Abstract
Oligodeoxynucleotides (ODN) with unmethylated deoxycytidyl-deoxyguanosine dinucleotides (CpG-ODNs) stimulate Toll-like receptor 9 (TLR9) in plasmacytoid dendritic cells (pDC) and B cells and activate innate and adaptive immunity. Three classes of synthetic CpG-ODNs, class A, B and C, activate cells through TLR9; our goal was to evaluate their effect on cells from human immunodeficiency virus (HIV)-1+ individuals. We compared the frequencies and the unstimulated activation status of immune effector cells in HIV-1+ and HIV-1– individuals. Fewer pDC, myeloid dendritic cells (mDC), B cells, natural killer (NK) cells and invariant natural killer T cells (iNKT) were present in HIV-1+ peripheral blood mononuclear cells (PBMC) and their baseline activation status was higher than HIV-1– PBMC. Exposure of HIV-1+ PBMC to all classes of CpG-ODNs led to activation and maturation of pDC based on CD86, CD80, and CD83 expression similar to that of cells from HIV-1– individuals. The percentage of CpG-ODN stimulated pDC that express CD40 was dramatically higher when cells were obtained from HIV-1+ than from HIV-1– individuals. B-lymphocytes were activated similarly in HIV-1+ and HIV-1– individuals. mDC, NK and iNKT cell, which lack TLR9, were indirectly activated. Interferon-α (IFN-α) and interferon inducible protein 10 (IP-10) secretion was induced by class A or C but not class B CpG-ODN, but the concentrations were less than those produced by HIV-1– PBMC. HIV-1 infected individuals have fewer innate effector cells that are chronically activated, but these cells can be further activated by CpG-ODN, which suggests that synthetic CpG-ODNs could be used to enhance the immune system in HIV-1 infected individuals.
Keywords: adjuvant, Toll-like receptors, TLR9, innate immunity, dendritic cells, pDc, HIV
Introduction
Advanced human immunodeficiency virus (HIV)-1 infection is associated with CD4+ T-cell loss and chronic immune activation which results in a diminished immune response to pathogens and increased susceptibility to opportunistic infections. It is also associated with markedly diminished numbers of plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC), invariant natural killer T cells (iNKT) and natural killer cells (NK).1–3 Lower numbers of pDC in chronic HIV-1 infection are also associated with a diminished innate immune response and decreased levels of IFN-α production, which is also associated with an increased risk of opportunistic infections.2,4 Given the central role played by dendritic cells in linking innate and adaptive immunity, abnormalities in pDC and mDC number and function may play a role in the deficient pathogen-specific response seen in HIV-1 disease. Activation of these innate effector cells could contribute to stronger innate and HIV-1-specific adaptive immune responses. One way to modulate the immune response to HIV-1 would be through pathogen associated molecular patterns (PAMPs).
Foreign pathogens are detected by innate immune cells using germ-line encoded pattern recognition receptors, also called toll-like receptors (TLRs). To date, 10 TLRs have been identified in humans that recognize PAMPs expressed in broad classes of pathogens. Viral and bacterial DNA with unmethylated CpG motifs and oligodeoxynucleotides with unmethylated CpG dinucleotides (CpG-ODNs) stimulate immune cells that express TLR9. In humans, these include B cells and pDC.5–7 Cellular uptake and endosomal maturation is a prerequisite for CpG-ODN induced TLR9 activation which triggers cell stimulation through the nuclear factor (NF)-κB and mitogen-activated protein (MAP) kinase activation pathways8 resulting in the production of pro-inflammatory cytokines and the activation and maturation of antigen presenting cells.9 PAMPs are generally absent from host tissues or inaccessible to TLRs; as a result, they can activate innate immunity when an individual is exposed to viruses or bacteria without stimulating an autoimmune response.10,11
Synthetic CpG-ODNs mimic oligonucleotide sequences common in bacterial and viral DNA, and are potent TLR9 agonists that induce pDC-mediated interferon-α (IFN-α) production12 and promote direct antiviral effects and adaptive cellular antiviral immune responses.13–15 Three distinct classes of CpG-ODNs have been identified based on structural differences. They are designated class A, B, and C CpG-ODN.16–19 In HIV-1– individuals, class A CpG-ODN stimulates pDC to produce high quantities of IFN-α, induces antigen-presenting cell maturation (APC), and indirectly (through IFN-α) activates natural killer (NK) cells.19–21 Class B CpG-ODN activates B cells and NK cells but stimulate little IFN-α secretion.19,20,22 Class C CpG-ODN combines the properties of both class A and B CpG-ODNs by being potent stimulators of pDC IFN-α production, APC activation and maturation, indirect NK cell activation, and direct B-cell stimulation.7,17
Different types of TLRs are expressed on APC including pDC, mDC, B-lymphocytes, monocytes and macrophages. Specific ligands for these receptors mediate a range of functions in these cells.23–28 TLR9, the TLR being evaluated in this study, is expressed in pDC and B cells.5,6,29,30 The goals of these studies were to determine the frequencies and activation status of the cells that participate in the innate immune response in HIV-1+ individuals and to characterize their response to class A, B and C CpG-ODNs with the hope that these TLR9 agonists could be used in the future to initiate both innate and acquired immunity in HIV-1 infected individuals.
Materials and methods
Study population
Fifty-two HIV-1+ individuals were recruited from the Mark Weiss Memorial Infectious Diseases Clinic of Rush University Medical Center. Twenty-five uninfected individuals were recruited from Rush University Medical Center. All of these individuals were used for studies of pDC, mDC, iNKT, and NK cell populations. Additionally, PBMC from a subset of 10 HIV-1+ and 10 HIV-1– individuals were used to assess innate immune cell responses to CpG-ODN. Because these functional studies required a large volume of blood, all but one of the HIV+ individuals who participated was on successful antiretroviral therapy. The one treatment-naïve subject had a relatively normal CD4 cell number and a viral load of 503 copies/ml. Most had moderate CD4 cell numbers with the exception of one individual who had 125 CD4 cells/ml, but had an undetectable viral load (Table 1). Only two had high viral loads; 35 900 and 49 500 copies/ml and they had 612 and 427 CD4 cells/ml, respectively. All treated individuals were receiving a highly active antiretroviral regimen consisting of at least three agents. This study was approved by the Rush University Medical Center Institutional Review Board.
