Abstract
Immunological memory is characterized by a quick and enhanced immune response after re-exposure to the same antigen. To explain the mechanism involved in generation and maintenance of immunological memory, we had earlier proposed a hypothesis involving the relay of memory by idiotypic and anti-idiotypic B cells. The peptidomimic present in the hypervariable region of anti-idiotypic antibody was hypothesized to carry forward immunological memory. In the present work, we provide evidence supporting a role for the anti-idiotypic antibody in eliciting antigen-specific B-cell and T-cell responses. Employing the idiotypic monoclonal antibody (Ab1) specific for haemagglutinin (H) protein of rinderpest virus, Ab2β was generated, which possesses an internal image of the H protein in the region between amino acids 527 and 556. We demonstrate that antigen-specific memory is perpetuated by immunization with Ab2, as shown by maintenance of antigen-specific T-cell responses upon restimulation in vitro of Ab2 immune splenocytes by antigen-presenting cells expressing H protein or pulsed with H-protein-derived peptides. We have also shown that boosting with antigen-specific anti-idiotypic B cells generates a memory response in antigen-primed mice. Evidence has been provided for the existence of an antigen-specific B-cell idiotypic network in the body that supports the perpetuation of immunological memory as proposed in the relay hypothesis.
Keywords: anti-idiotypic-B cells, idiopeptide, immunological memory, peptidomimics, relay hypothesis
Introduction
Immunological memory is an intrinsic property of the immune system. Factors governing the generation and perpetuation of immunological memory have been the subject of many investigations but these have not described any clear-cut mechanism to account for its perpetuation. The immune system of an individual can make millions of different kinds of antibodies. Each antibody can in turn be the target of other antibodies that recognize its unique molecular determinants. Idiotypes (sets of idiotopes) are determinants expressed in the variable region of the antibody molecules and T-cell receptors. Jerne's Network theory predicts that idiotypic and anti-idiotypic interactions constitute an immune network that is involved in the regulation of the immune responses.1
A mechanism has been proposed2 that explains the perpetuation of B-cell immunological memory indefinitely without requiring the presence of long-living memory cells or persisting antigen. The hypothesis combines the essential features of Burnet's clonal selection theory and Jerne's network hypothesis and considers both B-cell and T-cell memory. According to this hypothesis, the key event for generation of a memory response is the interaction of idiotypic (antigen-specific cells or Burnet cells) and anti-idiotypic cells (cells that are reactive to idiotypic determinant or Jerne cells). These idiotype and anti-idiotype B cells mutually stimulate and clonally expand, with either specific or bystander T-cell help. The anti-idiotypic cells carry a peptidomimic, which drives the memory response further by triggering idiotypic memory T cells. Because B cells can present antigen,3 they present ‘apparently foreign’ idiopeptides to T cells. The idiopeptides of de novo synthesized antibody are presented to CD8+ T cells through class I major histocompatibility complex (MHC), which recognizes the idiopeptide-presenting cells as targets and regulates their population. The recycling of immunoglobulins from the surface to the endosomal compartment of B cells leads to the presentation of idiopeptides to CD4+ T cells by class II MHC. Even if the majority of the clonally expanded cells die as a result of lack of stimulation, cytotoxic T-lymphocyte (CTL) lysis or for other reasons, the surviving cells are able to carry forward the memory. This mechanism also provides a means for affinity maturation through the idiotypic selection of somatically mutated high-affinity cells or those from the naive pool.
There is experimental evidence for the relay hypothesis descsribed above, and it has been shown that the idiotypic and anti-idiotypic B cells are generated in the same animal after immunization with antigen4 and that T cells are involved in the idiotype–anti-idiotype B-cell network.5 Anti-idiotypic B cells, which carry peptidomimic in their antigenic determinants, are thought to play a crucial role in the maintenance and regulation of B-cell and T-cell memory, as originally proposed in the relay hypothesis. A role for serum immunoglobulins in the perpetuation of immunological memory has also been proposed.6
The immune system is a functional idiotypic network and anti-idiotypic antibodies are components of the normal immune system.7–10 It has been proposed that one of the peripheral regulatory mechanisms involves recognition of internal image by the idiotypic determinants of specific antibodies or T lymphocytes, which regulate the immune response to both foreign and self-antigens.1,2,11 Anti-idiotypes have long been used as priming agents or sole immunogens, in conjunction with antigen or coupled with tetanus toxoid, interleukin-2 (IL-2) or granulocyte–macrophage colony-stimulating factor,12,13 for the production of antibodies reactive to virus, bacteria or tumour antigens. It has also been shown that part of transplacental immunoglobulin G (IgG) antibodies also contain anti-idiotypic antibodies that might prime the immune system of the offspring.14 However, the role of anti-idiotypic antibodies in the long-term perpetuation of antigen-specific immunological memory in the absence of an antigenic stimulus has not been established.
