adaptive immune response.2
The extravasation of circulating naive lymphocytes out of the bloodstream into the tissue matrix of peripheral lymph nodes, the so-called ‘homing’ process, occurs in the microcirculation of lymph nodes in site-specific postcapillary high endothelial venules (HEVs).3 The passage of lymphocytes through the HEV wall is a strictly sequential process, tightly orchestrated by the co-ordinated expression and function of adhesion molecules and chemotactic mediators.4–9 It is well recognized that the lymphocyte rolling is mediated by l-selectin, while the subsequent firm adhesion is controlled by the adhesive function of the heterodimeric integrin β2 lymphocyte function-associated antigen 1 (LFA-1; CD11a/CD18).6,10 The key role of LFA-1 for firm adhesion of lymphocytes to peripheral lymph node HEV endothelium has been clearly illustrated by mechanistic intravital microscopy studies using specific function blocking monoclonal antibodies directed against LFA-1.6,11 Further, mice lacking the α-subunit CD11a are devoid of LFA-1 and the corresponding phenotype presents with only small lymph nodes and decreased lymph node cellularity.12 These lines of evidence clearly indicate that the adhesive function of LFA-1 is of great importance during the physiological homing of naive lymphocytes to peripheral lymph nodes.
Virtually all leucocytes constitutively express LFA-1 in an inactive state and leucocyte activation provokes a change in LFA-1 avidity rather than quantitative up-regulation of this integrin β2.13,14 Proper binding of LFA-1 to its receptive counterpart intercellular adhesion molecule 1 (ICAM-1), as expressed for example on endothelial cells or antigen-presenting cells, requires a complex structural change of the extracellular domains of LFA-1 from a bent, non-adhesive form to an extended, highly adhesive conformation.15 Next to the prominent role for cell migration, LFA-1 additionally functions as a costimulatory molecule during lymphocyte activation upon recognition of cognate antigen. Costimulatory binding of LFA-1 to the antigen-presenting cell-expressed ICAM-1 boosts cytokine production and allows for clonal expansion of lymphocytes, which guarantees efficient adaptive immunity.16 Targeting LFA-1 therefore appears to be an attractive approach to control leucocyte responses during both innate and adaptive immune diseases.
Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors and are frequently prescribed to decrease the risk of cardiovascular disease because of their potent lipid-lowering effects. There is a growing body of evidence demonstrating that, besides lowering lipids, statins exhibit potent immunomodulatory effects.17–23 It was found that lovastatin binds a previously unknown site of the LFA-1 inserted (I)-domain, which was therefore termed the l-site.24 Because the l-site is located distant to the ligand binding site of LFA-1, the so-called metal-ion-dependent adhesion site (MIDAS), these data indicated that statins may inhibit LFA-1-dependent cell interactions via this allosteric antagonism. In fact, LFA-1-mediated lymphocyte adhesion to ICAM-1 was sensible to co-incubation with HMG-CoA reductase inhibitors in vitro.25 In addition, statins blocked the LFA-1-dependent costimulation of T cells in vitro and reduced the number of graft infiltrating mononuclear leucocytes (MNLs) in a model of experimental cardiac transplantation.25,26 Thus, statins may suppress LFA-1-dependent adaptive immune reactions at distinct levels. It is not known, however, if statins are also capable of inhibiting the LFA-1-dependent process of lymphocyte homing in HEVs of peripheral lymph nodes, thereby attenuating adaptive immune responses.
This study was meant to determine the effects of statin treatment on naive lymphocyte homing to peripheral lymph nodes in vivo. We used intravital fluorescence microscopy of the cervical lymph node preparation in mice to quantitatively analyse the lymphocyte–endothelial cell adhesive interactions in the lymph node HEVs under physiologically resting conditions.
Materials and methods
Animals
C57BL/6 (Charles River, Sulzfeld, Germany) and LFA-1-deficient mice12 with body weights ranging between 20 and 24 g were used for the experiments. Animals were housed in a 12 h : 12 h dark : light cycle and were allowed free access to standard pellet food (Altromin, Lage, Germany) and tap water. All experiments were performed in accordance with the legislation on the protection of animals and were approved by the Governmental Ethical Committee for Animal Experimentation. For all surgical interventions, animals were anaesthetized by an intraperitoneal (i.p.) injection of 7·5 mg ketamine hydrochloride (Parke Davis, Freiburg, Germany) and 2·5 mg xylacine (Bayer, Leverkusen, Germany) per 100 g body weight and were placed on a heating pad to maintain a mean body temperature of 37° throughout the experimental procedures. After experimentation, blood samples were drawn from the inferior vena cava for determination of systemic leucocyte counts and the anaesthetized animals were killed by midline sternotomy, diaphragmotomy and ventriculotomy.
Cervical lymph node preparation
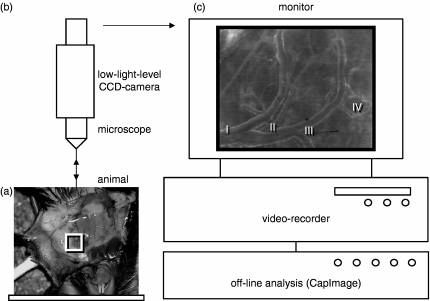
The anaesthetized animals were placed in a supine position onto a microsurgical stage and the left-sided, most cranial cervical lymph node was exteriorized for intravital fluorescence microscopy. A cutaneous flap was prepared on the entire ventral aspect of the neck with its base on the left postero-lateral side. After lateral eversion, the layer of fatty tissue, covering the submandibular gland, was carefully incised along the midline and everted to the left as a fatty tissue flap along the lateral microvascular pedicle. Great care was taken to manipulate neither the embedded cervical lymph nodes nor the supplying microvasculature. To prevent the preparation from drying, a cover-glass slip was gently placed onto the everted tissue. A 10-min equilibration period was allowed before transfer of the animals to the microscope stage (Fig. 1).