Table 1.
Demographics of CpG-ODN sub study participants
| ID | Gender | Age | Therapy months | CD4 nadir (cells/µl) | CD4 counts (cells/µl) | Viral load (copies/ml) | Therapy |
|---|---|---|---|---|---|---|---|
| 1 | Male | 43 | none | NA | 819 | 503 | tenofovir, emtricitabine, efavirenz |
| 2 | Male | 30 | 3 | 249 | 787 | 81 | zidovudine, lamivudine, nelfinavir |
| 3 | Female | 41 | 25 | 151 | 343 | 639 | zidovudine, lamivudine, efavirenz |
| 4 | Male | 36 | 36 | 389 | 612 | 35 900 | efavirenz, lopinavir/r, indinavir |
| 5 | Male | 41 | 101 | 187 | 620 | <50 | zidovudine, abacavir, lamivudine, efavirenz |
| 6 | Male | 50 | 112 | 378 | 747 | <50 | tenofovir, didanosine, lopinavir/r |
| 7 | Male | 49 | 103 | 383 | 509 | 734 | tenofovir, abacavir, nelfinavir |
| 8 | Male | 47 | 124 | 87 | 125 | <50 | tenofovir, abacavir, nelfinavir |
| 9 | Male | 46 | 132 | 255 | 427 | 49 500 | zidovudine, tenofovir, lamivudine, fosamprenavir/r |
| 10 | Male | 52 | 156 | 140 | 358 | 75 | zidovudine, tenofovir, lamivudine, fosamprenavir/r |
NA, not applicable.
Clinical data
All viral loads and CD4+ T-cell counts were done through CLIA-certified commercial laboratories, and were obtained at the same time as the samples for this study.
Antibodies and reagents
Mouse anti-human monoclonal antibodies against CD3, CD4, CD8, CD11c, CD16, CD38, CD40, CD56, CD69, CD80, CD83, CD123, HLA-DR, the CDR3 loop of the invariant α chain from the iNKT cell T-cell receptor (TCR), TCR (clone 6B11) and appropriate isotype-control antibodies which had been conjugated to either fluoroscein isothiocyanate (FITC), phycoerythrin (PE), peridinin-chlorophyll-protein (PerCP), PE-Cy5, or allophycocyanin (APC) were used for these studies (Becton Dickinson-Pharmingen, San Jose, CA). The Lin1-FITC antibody cocktail which contains anti-CD3, CD14, CD16, CD19, CD20 and CD56 was used as part of the identification of dendritic cells (Becton Dickinson-Pharmingen). Anti-Vβ11-FITC and anti-Vα24-PE were used to identify iNKT cells and were obtained from Beckman Coulter Immunotech (Miami, FL). Fcγ-receptor (FcR) blocking reagent which was used to prevent non-specific binding was obtained from Miltenyi Biotech (Auburn, CA).
Endotoxin-free class A CpG-ODN 2216 (5′-ggGGGACGATCGTCgggggG-3′), class B CpG-ODN 2006 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′), class C CpG-ODN 2395 (5′-TCGTCGTTT-TCG-GCGCGCGCCG-3′) and control CpG-ODN 2243 (5′-ggGGGAGCATGC TGGggggG-3′) CpG-ODN were provided by Coley Pharmaceutical Group, Inc. (Wellesley, MA). Class A and Control CpG-ODNs were used at a concentration of 4 µg/ml while the class B and class C CpG-ODN were used at a concentration of 1·5 µg/ml, based on previous experiments in which optimal concentrations for these CpG-ODN were determined.31
Isolation and culture of PBMC
In order to obtain sufficient numbers of cells for functional studies peripheral blood (80 ml) from each participant was collected in sodium heparin. PBMC were isolated by density gradient centrifugation using lymphocyte separation medium (BioWhittaker, Walkersville, MD). PBMC (1 × 106 cells/ml) were incubated in the presence of media, non-reactive CpG-ODN (most comparable to class A), or CpG-ODN in a volume of 6–8 ml in six-well tissue culture plates (Becton Dickinson, Franklin Lakes, NJ). A control CpG-ODN (2137) which is more comparable to classes B and C CpG-ODN contains phosphorothioate as a backbone. This was not an appropriate control for our studies because phosphorothioate also activates cells through the TLR9 receptor.32,33 Cultures were incubated in a humidified 5% CO2 environment at 37°. After 24 hr, the cultures were harvested. Cells were used immediately to assess activation marker expression by flow cytometry; supernatants were frozen at −20° for cytokine analysis.
Flow cytometric analysis
The frequency and activation status of pDC, mDC, iNKT, and CD4+ and CD8+ lymphocytes were performed on whole blood samples from HIV-1+ and HIV-1– individuals by three- or four-colour flow cytometry. Logical gating was used to identify the pDC (Lin1–/CD123+/HLA-DR+) and mDC (Lin1–/CD123+/HLA DR+), iNKT (CD3+/6B11+ or Vβ11+/Vα24+), and CD4+ or CD8+ lymphocyte populations. Cellular activation (pDC-CD86+, mDC-CD86+, iNKT-CD69+, CD4– CD38+/HLA DR+, CD8– CD38+/HLA-DR+) is expressed as a percentage of the parent population. We also evaluated the frequency of NK (CD3–/CD56+ 16+) cells in the samples. In brief, 100–150 µl aliquots of anticoagulated whole blood were incubated with the appropriate monoclonal antibodies for 20 min at room temperature. Erythrocytes were lysed with fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson). The remaining cells were washed twice with Dulbecco's phosphate-buffered saline without Ca2+ or Mg2+ (DPBS-CMF, BioWhittaker), fixed with 2% formaldehyde, and held at 4° for flow cytometric analysis.
