Abstract
It is now becoming apparent that the immune system undergoes age-associated alterations, which accumulate to produce a progressive deterioration in the ability to respond to infections and to develop immunity after vaccination, both of which are associated with a higher mortality rate in the elderly. Immunosenescence, defined as the changes in the immune system associated with age, has been gathering interest in the scientific and health-care sectors alike. The rise in its recognition is both pertinent and timely given the increasing average age and the corresponding failure to increase healthy life expectancy. This review attempts to highlight the age-dependent defects in the innate and adaptive immune systems. While discussing the mechanisms that contribute to immunosenescence, with emphasis on the extrinsic factors, particular attention will be focused on thymic involution. Finally, we illuminate potential therapies that could be employed to help us live a longer, fuller and healthier life.
General introduction
A functional immune system is considered vital for the host's continued survival against the daily onslaught of foreign organisms and pathogens. In humans, as well as in many other species, it is becoming recognized that the immune system declines with age, a term known as immunosenescence, which leads to a higher incidence of infections, neoplasia and autoimmune diseases.1
The impact of age-related changes in the immune system was clearly not an issue when the average human life span was approximately 40 years. However, over the past 150 years, advances in medical sciences and nutrition have resulted in a dramatic increase in life expectancy to an unprecedented 80 years. Currently, in the UK more than 18% of the population are 65 years or older, and this percentage is expected to rise to 25% by 2031; a trend predicted worldwide.2 Thus, in the absence of any major evolutionary pressure, an immune system that was designed to function for approximately 40 years now has to continue for an additional four decades. Therefore, it is unsurprising that the increased susceptibility to cancers and infections seen in older persons points to severe deficiencies in the immune system.
The activity of haematopoietic stem cells in the aged
A detailed overview addressing the impact of ageing on haematopoietic stem cell (HSC) function is beyond the scope of this article and more comprehensive reviews can be found elsewhere.3,4 Here we will discuss the more salient points and highlight recent findings. HSC possess the ability to differentiate into different blood-borne cells (Fig. 1), coupled with the capacity of self-renewal to prevent clonal exhaustion. Considering that all haematopoietic cells are derived from HSC, the age-dependent decline in immunity could be attributed to the functional activity of HSC in the aged. Earlier studies addressing the effect of ageing on HSC function were inconclusive. This was largely attributed to the different techniques, such as bone marrow transplantation (BMT),5 serial BMT,6 or colony-forming unit–spleen activity,7 that were employed to answer this question. However, recent evidence suggests that HSC show a decline in function that is associated with increasing age.3,4
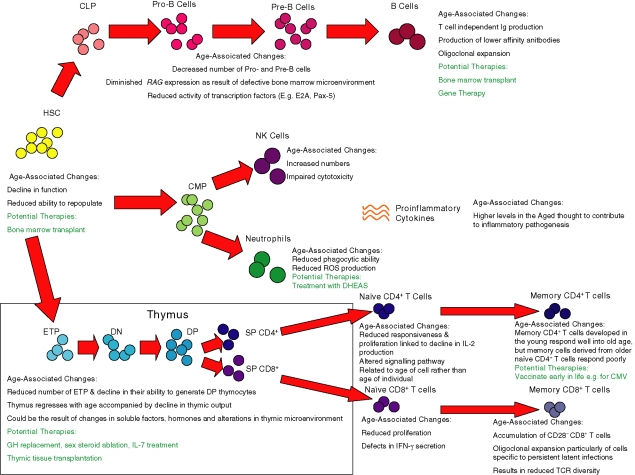
Figure 1.
The effect of age on the different components of the innate and adaptive immune systems. Stem cells from the bone marrow give rise to the haematopoietic progenitors under signals from the different microenvironments. Age-associated defects are highlighted in the different haematopoietic stages of development. In addition, potential therapies are described. CLP, common lymphoid precursor; CMP, common lymphoid progenitor; DHEAS, dehydroepiandrosterone sulphate; DN, double-negative; ETP, early thymic precursors; HSC, haematopoietic stem cell; Ig, immunoglobulin; IFN-γ, interferon-γ; NK, natural killer cell; RAG, recombination activating gene; ROS, reactive oxygen species; SP, single-positive; TCR, T-cell receptor.
It is now apparent that there are significant genetic component(s) that regulate stem cell ageing.4 For instance, studies in C57BL/6 mice revealed an increase in the number of HSC in aged mice.5 In contrast, the number of HSC in DBA mice markedly decreased with age. In competitive repopulation experiments, comparing young and old bone marrow transplanted into the same recipient, a reduced ability of old HSC to repopulate haematopoietic progenitors was apparent.8 This could be because of their inability to adhere within the bone marrow stromal environment, leading to the proposal that the homing potential of old HSC is reduced.9 More recently, gene array data showed that the transcript from old HSC reflected a reduced capacity to differentiate into the lymphoid lineage, while exhibiting a gene expression profile that reflected an increased myeloid potential.10 Furthermore, the expression of the cell-cycle inhibitor p16INKa appeared to increase with age in HSC and evidence suggests that this age-dependent increase contributes to the reduced repopulation potential of old HSC.11 Taken together these studies would support the effect of ageing on HSC to be a cell-intrinsic phenomenon, giving rise to these cells exhibiting reduced homing and reconstitution ability together with an altered programming of haematopoietic differentiation.
The impact of advancing age on innate immunity
Whereas age-related alterations of the components within the adaptive immune system are well documented, detailed analysis of the impact of advancing age on the innate immune system remains unresolved. The clinical features of immunosenescence clearly indicate a dysfunction in innate immunity and in the last few years several studies have tried to address this issue.12–14 Investigations now suggest that ageing is associated with the increased production of pro-inflammatory cytokines by macrophages and fibroblasts for example.15,16 Elevated levels of these mediators are believed to be responsible for most of the age-associated diseases such as diabetes, osteoporosis and atherosclerosis because they all share an inflammatory pathogenesis.15,16 Termed ‘inflamm-ageing’, it has been hypothesized that as a result of constant antigenic challenge, the continual production of inflammatory mediators could potentially trigger the onset of associated inflammatory diseases. Indeed, emerging evidence suggests that the balance between pro- and anti-inflammatory cytokines can be used as a profile to indicate frailty and mortality in older individuals.17
An increasing number of studies have also suggested that there is a decline in the phagocytic capacity and reduced superoxide anion production in macrophages and neutrophils in the aged.12–14 Other features of aged phagocytes include a reduced expression of Toll-like receptors on macrophages.18 Similarly, activation of neutrophils by formyl-methionyl-leucyl-phenylalanine (fMLP) is impaired in old subjects compared to young subjects, linked to a reduction of intracellular Ca2+ mobilization.19 As to the cause of such defects, recent data seem to suggest that the environment plays a key role, with hormones and cytokines influencing functional activity.20,21 Indeed, Butcher and Lord proposed that the high rate of infections following hip fracture in older individuals, could be the result of neutrophil dysfunction,21 which is attributed to the high level of circulating cortisol present in these subjects. The adrenal hormone dehydroepiandrosterone sulphate (DHEAS), which can enhance immune function and has an effect that is opposite to that of cortisol; indeed in young trauma patients the accompanied increase of cortisol level is controlled by the production of DHEAS.22 However, the production of DHEAS is markedly reduced with age and consequently the heightened immunosuppressive effect of cortisol is seen in the elderly after injury.