Figure 1.
Schematic illustration of the intravital microscope set-up. After preparation and eversion of the cutaneous flap at the ventral aspect of the neck, the experimental animals were placed in a supine position onto the microscope stage (a). A modified Zeiss Axiotech microscope, fixed vertically above the stage platform, was used for epi-illumination microscopy of the lymph node preparation (b). Microscopic images were televised and recorded on videotape (c) for off-line analysis of haemodynamics and leucocyte–endothelial cell adhesive interactions in individual cervical lymph node HEVs. The depicted microscopic image of the cervical lymph node microcirculation (c) shows the typical arrangement of four generations of HEVs.
Intravital fluorescence microscopy
After an intravenous injection of 0·1 ml 5% fluorescein isothiocyanate-dextran (molecular weight 150 000; Sigma Chemical Co., St Louis, MO, USA) and 0·1 ml 0·5% Rhodamine 6G (Sigma Chemical Co.), the HEV microvasculature of the cervical lymph node was visualized using a modified Zeiss Axiotech microscope (Zeiss, Oberkochen, Germany) equipped with a 100-W mercury lamp, filter sets for blue (450–490 nm excitation; > 520 nm emission wave length), green (530–560 nm; > 580 nm) and ultraviolet (330–390 nm; > 430 nm) light epi-illumination and long-distance objectives [10× numerical aperture (NA) = 0·30; 20× NA = 0·50, 50× NA = 0·55; Zeiss, Oberkochen, Germany]. Microscopic images were televised and recorded on a charge-coupled device video camera (FK6990, Pieper, Schwerte, Germany) and on video tape (Panasonic AG-7350-S-VHS, Matsushita, Tokyo, Japan) for subsequent off-line analysis of microcirculatory parameters. Red blood cell flow velocity, vascular diameters and wall shear rates were determined using CapImage Software (Zeintl, Heidelberg, Germany).
HEVs were differentiated depending on their branching order within the HEV microvascular tree (Fig. 1c). The cervical lymph node microcirculation regularly presents with four generations of HEVs arising from the smallest postcapillary subcortical HEVs (generation IV) and draining blood into mostly a single large venule leaving the lymph node (generation I). For quantitative assessment of leucocyte–endothelial cell adhesive interactions a magnification of 1100× was used on a 14-inch (35·5-cm) video screen. Under these conditions, the length of non-branching HEV segments ranged between 50 and 110 μm. Firm adhesion of leucocytes was assessed by counting the number of Rhodamine 6G-labelled leucocytes remaining stationary within HEV segments for at least 30 seconds and is expressed in cells/mm2 endothelial surface. Leucocyte rolling was assessed by counting the number of Rhodamine 6G-labelled leucocytes passing a reference point in the centre of the individual microvascular HEV segments for 30 seconds and is expressed as the number of rolling cells/min/mm circumference. The nuclear staining elicited by Rhodamine 6G allows differentiation between polymorphonuclear cells and mononuclear cells, because polymorphonuclear and mononuclear characteristics can be identified. Others have previously demonstrated that almost all leucocytes interacting with the HEV endothelium in peripheral lymph nodes are MNLs, indicating their lymphocytic nature.6 Indeed, not all MNLs observed may be lymphocytes. However, presuming that almost exclusively lymphocytes home to peripheral lymph nodes, we refer to the analysed MNLs as lymphocytes throughout the text of the manuscript.
Subsequent to intravital microscopy, the lymph nodes were harvested and fixed in 4% formaldehyde overnight for histological examination.
Flow cytometry
For confirmation that leucocytes of CD11a gene-targeted animals lack the surface expression of LFA-1, blood samples of C57BL/6 wild-type and LFA-1-deficient mice were drawn from the vena cava inferior into EDTA tubes. Fifty microlitres of blood were incubated for 30 min with 1·5 μl of a rat anti-mouse CD11a monoclonal antibody (Clone I21.7; Immunotech, Luminy, France). Excessive antibodies were washed off with 1 ml phosphate-buffered saline by centrifugation for 3 min at 400 g before incubation with a Cy™3-conjugated AffiniPure goat anti-rat immunoglobulin G (Jackson ImmunoResearch, West Grove, PA), which served as a secondary antibody and for controls. Next, red blood cells were lysed and washed off before the cell pellet was resuspended in 500 μl Cell Fix (BD Bioscience, San Jose, CA) and kept on ice until flow cytometrical analysis (BD Bioscience). Samples were protected from light during all incubation steps. Leucocytes were differentiated upon forward and side scatter characteristics.
Experimental protocol
To confirm the dependence of lymphocyte–HEV endothelial cell interactions on the function of LFA-1, we compared firm adhesion of lymphocytes in cervical lymph node HEVs of C57BL/6 wild-type and LFA-1-deficient mice. Next, we determined whether or not treatment with statins could affect firm lymphocyte adhesion in HEVs. Therefore, wild-type mice were pretreated by one i.p. injection (at 2 hr prior to IVM) of either simvastatin (0·5 mg/kg body weight dissolved in 5% ethanol; Calbiochem/EMD Biosciences Inc., La Jolla, CA) or ethanol alone. The dose of 0·5 mg simvastatin/kg body weight was used in accordance with established clinical treatment regimens.27 In a separate set of experiments, animals were daily treated i.p. with this statin or ethanol and lymph nodes were harvested for histological examination of the lymph node cellular density after a 10-day treatment period. Additionally, we determined the efficacy of the statin-based small molecule inhibitors of LFA-1, LFA-878 and LFA-703 (30 mg/kg dissolved in ethanol/CremophorEL and further diluted 1 : 3 with phosphate-buffered saline, i.p. 2 h before intravital microscopy; Novartis Institutes for BioMedical Research, Basel, Switzerland) in inhibiting firm lymphocyte adhesion to peripheral lymph node HEV endothelium in mice. Corresponding controls received the vehicle alone.