To evaluate innate immune cell activation (pDC-CD86, pDC-CD40, pDC80, mDC-CD86, mDC-CD40, mDC-CD80, iNKT-CD69, NK-CD69, CD19-CD86) and maturation (pDC-CD83, mDC-CD83) after CpG-ODN stimulation, PBMC were harvested and washed with cold PBS without Ca2+ or Mg2+. Because incubation with synthetic TLR9 agonists can induce expression of FcR, non-specific antibody binding was routinely blocked by incubating experimental and isotype control antibodies with FcR blocking reagent for 20 min at 4°. Mean channel fluorescence values for isotype- and fluorochrome-matched controls generally ranged from 3·47 to 6·00. This was similar to values for negative cells. Cells that were positive for experimental markers had mean channel fluorescence values ranging from 10- to more than 20-fold higher.
PBMC were then washed, incubated with appropriate antibodies for 20 min, washed, fixed with 2% formaldehyde and stored at 4° for analysis. Samples were analysed on a FACS CaliburTM flow cytometer using CellQuestTM v2.1 software (BD). Logical gating was used to identify specific innate immune cell populations (pDC, iNKT, NK, B cell) and the activation or maturation markers expressed by these cells are expressed as a percentage of the parent population.
Measurement of cytokines by enzyme-linked immunosorbent assay (ELISA)
Commercial ELISA kits were used to measure the concentration of IFN-α (PBL Biomedical Laboratories, Piscataway, NJ) or IP-10 (R & D Systems, Minneapolis, MN) in cell culture supernatants following CpG-ODN stimulation of PBMC from HIV-1+ and HIV-1– individuals. ELISA assays were performed according to the manufacturer's guidelines.
Statistical analysis
Results are presented as mean ± standard deviation (SD). Groups were compared using Student's t-test. Differences were considered significant when P < 0·05.
Results
Comprehensive evaluation of PBMC subpopulations in HIV-1 infected individuals
In order to determine the impact of TLR agonists on innate immune cells of HIV-1+ individuals, we first assessed the frequency of innate immune cells that could potentially respond to class A, B and C CpG-ODNs. We evaluated the frequency of peripheral pDC, mDC, iNKT, NK, CD4+ T cells and CD8+ T cells in whole peripheral blood samples from 52 HIV-1+ and 25 HIV-1– individuals. A description of this cohort is presented in Table 2. When groups with all HIV-1+ individuals or groups of individuals who were on antiretroviral therapy (ART) were considered, both had a significantly lower proportion of pDC (∼1·9-fold), mDC (∼1·5-fold), iNKT (∼7·5-fold), NK (∼1·4-fold), and CD4+ T cells (∼1·8-fold) cells than HIV-1– controls (Table 3). These same trends were seen for treatment-naïve individuals, although the differences between HIV-1– and treatment-naïve HIV-1+ individuals were less marked and less likely to be significant, perhaps because as a group they were healthier, with a median CD4+ T-cell count of 504 cells/µl, and there were fewer treatment-naïve participants (Table 2).
Table 2.
Demographics of study participants
| HIV-1 status | HIV-1– | HIV-1+ On ART | HIV-1+ On ART | HIV-1+ |
|---|---|---|---|---|
| Treatment and status | Undetectable viral load | Detectable viral load | Untreated | |
| Number of participants | 25 | 21 | 26 | 5 |
| Male | 12 | 19 | 22 | 4 |
| Female | 13 | 2 | 4 | 1 |
| Age in years | 31 (21–48) | 48 (35–65) | 44 (28–59) | 46 (43–52) |
| Months of therapy | NA | 80 (4–124) | 84 (0·4–156) | NA |
| CD4+ T cells (cells/µl) | ND | 620 (12–1328) | 351 (10–1040) | 504 (275–819) |
| Viral load (copies/ml) | NA | <50 | 687 (64–160 300) | 22 400 (503–38 092) |
ART, antiretroviral therapy; NA, not applicable; ND, not done.
Data is expressed as median (range).
Table 3.
Percentages of cell subpopulations and activation markers in HIV-1+ individuals
| HIV-1 status | HIV-1– | HIV-1+ | HIV-1+ | HIV-1+ | HIV-1+ CD4 | HIV-1+ CD4 | HIV-1+ CD4 |
|---|---|---|---|---|---|---|---|
| Description (number) | 25 | All 52 | ART 47 | No ART 5 | low 10 | moderate 21 | high 21 |
| Cell type | |||||||
| pDC | 0·15 | 0·081 | 0·08* | 0·08 | 0·081 | 0·071 | 0·091 |
| mDC | 0·25 | 0·171 | 0·171 | 0·15 | 0·141 | 0·161 | 0·19 |
| iNKT | 0·12 | 0·021 | 0·021 | 0·10 | 0·011 | 0·021 | 0·041 |
| NK | 11·17 | 8·011 | 8·251 | 5·731 | 7·061 | 8·70 | 7·771 |
| CD4 | 41·54 | 22·631 | 23·021 | 18·971 | 10·281 | 19·851 | 31·161 |
| CD8 | 23·05 | 46·681 | 47·271 | 41·12 | 58·211 | 48·861 | 39·011 |
| Percentage activated | |||||||
| pDC/CD86+ | 0·37 | 2·641 | 2·741 | 1·91 | 3·451 | 3·09 | 1·771 |
| mDC/CD86+ | 2·30 | 20·851 | 21·701 | 13·271 | 29·811 | 19·521 | 17·701 |
| iNKT/CD69+ | 5·47 | 27·941 | 28·611 | 21·66 | 34·741 | 28·931 | 23·711 |
| CD4/CD38+, HLA-DR+ | 2·39 | 7·911 | 7·981 | 7·31 | 15·121 | 8·701 | 3·721 |
| CD8/CD38+,HLA-DR+ | 4·68 | 16·771 | 15·441 | 29·321 | 23·221 | 17·511 | 12·961 |
pDC, plasmacytoid dendritic cells; mDC, myeloid dendritic cells; NK, natural killer cells; iNKT, CD1d-restricted, TCR-invariant natural killer T cells; ART, antiretroviral therapy.