Given the essential role that natural killer (NK) cells play in immunity, it is not unreasonable to assume that the clinical manifestations attributed to immunosenescence could also be the result of age-dependent alterations in NK-cell number and function. It is now regarded that the number of NK cells significantly increases with age, but changes in NK-cell function are less clear and in some cases there have been conflicting reports.13,23,24 Nevertheless, the overwhelming evidence indicates depressed NK function in old individuals. Such reports have demonstrated impaired NK-cell cytotoxicity, as well as reduced production of cytokines and chemokines by activated NK cells.13,23,24 This could be attributed to the expansion of different NK-cell subsets because there are reports that in the elderly there is an increased proportion of the CD56– NK-cell subset; cells which exhibit lower cytolytic activity and have a reduced ability to secrete cytokines in comparison to the more abundant CD56+ NK-cell subset.25
Impaired B-cell development and function in the aged
Essentially, HSC in the bone marrow give rise to early B cells through common lymphocyte precursors. These common lymphocyte precursors become Pro-B cells in the bone marrow by successful immunoglobulin heavy-chain gene rearrangements and subsequently differentiate into Pre-B cells (Fig. 1), which then migrate to the periphery.26 Transition to Pro-B cell and Pre-B cell stages are dependent upon the activity of recombination activating gene 1 (RAG1) and RAG2.27 Humoral immunity in aged individuals is severely compromised as the result of mainly two mechanisms: (1) decreased production of long-term immunoglobulin-producing B lymphocytes because of intrinsic and microenvironmental defects, and (2) the loss of immunoglobulin diversity and affinity as a result of disrupted germinal centre formation.28
Whereas the decline in frequencies of pre-B cells has been well established for some time, it was presumed to be primarily the consequence of the diminished capacity that Pro-B cells have to differentiate. However, this may not be the sole reason because Miller and Allman reported a decay in frequency and absolute numbers of Pro-B cells and in the progenitor pools for the B-cell lineage, with marked reductions observed as early as 7 months of age in mice.29 More recently, Min et al. have shown that both Pro-B-cell and Pre-B-cell production is severely impaired in aged mice recovering from induced 5-fluorouracil treatment30 and such age-associated defects in Pro-B cells may help explain a reduction in the numbers of Pre-B cells generated.31 Several studies have also underlined the loss of RAG mRNA in total bone marrow preparations from old mice.32,33 Elegant studies using reciprocal bone marrow chimeras have led Labrie et al. to hypothesize that RAG expression in Pro-B cells is controlled by the microenvironment itself rather than being an intrinsic defect of senescent B-cell progenitors.34 Additional evidence supporting this hypothesis has come from stromal cell cultures, because cultures from old individuals are less efficient in supporting B-cell proliferation than those from young counterparts.31,35,36 Nevertheless, other reports have suggested defects in aged B-cell precursor transcription factors. E2A and Pax-5 are crucial to B lymphopoiesis because they accompany differentiation, proliferation and survival of early B cells following interleukin-7 (IL-7) receptor interaction.37 The reduced expression of the downstream products of E2A (E47 and E12) and Pax-5 (B-cell-specific activator protein; BSAP) have also been shown to accompany old age in Pro-B cells.38–40 More recently these defects have also been shown to be present in peripheral B cells from older mice.41
The impact of ageing on peripheral B cells is multifaceted because the numbers of B cells exported from the bone marrow is already reduced, exacerbating downstream defects. Despite a fourfold to fivefold decline in B-cell production in the aged mice, peripheral B-cell numbers remain relatively constant. One reason might be that the peripheral B-cell pool is already ‘saturated’, in a manner that is similar to what happens in T-cell homeostasis in the old.42 However, another possible explanation is that peripheral B cells in the mouse reflect a decreased B-cell generation and a fivefold increase in peripheral B-cell longevity.43,44 In addition, the oligoclonal expansions of B cells associated with CD5 expression, T-cell-independent immunoglobulin production and production of low-affinity auto-antibodies are known to occur in old individuals45,46 and to occupy niches, which then cannot be occupied by other B cells.47,48 Many other intrinsic B-cell defects have also been reported in aged mice and humans, including reduction of costimulatory molecules,49 defects in B-cell receptor signalling50,51 and low immunoglobulin titre and affinity.52–54 In addition, T-cell/B-cell interactions are known to be disrupted both in aged mice55 and in aged humans.56 Such defects in T-cell helper function, which are known to occur during ageing,57 significantly affect humoral immunity because they are required for germinal centre formation and production of soluble factors.
The thymus, T-cell development and ageing
Of all age-associated changes in the immune system, regression of the thymus must be the most dramatic, ubiquitous and recognizable. Reduced thymic size during ageing was documented even before the function of the thymus was established. Paradoxically the reduced thymus size observed in older people and in those who have died from fatal illnesses was considered normal, whereas sudden deaths on the operating table were commonly attributed to the ‘large’ thymus thought to impede breathing.58 Chronic thymic atrophy is now accepted as an ancient and conserved evolutionary process59 and the impact on immunosenescence along with characterization of the stages and mechanisms concerned are under increasing scrutiny.
The thymus is the primary site of T-cell development capable of generating self-tolerant, self major histocompatibility complex-restricted, immunocompetent T cells58,60. Highly keratinized thymic epithelial cells (TEC) constitute the major subcomponent of the thymic stroma accredited with providing the favourable microenvironment that encourages T-cell development.61,62 Through a combination of cell-to-cell contact and production of soluble factors, TEC create discrete niches in the thymus to direct the many stages of thymopoiesis as reflected by the distribution of developing thymocytes.