In the present work, we describe the generation and characterization of syngeneic monoclonal anti-idiotypic antibodies against a monoclonal antibody that recognizes the envelope glycoprotein haemagglutinin (H) of rinderpest virus. The main purpose of this study was the generation and analysis of the internal image of H protein in the form of an anti-idiotypic antibody, which may have the potential to elicit virus-specific immune responses and may maintain the immunological memory. Anti-idiotypic monoclonal antibody D9D8 (Ab2) was produced against a monoclonal idiotypic antibody A12A9 (Ab1, which had been generated earlier)4 for which the antigenic site on H protein lies between amino acid residues 527 and 556. We show that mimicry of the H antigen by anti-idiotypic antibody D9D8 is associated with a 12-amino-acid sequence on its heavy-chain hypervariable region. This sequence is partially homologous with the epitope on H protein, which is conserved in the H protein of viruses in the morbillivirus genus. The anti-idiotypic antibody was able to elicit antigen-specific B-cell and T-cell responses when given as soluble or cell-bound protein. In addition, the boosting of antigen-primed animals with anti-idiotypic B cells generates antigen-specific memory B-cell and T-cell responses. We demonstrate that an idiotypic–anti-idiotypic network of antigen-specific B cells, which also involves T cells, exists in the body of immunized animals. This network is capable of relaying the memory in the absence of antigen to succeeding generations of B and T cells.
Materials and methods
Animals
BALB/c mice and Sprague Dawley rats were bred and maintained in the Central Animal Facility, Indian Institute of Science, Bangalore, in accordance with the institutional guidelines. Female mice, 8–12 weeks old, were used in all experiments.
Cell lines
A12A9 (Ab1), a B-cell hybridoma cell line of BALB/c origin, was generated against the purified recombinant haemagglutinin protein of rinderpest virus.15 The murine myeloma cell line sp2/0 and, the hybridoma cell line A12A9 (Ab1) and Ab2 cell line (D9D8) generated in this work were maintained in Iscove's modified Dulbecco's medium supplemented with 10% heat inactivated fetal bovine serum (FBS) (Life Technologies, Grand Island, NY), 2 mm/l l-glutamine, 100 U/ml penicillin (Gibco-BRL, New York, NY), and 100 μg/ml streptomycin (Gibco). P815 (H-2d) murine mastocytoma cells were cultured in RPMI-1640 medium (Gibco-BRL) supplemented with 5% FBS (Life Technologies). Sf-21 cells were maintained in TC-100 medium (Gibco-BRL) supplemented with 10% FBS (Life Technologies). Baculovirus expressing full-length H protein as secretory protein16 was used to infect these cells at a multiplicity of infection of 20 and the secreted H protein was collected from the medium and purified by immunoaffinity chromatography. The purified H protein was used as a source of antigen.
Cytokines
All cytokine standards and cytokine-specific antibodies were purchased from E-bioscience (San Diego, CA). Recombinant murine IL-4 was purchased from Pharmingen (San Diego, CA).
Peptides
Peptides were designed based on the amino acid sequence homology of D9D8 and H protein. They were procured from Peptron (Daejeon, South Korea). The purity of peptides was > 90% by high-performance liquid chromatography analysis. The sequences of the peptides were YYYPFKLPI (H-1), IYSTTGRLS (H-2), GSTYYPDSV (VH), RLLIYLVSL (VL).
Immunization and hybridoma production
Three female 8-week-old BALB/c mice were injected subcutaneously with monoclonal anti-haemagglutinin hybridoma cells (secreting A12A9 antibody; 20 × 106) irradiated at 3500 rads and boosted twice with the same number of cells at a 1-week interval. Sera collected from the immune mice were tested for the presence of anti-idiotypic antibody. Hybridoma cells were generated by polyethyleneglycol-mediated fusion of immune splenocytes with Sp2/0 cells followed by two cycles of subcloning by limiting dilution.17
Detection of anti-idiotypic antibody
The anti-idiotypic antibody was detected by enzyme-linked immunosorbent assay (ELISA) employing purified polyclonal anti-H antibody (200 μg/well) raised in rabbit. The presence of anti-idiotypic antibody in culture supernatants was also monitored by FACScan flow cytometry (B&D, San Diego, CA) using monoclonal Ab1 cells bearing surface immunoglobulin and anti-mouse IgG fluorescein isothiocyanate conjugate. The clone D9D8, which consistently produced high titre antibodies as determined by ELISA and fluorescence-activated cell sorting, was chosen for further experiments and the isotype of this clone was determined using an Isotype ELISA kit (Boehringer-Mannheim, GmbH, Mannheim, Germany) following the kit protocol. IgG for D9D8 was purified from ascitic fluid by affinity chromatography on a protein A–Sepharose 4B column (Pharmacia, Uppsala, Sweden). The purity of the isolated immunoglobulin (> 99%) was determined by sodium dodecyl sulphate polyacrylamide gel electrophoresis.
Generation of anti-anti-idiotypic antibody (Ab3)
Sprague Dawley female rats (12 weeks old) were immunized by two intraperitoneal injections of purified D9D8 (200 μg) in complete Freund's adjuvant with a 1-week interval. After 2 weeks, sera were collected and booster was given with the same amount of antigen in incomplete Freund's adjuvant. The 2-week post-booster serum was checked for the presence of anti-anti-idiotypic antibody by ELISA.
Competitive ELISA
Purified Ab1 was biotinylated using ImmunoProbeTM Biotinylation Kit (Sigma, St. Louis, MO) following the manufacturer's instructions. Microtitre plates (high-binding, Costar, Cambridge, MA) were coated with 200 μg H protein, blocked with 2% bovine serum albumin, reacted with a mixture of 50 μl biotinylated Ab1 (A12A9) (1 : 1000 dilution) and 50 μl varying concentrations Ab2 (10 μg to 0·01 μg) or control anti-bodies (normal mouse IgG and purified X2A5, an isotype-matched non-specific antibody). Following a 1-hr incubation, avidin–horseradish peroxidase was added and colour was developed by addition of H2O2 and o-phenylenediamine. The per cent inhibition by Ab2 of the binding of Ab1 to antigen was calculated by taking the optical density of the well containing no competitor as 100%.