Data is expressed as means of percentages
indicates that value is significantly different from HIV-1– mean at P < 0·05.
A better measure of relative health in HIV-1 infected individuals is CD4+ T-cell number. We evaluated the proportions of peripheral pDC, mDC, iNKT, NK, CD4+ T cells and CD8+ T cells in HIV-1+ individuals who where grouped based on CD4+ T-cell numbers. Individuals with <200 CD4+ T cells/µl were considered CD4 low, those with 200–500 CD4+ T cells/µl were considered CD4 moderate and those with >500 CD4+ T cells/µl were called CD4 high. All HIV-1+ groups, even the CD4 high individuals had significantly lower percentages of pDC, iNKT and NK cells than those seen in HIV-1– individuals, suggesting that the defect in these cells occurs relatively early in HIV-1 disease. The exception to this was the percentage of mDC in CD4 high HIV-1+ individuals which approximated the percentages found in HIV-1– individuals (P = 0·11), suggesting that the reduction in mDC may occur only when there is a substantial decline in CD4+ T cells. The proportion of activated mDC, pDC, and iNKT cells in HIV-1+ individuals was consistently elevated regardless of CD4+ T-cell count (Table 2).
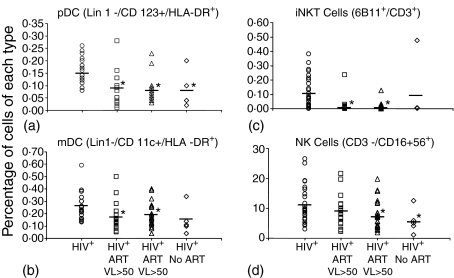
In order to assess the impact of HIV-1 viral replication on the percentages of pDC, mDC, NK and iNKT cells, we stratified ART-treated HIV-1+ subjects into groups: (1) those with detectable viraemia (HIV-1 viral loads >50 copies/ml) and (2) those with undetectable viral loads (HIV-1 viral load <50 copies/ml). The median length of ART treatment was similar between groups; 80 months for those with controlled viral loads and 84 months for those with detectable virus (Table 2). HIV-1 infected individuals who were not on ART had a mean viral load of 17 551 ± 15 563 copies/ml. HIV-1+ individuals with detectable viraemia had significantly lower frequencies of pDC, mDC, NK and iNKT cells than HIV-1– controls (Fig. 1). pDC, mDC, and iNKT frequencies in HIV-1+ individuals with undetectable virus (n = 21) were also significantly different from HIV-1– controls and were indistinguishable from those of HIV-1+ individuals with viraemia. Interestingly, the NK cell population in HIV-1+ individuals with undetectable viral loads was more comparable to the HIV-1+ controls. With the exception of NK cells, innate immune dysfunction appears to persist during successful ART.
Figure 1.
Impact of viral load on frequency of pDC, mDC, iNKT and NK cell subpopulations in HIV-1+ individuals. Cell frequency in the peripheral blood was evaluated by flow cytometry. Each point represents one individual. Percentages of the following were evaluated: (a) pDC (Lin1–/CD123+/HLA-DR+) (b) mDC (Lin1–/CD11c+/HLA-DR+) (c) iNKT (6B11+/CD3+), and (d) NK (CD3–/CD56 16+). Groups included: HIV-1– (n = 25), HIV-1+ individuals treated with antiretroviral therapy who had a viral load <50 copies/ml (HIV-1+ART VL < 50) (n = 21), HIV-1+ individuals treated with antiretroviral therapy who had a viral load >50 copies/ml (HIV-1+ART VL > 50) (n = 26), or HIV-1+ treatment-naive (HIV-1+ No ART) (n = 5). The horizontal bar corresponds to mean percentage of cells. Statistical comparisons were made using the Student's t-test. *Significance at P < 0·05.
The activation status of innate immune effectors in the absence of stimulation was evaluated to determine whether HIV-1 infection itself lead to activation of these cells. Nearly all subpopulations of cells from HIV-1+ individuals evaluated had significant activation, as measured by CD86, CD69, or coexpression of CD38 and class II major histocompatibility complex (MHC, HLA-DR), when compared to the same subpopulation in HIV-1 uninfected healthy individuals. The exceptions to this were (1) the HIV-1+ treatment-naive group where the number of participants in this group was low and (2) pDC in the moderate CD4 group where the variability between individuals was high (Table 3).
Effect of CpG-ODN class A, B and C on cellular activation and maturation markers on pDC and B cells from HIV-1+ individuals
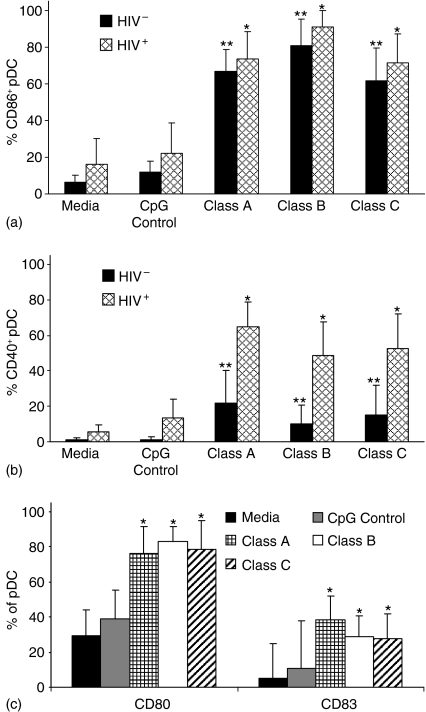
PBMC were isolated from peripheral blood obtained from HIV-1+ and HIV-1– individuals. These PBMC were then incubated for 24 hr in the presence of optimal concentrations of each class of CpG-ODN and appropriate activation and maturation markers were evaluated (Fig. 2). Expression of CD86 (B7-2) is up-regulated in pDC from both uninfected and HIV-1-infected individuals when PBMC were exposed to classes A, B, and C CpG-ODNs (Fig. 2a). All classes of CPG-ODN caused a similar increase in CD80 (B7-1) expression in HIV-1+ individuals (Fig. 2c). CD40 expression increased following stimulation with CpG-ODNs in both infected and uninfected individuals, but significantly more cells from HIV-1+ individuals expressed CD40 (Fig. 2b) after incubation with CpG-ODNs. All classes of CpG-ODNs triggered maturation of pDC in HIV-1+ individuals based on CD83 expression (Fig. 2c).