Briefly, the HSC that are termed double-negative (DN), which do not express CD4 or CD8, enter the thymus through the cortical–medullary junction and migrate to the outermost cortical zone. The DN subset may be further divided on the expression of CD44 and CD25 with the maturation sequence CD44+ CD25– (DN1), CD44+ CD25+ (DN2), CD44– CD25+ (DN3) and CD44– CD25– (DN4) identifying stages of expansion, commitment to the T-cell lineage and rearrangement of T-cell receptor (TCR) genes.63,64 The majority of thymocytes are found in the cortex following up-regulation of CD4 and CD8 to become double-positive (DP) thymocytes and undergo stringent selection processes; they then continue into the medulla where they differentiate into either the single-positive (SP) CD4+ or SP CD8+ T cells and await export into the periphery (Fig. 1).65
With age, there is a decrease in thymic epithelial space and thymic cellularity, collectively called thymic involution. In mice, loss of thymic epithelial space is caused by a gross reduction in thymus size,66,67 whereas in the human thymus there is an increase in perivascular space, which is progressively replaced with fat in the ageing thymus.68,69 Despite the reduction in functional thymic area, the ageing thymus still demonstrates T-cell output, although at decreased rates.70 Continual persistence of T-cell receptor excision circle-positive (TREC+) T cells, representing recent thymic emigrants (RTE), was found in the peripheral blood of elderly people.71 The drawbacks of using TREC analysis including the inclusion of long-lived naive cells were overcome by a transgenic mouse model with a green fluorescent protein (GFP) transgene under the expression of the RAG-2 promoter where RTE retain high GFP levels that fade over a 3-week period.72 RTE were clearly detectable in 2-year-old mice and, interestingly, controlling for loss of thymic size, output is relatively age-independent as calculated by the number of splenic RTE per 100 DP thymocytes.73
There is consistently emerging evidence that thymic involution does not correspond with the onset of puberty as was previously assumed.74 In the mouse thymus a significant decline in thymic cellularity has been observed at 6 weeks of age.75 In humans a decrease in thymic cellular density begins as early as 9 months old76 and appears to go through several phases of rapid regression (in those under 10 years of age and between the ages of 25 and 40 years) and slower atrophy (between 10 and 25 years of age and in those over 40 years).68 Despite these insights into the events of thymic atrophy, the mechanisms controlling the process remain obscure. A number of candidates have been proposed, which are to be discussed below.
Do the defects stem from the bone marrow?
The impact of HSC on thymic involution is a contentious debate given the conflicting data. Originally, Tyan reported a decline in the ability of aged bone marrow to reconstitute T-cell populations in lethally irradiated hosts.77 Adding credence to these studies, purified HSC from old mice also exhibited decreased differentiation potential towards lymphoid lineages in vivo and in vitro.78 Within DN1 cells are the early thymic progenitors (ETP) that were found to decline in frequency and total number in ageing mice. Moreover, ETP from older mice were inefficient at seeding fetal thymic lobes and generating DP and SP thymocytes.79 However, a number of studies transferring young bone marrow into aged lethally irradiated hosts have shown that thymic and splenic repopulation and mitogenic responses were consistently lower in the aged recipients.80 Furthermore, young bone marrow injected into aged mice failed to restore histological abnormalities of the thymus.81 Therefore, it has been suggested that there are also age-associated defects in the stromal cells.
Is IL-7 responsible?
IL-7, produced by TEC, is a vital cytokine for thymocyte development; it controls the early stages of thymopoiesis and has been shown to decline with age.82 Interestingly, treatment of mice with antibodies against IL-7 resulted in a phenotype similar to thymic involution.83 In contrast, injecting aged mice with exogenous IL-7 increased thymic weight and cellularity. Yet, although other groups have described an increase in TREC+ CD8+ T cells in the periphery after 14 days of IL-7 treatment, they failed to observe an increase in thymic numbers.66 There is also the difficulty of distinguishing the effects of IL-7 on thymopoiesis from peripheral responses, therefore thymic stromal cells engineered to constitutively express IL-7 were transplanted into mice and thymic atrophy was monitored.84 Despite the significant rise in the percentage of CD25+ DN thymocytes in older implanted mice, no change in the rate or degree of thymic involution was found and the total number of thymocytes and thymic output were similar in transplanted and control mice.84 Consequently, IL-7 may rescue the early defect in thymopoiesis of ageing mice but it fails to successfully regenerate the thymus.
A hormonal problem?
In association with generating T cells, the thymus is recognized as an endocrine gland, sensitive to hormonal control and capable of endogenous production of some hormones with various receptors expressed on the thymic stroma and thymocytes.85 Given the circumstantial evidence that decline in circulating levels of growth hormone (GH) coincides with the presumed onset of thymic atrophy it has been proposed that GH could be involved. Indeed, GH and its mediator insulin-like growth factor-1 (IGF-1) have been shown to stimulate thymopoiesis in young animals. Using a rat model with GH3 pituitary adenoma cells (which secrete GH) implanted into 22-month-old rats, thymus size increased and cellularity was enhanced.86 In older mice thymus size and cellularity were increased after administration of GH; however, recovery was still far below the numbers seen in young mice, implying that the role of GH in thymic involution may be limited.87 In conjunction, studies of little mice (with a 90% deficiency in serum GH and IGF-1 do not display any changes in the rate of involution.88
By contrast, sex steroids are renowned to have detrimental effects on thymocytes. In the absence of sex hormones by castration or ovariectomy, regeneration of the thymus was observed. Intriguingly, chimeric mice with androgen receptor-defective stroma but wild-type thymocytes did not undergo thymic atrophy, suggesting that the stroma is the target of androgen-induced regression.89 Two recent studies have attempted to further characterize the influences of sex steroids on the thymus. ETP number but not proportion was amplified in middle-aged castrated mice, implying that enhancement is at progenitor entry level rather than the result of replication.90 Additionally, aberrant thymic architecture is restored after castration.91 Nonetheless, there is evidence to suggest that testosterone is not the sole contributor to involution, including a recent examination of thymic atrophy in hypogonadal mice with diminished sex steroid production, which presented no changes in cellularity or cellular distribution compared to wild-type littermates.88 Correspondingly, this group showed that the effects of sex hormone removal are transient in the wild-type mouse, with positive effects lost after 20 weeks.
Are changes to TEC involved?
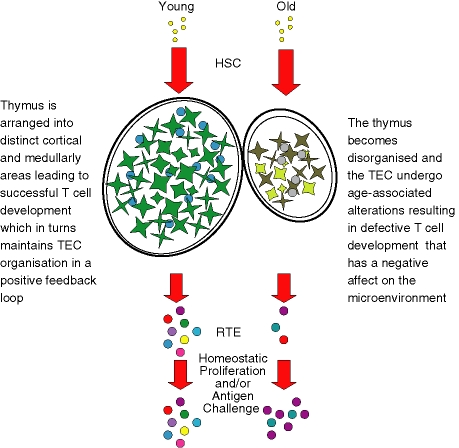
A largely overlooked potential candidate is the TEC. Given that TEC constitute an integral compartment of the thymic stroma and are the major driving force of thymopoiesis further investigation into age-related changes is required.61,92 Whether there is a decline in the number of TEC with age is hotly contested;93,94 however, in vitro data suggest that the proliferative rate is reduced in older mice. A recent study has shown that CD45– cells decline in number with age and that the proportion of proliferating TEC, as measured by Ki67 expression, decreases in older mice.95 Alterations in thymic architecture though have been consistently observed in both mice and humans. There appear to be accumulations of fibroblasts and a decrease in keratin-positive areas in the human thymus with age.76 This is accompanied by a distortion at the cortical–medullary junction.67–69,96 In addition, there is a decrease in the TEC genes FoxNI and subunit 8 of keratin in the ageing mouse.75 Collectively these suggest a qualitative and/or quantitative loss of TEC with age. Indeed our group has found that the gross morphological changes are associated with alterations in the expression of critical molecules such as major histocompatibility complex class II and defining molecules of cortical and medullary TEC, which appear to decline with age (Aw et al., manuscript in preparation). Considering the exacerbated rate at which the thymus demonstrates signs of age-associated atrophy, we propose that it is under different parameters to those controlling the ageing process in other organs and tissues. This is supported by microarray analysis demonstrating that the majority of specific genes found to change with thymic ageing are distinct from those in other systems.97 Thus, we suggest that the deficiency begins within the thymus itself as, according to the disposable soma theory,98 it becomes redundant once it has generated a significant TCR repertoire,99 which occurs early in life. These changes result in defective T-cell development,100 which in turn has a negative effect on the TEC because the maintenance of the thymic architecture is dependent on the presence of functionally maturing thymocytes (Fig. 2).101,102 Undoubtedly, the process of thymic involution is a multifactorial one and all the mechanisms discussed here could be involved with grave repercussions for the peripheral T cells.