Polymerase chain reaction amplification and sequence analysis of variable region of heavy and light chains
Cytoplasmic RNA was extracted from D9D8 hybridoma cells (3 × 106 cells) by the TRIZOL method. Reverse transcription and polymerase chain reactions were performed. Variable regions of the heavy (VH) and light (VL) chains of immunoglobulin were amplified in two different reactions using IgG-specific primers (forward primer: TGAGGAGACGGTGACCGTGGTCCCTTGGCCCC; reverse primer: AGGTSMARCTGCAGSAGTCWGG) and κ-chain-specific primers (forward primer: GACATTGAGCTCACCCAGTCTCN; reverse primer: CTCGAGTTTGAGTTCCAGCTTN), respectively. Polymerase chain reaction products were subsequently cloned into pGEM-T EASY (Promega, Madison, WI) vector and were sequenced by standard DNA sequencing techniques. The amino acid sequence of VH and VL was deduced from the nucleotide sequence using a DNA-translate tool (http://www.expasy.org/tools/dna.html). The VH and VL sequences were aligned with the known sequence of H antigen using the Global Alignment Program (GAP) algorithm18 to check for homologous regions.
Generation of a P815 cell line stably expressing H protein
P815 cells were plated in 60-mm tissue culture Petri plates and transfected with 10 μg H-pFLAG-CMV-3 plasmid DNA (Sigma) (harbouring a full-length H gene subcloned from the pBluescript (PBS) vector16 into eukaryotic pFLAG cytoplasmic expression vector) in serum-free medium using lipofectamine (Life Technologies). Stable cell lines were made following the protocol mentioned in Nilsson and Nilsson.19 Positive clones (P815-H cells) were maintained in complete medium containing 400 μg/ml of G418 (Calbiochem, Lajolla, CA). Protein expression in the cells was confirmed by fluorescence microscopy, sodium dodecyl sulphate–polyacrylamide gel electrophoresis and enhanced chemiluminescence Western blotting of the cell lysates using H-protein-specific rabbit polyclonal antibody and anti-flag antibody.
Lymphocyte proliferation assay
Responder T cells (T cells from H-protein-immune and Ab2-immune mice) were isolated as described earlier20 and were plated at a density of 5 × 105 cells/well in 96-well flat-bottomed tissue culture plates in the presence of either P815-H cells, purified H protein, soluble Ab2 or P815 cells pulsed with synthetic peptides derived from the mimic regions of H protein, VH or VL. Anti-rinderpest virus N-protein-specific monoclonal antibody and purified rinderpest virus P protein were used as negative controls. Cells were cocultured for 72 hr and then pulsed for 16 hr with [3H]thymidine (1 μCi/well). Cells were harvested on glass fibre filters using a semi-automated cell harvester (Nunc, Roskilde, Denmark). [3H]Thymidine incorporation was measured in a liquid scintillation spectrophotometer and T-cell proliferation was measured as mean counts/min of triplicate wells. For recall response experiments, lymphocytes isolated from H-protein-immune and differentially boosted mice were restimulated in vitro with purified H protein (50 μg/ml) and a proliferation assay was carried out as described above.
Cytotoxic T-cell assay
Single cell suspensions from lymph nodes isolated from H-protein-immune and differentially boosted mice were seeded in complete medium at a concentration of 2 × 106 cells/well of 24-well plates. P815-H cells (1 × 106 cells/well) irradiated at 4000 rads were used as antigen-presenting cells. The cells were cocultured for 10 days in the presence of antigen-presenting cells and IL-2 (100 units/ml). Viable cells were harvested and were used as effector cells in a CTL assay. Antigen-specific CTL activity was assessed employing P815-H cells as targets at different effector : target ratios. CTL activity was measured with a cytotoxic assay kit (Roche, Mannheim, Germany) following the manufacturer's instructions.
Cytokine ELISA
Splenocytes from the immune mice were incubated with various restimulants for 72 hr as described above for cell proliferation assays. Supernatants from the culture were collected for cytokine assays. IL-2, IL-4 and interferon-γ (IFN-γ) were assayed using cytokine ELISA kits (e-Bioscience).
Intracellular cytokine staining
Lymph node cells from H-protein-immunized mice and P815-H cells were cocultured for 4 hr. Brefeldin-A was added at a final concentration of 10 μg/ml 6 hr later, cells were harvested and after permeabilization with 0·5% saponin, intracellular staining was performed using anti-mouse IFN-γ–biotin-conjugated antibody and streptavidin–phycoerythrin (Pharmingen).
Results
Anti-idiotypic antibody response in mice
There were 180 clones as a result of the fusion of Sp2/0 myeloma cells and splenocytes from BALB/c mouse immunized with Ab1 (A12A9), of which 14 clones produced antibodies that reacted with Ab1. Two clones (D9D8 and D9E6) secreting antibodies that reacted best with Ab1 both in ELISA and flow cytometry were selected for further study. Both produced antibodies of IgG heavy and κ light chain isotypes. The D9D8 hybridoma was selected for all further studies.