Figure 2.
Impact of HIV-1 on activation of pDC stimulated with CpG-ODN. PBMC from 10 HIV-1+ and 10 HIV-1– individuals were incubated for 24 hr with control CpG-ODN, class A, class B or class C CpG-ODN or media alone. Flow cytometry was used to determine expression of: (a) CD86, (b) CD40 and (c) CD80 and CD83 on pDC. Results are expressed as the mean ± standard deviation of the percentage of cells that express each cell surface marker. Statistical comparisons were made between the media control and the corresponding class A, B, or C CpG-ODN stimulated cultures using Student's t-test; values were considered significant if P < 0·05. *Significantly different from HIV-1+ controls; **significantly different from HIV-1– controls.
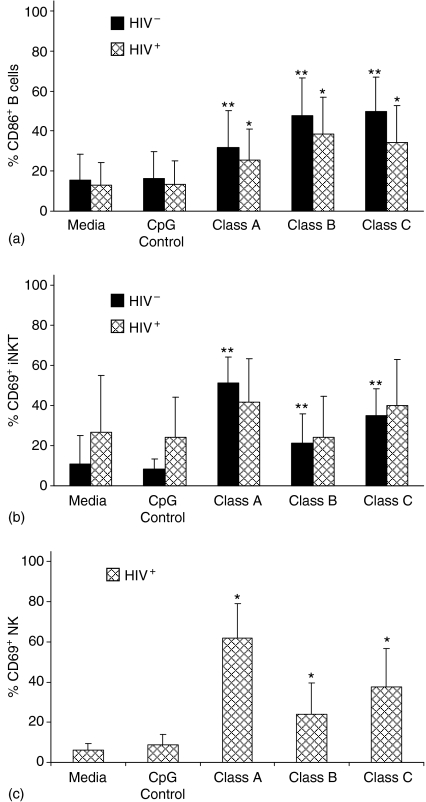
Previous studies of CpG-ODN activation in HIV-1 uninfected individuals indicates that all three classes activate B cells but classes B and C activate more than class A.7,34 Our data in HIV-1 uninfected individuals confirms this and extends it to show that similar activation was seen in B cells from HIV-1 infected individuals. B-cell activation (as measured by CD86 expression) was significant and CpG-ODN class specific activation was similar, but the magnitude of activation was less than seen in uninfected individuals in every case (Fig. 3a).
Figure 3.
Impact of HIV-1 on activation of B cells, iNKT cells and NK cells stimulated with CpG-ODN. PBMC from 10 HIV-1+ and 10 HIV-1– individuals were incubated for 24 hr with control CpG-ODN, class A, class B or class C CpG-ODN or media alone. Flow cytometry was used to determine expression of: (a) CD86 on B cells, (b) CD69 on iNKT cells and (c) CD69 on NK cells. Cell populations were defined using the cell surface markers described in Figure 1. PBMC were evaluated by flow cytometry. Results are expressed as the mean ± standard deviation of the percentage of cells expressing the indicated cellular activation marker. Statistical comparisons were made using Student's t-test; values were considered significant if P < 0·05. *Significantly different from HIV-1+ controls; **significantly different from HIV-1– controls.
These results indicate that both of the cell subpopulations that reportedly express TLR9 receptors, pDC and B cells, are responsive to TLR9 stimulation even when an individual is infected with HIV-1. pDC from HIV-1 infected individuals appear to be somewhat more responsive to TLR9 agonists than pDC from uninfected individuals, especially with respect to CD40. In contrast, B cells from HIV-1 infected individuals appear to be less responsive to TLR9 agonists than B cells from uninfected individuals (Fig. 3a).
Indirect impact of CpG-ODN stimulation of PBMC on TLR9 negative subpopulations in HIV-1+ individuals
NK, iNKT, and mDC are potent mediators of innate and adaptive immune responses through targeted killing of virus infected cells, cytokine production, and antigen presentation. Although these cell types lack TLR9 receptors, treatment of PBMC with CpG-ODNs in uninfected individuals can induce activation through pDC-induced cytokines.31Figure 3(b) compares the impact of incubating CpG-ODNs with PBMC on activation of iNKT cells in HIV-1+ and HIV-1– individuals based on CD69 expression. All classes of CPG-ODNs induced statistically significant increases in the percentages of activated cells from HIV-1– individuals and levels of activation in the presence of CPG-ODN were similar in HIV-1+ individuals. However, activation in HIV-1+ individuals was not statistically significant, probably because percentages of CD69 iNKT cells from HIV-1 infected individuals are higher than in uninfected cells in the absence of CPG-ODN stimulation (Fig. 3b). Incubation of Class A, B, and C CpG-ODN with PBMC significantly up-regulated the early cellular activation marker CD69 on NK cells compared to the controls (Fig. 3c). NK activation was significantly greater using the class A CpG-ODN compared with the class B and C CpG-ODN. NK activation was similar between the latter two classes. We also evaluated CpG-ODN induction of activation and maturation markers on mDC, but did not detect any stimulation in the HIV-1+ PBMC (data not shown).