Histological analysis
Formaldehyde-incubated lymph node specimens were embedded in standard paraffin and 4-μm mid-sections were simultaneously stained with haematoxylin before subsequent transillumination light microscopy. Using an identical power light source for all specimens, images were recorded on videotape for subsequent determination of the lymph node cellularity by CapImage software. A static grey level was defined, which allowed the measurement of the summarized area of all visible and haematoxylin-stained cell nuclei, which is given as a percentage of the entire high-power field.
Statistical analysis
Data are given as mean values ± standard error of the mean and n represents the number of animals used per group. Statistical differences were calculated by means of analysis of variance followed by appropriate post hoc testing, including the correction of the α error to compensate for multiple comparisons (SigmaStat 4·0, Jandel Scientific, San Rafael, CA). Differences were considered significant at a P < 0·05.
Results
Lymphocyte arrest in cervical lymph node HEVs is dependent on LFA-1
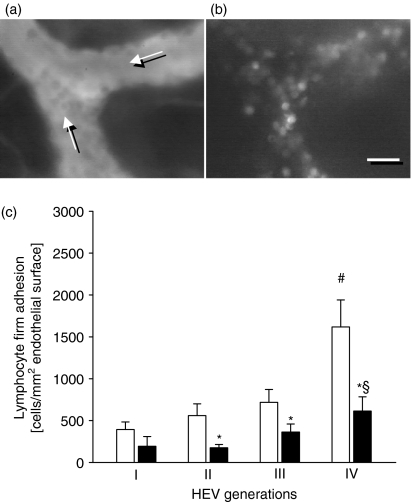
Intravital fluorescence microscopy of the cervical lymph node microcirculation revealed that blood-borne lymphocytes interacted with the HEV endothelium under physiologically resting conditions (Fig. 2a,b). Firm lymphocyte adhesion was found to be most prominent in the smallest post-capillary HEVs of generation III and IV, while fewer lymphocytes were attached to the endothelium of larger HEVs (Fig. 2c). Confirming that firm lymphocyte attachment to the HEV endothelium was site-specific for secondary lymphoid organs and critically dependent on the adhesive function the integrin β2 LFA-1, we found markedly fewer firmly adherent lymphocytes in cervical lymph node HEVs of LFA-1-deficient mice (Fig. 2c). The overall firm lymphocyte adhesion was significantly reduced by 58% in the mutant animals (P < 0·05 versus C57BL/6 wild-type mice, n = 6 or n = 7). Detailed analysis demonstrated that firm lymphocyte adhesion to HEV endothelium was LFA-1-dependent in HEVs of generation II–IV (Fig. 2c; P < 0·05 versus C57BL/6 wild-type mice, n = 6 or n = 7). In contrast, the numbers of firmly adherent lymphocytes in the larger generation I HEVs were comparable between wild-type and LFA-1-deficient mice. Of note, flow cytometry confirmed that leucocytes of C57BL/6 wild-type mice, but not of mutant mice, expressed LFA-1 (data not shown). Systemic leucocyte counts did not differ significantly between LFA-1-deficient and C57BL/6 wild-type mice (Table 1). Furthermore, there was no significant difference in the HEV microhaemodynamics between LFA-1-deficient and C57BL/6 wild-type mice (Table 2), indicating that the differences in lymphocyte adhesion are not the result of differences in microvascular flow conditions.
Figure 2.
Firm adhesion by lymphocytes in HEVs of the cervical lymph node is LFA-1-dependent. Intravital fluorescence microscopy of the cervical lymph node allows for visualization of individual HEVs and intravascularly adherent leucocytes, using blue-light epi-illumination and contrast enhancement of the intravascular plasma by intravenously applied fluorescein isothiocyanate-dextran (a, direction of blood flow is as indicated), as well as green-light epi-illumination and contrast enhancement by Rhodamine 6G (b), respectively. Notably, the majority of the Rhodamine 6G-stained leucocytes appear with homogeneously stained and round-shaped nuclei, indicating their lymphocytic nature. Quantitative analysis of firm lymphocyte adhesion in four generations of cervical lymph node HEVs (c) in C57BL/6 wild-type mice (white bars) and LFA-1-deficient animals (black bars) under resting conditions. Data are mean values ± SEM, n = 6 or n = 7, *P < 0·05 versus C57BL/6 wild-type mice, #P < 0·05 versus HEV generation I, §P < 0·05 versus HEV generation II. Scale bar represents 15 μm (a,b).
Table 1.
Systemic leucocyte counts
| Cells (× 103/μl blood) | ||
|---|---|---|
| C57BL/6 wild-type | (n = 6) | 7·1 ± 1·5 |
| LFA-1-deficient | (n = 7) | 8·6 ± 1·3 |
| Ethanol-treated | (n = 9) | 6·1 ± 0·6 |
| Simvastatin-treated | (n = 9) | 4·5 ± 0·9 |
| Vehicle-treated | (n = 8) | 5·3 ± 0·7 |
| LFA878-treated | (n = 8) | 3·4 ± 0·3* |
| LFA703-treated | (n = 8) | 3·5 ± 0·3* |
Blood samples were drawn from the inferior vena cava into EDTA-containing tubes and the number of white blood cells was counted using a haemocytometer. Data are mean values ± standard error of the mean and n represents the number of animals per group.
P < 0·05 versus vehicle-treated wild-type controls.
Table 2.