Anti-anti-idiotypic antibody response
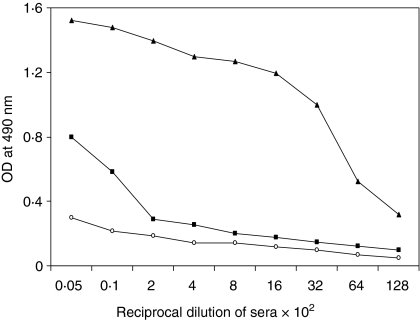
To test whether D9D8 Ab2 is an antigen mimic (Ab2β), purified Ab2 protein was used to immunize rats. The reactivity of rat serum with antigen H was measured in ELISA. The results illustrated in Fig. 1 show that Ab3 made in rats reacts with the antigen and thus is similar to Ab1. Thus, Ab2 D9D8 induces Ab3, which recognizes the original antigen H, indicating that D9D8 is Ab2β.
Figure 1.
Determination of D9D8 as Ab2β by ELISA. Plates were coated with purified recombinant H protein (200 ng/well) and incubated with rat sera (pre-immune, ○; pre-boost, ▪ and post-boost, ▴) raised against purified Ab2 at different dilutions. Donkey anti-rat–horseradish peroxidase was used as the secondary antibody. Data shown are the average optical density (OD) of triplicate wells.
Ab2 recognizes Ab1 from different animal species
D9D8 (Ab2) recognized antibodies made in different animal species against rinderpest virus infection or H protein immunization (Table 1). These results confirmed that Ab2 is indeed a functional H protein mimic.
Table 1.
Reactivity of purified Ab2 (D9D8) with heterologous sera as the source of Ab11
| Immunizing agent | Species | Titre of Ab1 in immune in sera2 |
|---|---|---|
| Recombinant Baculo H protein (soluble) | Cattle | 6·4 |
| H protein displayed on recombinant extracellular baculovirus | Cattle | > 3·2 |
| Recombinant baculo H protein (membrane-bound form) | Rabbit | 3·2 |
| Peste des petits ruminants virus | Goat3 | > 6·4 |
| Rinderpest virus | Cattle4 | > 3·2 |
Microtitre plates were coated with 200 ng/well of Ab2 and dilutions of H-specific immune sera from different sources was added.
Data shown are titre (× 103) of Ab1 recognized by Ab2 and are calculated from average optical density (OD) of triplicate wells on comparison with OD of respective dilutions of pre-immune sera. Normal rabbit, bovine and goat serum were used as controls and the OD values of controls were subtracted from the experimental values.
Hyper-immunized goats.
Convalescent cattle.
Anti-idiotypic antibody competes with H protein for binding to idiotypic antibody
Ab2 (D9D8) inhibited the binding of Ab1 to antigen in a dose-dependent manner (Fig. 2). D9D8 at a concentration of 10 μg/ml inhibited the binding of A12A9 to H protein by 76% while the control antibodies did not cause any significant inhibition over a range of concentrations. This indicated that D9D8 has the functional epitope for Ab1, which is similar to the epitope on H protein.
Figure 2.
Inhibition of Ab1 (A12A9) binding to Sec H by Ab2 (D9D8) in ELISA. Sec H protein 100 ng/well was the coating antigen. Inhibition of binding of biotinylated A12A9 to Sec H was tested in the presence of different concentrations of D9D8 (♦). Normal mouse IgG (•) and X2A5 (▴), an isotype-matched control antibody (purified) raised against rinderpest virus nucleocapsid protein, were used as controls at the same concentrations. Data represent average optical density (OD) of triplicate wells.
Sequence analysis of anti-idiotypic antibody
The sequences of VH and VL are given in Fig. 3(a). Hypervariable regions (CDRs) and framework regions (FRs) on these sequences were located using immunoglobulin BLAST of NCBI (http://www.ncbi.nlm.nih.gov/igblast/). The epitopic site on H protein for Ab1 was mapped to the region between amino acids 525 and 556 employing deletion mutants of H protein. Alignment of the Sec-H-epitope-containing sequence with that of D9D8 (Fig. 3b) using the global alignment programme (GAP) indicated a significant homology with heavy chain CDR2. Fifty-two per cent similarity and 48% identity were seen between the epitopic region and CDR2 (amino acids 32–54) of the D9D8 VH (in a 15-amino-acid stretch, nine amino acids were identical and one was similar) while between VL and the epitopic region 35% similarity and 28% identity were seen, corresponding to part of the CDR2 and part of the FR2 regions (a two-amino-acid similarity and a one-amino-acid identity).
Figure 3.
(a) Sequences of variable regions of heavy (VH) and light (VL) chains of D9D8. (b) Alignment of homology regions between VH/VL sequences and antigenic region carrying the epitope on rinderpest virus H protein showing the ‘Internal Image’.
Anti-idiotypic Ab immunization generates an antigen-specific immune response
To test the generation of an antigen-specific T-cell response elicited by anti-idiotypic antibody, T-cell proliferation assays using T cells from anti-idiotypic-antibody-immune mice with various in vitro restimulants were performed. P815-H cells, which express H protein endogenously, were used as antigen-presenting cells and proliferation of immune T cells was monitored (Fig. 4). The proliferation was equally good, in response to soluble H, although the immune animals had never been exposed to the H protein. This response is 70% of the response generated when H-immunized splenocytes were restimulated with P815-H cells (data not shown). No cross-reaction was observed when immune cells were restimulated with irrelevant antibody or irrelevant protein. As can be seen from thymidine incorporation (Fig. 5a), the Ab2 immune cell population had substantial levels of antigen-primed T cells by virtue of selective proliferation of T cells by the antigen mimic present on the anti-idiotypic B cells. The cells were also stimulated with synthetic peptides from the mimic regions on H and VH and VL of Ab2. Cells stimulated with the soluble H as well as H-derived synthetic peptide (IYSTTGRLS) exhibited greater proliferation, as shown by increased thymidine incorporation when compared to Ab2-stimulated cells. The presence of idiotypic cascade was monitored using Ab1 for in vitro restimulation, which also showed a substantial level of cell proliferation. Thus, it is evident that antigen-specific and idiotype-specific T cells are generated in vivo and coexist, probably as a result of the operation of an idiotypic–anti-idiotypic network.