Figure 2.
Crumbling architecture with faulty foundations – a proposed model of thymic involution. T-cell development initiates when HSC enter into the thymus. It is still unknown whether the number of HSC entering the thymus is reduced with age and there are still debates over the development potential of these cells. HSC are instructed by the TEC through the developmental pathway of successful T-cell differentiation by a combination of cell–cell contact and soluble factors. In the left diagram, the young thymus is distinctly orientated into the cortex and medulla directing the early and late stages of thymopoiesis, respectively, generating functional T cells. The presence of immunocompetent T cells is imperative to the maintenance of the thymic architecture. However, with increasing age the thymus shrinks, becomes disorganized and the TEC lose defining molecules such as keratin (right diagram) contributing to aberrant T-cell development. As the nature of the relationship between TEC and T cells is symbiotic, these defective T cells have a negative influence on the already age-altered TEC. This leads to a decrease in the number of RTE exiting the thymus and entering the peripheral pool, which in turn results in a constriction of the TCR repertoire in the older individuals by expansion of specific memory clones and decreased naive T-cell numbers. HSC, haematopoietic stem cells; RTE, recent thymic emigrants; TCR, T-cell receptor; TEC, thymic epithelial cells.
Age-dependent defects in peripheral T cells
Surprisingly there is little change in the number of peripheral T cells with age, especially given the reduction in thymic output in the aged.103 The size of the peripheral T-cell pool is tightly regulated by several variables including homeostatic mechanisms.104 Both memory and naive T cells undergo homeostatic control and in humans steady-state proliferation significantly contributes to the naive TCR repertoire.104 It had been presumed that naive and memory T-cell pools were maintained separately with different survival requirements that are considerably stricter for naive T cells.105 Yet an innovative study revealed that clonal expansion of CD8+ T cells is the consequence of the diversity of the remaining T cells, particularly those that share the same TCR Vβ element.106 This could have a profound impact on TCR diversity. Analysis of the TCR Vβ chain presented a decreased antigen-recognition repertoire from approximately 108 in young adults to 106 in older individuals107 with a drastic contraction in CD4+ T-cell diversity in the seventh and eighth decades of life.108 Studies in mice have determined that a twofold to 10-fold decrease in diversity is sufficient to jeopardize a T-cell-mediated immune response,109 thereby leaving the elderly more susceptible to new pathogens.
As the emphasis in the ageing peripheral T-cell pool is perpetuation through replication, this has gross implications for the individual cell. Examination of lymphocyte lifespan has shown variations in subsets, but all are finite. Human CD4+ T cells have around 33 population doublings in culture110 whereas CD8+ T cells have only around 23.111 The restraint dictating lifespan is believed to be telomere-dependent and analysis of telomere length displays significantly shorter telomeres in old individuals among all T-cell subsets.112 These cells, which have undergone replicative senescence, accumulate with age and many, particularly in the CD8+ memory subset, are specific to only certain persistent infections.113 One virus that has attracted specific attention is cytomegalovirus (CMV) with a large expansion of CMV-specific CD8+ T-cell clones in the elderly and it has now been verified through longitudinal studies that CMV seropositivity identifies those with an immune risk phenotype.114 Shortened telomeres have been correlated with changes in phenotypes (reviewed by Weng in 2006107), modifications in responses115 and resistance to apoptosis.116
Can immunosenescence/immunodeficiencies be reversed?
Along with advances in research into immunosenescence and immunodeficiencies, potential therapies to counteract these deficits in older people and immunocompromised individuals are being studied (summarized in Fig. 1). Since age-associated defects appear to be partially within progenitor cells, in theory some of the problems associated with immunosenescence could be reversed by improving the quality and numbers of naive T cells and immunoglobulin-producing B cells. One successful therapy is BMT, however, the occurrence of graft-versus-host disease and graft-versus-leukaemia encountered by BMT recipients remains a major hurdle to be overcome.117 In mice, the administration of growth factors and cytokines such as IGF-I and IL-15 after allogeneic BMT has been proven to enhance immune reconstitution, including NK cells, NK T cells and T cells associated with enhanced T-cell and NK-cell function while IGF-I in combination with IL-7 greatly enhances B-cell lymphopoiesis.118,119 Indeed, when using soluble factor therapies, IL-7 might prove to be a good candidate for immune reconstitution after BMT because Bolotin et al. reported the enhancement of thymopoiesis after syngeneic BMT by in vivo administration of IL-7 without aggravating graft-versus-host-disease.120 Similar results were found for B cells, NK cells, monocytes and macrophage expansion following BMT, including in middle-age recipient mice,121 introducing the hope of immune reconstitution in the aged using post-transplant cytokine conditioning.
As a result of a dysfunctional microenvironment, altered cytokine profiles and signal transduction defects are critical in aged individuals. Research suggests that IL-7 therapy alone in old mice can rejuvenate the thymus,122 although never to the point of thymic size and output that are seen in the young.123 Additionally, IL-7 receptor components seem to be expressed in Pro- and B cells derived from old mice at comparable levels to young mice.124 Thus, IL-7 therapy alone might not work because of the inability to maintain this cytokine in the particular niche and/or signalling defects in the aged mice. In fact phosphorylated P-STAT5, a signal transducer from IL-7 and IL-2 receptor JAK activation, is likely to be crucial in anti-immunosenescence therapies because its presence is much reduced in both aged B-cell precursors40 and ageing T cells.125 However, one must remember that even in the event of B-cell and T-cell IL-7/IL-2-driven reconstitution of thymopoiesis and B-cell development in aged mice, the same would not necessarily apply in humans because, for example, IL-7 does not have the same properties in humans as it does in mice.126
The prospect of whole thymic transplantation as a means to increase the number of naive T cells has proved promising in both mice and humans suffering from immune dysfunction.127,128 Indeed, transplantation of cultured pieces of thymic tissue into paediatric patients suffering from DiGeorge syndrome has proved successful in restoring immune function129 for up to 10 years postsurgery.130 The implications of this approach for the treatment of immune dysfunction in the elderly are however, more complex because of the limited amount of tissue, the invasive surgery involved and tissue rejection. In the mouse, several groups have now identified a progenitor multipotent TEC that can grow into a three-dimentional thymus and support normal T-cell development when transplanted onto the kidney capsule of normal and nude mice.131–133 In humans, such microenvironmental progenitor epithelial cells have not yet been identified, but advances in finding such undifferentiated epithelial cells in the murine postnatal thymus134 make this research an exciting and promising avenue to the restoration of thymic function.