Haemodynamics in the HEV microcirculation of murine cervical lymph nodes
| RBC flow velocity | Wall shear rate | ||
|---|---|---|---|
| C57BL/6 wild-type | (n = 6) | 0·8 ± 0·1 | 82 ± 10 |
| LFA-1-deficient | (n = 7) | 0·7 ± 0·1 | 81 ± 9 |
| Ethanol-treated | (n = 9) | 0·8 ± 0·1 | 78 ± 15 |
| Simvastatin-treated | (n = 9) | 0·7 ± 0·1 | 66 ± 19 |
| Vehicle-treated | (n = 8) | 0·7 ± 0·1 | 78 ± 12 |
| LFA878-treated | (n = 8) | 0·6 ± 0·1 | 66 ± 13 |
| LFA703-treated | (n = 8) | 0·7 ± 0·1 | 81 ± 7 |
Intravital microscopic images were televised and centreline red blood cell (RBC) flow velocity (mm/second) as well as wall shear rates (per second) were quantitatively analysed off-line. Data are mean values ± standard error of the mean and n represents the number of animals per group.
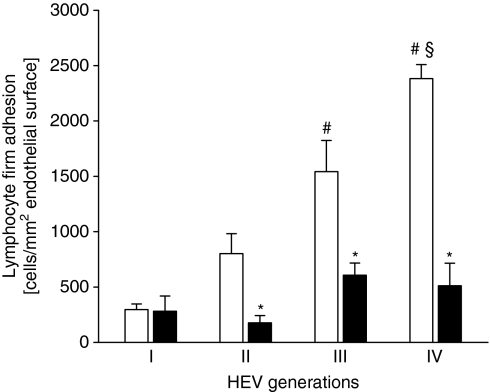
Simvastatin inhibits firm lymphocyte adhesion in cervical lymph node HEVs
In wild-type mice, firm adhesion by lymphocytes to cervical lymph node HEV endothelium was significantly reduced by 63% after the 2-hr pretreatment with simvastatin, i.e. the overall number of firmly adherent lymphocytes was 1209 ± 165 and 452 ± 77 cells/mm2 endothelial surface in ethanol-treated controls and simvastatin-treated animals, respectively (Fig. 3; P < 0·05, n = 9). Similar to the findings in LFA-1-deficient mice, simvastatin reduced firm lymphocyte adhesion in HEVs of generations II–IV (P < 0·05 versus ethanol-treated controls, n = 9), while there was no significant effect on lymphocyte–endothelium adhesive interactions in generation I HEVs (Fig. 3). Of note, simvastatin treatment did not affect lymphocyte rolling. The overall numbers of rolling lymphocytes in cervical lymph node HEVs were 239 ± 33 and 277 ± 39 cells/min/mm circumference in simvastatin-treated and ethanol-treated controls, respectively (P > 0·05, n = 9). There was no significant difference in systemic leucocyte counts and HEV microhaemodynamics between simvastatin- and ethanol-treated mice (Tables 1, 2).
Figure 3.
Firm adhesion of lymphocytes in HEVs of the cervical lymph node is sensitive to treatment with simvastatin. Quantitative analysis of firm lymphocyte adhesion in four generations of cervical lymph node HEVs in ethanol-treated (white bars) and simvastatin-treated (black bars) C57BL/6 wild-type mice. Data are mean values ± SEM, n = 9, *P < 0·05 versus ethanol-treated C57BL/6 wild-type mice, #P < 0·05 versus HEV generation I, §P < 0·05 versus HEV generation II.
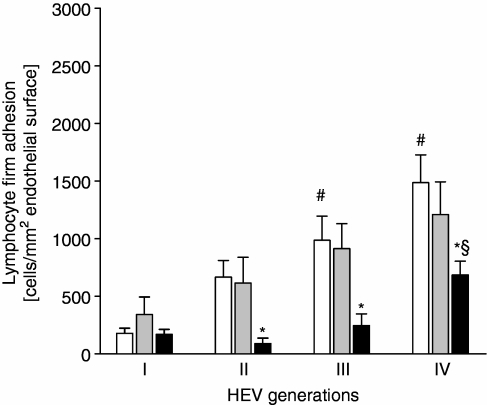
The statin-based LFA-1 antagonist LFA878 inhibits firm lymphocyte adhesion in HEVs
Treatment with the statin analogue LFA703 had no significant impact on the firm adhesion of lymphocytes to HEV endothelium in cervical lymph nodes, when compared to vehicle-treated wild-type controls (Fig. 4). In contrast, treatment with LFA878 significantly reduced firm lymphocyte adhesion. The overall number of firmly attached lymphocytes was reduced by 63% in LFA878-treated mice (P < 0·05 versus vehicle-treated wild-type controls, n = 8). We found that LFA878 inhibited firm adhesion by lymphocytes in HEVs of generation II, III and IV (Fig. 4; P < 0·05 versus vehicle-treated controls, n = 8) without affecting lymphocyte–HEV endothelial cell interactions in generation I HEVs. Neither treatment with the statin-derivate LFA703, nor with LFA878 affected the lymphocyte rolling in cervical lymph node HEVs, i.e. the overall numbers of rolling lymphocytes in cervical lymph node HEVs were 181 ± 21, 235 ± 38 and 212 ± 34 cells/min/mm circumference in wild-type mice treated with LFA703, LFA878 and the vehicle, respectively (P > 0·05, n = 8). LFA878 reduced the total number of circulating leucocytes, but so did LFA703 (Table 1), which in turn had no effect on firm lymphocyte adhesion. There was no significant difference in the HEV microhaemodynamics between LFA703-, LFA878- and placebo-treated mice (Table 2).
Figure 4.
Firm adhesion of lymphocytes in cervical lymph node HEVs is sensitive to treatment with LFA878, a statin-based small molecule inhibitor of LFA-1. C57BL/6 wild-type mice received an i.p. injection of either LFA703 (grey bars), LFA878 (black bars), or the vehicle alone for controls (white bars) and intravital fluorescence microscopy of the cervical lymph node was performed after 2 hr. Firm lymphocyte adhesion was quantified in four generations of the HEV microcirculation. Data are mean values ± SEM, n = 8, *P < 0·05 versus vehicle-treated controls, #P < 0·05 versus HEV generation I, §P < 0·05 versus HEV generation II.