Figure 4.
Proliferation of Ab2 immune splenocytes (5 × 105 cells/well) on in vitro restimulation by P815 cells stably expressing H (stippled bars) and wild-type P815 (striped bars) cells. The data shown are the mean counts/min of triplicate wells.
Figure 5.
(a) Proliferation of Ab2 immune splenocytes on in vitro restimulation with purified Ab1, H protein, H-specific synthetic peptides [H-1, H-2], VH peptide and VL peptide (10 μg/ml) derived from the homology regions. The data shown are the mean counts/min of triplicate wells. (b) IL-2 and IFN-γ production by in vitro restimulated Ab2 immune splenocytes in response to stimulation by full-length antigen and antigen-derived peptides (8 μg/ml). The results shown are representative of three independent experiments.
When cytokine patterns were evaluated, it was observed that in vitro stimulation of Ab2 immune cells with soluble Ab2 (Fig. 5b) produced high levels of IL-2 and IFN-γ. H protein and H-2 peptide (IYSTTGRLS) restimulated cells also secreted relatively higher amounts of IFN-γ and IL-2, consistent with the proliferation assay results.
Generation of B- and T-cell memory response after boosting of antigen-H-primed animals with anti-idiotypic B cells
To evaluate the role of anti-idiotypic B cells in the maintenance of B-cell and T-cell memory, mice were immunized with H protein in Freund's complete adjuvant and, after 72 days, were boosted with H protein or irradiated H-specific idiotypic B cells or anti-idiotypic B cells. The memory T-cell recall response was monitored after 1 week of booster, using the splenocytes of these mice and in vitro proliferation assay, cytotoxic assay and cytokine response. The results showed that splenocytes from anti-idiotypic B-cell-boosted mice exhibited a secondary immune response similar to those mice that were boosted with antigen (Fig. 6a), whereas the cells from animals that did not receive booster immunization showed a smaller antigen-specific proliferative response. CD8+ T cells from the lymph node cells of boosted mice, in vitro enriched in the presence of P815-H cells and IL-2, when used as effector cells, recognized the antigen-presenting cells and exhibited killing activity. Cells from H-boosted, anti-idiotype-boosted mice showed the same levels of cytotoxicity followed by cells from idiotype-boosted mice (Fig. 6b). CD8+ T cells from the lymph node cells of boosted mice secreted IFN-γ after in vitro restimulation with P815-H cells, as shown by the intracellular cytokine staining (Fig. 6c). On restimulation with H protein, the splenocytes secreted high levels of IFN-γ and considerable amount of IL-2, whereas IL-4 was not detected in significant amounts (Fig. 6d). The memory B-cell recall response was monitored by checking the presence of H-specific antibodies in the serum from immune animals. Serum from mice boosted with anti-idiotypic cells showed high titres of H-specific antibody, which was more than that in antigen-boosted mice (Table 2). Cumulatively, boosting of antigen-immunized mice with anti-idiotypic B cells produced a secondary response comparable to that upon antigen boosting. Boosting with normal B cells did not result in a secondary response in animals demonstrating the specificity of recall response.
Figure 6.
Generation of memory T-cell response by anti-idiotypic B cells. Mice were immunized with H protein (50 μg/mouse). After 72 days, a booster dose was given with H protein, irradiated H-specific idiotypic B cells (Id; 2 × 107), anti-idiotypic B cells (anti-Id; 2 × 107), normal B cells (2 × 107) or only PBS in Freund's incomplete adjuvant. After 1 week (a) in vitro proliferation of splenocytes was performed in the presence of H protein (50 μg/ml). Data shown are the mean counts/min of triplicate wells. The result shown is representative of three independent experiments (b) Draining lymph nodes were collected from the above mentioned immune mice 1 week after booster. Lymph node cells (4 × 106 cells/well) (boosted with antigen, ♦; anti-Id, ▪; Id, ▴; normal cells, ○) were restimulated in vitro with irradiated P815-H cells and IL-2 (100 units/ml) as mentioned in the Materials and methods. A CTL assay was performed using these as effector cells and P815-H cells as targets. (c) Lymph node cells (4 × 106 cells/well) were restimulated in vitro with P815 cells expressing H protein or P815 cells (5 × 106 cells/well) transfected with vector alone for 8 hr in complete medium. Brefeldin-A was added in the last 6 hr. Surface staining for CD8 and intracellular cytokine staining for IFN-γ were performed and analysed by fluorescence-activated cell sorting. The results shown are the percentages of CD8 and IFN-γ double-positive cells. (d) From the in vitro proliferated splenocyte cultures (crossed bar, H; striped bar, anti-Id antibody; dotted bar, Id antibody; closed dotted bar, normal cells; open bar, PBS-boosted) cell-free supernatants were collected and were assayed for IL-2, IL-4 and IFN-γ. Data shown are means of triplicate wells and the amount of cytokine (pg/ml) was calculated using cytokine standards.