There are also a considerable number of studies reporting cross-talk between the immune and neuroendocrine systems, placing the thymus as a target for neuroendocrine control.135,136 Data from our group seem to support this hypothesis because we have shown that neuropeptide and thymic hormone expression in the thymus seems to be affected by ageing and have a role in T-cell development across different evolutionary species.137,138 Treatment with GH in HIV-1-infected patients has proved successful in the reversal of thymic atrophy.139 Of late, there has been a re-emergence of interest in zinc supplementation as a therapeutic adjuvant. The benefits of zinc on the immune system have been reported for a number of years,140 and the corresponding decrease in zinc absorption in older individuals141 has led to the proposal of zinc supplementation for the elderly. However, this approach should be used with caution, a recent report observed that while zinc can decrease spontaneous apoptosis in peripheral blood mononuclear cells there was an increase in oxidative stress-induced apoptosis that was age-dependent.142 Another therapeutically exciting approach is to target sex steroids, which are known to have a dramatic effect on thymus size and function during ageing143–145 as well as in modulating differentiation, proliferation and survival of B-cell precursors.146 Boyd's group has recently shown that castration of 9-month-old mice enhanced the number of ETP as well as thymic proliferation.90 While this technique might have very limited applications in humans, analysis of male patients undergoing sex steroid ablation therapy for prostatic cancer has shown that this technique might prove useful in increasing circulating naive T cells in old individuals.91 Furthermore, hormone therapies could prove useful in the treatment of innate age-associated dysfunctions, for example DHEAS supplements are able to enhance superoxide production in neutrophils and can increase NK-cell activity.21
Without the need for surgery or sex steroid ablation and their intrinsic side-effects, gene therapy alone or combined with other therapies could prove to be more effective in restoring immune function in immunocompromised patients as well as in old individuals. Although short-lived, infants with X-linked severe combined immunodeficiency who received retroviral gene addition to cells from their bone marrow developed impressive immune reconstitution.147 This shows that although still in its early stages, gene therapy can in theory treat immunological disorders in old and immunodeficient individuals.
Concluding remarks
Despite the relative infancy of the study of immunosenescence, much has been achieved. The move away from the descriptive phase of the research into finding causative effects can only be of benefit to a world population with an increasing average lifespan that is projected to keep rising. A better understanding of the signals dictating immune dysfunction and senescence, particularly from the microenvironment, could be the key to successful immune reconstitution in the future.
Acknowledgments
We thank Dr Julian Dyson, Dr Sian Henson, Dr Imelda McGonnell and Dr Steve Allen for critical review of the manuscript. D.A. is supported by Research into Ageing and A.B.S. is supported by the Thomas Brown Fellowship (University of London). D.B.P. is a recipient of a Strategic Promotion of Ageing Research Capacity (SPARC) award.
Glossary
Abbreviations:
- BSAP
B-cell-specific activator protein
- BMT
bone marrow transplantation
- CLP
common lymphocyte precursor
- CMV
cytomegalovirus
- DHEAS
dehydroepiandrosterone sulphate
- DN
double negative
- DP
double positive
- ETP
early thymic progenitors
- fMLP
formyl-methionyl-leucyl-phenylalanine
- GFP
green fluorescent protein
- GH
growth hormone
- HIV-1
human immunodeficiency virus-1
- HSC
haematopoietic stem cells
- IFN-γ
interferon-γ
- Ig
immunoglobulin
- IGF-1
insulin-like growth factor-1
- IL
interleukin
- NK
natural killer
- RAG
recombination activating gene
- ROS
reactive oxygen species
- RTE
recent thymic emigrants
- SP
single positive
- TCR
T-cell receptor
- TEC
thymic epithelial cells
- TREC
T-cell receptor excision circle
References
- 1.Pawelec G. Immunosenescence: impact in the young as well as the old? Mech Ageing Dev. 1999;108:1–7. doi: 10.1016/s0047-6374(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 2.Office for National Statistics. Theme: Population. http://www.statistics.gov.uk/cci/nugget.asp?id=949.
- 3.Kamminga LM, de Haan G. Cellular memory and hematopoietic stem cell aging. Stem Cells. 2006;24:1143–9. doi: 10.1634/stemcells.2005-0345. [DOI] [PubMed] [Google Scholar]
- 4.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–33. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 5.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–16. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 6.Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr Biol. 1997;7:805–8. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 7.Albright JW, Makinodan T. Decline in the growth potential of spleen-colonizing bone marrow stem cells of long-lived aging mice. J Exp Med. 1976;144:1204–13. doi: 10.1084/jem.144.5.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison DE. Long-term erythropoietic repopulating ability of old, young, and fetal stem cells. J Exp Med. 1983;157:1496–504. doi: 10.1084/jem.157.5.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing Z, Ryan MA, Daria D, Nattamai KJ, Van Zant G, Wang L, Zheng Y, Geiger H. Increased hematopoietic stem cell mobilization in aged mice. Blood. 2006;108:2190–7. doi: 10.1182/blood-2005-12-010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102:9194–9. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–6. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 12.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–62. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–9. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- 14.Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122:1521–35. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 15.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–9. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 16.Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Biggelaar AH, Huizinga TW, de Craen AJ, Gussekloo J, Heijmans BT, Frolich M, Westendorp RG. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004;39:1407–14. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 19.Schroder AK, Rink L. Neutrophil immunity of the elderly. Mech Ageing Dev. 2003;124:419–25. doi: 10.1016/s0047-6374(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 20.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3:151–60. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- 22.Butcher SK, Killampalli V, Lascelles D, Wang K, Alpar EK, Lord JM. Raised cortisol: DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell. 2005;4:319–24. doi: 10.1111/j.1474-9726.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 23.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18:1613–20. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- 24.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–84. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 25.Borrego F, Alonso MC, Galiani MD, Carracedo J, Ramirez R, Ostos B, Pena J, Solana R. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–65. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 26.Allman D, Miller JP. B cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:463–7. doi: 10.1016/j.coi.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–70. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 28.Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, Zheng B. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol. 2003;170:1267–73. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- 29.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–30. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 30.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on the common lymphoid progenitor to pro-B cell transition. J Immunol. 2006;176:1007–12. doi: 10.4049/jimmunol.176.2.1007. [DOI] [PubMed] [Google Scholar]