Simvastatin inhibits lymphocyte homing to peripheral lymph nodes
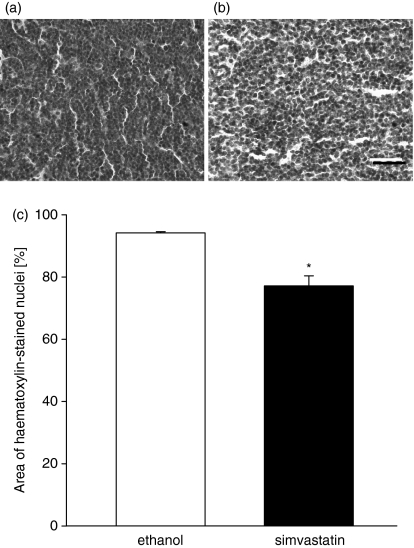
Histological analysis revealed that the 10-day treatment of mice with simvastatin markedly reduced the cellularity of cervical lymph nodes (Fig. 5a,b). The area of haematoxylin-stained cell nuclei was significantly smaller in cervical lymph node cross-sections of simvastatin-treated mice, when compared to specimens harvested from ethanol-treated controls (Fig. 5c; P < 0·05, n = 6).
Figure 5.
Simvastatin reduces the cellularity in cervical lymph nodes. Histological analysis of the cervical lymph node cellularity in C57BL/6 wild-type mice, which were daily treated i.p. with simvastatin (b, c black bar) or the ethanol alone (a, c white bar). Cervical lymph nodes were harvested on day 10, stored overnight in 4% formaldehyde and embedded into paraffin. Midline cross-sections of the identical thickness of 4 μm were stained simultaneously with haematoxylin and central high-power fields were recorded on tape, using a defined light source. The relative area of haematoxylin-stained cell nuclei was determined by means of a computer-assisted image analysis program and is given in per cent. Data are mean values ± SEM, n = 6 or n = 7, *P < 0·05 versus ethanol-treated C57BL/6 wild-type mice. Scale bar represents 30 μm (a,b).
Discussion
This study provides novel data on the immunomodulatory properties of statin compounds. Using intravital fluorescence microscopy in the cervical lymph node preparation in mice, we found that the HMG-CoA reductase inhibitor simvastatin at a clinically relevant dose and the statin-based small molecule inhibitor of LFA-1 LFA878 inhibit the firm adhesion of lymphocytes in the cervical lymph node HEVs. Moreover, our data illustrate that treatment with simvastatin reduces the cellularity of murine cervical lymph nodes, indicating that LFA-1-dependent firm adhesion of lymphocytes is a prerequisite for the extravasation into the lymph node matrix. Thus, the current analysis provides evidence that statin compounds exert immunomodulatory functions by inhibiting LFA-1-dependent lymphocyte homing to peripheral lymph nodes.
The recruitment of leucocytes is a hallmark of inflammation and consequently it was investigated whether statins would interfere with the leucocyte extravasation cascade, thereby exhibiting their anti-inflammatory effects. There is a great body of evidence demonstrating that clinically relevant statins inhibit the extravasation cascade during acute inflammatory tissue infiltration of granulocytes, for example in non-specific pulmonary inflammation, chemically induced peritonitis and myocardial ischaemia/reperfusion injury.28–30 In contrast, there are only limited data on the inhibitory effects of HMG-CoA reductase inhibitors on immune responses involving the recruitment of MNLs, such as lymphocytes.26,31,32 Such data, however, are of particular interest when discussing the potential value of statins as an additional pharmacological tool to treat adaptive immune responses. Expanding on previous findings demonstrating that statins are capable of modulating adaptive immunity, our data show that statins were capable of inhibiting firm adhesion by lymphocytes to HEV endothelial cells in vivo. This is further supported by the fact that simvastatin treatment also reduced the cellularity of lymph nodes, because firm adhesion of lymphocytes to the HEV endothelium is a critical prerequisite and a rate-limiting step during lymphocyte homing.6,10 Moreover, our data underscore the fact that the immunomodulatory effects of statins target LFA-1-dependent events first, because it was demonstrated that firm lymphocyte adhesion was critically dependent on the adhesive function of LFA-1, and second, because the inhibition of firm lymphocyte adhesion exerted by both simvastatin and the statin-based LFA-1 inhibitor LFA878, which does not inhibit the HMG-CoA reductase, resembled the reduction of firm adhesion in lymph node HEVs of LFA-1-deficient animals. Moreover, demonstrating that statins interfere with the LFA-1-dependent extravasation cascade during MNL migration, it is also tempting to speculate that statin-based cardiovascular protection is, despite lipid lowering, the result of direct inhibition of the LFA-1-dependent firm adhesion of MNLs to the arterial endothelium.33 In fact, it is a well-established concept that monocytes and T-lymphocytes are recruited to developing atherosclerotic plaques.34,35
We found that lymphocytes predominantly adhere to the endothelium of generation III and IV HEVs, while fewer cells attached firmly to the endothelium of the large generation I HEVs. This is in line with previous data on lymphocyte homing in the murine inguinal lymph node, highlighting that the extravasation of lymphocytes in peripheral lymph nodes is highly site-specific and occurs predominantly in the smallest paracortical HEVs.6,10 Indeed, there are regional differences in the ultrastructural anatomical features of HEV endothelium.36 The smallest post-capillary HEVs, which may correspond to the herein defined generation III and IV HEVs, present with site-specific cuboidal endothelium carrying finger-like protrusions into the vessel lumen. Further downstream, HEVs, which may correspond to the herein defined generation I and II HEVs, exhibit an endothelial surface structure that is more flattened out and is almost similar to the endothelium in non-lymphoid microvessels.36 These regional differences in the HEV microanatomy may thus also help to explain why we found that statin compounds inhibit firm lymphocyte adhesion selectively in cervical lymph node generation III and particularly in generation IV HEVs, while lymphocyte–endothelial cell adhesive interactions in the larger generation I and II HEVs were almost unaffected.