Table 2.
Generation of memory B-cell response by anti-idiotypic B cells as detected by measuring the amount of secreted antibody1
| Antigen used for booster immunization | Antibody titre (× 103)2 |
|---|---|
| H protein | > 16 |
| Anti-idiotypic B cells | > 64 |
| Idiotypic B cells | 32 |
| Irrelevant B cells | < 2 |
| None | < 2 |
Dilutions of sera collected from mice (as mentioned in legend of Fig. 6) were reacted with antigen (200 μ g/well of H protein).
Data shown as titre are from the average optical density of triplicate wells normalized with readings for pre-immune sera.
Discussion
Anti-idiotypic antibodies with homologous sequences to specific regions of the immunogenic proteins of infectious organisms have been shown to functionally mimic these epitopes and to induce protective immune responses;21,22 they have been used in experimental systems as surrogate vaccines against several bacterial,23 viral22,24–27 and parasitic organisms21,28 and as several tumour-associated antigens.13,29 It has been shown that a murine Ab2 mimicking a peptide of hepatitis B surface antigen expressed epitopes for both B and T cells. The Ab2 appeared to activate T cells through the same mechanisms as used by the nominal antigen.30 Moreover, the Ab2 elicited cellular and humoral responses in an out-bred strain of mice and also in a strain that was not responsive to the nominal antigen. Thus, the authors have postulated that Ab2 vaccines containing specific epitopes capable of stimulating T and B cells might well induce a specific cellular and humoral immunity against the nominal antigen in a genetically diverse human population that has never been confronted with the antigen.
Operation of the idiotypic network has been postulated in the control of autoimmune disease in animal models and patients.31–35 Several studies36,37 have shown that iodiotypic networks have important roles in the control of a host's response to an antigen: regulating, enhancing or suppressing feedback mechanisms. Monoclonal anti-idiotype antibodies fused to granulocyte–macrophage colony-stimulating factor have been shown to be capable of breaking tolerance to carcinoembryonic antigen in carcinoembryonic antigen transgenic mice.12 Antigen-specific protective T-cell and B-cell immunity by an amplifying anti-idiotypic response in multiple myeloma patients has also been demonstrated.38
The role of B cells in the presentation of idiotype to T cells has been shown in a transgenic mouse model where B cells present self-peptides in the absence of conventional antigen.39 For the generation and regulation of specific immunity by anti-idiotypic and anti-idiotype-derived peptidomimics, we postulate four possible mutually non-exclusive pathways.
Antigen-specific idiotypic B cells, as well as their complementary anti-idiotypic B cells (carrying immunoglobulins on their surface that are specifically reactive against the idiotypic determinant on the antigen-specific B cells) can undergo terminal differentiation into plasma cells upon recognition and contact with the idiotypic determinants of the B-cell membrane-bound immunoglobulin.40 Regulation of these cells is brought about by the engagement of their B-cell antigen receptor, leading to proliferation, differentiation and/or programmed cell death.41,42
Anti-idiotypic B cells can present peptide mimics of antigen to T helper and T cytotoxic cells thus priming them in the absence of antigen. Cytotoxic T cells complementary to the idiopeptide may recognize the cognate antigen or the idiopeptides derived from the antigen mimic or the anti-idiotypic immunoglobulin and kill the B cells presenting such idiopeptides.43–46
Receptor-mediated endocytosis followed by endosomal cleavage of the idiotype–anti-idiotype complex can take place and peptides generated in the process can then be loaded onto MHC class II molecules and presented to CD4+ T cells, switching on the T-cell and B-cell interactive idiotypic cascade.
Apart from these, the idiopeptides can also be processed and presented in vivo by dendritic cells and by follicular dendritic cells in an MHC-restricted manner.6
To provide experimental evidence that supports the role played by the anti-idiotypic B cells in the generation of antigen-specific B-cell and T-cell memory responses and the regulation of B-cell homeostasis, it was imperative to study a single B-cell clone that was specific for a well-defined antigen. However, the isolation of such B-cell populations is difficult because of the presence of small numbers of idiotypic and anti-idiotypic B cells, which limits their enrichment from the total cell population. The second alternative, which was ruled out, was the use of antigen-primed primary B-cell clones maintained in vitro, which cease to proliferate after a finite number of divisions in tissue culture. Use of Epstein–Barr-virus-transformed B-cell cultures was avoided because such cell populations are expected to present viral antigens that shift the experimental set-up away from natural conditions. We have therefore, used anti-idiotypic B-cell hybridoma as the surrogate B cells in this study.
In the present work, we have shown that on adoptive transfer of anti-idiotypic B cells carrying peptidomimics, antigen-specific B-cell and T-cell memory can be perpetuated. We have shown that anti-anti-idiotypic CD4+ and CD8+ T cells are generated in the body after the transfer of monoclonal anti-idiotypic B cells in syngeneic mice. We observed that when Ab1 was introduced as a restimulant, significant amounts of cell proliferation occurred. This could be the result either of the anti-idiotypic cells used for primary immunization persisting in the body at the time of detection of anti-anti-idiotypic T and B cells, or of the generation of anti-anti-anti-idiotypic cells, which is the next stage in the cascade. The results show that idiotypic and anti-idiotypic T and B cells can coexist in the body and may form a dynamic system as a B-cell and T-cell idiotypic network. The anti-idiotype-specific CD4+ and CD8+ T cells did proliferate relatively less efficiently against the synthetic peptides derived from anti-idiotypic antibody sequences, which is expected because the fragments of anti-idiotypic antibody may contain many fewer processed and presented epitopes compared to H protein or full-length anti-idiotypic antibody.