CpG-ODN stimulation of PBMC from HIV-1+ individuals leads to production of IFN-α and IFN-γ-inducible protein 10 (IP-10 or CXCL10)
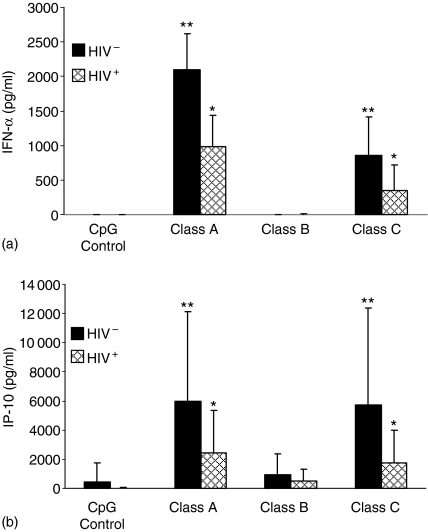
When CpG-ODN binds to TLR9 it stimulates pDC secretion of IFN-α.7,35 IFN-α then induces IP-10 production.36 If IFN-α is produced in response to CpG-ODN-TLR9 binding we would expect to also see production of IP-10.
While others have looked at the effects of class A CpG-ODN on cytokine production in HIV-1 infected and uninfected individuals37,38 we extended this to look at all three classes of these TLR9 agonists. We measured the concentrations of IFN-α in the cell culture supernatants of PBMC from HIV-1+ and HIV-1– individuals. While there was consistently more cytokine secretion by PBMC from HIV-1– individuals, cells from HIV-1+ individuals were also stimulated to produce both IFN-α and IP-10. Profiles of cytokine release stimulated in both populations of cells were similar. Class A CpG-ODN induced significantly more IFN-α than either class B or class C. IFN-α was detected in only 1 of 10 cultures from HIV-1+ PBMC incubated with class B CpG-ODN and the concentration of IFN-α in the positive culture was 20·1 pg/ml. IFN-α secretion induced by class C CpG-ODN was intermediate to class A and class B. Control CpG-ODN did not induce IFN-α production (Fig. 4a). We also measured IP-10 cytokine secretion in the cell culture supernatants of PBMC from HIV-1+ and HIV-1– individuals after CpG-ODN stimulation (Fig. 4b). Again there was consistently more cytokine secretion by PBMC from HIV-1– individuals, but PBMC from HIV-1+ individuals were able to stimulate the release of IP-10. Class A and class C CpG-ODN induced significantly higher levels of IP-10 secretion than class B CpG-ODN. Control CpG-ODN did not induce the production of IP-10.
Figure 4.
CpG-ODN induced IFN-α and IP-10 secretion by PBMC from HIV–1+ individuals. PBMC from 10 HIV-1+ and 10 HIV-1– individuals were incubated for 24 hr with control CpG-ODN, class A, class B or class C CpG-ODN or media alone. Culture supernatants were collected and concentrations of IFN-α and IP-10 were measured by ELISA. Results are expressed as mean ± standard deviation: (a) IFN-α and (b) IP-10. Statistical comparisons between groups were made using the Student's t-test. *P < 0·05 greater than the HIV+ CpG control; **P < 0·05 greater than the HIV− CpG control.
Discussion
The innate immune response to foreign pathogens mediates direct antiviral effects and leads to development of an adaptive immune response. Early in HIV-1 disease, pDC, mDC, NK, and iNKT cells are either diminished because of direct cytopathic effects of HIV-1 or are dysfunctional because of dysregulation of the cytokine network.1–3 These abnormalities correlate with the level of viraemia and the risk of clinical disease progression.2,4 Innate immune cell numbers are lower than normal in HIV-1 infected individuals in spite of effective ART.39 Treatments are needed that could either increase the number or function of these important innate cell populations. These studies show that even though fewer innate immune cells are present in HIV-1 infected individuals, these cells remain responsive to CpG-ODN stimulation. CpG-ODN treatment may help restore innate and antigen-specific acquired immune responses in HIV-1 infected individuals.
This is the first comprehensive study to evaluate innate immune cell activation and cytokine production in PBMC from HIV-1+ individuals in response to all three classes of synthetic CpG-ODN. CpG-ODNs are promising molecules that could be used to activate or maintain immune cell function.40 They can increase antigen presentation by up-regulating costimulatory (CD86, CD80), activation (CD40) and maturation (CD83) markers in pDC and B lymphocytes and by inducing the synthesis of pro-inflammatory cytokines including IFN-α. In these studies all three classes of CpG-ODN were equally able to mediate pDC activation and maturation as seen by up-regulation of CD86, CD80 and CD83. Interestingly, many more pDC from HIV-1+ individuals expressed CD40 when stimulated with any class of CpG-ODNs. Because CD40/CD40L interactions contribute to activation of acquired immunity, CpG-ODN might increase the HIV-1 specific immune response in ART-treated individuals.
pDC can produce IFN-α in healthy individuals which leads to indirect activation of NK and iNKT cells.41–43 Alternatively, they may be activated through a glucocorticoid-induced tumour necrosis factor receptor-ligand (GITRL).44 In NK cells from uninfected individuals, class A CpG-ODN stimulates IFN-γ secretion45,46 and class C induces both IFN-γ secretion and cytolytic activity against K562 target cells.7 We did not see CpG-ODN induced IFN-γ production by PBMC from HIV-1+ individuals (data not shown). Class A and C CpG-ODNs did induce IFN-α and IP-10 secretion in PBMC from HIV-1+ individuals. The amount of cytokine produced in these PBMC cultures was probably lower because fewer pDC were present in the HIV-1+ than HIV-1– individuals. Class B and C CpG-ODNs are the strongest activators of CD19+ B-lymphocytes in HIV-1+ patients. Class A CpG-ODN also induced significant B-lymphocyte activation as demonstrated by up-regulation of the costimulatory molecule CD86.