The inhibitory mechanisms of action of simvastatin in our experimental setting are probably unrelated to lipid-lowering per se, because we applied simvastatin acutely and found a prompt decrease in firm lymphocyte adhesion already after 2 hr. Significant reduction of plasma lipid levels by statins has its onset later.26,37 However, the inhibition of the HMG-CoA reductase not only results in the long-term decrease of plasma lipid levels but the prevention of β-hydroxy-β-methylglutaryl conversion to mevalonic acid during the isoprenoid production also prevents the post-translational lipid modification of proteins, the so-called ‘prenylation’.25,38 Inhibition of prenylation of the Rho family of GTPases by statins was shown to correlate with impaired integrin binding, including LFA-1.39,40 In endothelial cells, statin blockade of Rho GTPase activity resulted in a reduction of activated ICAM-1 expression.41,42 One explanation for the simvastatin effect on lymphocyte homing observed here may be the HMG-CoA reductase inhibition-dependent reduction of protein prenylation and the subsequently limited expression and function of lymphocytic LFA-1 and HEV endothelial ICAM-1. This is in line with previous in vitro data on lovastatin-inhibited immunological costimulation of T cells, which was specific to LFA-1–ICAM-1 binding and was partially reversible by addition of mevalonic acid.25 Nevertheless, such data do not per se exclude the possibility that simvastatin-reduced lymphocyte homing may at least partly apply to HMG-CoA reductase inhibition-independent antagonism of LFA-1 function. Of note, simvastatin not only blocks the HMG-CoA reductase, but also binds to the l-site of LFA-1, thereby inhibiting the adhesive function of this integrin β2 with the same potency as lovastatin.24,25 Further, the in vitro costimulation of T cells was prevented by the synthetic des-oxo-lovastatin, which retains the inhibitory effects on LFA-1 function mediated by l-site binding, but does not affect the HMG-CoA reductase-driven isoprenoid synthesis required for protein prenylation.25 Thus, the simvastatin-based reduction of lymphocyte homing observed in this study may rely on both HMG-CoA reductase inhibition-dependent and -independent pathways, affecting the adhesive function of LFA-1.
Statin-based small molecule inhibitors of LFA-1, such as LFA878 and LFA703, allow for a more detailed analysis of those immunomodulatory effects of statin compounds, which are solely driven by the l-site binding-mediated allosteric antagonism of LFA-1. Neither LFA878, nor LFA703 inhibits the HMG-CoA reductase.25,43 We found that LFA878 blocked the firm adhesion of lymphocytes to the endothelium of peripheral lymph node HEVs. These data may indicate that the simvastatin-based inhibition of lymphocyte extravasation is the result of l-site binding-mediated allosteric antagonism of LFA-1. This notion is further supported by the fact that there were profound pharmacodynamic similarities between LFA878 and simvastatin in reducing the LFA-1-dependent firm adhesion of lymphocytes in the distinct generations of peripheral lymph node HEVs. In fact, the reduction in firm lymphocyte adhesion exerted by both LFA878 and simvastatin was comparable to the reduction of firm lymphocyte adhesion in LFA-1-deficient animals. Nevertheless, several metabolites of statins are found in vivo after administration with and without inhibitory activity to the HMG-CoA reductase.44,45 It can therefore not be excluded that HMG-CoA reductase inhibition-dependent mechanisms of inhibition of LFA-1 function by simvastatin might have paralleled or even superseded the HMG-CoA reductase inhibition-independent effects on lymphocyte homing.46
Ethanol-treated controls showed a more pronounced firm lymphocyte adhesion when compared to normal wild-type animals. Because simvastatin treatment required the drug to be dissolved in 5% ethanol, this relatively high dose of ethanol has also to be used in the matching control group. Because ethanol is known to increase leucocyte adhesive interactions in vivo,47 this may represent the cause of the higher firm adhesion of the lymphocytes observed in this control group.
Treatment with both statin analogues, LFA703 and LFA878, led to a significant decrease of circulating leucocytes when compared to the matching vehicle-treated controls. Simvastatin also showed a lowering (although not significant) of circulating leucocyte counts compared to its corresponding control group. This may reflect one anti-inflammatory action of statins with reduction of leucocyte counts, as also described in large clinical trials with patients with coronary artery and peripheral artery disease.48,49
In contrast to LFA878, we did not find a significant inhibitory effect of the statin analogue LFA703 on the LFA-1-dependent firm adhesion of lymphocytes in peripheral lymph node HEVs. Notably, it was demonstrated in vivo that LFA703 effectively blocked the acute inflammatory and LFA-1-dependent recruitment of granulocytes, for example during peritonitis, intestinal ischaemia/reperfusion injury and endotoxaemic liver injury.25,50,51 Both statin analogues, LFA878 and LFA703, bind to the l-site of LFA-1.43 Distinct occupancy of certain l-site subpockets, however, leads to differential effects on the extracellular LFA-1 conformation and, thereby, to a more efficient LFA-1 blockade by LFA878.43 The overtly specific inhibitory effect of LFA878 on firm lymphocyte adhesion is thus explained by the more efficient LFA-1 blockade by LFA878 and is not simply the result of the decrease of systemic leucocyte counts in statin-analogue-treated animals.
The diverse functions of LFA-1 mediating lymphocyte trafficking on one hand and costimulatory signalling on the other make this integrin β2 a promising target to modulate adaptive immune reactions, for example during autoimmune disease and transplant rejection. Current clinical immunosuppressive approaches targeting LFA-1 function are however, limited by the requirement for parenteral application.44 HMG-CoA reductase inhibitors are relatively safe and orally active. Our data thus underline the concept of using LFA-1 inhibition by statin compounds as a supplemental tool for the clinical treatment of adaptive immune reactions.44 The additional lipid-lowering capacity of HMG-CoA reductase inhibitors may further help to attenuate corticoid treatment-associated side-effects during immunosuppression.