Furthermore, boosting with anti-idiotypic B cells generated antigen-specific memory T-cell and B-cell immune responses. This could be operative through direct B-cell–B-cell interactions or through presentation of idiopeptides by phagocytic antigen-presenting cells after phagocytosis of idiotypic T and B cells. The anti-idiotypic B and T cells generated after immunization with antigen are directed against antigen-reactive T cells, implying that the presence of an idiotypic B-cell–anti-idiotypic B-cell cascade is natural to the system after infection or vaccination.
Significant levels of IFN-γ were secreted by Ab2-primed T cells upon in vitro restimulation with H, H-derived peptides or Ab2, although IL-4 was not detected, suggesting that CD4+ cells induced by H were probably T helper type 1. Traditionally CD4+ T cells function as helper cells for antibody production. Therefore D9D8 immunization/vaccination may prime T helper type 1 cells, which in turn may induce cytotoxic T-cell proliferation by secretion of cytokines such as IFN-γ.
T-cell epitopes derived from the naturally processed self-peptides are the peptides most abundantly presented by MHC,47,48 more than the peptides from pathogens. The anti-idiotypic antibodies, being self-generated, thus have more chance to act as elicitors of effective antigen-specific T-cell responses. The observed cytotoxic and lymphoproliferative responses indicate that anti-idiotypic B cells (Jerne cells) stimulate both T helper and T cytotoxic cells by virtue of their ability to present recycled or regurgitated peptidomimics of antigen to T helper cells through class II MHC and de novo synthesized peptidomimics of antigens to CTLs through class I MHC. Thus, the T-cell memory response can be perpetuated by anti-idiotypic Jerne B cells and these findings lend support to the earlier proposed relay hypothesis for the perpetuation of immunological memory.2 The results provide a mechanism for the maintenance of both idiotypic and anti-idiotypic B cells, which are generated in a cyclic fashion as has been proposed earlier.2 This also means that B-cell and T-cell memory of longer duration could arise as the idiotypic cascade is triggered. Based on the structural information and on the recent study of altered peptide ligands,49,50 we propose that peptidomimics in the CDRs of anti-idiotypic B cells, which may not be completely homologous to the original antigen peptide but which carry the structural complementarities,51 will trigger idiotypic antigen-specific T cells.
In summary, the antibody repertoire contains the antigenic universe with an innate ability to react with the B and T cells of the self. The anti-idiotypic antibody functions as a surrogate antigen and idiotypic selection ensures that relevant B-cell clones are always maintained. The peptidomimics derived as a result of the degradation of anti-idiotypic antibody bring about idiotypic selection and activation of cognate CD4+ and CD8+ T cells, so an elevated population of activated T cells, the memory T cells, is always maintained. Thus the idiotypic selection of B cells and T cells containing the already randomly rearranged and mutated antibody and T-cell receptor genes ensures a specific memory response. The duration of the memory is dependent on the strength of the interaction of idiotypic and anti-idiotypic B-cell receptors and on the effective presentation of idiopeptides (peptidomimics) to cognate T cells.
Acknowledgments
This research was supported in part by the Department of Science and Technology, Government of India. The infrastructural support provided by the Department of Biotechnology, Government of India, under support of the Basic Biology of Microbial Pathogens programme, is gratefully acknowledged.
Abbreviations
- Ab
antibody
- APC
antigen-presenting cell
- APL
altered peptide ligand
- BCR
B-cell receptor
- CDR
complementarity-determining region
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- H protein
haemagglutinin protein
- Id
idiotype
- IFN-γ
interferon-γ
- Ig
immunoglobulin
- IL
interleukin
- MHC
major histocompatibility complex
- OD
optical density
- VH/VL
variable region of heavy/light chains of immunoglobulin
References
- 1.Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) 1974;125C:373–89. [PubMed] [Google Scholar]
- 2.Nayak R, Mitra-Kaushik S, Shaila MS. Perpetuation of immunological memory: a relay hypothesis. Immunology. 2001;102:387–95. doi: 10.1046/j.1365-2567.2001.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottomly K, Janeway CA., Jr Antigen presentation by B cells. Nature. 1989;337:24. doi: 10.1038/337024a0. [DOI] [PubMed] [Google Scholar]
- 4.Mitra-Kaushik S, Shaila MS, Karande AK, Nayak R. Idiotypic–anti-idiotypic B cell interactions generated against a protective antigen of a morbillivirus in mice. Cell Immunol. 2001;209:10–18. doi: 10.1006/cimm.2001.1788. [DOI] [PubMed] [Google Scholar]