Previous studies reported impaired IFN-α production by pDC and deficient monocyte maturation when PBMC from HIV-1+ individuals were cultured with class A CpG-ODN.37,38 While our results support the observation that PBMC from HIV-1+ individuals make lower concentrations of cytokine than PBMC from uninfected individuals in response to CPG-ODNs, we also show that there are fewer pDC in these PBMC cultures that would be able to produce IFN-α in response to CPG-ODNs. CpG-ODNs were able to stimulate production of IFN-α and activate pDC, mDC and iNKT cells within the PBMC. The pattern of activation of the different classes of CpG-ODNs in HIV-1+ individuals was consistent with that seen in uninfected cell donors.
Our results are consistent with previous reports of increased dendritic cell activation/maturation,47–49 B-lymphocyte activation50–52 and expression of pro-inflammatory cytokines53–57 following CpG-ODN stimulation. We highlight the potential of CpG-ODNs, particularly class A and C CpG-ODNs, through the induction of pDC activation, maturation and IFN-α production to suppress viral replication in the setting of HIV-1 disease. HIV-1-infected CD4+ lymphocytes can induce pDC secretion of IFN-α, which, in turn, suppresses viral replication in infected T lymphocytes.58 By inducing greater levels of pDC activation and IFN-α production, CpG-ODNs may augment this natural antiviral mechanism. The relative contribution of CpG-ODNs to the control of HIV-1 replication and enhancement of HIV-1-specific immunity in an in vitro setting are currently being studied.
CpG-ODNs are used as adjuvants in vaccines, insuring the activation of both innate and acquired immune responses. A class B CpG-ODN (CpG 7909) used as an adjuvant was shown to increase the antibody response to hepatitis B vaccination in HIV-1-infected individuals.59 Our data suggests that class A CpG-ODN is likely to stimulate a stronger T helper 1 (Th1) response and class C may stimulate both humoral (Th2) and cell-mediated immunity (Th1). When vaccinating against a pathogen, the goal is not only to stimulate a response against the particular pathogen, but to stimulate the class of immune response that is most likely to protect the vaccinated individual against that pathogen. Successful vaccines have historically been protective by generating strong antibody responses that are mediated by Th2. Generation of Th2 against HIV-1 has not provided adequate protection. A combination of the right class of CpG-ODN and HIV-1 vaccine is more likely to provide a Th1 response and, hopefully, protective defence against HIV-1. A similar approach could be used in development of vaccines against other viral pathogens.
Acknowledgments
This work was supported by NIH Research Grant AI055793. Arthur M. Krieg has a potential conflict of interest since he is an employee of Coley Pharmaceutical Group who provided the CpG-ODNs used in this study. We would like to thank the physicians and nurses in the Mark Weiss Memorial Infectious Disease Clinic at Rush University Medical Center for enrolling the participants of this study.
Glossary
Abbreviations:
- ART
antiretroviral therapy
- CpG-ODN
oligodeoxynucleotides with unmethylated deoxycytidyl-deoxyguanosine dinucleotides
- IFN-α
interferon-α
- iNKT
invariant natural killer T cells
- IP-10
interferon inducible protein 10
- mDC
myeloid dendritic cells
- NK
natural killer cells
- pDC
plasmacytoid dendritic cells
- TLR9
Toll-like receptor 9
References
- 1.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–71. [PubMed] [Google Scholar]
- 2.Feldman S, Stein D, Amrute S, et al. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–10. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 3.Sandberg JK, Fast NM, Palacios EH, et al. Selective loss of innate CD4+ V{alpha}24 natural killer T Cells in human immunodeficiency virus infection. J Virol. 2002;76:7528–34. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–12. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 5.Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadowaki N, Ho S, Antonenko S, de Waal Malefyt R, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–70. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vollmer J, Jurk M, Samulowitz U, et al. CpG oligodeoxynucleotides stimulate IFN-gamma-inducible protein-10 production in human B cells. J Endotoxin Res. 2004;10:431–8. doi: 10.1179/096805104225006534. [DOI] [PubMed] [Google Scholar]
- 8.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 9.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Ann Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 11.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9:831–5. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993;178:1655–63. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum Immunol. 2002;63:1126–32. doi: 10.1016/s0198-8859(02)00751-6. [DOI] [PubMed] [Google Scholar]
- 15.O'Shea JJ, Visconti R. Type 1 IFNs and regulation of TH1 responses: enigmas both resolved and emerge. Nat Immunol. 2000;1:17–9. doi: 10.1038/76872. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, Rothenfusser S, Endres S. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633–41. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 17.Marshall J, Fearn K, Abbate C, Subramanian S, Yee P, Gregorio J, Coffman RL, Van Nest G. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J Leukoc Biol. 2003;73:781–92. doi: 10.1189/jlb.1202630. [DOI] [PubMed] [Google Scholar]
- 18.Rothenfusser S, Tuma E, Endres S, Hartmann G. Plasmacytoid dendritic cells: the key to CpG. Hum Immunol. 2002;63:1111–9. doi: 10.1016/s0198-8859(02)00749-8. [DOI] [PubMed] [Google Scholar]
- 19.Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J Immunol. 2001;166:2372–7. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164:1617–24. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 21.Sivori S, Carlomagno S, Moretta L, Moretta A. Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells. Eur J Immunol. 2006;36:961–7. doi: 10.1002/eji.200535781. [DOI] [PubMed] [Google Scholar]
- 22.Gursel M, Verthelyi D, Gursel I, Ishii KJ, Klinman DM. Differential and competitive activation of human immune cells by distinct classes of CpG oligodeoxynucleotide. J Leukoc Biol. 2002;71:813–20. [PubMed] [Google Scholar]