Taken together, this study provides new insights into the immunomodulatory mechanisms of action of clinically relevant HMG-CoA reductase inhibitors. We demonstrated in vivo that the lymphocyte homing to peripheral lymph nodes is sensitive to treatment with the HMG-CoA reductase inhibitor simvastatin and the synthetic statin analogue LFA878 under physiological resting conditions. The mechanisms of action of simvastatin may involve HMG-CoA reductase inhibition-dependent and -independent inhibitory effects on the adhesive function of LFA-1. Considered collectively with previous findings on statin-based inhibition of LFA-1 function in adaptive immunity and in view of the potential use of HMG-CoA reductase inhibitors as complementary immunosuppressive agents, we may therefore propose an at least three-stage concept of statin-based effects on adaptive immunity by inhibition of LFA-1, including (1) blockade of lymphocyte homing and, thus, prevention of naive lymphocyte exposure to foreign antigen in secondary lymphoid organs, (2) repression of lymphocyte costimulation, and (3) inhibition of peripheral tissue infiltration of reactive effector lymphocytes.
References
- 1.Marchesi VT, Gowans JL. The migration of lymphocytes through the endothelium of venules in lymph nodes: an electron microscope study. Proc R Soc Lond B Biol Sci. 1964;159:283–90. doi: 10.1098/rspb.1964.0002. [DOI] [PubMed] [Google Scholar]
- 2.Wiedle G, Dunon D, Imhof BA. Current concepts in lymphocyte homing and recirculation. Crit Rev Clin Laboratory Sci. 2001;38:1–31. doi: 10.1080/20014091084164. [DOI] [PubMed] [Google Scholar]
- 3.Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–57. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 4.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 5.Kunkel EJ, Ramos CL, Steeber DA, Muller W, Wagner N, Tedder TF, Ley K. The roles of 1-selectin, beta 7 integrins, and P-selectin in leukocyte rolling and adhesion in high endothelial venules of Peyer's patches. J Immunol. 1998;161:2449–56. [PubMed] [Google Scholar]
- 6.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–16. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MB, Butcher EC. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–52. [PubMed] [Google Scholar]
- 8.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 9.Warnock RA, Campbell JJ, Dorf ME, Matsuzawa A, McEvoy LM, Butcher EC. The role of chemokines in the microenvironmental control of T versus B cell arrest in Peyer's patch high endothelial venules. J Exp Med. 2000;191:77–88. doi: 10.1084/jem.191.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Andrian UH. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation. 1996;3:287–300. doi: 10.3109/10739689609148303. [DOI] [PubMed] [Google Scholar]
- 11.Diacovo TG, Catalina MD, Siegelman MH, von Andrian UH. Circulating activated platelets reconstitute lymphocyte homing and immunity in 1-selectin-deficient mice. J Exp Med. 1998;187:197–204. doi: 10.1084/jem.187.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmits R, Kundig TM, Baker DM, et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–26. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- 14.Ding ZM, Babensee JE, Simon SI, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163:5029–38. [PubMed] [Google Scholar]
- 15.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin αLβ2. J Cell Biol. 2004;167:1241–53. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins MK, Johnson JG. Molecules involved in T-cell costimulation. Curr Opin Immunol. 1993;5:361–7. doi: 10.1016/0952-7915(93)90054-v. [DOI] [PubMed] [Google Scholar]
- 17.Vaughan CJ, Murphy MB, Buckley BM. Statins do more than just lower cholesterol. Lancet. 1996;348:1079–82. doi: 10.1016/S0140-6736(96)05190-2. [DOI] [PubMed] [Google Scholar]
- 18.Buchwald H, Campos CT, Boen JR, Nguyen PA, Williams SE. Disease-free intervals after partial ileal bypass in patients with coronary heart disease and hypercholesterolemia: report from the Program on the Surgical Control of the Hyperlipidemias (POSCH) J Am Coll Cardiol. 1995;26:351–7. doi: 10.1016/0735-1097(95)80006-3. [DOI] [PubMed] [Google Scholar]
- 19.Strandberg TE, Vanhanen H, Tikkanen MJ. Effect of statins on C-reactive protein in patients with coronary artery disease. Lancet. 1999;353:118–19. doi: 10.1016/S0140-6736(05)76154-7. [DOI] [PubMed] [Google Scholar]
- 20.Strandberg TE, Vanhanen H, Tikkanen MJ. Associations between change in C-reactive protein and serum lipids during statin treatment. Ann Med. 2000;32:579–83. doi: 10.3109/07853890008998838. [DOI] [PubMed] [Google Scholar]
- 21.Plutzky J, Ridker PM. Statins for stroke: the second story? Circulation. 2001;103:348–50. doi: 10.1161/01.cir.103.3.348. [DOI] [PubMed] [Google Scholar]
- 22.Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333:621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 23.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 24.Kallen J, Welzenbach K, Ramage P, et al. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J Mol Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- 25.Weitz-Schmidt G, Welzenbach K, Brinkmann V, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu K, Aikawa M, Takayama K, Libby P, Mitchell RN. Direct anti-inflammatory mechanisms contribute to attenuation of experimental allograft arteriosclerosis by statins. Circulation. 2003;108:2113–20. doi: 10.1161/01.CIR.0000092949.67153.74. [DOI] [PubMed] [Google Scholar]
- 27.Todd PA, Goa KL. Simvastatin. A review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs. 1990;40:583–607. doi: 10.2165/00003495-199040040-00007. [DOI] [PubMed] [Google Scholar]