- 31.Stephan RP, Sanders VM, Witte PL. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–18. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- 32.Szabo P, Zhao K, Kirman I, Le Maoult J, Dyall R, Cruikshank W, Weksler ME. Maturation of B cell precursors is impaired in thymic-deprived nude and old mice. J Immunol. 1998;161:2248–53. [PubMed] [Google Scholar]
- 33.Ben-Yehuda A, Szabo P, Weksler ME. Age-associated changes in the B-cell repertoire. effect of age on RAG-1 gene expression in murine bone marrow. Immunol Lett. 1994;40:287–9. doi: 10.1016/0165-2478(94)00067-0. [DOI] [PubMed] [Google Scholar]
- 34.Labrie JE, Sah AP, Allman DM, Cancro MP, Gerstein RM. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–23. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–609. [PubMed] [Google Scholar]
- 36.Van der Put E, Sherwood EM, Blomberg BB, Riley RL. Aged mice exhibit distinct B cell precursor phenotypes differing in activation, proliferation and apoptosis. Exp Gerontol. 2003;38:1137–47. doi: 10.1016/j.exger.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Corcoran LM. Transcriptional control of B cell activation. Curr Top Microbiol Immunol. 2005;290:105–46. doi: 10.1007/3-540-26363-2_6. [DOI] [PubMed] [Google Scholar]
- 38.Van der Put E, Frasca D, King AM, Blomberg BB, Riley RL. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. J Immunol. 2004;173:818–27. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]
- 39.Sherwood EM, Xu W, King AM, Blomberg BB, Riley RL. The reduced expression of surrogate light chains in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech Ageing Dev. 2000;118:45–59. doi: 10.1016/s0047-6374(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 40.Riley RL, Van der Put E, King AM, Frasca D, Blomberg BB. Deficient B lymphopoiesis in murine senescence. Potential roles for dysregulation of E2A, Pax-5, and STAT5. Semin Immunol. 2005;17:330–6. doi: 10.1016/j.smim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Frasca D, Van der Put E, Landin AM, Gong D, Riley RL, Blomberg BB. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–44. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- 42.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on early B- and T-cell development. Immunol Rev. 2005;205:7–17. doi: 10.1111/j.0105-2896.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 43.Huppert FA, Solomou W, O'Connor S, Morgan K, Sussams P, Brayne C. Aging and lymphocyte subpopulations: whole-blood analysis of immune markers in a large population sample of healthy elderly individuals. Exp Gerontol. 1998;33:593–600. doi: 10.1016/s0531-5565(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 44.Kline GH, Hayden TA, Klinman NR. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162:3342–9. [PubMed] [Google Scholar]
- 45.Cancro MP, Smith SH. Peripheral B cell selection and homeostasis. Immunol Res. 2003;27:141–8. doi: 10.1385/IR:27:2-3:141. [DOI] [PubMed] [Google Scholar]
- 46.Johnson SA, Rozzo SJ, Cambier JC. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire. J Immunol. 2002;168:5014–23. doi: 10.4049/jimmunol.168.10.5014. [DOI] [PubMed] [Google Scholar]
- 47.Eaton-Bassiri AS, Mandik-Nayak L, Seo SJ, Madaio MP, Cancro MP, Erikson J. Alterations in splenic architecture and the localization of anti-double-stranded DNA B cells in aged mice. Int Immunol. 2000;12:915–26. doi: 10.1093/intimm/12.6.915. [DOI] [PubMed] [Google Scholar]
- 48.LeMaoult J, Delassus S, Dyall R, Nikolic-Zugic J, Kourilsky P, Weksler ME. Clonal expansions of B lymphocytes in old mice. J Immunol. 1997;159:3866–74. [PubMed] [Google Scholar]
- 49.Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 50.Whisler RL, Grants IS. Age-related alterations in the activation and expression of phosphotyrosine kinases and protein kinase C (PKC) among human B cells. Mech Ageing Dev. 1993;71:31–46. doi: 10.1016/0047-6374(93)90033-n. [DOI] [PubMed] [Google Scholar]
- 51.Souvannavong V, Lemaire C, Andreau K, Brown S, Adam A. Age-associated modulation of apoptosis and activation in murine B lymphocytes. Mech Ageing Dev. 1998;103:285–99. doi: 10.1016/s0047-6374(98)00051-7. [DOI] [PubMed] [Google Scholar]
- 52.Zharhary D, Segev Y, Gershon H. The affinity and spectrum of cross reactivity of antibody production in senescent mice: the IgM response. Mech Ageing Dev. 1977;6:385–92. doi: 10.1016/0047-6374(77)90040-9. [DOI] [PubMed] [Google Scholar]
- 53.Goidl EA, Innes JB, Weksler ME. Immunological studies of aging. II. Loss of IgG and high avidity plaque-forming cells and increased suppressor cell activity in aging mice. J Exp Med. 1976;144:1037–48. doi: 10.1084/jem.144.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicoletti C, Yang X, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibodies from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–9. [PubMed] [Google Scholar]
- 55.Yang X, Stedra J, Cerny J. Relative contribution of T and B cells to hypermutation and selection of the antibody repertoire in germinal centers of aged mice. J Exp Med. 1996;183:959–70. doi: 10.1084/jem.183.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 58.Miller JF. Events that led to the discovery of T-cell development and function – a personal recollection. Tissue Antigens. 2004;63:509–17. doi: 10.1111/j.0001-2815.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- 59.Torroba M, Zapata AG. Aging of the vertebrate immune system. Microsc Res Tech. 2003;62:477–81. doi: 10.1002/jemt.10409. [DOI] [PubMed] [Google Scholar]
- 60.Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–9. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 61.Boyd RL, Tucek CL, Godfrey DI, et al. The thymic microenvironment. Immunol Today. 1993;14:445–59. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 62.Anderson G, Jenkinson EJ. Lymphostromal interactions in thymic development and function. Nat Rev Immunol. 2001;1:31–40. doi: 10.1038/35095500. [DOI] [PubMed] [Google Scholar]
- 63.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3–CD4–CD8– triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–52. [PubMed] [Google Scholar]
- 64.Fehling HJ, von Boehmer H. Early alpha beta T cell development in the thymus of normal and genetically altered mice. Curr Opin Immunol. 1997;9:263–75. doi: 10.1016/s0952-7915(97)80146-x. [DOI] [PubMed] [Google Scholar]
- 65.Berg LJ, Kang J. Molecular determinants of TCR expression and selection. Curr Opin Immunol. 2001;13:232–41. doi: 10.1016/s0952-7915(00)00209-0. [DOI] [PubMed] [Google Scholar]