- 23.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 25.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 27.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 28.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–9. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 29.Dalpke AH, Schafer MKH, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–63. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- 30.Rehli M. Of mice and men: species variations of Toll-like receptor expression. Trends Immunol. 2002;23:375–8. doi: 10.1016/s1471-4906(02)02259-7. [DOI] [PubMed] [Google Scholar]
- 31.Montoya CJ, Jie HB, Al-Harthi L, et al. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J Immunol. 2006;177:1028–39. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer J, Janosch A, Laucht M, Ballas ZK, Schetter C, Krieg AM. Highly immunostimulatory CpG-free oligodeoxynucleotides for activation of human leukocytes. Antisense Nucl Acid Drug Dev. 2002;12:165–75. doi: 10.1089/108729002760220761. [DOI] [PubMed] [Google Scholar]
- 33.Vollmer J, Weeratna RD, Jurk M, et al. Oligodeoxynucleotides lacking CpG dinucleotides mediate Toll-like receptor 9 dependent T helper type 2 biased immune stimulation. Immunology. 2004;113:212–23. doi: 10.1111/j.1365-2567.2004.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 35.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 36.Blackwell SE, Krieg AM. CpG-A-induced monocyte IFN-gamma-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-alpha. J Immunol. 2003;170:4061–8. doi: 10.4049/jimmunol.170.8.4061. [DOI] [PubMed] [Google Scholar]
- 37.Jiang W, Lederman MM, Salkowitz JR, Rodriguez B, Harding CV, Sieg SF. Impaired monocyte maturation in response to CpG oligodeoxynucleotide is related to viral RNA levels in human immunodeficiency virus disease and is at least partially mediated by deficiencies in alpha/beta interferon responsiveness and production. J Virol. 2005;79:4109–19. doi: 10.1128/JVI.79.7.4109-4119.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saez R, Echaniz P, de Juan MD, Iribarren JA, Cuadrado E. HIV-infected progressors and long-term non-progressors differ in their capacity to respond to an A-class CpG oligodeoxynucleotide. AIDS. 2005;19:1924–5. doi: 10.1097/01.aids.0000191229.52385.5f. [DOI] [PubMed] [Google Scholar]
- 39.Azzoni L, Papasavvas E, Chehimi J, Kostman JR, Mounzer K, Ondercin J, Perussia B, Montaner LJ. Sustained impairment of IFN-γ secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol. 2002;168:5764–70. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 40.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 41.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–34. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 42.Krug A, French AR, Barchet W, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Marshall JD, Heeke DS, Abbate C, Yee P, Van Nest G. Induction of interferon-gamma from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-alpha and tumour necrosis factor-alpha. Immunology. 2006;117:38–46. doi: 10.1111/j.1365-2567.2005.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanabuchi S, Watanabe N, Wang YH, Ito T, Shaw J, Cao W, Qin FX, Liu YJ. Human plasmacytoid pre-dendritic cells activate NK cells through glucocorticoid-induced tumor necrosis factor receptor-ligand (GITRL) Blood. 2006;107:3617–23. doi: 10.1182/blood-2005-08-3419. [DOI] [PubMed] [Google Scholar]
- 45.Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–5. [PubMed] [Google Scholar]
- 46.Iho S, Yamamoto T, Takahashi T, Yamamoto S. Oligodeoxynucleotides containing palindrome sequences with internal 5′-CpG-3′ act directly on human NK and activated T cells to induce IFN-γ production in vitro. J Immunol. 1999;163:3642–52. [PubMed] [Google Scholar]
- 47.Schattenberg D, Schott M, Reindl G, Krueger T, Tschoepe D, Feldkamp J, Scherbaum WA, Seissler J. Response of human monocyte-derived dendritic cells to immunostimulatory DNA. Eur J Immunol. 2000;30:2824–31. doi: 10.1002/1521-4141(200010)30:10<2824::AID-IMMU2824>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Martin-Orozco E, Kobayashi H, Van Uden J, Nguyen MD, Kornbluth RS, Raz E. Enhancement of antigen-presenting cell surface molecules involved in cognate interactions by immunostimulatory DNA sequences. Int Immunol. 1999;11:1111–8. doi: 10.1093/intimm/11.7.1111. [DOI] [PubMed] [Google Scholar]
- 49.Askew D, Chu RS, Krieg AM, Harding CV. CpG DNA induces maturation of dendritic cells with distinct effects on nascent and recycling MHC-II antigen-processing mechanisms. J Immunol. 2000;165:6889–95. doi: 10.4049/jimmunol.165.12.6889. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi H, Horner AA, Takabayashi K, Nguyen MD, Huang E, Cinman N, Raz E. Immunostimulatory DNA pre-priming: a novel approach for prolonged Th1-biased immunity. Cell Immunol. 1999;198:69–75. doi: 10.1006/cimm.1999.1572. [DOI] [PubMed] [Google Scholar]
- 51.Davis HL, Weeranta R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–6. [PubMed] [Google Scholar]
- 52.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 53.Gilkeson GS, Conover J, Halpern M, Pisetsky DS, Feagin A, Klinman DM. Effects of bacterial DNA on cytokine production by (NZB/NZW) F1 mice. J Immunol. 1998;161:3890–5. [PubMed] [Google Scholar]
- 54.Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides. a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–9. [PubMed] [Google Scholar]
- 55.Lipford GB, Sparwasser T, Bauer M, Zimmermann S, Koch ES, Heeg K, Wagner H. Immunostimulatory DNA. Sequence-dependent production of potentially harmful or useful cytokines. Eur J Immunol. 1997;27:3420–6. doi: 10.1002/eji.1830271242. [DOI] [PubMed] [Google Scholar]
- 56.Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur J Immunol. 1997;27:1671–9. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 57.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of interleukin-12 and tumor necrosis factor-alpha. Cell Immunol. 1996;167:72–8. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt B, Ashlock BM, Foster H, Fujimura SH, Levy JA. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology. 2005;343:256–66. doi: 10.1016/j.virol.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 59.Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, Krieg AM, Cameron DW. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS. 2005;19:1473–9. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]