- 28.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, Worthen GS. A role for hydroxy-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med. 2005;171:606–15. doi: 10.1164/rccm.200406-729OC. [DOI] [PubMed] [Google Scholar]
- 29.Fischetti F, Carretta R, Borotto G, Durigutto P, Bulla R, Meroni PL, Tedesco F. Fluvastatin treatment inhibits leucocyte adhesion and extravasation in models of complement-mediated acute inflammation. Clin Exp Immunol. 2004;135:186–93. doi: 10.1111/j.1365-2249.2003.02358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefer AM, Campbell B, Shin YK, Scalia R, Hayward R, Lefer DJ. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation. 1999;100:178–84. doi: 10.1161/01.cir.100.2.178. [DOI] [PubMed] [Google Scholar]
- 31.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–8. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 32.Wagner AH, Gebauer M, Guldenzoph B, Hecker M. 3-hydroxy-3-methylglutaryl coenzyme A reductase-independent inhibition of CD40 expression by atorvastatin in human endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:1784–9. doi: 10.1161/01.atv.0000037098.20829.31. [DOI] [PubMed] [Google Scholar]
- 33.Schramm R, Menger MD, Schaefers HJ, Thorlacius H. Leukocyte adhesion in aorta and femoral artery in vivo is mediated by LFA-1. Inflamm Res. 2004;53:523–7. doi: 10.1007/s00011-004-1285-x. [DOI] [PubMed] [Google Scholar]
- 34.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:419–20. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, Fan J. Atherosclerosis and inflammation mononuclear cell recruitment and adhesion molecules with reference to the implication of ICAM-1/LFA-1 pathway in atherogenesis. Int J Cardiol. 1998;66:45–53. doi: 10.1016/s0167-5273(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 36.De Bruyn PP, Cho Y. Structure and function of high endothelial postcapillary venules in lymphocyte circulation. Curr Top Pathol. 1990;84:85–101. doi: 10.1007/978-3-642-75519-4_4. [DOI] [PubMed] [Google Scholar]
- 37.Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin Pharmacokinet. 1997;32:403–25. doi: 10.2165/00003088-199732050-00005. [DOI] [PubMed] [Google Scholar]
- 38.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–69. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Moesner P, Kovach NL, Bailey R, Hamilton AD, Sebti SM, Harlan JM. Integrin-dependent leukocyte adhesion involves geranylgeranylated protein(s) J Biol Chem. 1999;274:33334–40. doi: 10.1074/jbc.274.47.33334. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida M, Sawada T, Ishii H, Gerszten RE, Rosenzweig A, Gimbrone MA, Jr, Yasukochi Y, Numano F. Hmg-CoA reductase inhibitor modulates monocyte–endothelial cell interaction under physiological flow conditions in vitro. Involvement of Rho GTPase-dependent mechanism. Arterioscler Thromb Vasc Biol. 2001;21:1165–71. doi: 10.1161/hq0701.092143. [DOI] [PubMed] [Google Scholar]
- 41.Takeuchi S, Kawashima S, Rikitake Y, Ueyama T, Inoue N, Hirata K, Yokoyama M. Cerivastatin suppresses lipopolysaccharide-induced ICAM-1 expression through inhibition of Rho GTPase in BAEC. Biochem Biophys Res Commun. 2000;269:97–102. doi: 10.1006/bbrc.2000.2238. [DOI] [PubMed] [Google Scholar]
- 42.Stalker TJ, Lefer AM, Scalia R. A new HMG-CoA reductase inhibitor, rosuvastatin, exerts anti-inflammatory effects on the microvascular endothelium: the role of mevalonic acid. Br J Pharmacol. 2001;133:406–12. doi: 10.1038/sj.bjp.0704070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weitz-Schmidt G, Welzenbach K, Dawson J, Kallen J. Improved lymphocyte function-associated antigen-1 (LFA-1) inhibition by statin derivatives. molecular basis determined by x-ray analysis and monitoring of LFA-1 conformational changes in vitro and ex vivo. J Biol Chem. 2004;279:46764–71. doi: 10.1074/jbc.M407951200. [DOI] [PubMed] [Google Scholar]
- 44.Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995;31:9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 45.Duggan DE, Vickers S. Physiological disposition of HMG-CoA-reductase inhibitors. Drug Metab Rev. 1990;22:333–62. doi: 10.3109/03602539009041088. [DOI] [PubMed] [Google Scholar]
- 46.Weitz-Schmidt G. Lymphocyte function-associated antigen-1 blockade by statins: molecular basis and biological relevance. Endothelium. 2003;10:43–7. doi: 10.1080/10623320303360. [DOI] [PubMed] [Google Scholar]
- 47.Kvietys PR, Perry MA, Gaginella TS, Granger DN. Ethanol enhances leukocyte–endothelial cell interactions in mesenteric venules. Am J Physiol. 1990;259:G578–83. doi: 10.1152/ajpgi.1990.259.4.G578. [DOI] [PubMed] [Google Scholar]
- 48.Schillinger M, Exner M, Mlekusch W, et al. Statin therapy improves cardiovascular outcome of patients with peripheral artery disease. Eur Heart J. 2004;25:742–8. doi: 10.1016/j.ehj.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 49.Bickel C, Rupprecht HJ, Blankenberg S, et al. Influence of HMG-CoA reductase inhibitors on markers of coagulation, systemic inflammation and soluble cell adhesion. Int J Cardiol. 2002;82:25–31. doi: 10.1016/s0167-5273(01)00576-9. [DOI] [PubMed] [Google Scholar]
- 50.Wan MX, Schramm R, Klintman D, Welzenbach K, Weitz-Schmidt G, Thorlacius H. A statin-based inhibitor of lymphocyte function antigen-1 protects against ischemia/reperfusion-induced leukocyte adhesion in the colon. Br J Pharmacol. 2003;140:395–401. doi: 10.1038/sj.bjp.0705432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Klintman D, Weitz-Schmidt G, Schramm R, Thorlacius H. Lymphocyte function antigen-1 mediates leukocyte adhesion and subsequent liver damage in endotoxemic mice. Br J Pharmacol. 2004;141:709–16. doi: 10.1038/sj.bjp.0705634. [DOI] [PMC free article] [PubMed] [Google Scholar]