- 66.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–8. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 67.Li L, Hsu HC, Grizzle WE, et al. Cellular mechanism of thymic involution. Scand J Immunol. 2003;57:410–22. doi: 10.1046/j.1365-3083.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- 68.Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–75. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 69.Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the human thymic perivascular space during aging. J Clin Invest. 1999;104:1031–9. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 71.Jamieson BD, Douek DC, Killian S, et al. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–75. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 72.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–25. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 73.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–52. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montecino-Rodriguez E, Dorshkind K. Evolving patterns of lymphopoiesis from embryogenesis through senescence. Immunity. 2006;24:659–62. doi: 10.1016/j.immuni.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Ortman CL, Dittmar KA, Witte PL, Le PT. Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int Immunol. 2002;14:813–22. doi: 10.1093/intimm/dxf042. [DOI] [PubMed] [Google Scholar]
- 76.Bertho JM, Demarquay C, Moulian N, Van Der Meeren A, Berrih-Aknin S, Gourmelon P. Phenotypic and immunohistological analyses of the human adult thymus: evidence for an active thymus during adult life. Cell Immunol. 1997;179:30–40. doi: 10.1006/cimm.1997.1148. [DOI] [PubMed] [Google Scholar]
- 77.Tyan ML. Impaired thymic regeneration in lethally irradiated mice given bone marrow from aged donors. Proc Soc Exp Biol Med. 1976;152:33–5. doi: 10.3181/00379727-152-39321. [DOI] [PubMed] [Google Scholar]
- 78.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–80. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–50. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 80.Doria G, Mancini C, Utsuyama M, Frasca D, Hirokawa K. Aging of the recipients but not of the bone marrow donors enhances autoimmunity in syngeneic radiation chimeras. Mech Ageing Dev. 1997;95:131–42. doi: 10.1016/s0047-6374(97)01871-x. [DOI] [PubMed] [Google Scholar]
- 81.Mackall CL, Punt JA, Morgan P, Farr AG, Gress RE. Thymic function in young/old chimeras: substantial thymic T cell regenerative capacity despite irreversible age-associated thymic involution. Eur J Immunol. 1998;28:1886–93. doi: 10.1002/(SICI)1521-4141(199806)28:06<1886::AID-IMMU1886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 82.Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol. 2002;37:455–63. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 83.Aspinall R, Andrew D. Thymic atrophy in the mouse is a soluble problem of the thymic environment. Vaccine. 2000;18:1629–37. doi: 10.1016/s0264-410x(99)00498-3. [DOI] [PubMed] [Google Scholar]
- 84.Phillips JA, Brondstetter TI, English CA, Lee HE, Virts EL, Thoman ML. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J Immunol. 2004;173:4867–74. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- 85.Savino W, Postel-Vinay MC, Smaniotto S, Dardenne M. The thymus gland: a target organ for growth hormone. Scand J Immunol. 2002;55:442–52. doi: 10.1046/j.1365-3083.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- 86.Kelley KW, Brief S, Westly HJ, Novakofski J, Bechtel PJ, Simon J, Walker EB. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci USA. 1986;83:5663–7. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Montecino-Rodriquez E, Min H, Dorshkind K. Reevaluating current models of thymic involution. Semin Immunol. 2005;17:356–61. doi: 10.1016/j.smim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Min H, Montecino-Rodriguez E, Dorshkind K. Reassessing the role of growth hormone and sex steroids in thymic involution. Clin Immunol. 2006;118:117–23. doi: 10.1016/j.clim.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 89.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology. 2001;142:1278–83. doi: 10.1210/endo.142.3.8032. [DOI] [PubMed] [Google Scholar]
- 90.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–93. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 91.Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–53. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- 92.Ritter MA, Palmer DB. The human thymic microenvironment: new approaches to functional analysis. Semin Immunol. 1999;11:13–21. doi: 10.1006/smim.1998.0148. [DOI] [PubMed] [Google Scholar]
- 93.Nakahama M, Mohri N, Mori S, Shindo G, Yokoi Y, Machinami R. Immunohistochemical and histometrical studies of the human thymus with special emphasis on age-related changes in medullary epithelial and dendritic cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58:245–51. doi: 10.1007/BF02890079. [DOI] [PubMed] [Google Scholar]
- 94.Mocchegiani E, Giacconi R, Cipriano C, Muti E, Gasparini N, Malavolta M. Are zinc-bound metallothionein isoforms (I+II and III) involved in impaired thymulin production and thymic involution during ageing? Immun Ageing. 2004;1:5. doi: 10.1186/1742-4933-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL. Developmental kinetics, turnover and stimulatory capacity of thymic epithelial cells. Blood. 2006;108:3777–85. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 96.Brelinska R. Thymic epithelial cells in age-dependent involution. Microsc Res Tech. 2003;62:488–500. doi: 10.1002/jemt.10410. [DOI] [PubMed] [Google Scholar]
- 97.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 98.Kirkwood TB, Franceschi C. Is aging as complex as it would appear? New perspectives in aging research. Ann N Y Acad Sci. 1992;663:412–17. doi: 10.1111/j.1749-6632.1992.tb38685.x. [DOI] [PubMed] [Google Scholar]
- 99.George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today. 1996;17:267–72. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 100.Thoman ML. The pattern of T lymphocyte differentiation is altered during thymic involution. Mech Ageing Dev. 1995;82:155–70. doi: 10.1016/0047-6374(95)01597-s. [DOI] [PubMed] [Google Scholar]
- 101.van Ewijk W, Hollander G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127:1583–91. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 102.Palmer DB, Viney JL, Ritter MA, Hayday AC, Owen MJ. Expression of the alpha beta T-cell receptor is necessary for the generation of the thymic medulla. Dev Immunol. 1993;3:175–9. doi: 10.1155/1993/56290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hulstaert F, Hannet I, Deneys V, Munhyeshuli V, Reichert T, De Bruyere M, Strauss K. Age-related changes in human blood lymphocyte subpopulations. II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994;70:152–8. doi: 10.1006/clin.1994.1023. [DOI] [PubMed] [Google Scholar]
- 104.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–75. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 105.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–62. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 106.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–58. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–9. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–52. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 109.Nanda NK, Apple R, Sercarz E. Limitations in plasticity of the T-cell receptor repertoire. Proc Natl Acad Sci USA. 1991;88:9503–7. doi: 10.1073/pnas.88.21.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pawelec G, Sansom D, Rehbein A, Adibzadeh M, Beckman I. Decreased proliferative capacity and increased susceptibility to activation-induced cell death in late-passage human CD4+ TCR2+ cultured T cell clones. Exp Gerontol. 1996;31:655–68. doi: 10.1016/s0531-5565(96)00097-6. [DOI] [PubMed] [Google Scholar]
- 111.Perillo NL, Naeim F, Walford RL, Effros RB. In vitro cellular aging in T-lymphocyte cultures. analysis of DNA content and cell size. Exp Cell Res. 1993;207:131–5. doi: 10.1006/excr.1993.1171. [DOI] [PubMed] [Google Scholar]
- 112.Akbar AN, Beverley PC, Salmon M. Will telomere erosion lead to a loss of T-cell memory? Nat Rev Immunol. 2004;4:737–43. doi: 10.1038/nri1440. [DOI] [PubMed] [Google Scholar]
- 113.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–5. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 114.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–10. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 115.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–6. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsu HC, Scott DK, Mountz JD. Impaired apoptosis and immune senescence – cause or effect? Immunol Rev. 2005;205:130–46. doi: 10.1111/j.0105-2896.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 117.Ferrara JL, Levine JE. Graft-versus-host disease in the 21st century: new perspectives on an old problem. Semin Hematol. 2006;43:1–2. doi: 10.1053/j.seminhematol.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 118.Alpdogan O, Muriglan SJ, Kappel BJ, et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation. 2003;75:1977–83. doi: 10.1097/01.TP.0000070167.81584.A2. [DOI] [PubMed] [Google Scholar]
- 119.Alpdogan O, Eng JM, Muriglan SJ, et al. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005;105:865–73. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 120.Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood. 1996;88:1887–94. [PubMed] [Google Scholar]
- 121.Alpdogan O, Schmaltz C, Muriglan SJ, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–65. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 122.Plum J, De Smedt M, Leclercq G. Exogenous IL-7 promotes the growth of CD3–CD4–CD8–CD44+CD25+/– precursor cells and blocks the differentiation pathway of TCR-alpha beta cells in fetal thymus organ culture. J Immunol. 1993;150:2706–16. [PubMed] [Google Scholar]
- 123.Aspinall R. T cell development, ageing and interleukin-7. Mech Ageing Dev. 2006;127:572–8. doi: 10.1016/j.mad.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 124.Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B-lymphopoiesis with age. Blood. 1998;91:75–88. [PubMed] [Google Scholar]
- 125.Pawelec G, Hirokawa K, Fulop T. Altered T cell signalling in ageing. Mech Ageing Dev. 2001;122:1613–37. doi: 10.1016/s0047-6374(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 126.LeBien TW. Fates of human B-cell precursors. Blood. 2000;96:9–23. [PubMed] [Google Scholar]
- 127.Berzins SP, Uldrich AP, Sutherland JS, Gill J, Miller JF, Godfrey DI, Boyd RL. Thymic regeneration: teaching an old immune system new tricks. Trends Mol Med. 2002;8:469–76. doi: 10.1016/s1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
- 128.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 129.Markert ML, Boeck A, Hale LP, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. N Engl J Med. 1999;341:1180–9. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 130.Rice HE, Skinner MA, Mahaffey SM, Oldham KT, Ing RJ, Hale LP, Markert ML. Thymic transplantation for complete DiGeorge syndrome: medical and surgical considerations. J Pediatr Surg. 2004;39:1607–15. doi: 10.1016/j.jpedsurg.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 131.Gill J, Malin M, Hollander GA, Boyd R. Generation of a complete thymic microenvironment by MTS24(+) thymic epithelial cells. Nat Immunol. 2002;3:635–42. doi: 10.1038/ni812. [DOI] [PubMed] [Google Scholar]
- 132.Bennett AR, Farley A, Blair NF, Gordon J, Sharp L, Blackburn CC. Identification and characterization of thymic epithelial progenitor cells. Immunity. 2002;16:803–14. doi: 10.1016/s1074-7613(02)00321-7. [DOI] [PubMed] [Google Scholar]
- 133.Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;441:988–91. doi: 10.1038/nature04813. [DOI] [PubMed] [Google Scholar]
- 134.Bleul CC, Corbeaux T, Reuter A, Fisch P, Monting JS, Boehm T. Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature. 2006;441:992–6. doi: 10.1038/nature04850. [DOI] [PubMed] [Google Scholar]
- 135.Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- 136.Savino W, Dardenne M. Neuroendocrine control of thymus physiology. Endocr Rev. 2000;21:412–43. doi: 10.1210/edrv.21.4.0402. [DOI] [PubMed] [Google Scholar]
- 137.Silva AB, Aw D, Palmer DB. Evolutionary conservation of neuropeptide expression in the thymus of different species. Immunology. 2006;118:131–40. doi: 10.1111/j.1365-2567.2006.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Solomou K, Ritter MA, Palmer DB. Somatostatin is expressed in the murine thymus and enhances thymocyte development. Eur J Immunol. 2002;32:1550–9. doi: 10.1002/1521-4141(200206)32:6<1550::AID-IMMU1550>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 139.Napolitano LA, Lo JC, Gotway MB, et al. Increased thymic mass and circulating naive CD4 T cells in HIV-1-infected adults treated with growth hormone. Aids. 2002;16:1103–11. doi: 10.1097/00002030-200205240-00003. [DOI] [PubMed] [Google Scholar]
- 140.Mocchegiani E, Costarelli L, Giacconi R, Cipriano C, Muti E, Malavolta M. Zinc-binding proteins (metallothionein and alpha-2 macroglobulin) and immunosenescence. Exp Gerontol. 2006;41:1094–107. doi: 10.1016/j.exger.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 141.Mitchell WA, Meng I, Nicholson SA, Aspinall R. Thymic output, ageing and zinc. Biogerontology. 2006;7:461–70. doi: 10.1007/s10522-006-9061-7. [DOI] [PubMed] [Google Scholar]
- 142.Ostan R, Alberti S, Bucci L, et al. Effect of zinc ions on apoptosis in PBMCs from healthy aged subjects. Biogerontology. 2006;7:437–47. doi: 10.1007/s10522-006-9059-1. [DOI] [PubMed] [Google Scholar]
- 143.Marchetti B, Guarcello V, Morale MC, Bartoloni G, Farinella Z, Cordaro S, Scapagnini U. Luteinizing hormone-releasing hormone-binding sites in the rat thymus: characteristics and biological function. Endocrinology. 1989;125:1025–36. doi: 10.1210/endo-125-2-1025. [DOI] [PubMed] [Google Scholar]
- 144.Utsuyama M, Hirokawa K. Hypertrophy of the thymus and restoration of immune functions in mice and rats by gonadectomy. Mech Ageing Dev. 1989;47:175–85. doi: 10.1016/0047-6374(89)90030-4. [DOI] [PubMed] [Google Scholar]
- 145.Greenstein BD, de Bridges EF, Fitzpatrick FT. Aromatase inhibitors regenerate the thymus in aging male rats. Int J Immunopharmacol. 1992;14:541–53. doi: 10.1016/0192-0561(92)90115-2. [DOI] [PubMed] [Google Scholar]
- 146.Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood. 2000;95:2059–67. [PubMed] [Google Scholar]
- 147.Puck JM, Malech HL. Gene therapy for immune disorders: good news tempered by bad news. J Allergy Clin Immunol. 2006;117:865–9. doi: 10.1016/j.jaci.2006.01.041. [DOI] [PubMed] [Google Scholar]