- 5.Mitra-Kaushik S, Shaila MS, Karande AK, Nayak R. Idiotype and anti-idiotype specific T cell responses on transplantation with hybridomas reactive to viral hemagglutinin. Immunol Lett. 2002;80:81–7. doi: 10.1016/s0165-2478(01)00312-1. [DOI] [PubMed] [Google Scholar]
- 6.Nayak R, Lal G, Shaila MS. Perpetuation of immunological memory: role of serum antibodies and accessory cells. Microbes Infect. 2005;7:1276–83. doi: 10.1016/j.micinf.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Urbain J, Wikler M, Franssen Collignon C. Idiotypic regulation of the immune system by the induction of antibodies against anti-idiotypic antibodies. Proc Natl Acad Sci USA. 1977;74:5126–30. doi: 10.1073/pnas.74.11.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichmann K, Rajewsky K. Induction of T and B cell immunity by anti-idiotypic antibody. Eur J Immunol. 1975;5:661–6. doi: 10.1002/eji.1830051002. [DOI] [PubMed] [Google Scholar]
- 9.Rundles CC, Feng ZK, Zhou Z, Woods KR. Relationship between naturally occurring human antibodies to casein and autologous anti-idiotypic antibodies. Implications for the network theory. J Clin Immunol. 1991;11:279–90. doi: 10.1007/BF00918186. [DOI] [PubMed] [Google Scholar]
- 10.Gilles JG, Remy JS. Healthy subjects produce both anti-factor VIII and specific anti-idiotypic antibodies. J Clin Invest. 1994;94:1496–505. doi: 10.1172/JCI117489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong J, Zang YC, Tejada-Simon MV, et al. Reactivity and regulatory properties of human anti-idiotypic antibodies induced by T cell vaccination. J Immunol. 2000;165:6858–64. doi: 10.4049/jimmunol.165.12.6858. [DOI] [PubMed] [Google Scholar]
- 12.Schwegler C, Dorn-Beineke A, Nittka S, Stocking C. Monoclonal anti-idiotype antibody 6G6.C4 fused to GM-CSF is capable of breaking tolerance to carcinoembryonic antigen (CEA) in CEA–transgenic mice. Cancer Res. 2005;65:1925–33. doi: 10.1158/0008-5472.CAN-04-3591. [DOI] [PubMed] [Google Scholar]
- 13.Wagner S, Hafner C, Allwardt D, et al. Vaccination with a human high molecular weight melanoma-associated antigen mimotope induces a humoral response inhibiting melanoma cell growth in vitro. J Immunol. 2005;174:976–82. doi: 10.4049/jimmunol.174.2.976. [DOI] [PubMed] [Google Scholar]
- 14.Hanson A, Korotkova M, Lundin S, Haversen I, Silfverdal SA, Mattsby-Baltzer I, Strandvik B, Telemo E. The transfer of immunity from mother to child. Ann N Y Acad Sci. 2003;987:199–206. doi: 10.1111/j.1749-6632.2003.tb06049.x. [DOI] [PubMed] [Google Scholar]
- 15.Mitra-Kaushik S, Shaila MS, Karande AK, Nayak R. Idiotype and antigen-specific T cell responses in mice on immunization with antigen, antibody, and anti-idiotypic antibody. Cell Immunol. 2001;209:109–19. doi: 10.1006/cimm.2001.1794. [DOI] [PubMed] [Google Scholar]
- 16.Naik S, Shaila MS. Characterization of membrane-bound and membrane anchor-less forms of hemagglutinin glycoprotein of Rinderpest virus expressed by baculovirus recombinants. Virus Genes. 1997;14:95–104. doi: 10.1023/a:1007957015953. [DOI] [PubMed] [Google Scholar]
- 17.Renukaradhya GJ, Mitra-Kaushik S, Sinnathamby G, Rajasekhar M, Shaila MS. Mapping of B-cell epitopes of hemagglutinin protein of rinderpest virus. Virology. 2002;298:214–23. doi: 10.1006/viro.2002.1465. [DOI] [PubMed] [Google Scholar]
- 18.Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–53. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson MO, Nilsson T. Stable transfection and selection of mammalian cells. In: Higgins SJ, Hames BD, editors. Protein Expression – a Practical Approach. Oxford: Manzar Khan; 2004. pp. 18–19. [Google Scholar]
- 20.Naik S, Renukaradhya GJ, Rajasekhar M, Shaila MS. Immunogenic and protective properties of haemagglutinin protein (H) of rinderpest virus expressed by a recombinant baculovirus. Vaccine. 1997;15:603–7. doi: 10.1016/s0264-410x(96)00244-7. [DOI] [PubMed] [Google Scholar]
- 21.Nisonoff A, Lamoyi E. Implications of the presence of an internal image of the antigen in anti-idiotypic antibodies: possible application to vaccine production. Clin Immunol Immunopathol. 1981;21:397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- 22.Thanavala YM, Bond A, Tedder R, Hay FC, Roitt IM. Monoclonal ‘internal image’ anti-idiotypic antibodies of hepatitis B surface antigen. Immunology. 1985;55:197–204. [PMC free article] [PubMed] [Google Scholar]
- 23.Buchwald UK, Lees A, Steinitz M, Pirofski LA. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infection Immunity. 2005;73:325–33. doi: 10.1128/IAI.73.1.325-333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osterhaus AD, Bunschoten EJ, Weijer K, Uytdehaag FG. Modulation of the immune system towards antiviral immune response using anti-idiotypic structures. In: Bona CA, editor. Biological Applications of Anti-Idiotypes. II. Boca Raton: CRC Press; 1988. pp. 13–30. [Google Scholar]
- 25.Betakova T, Vareckova E, Kostolansky F, Mucha V, Daniels RS. Monoclonal anti-idiotypic antibodies mimicking the immunodominant epitope of influenza virus haemagglutinin elicit biologically significant immune responses. J Gen Virol. 1998;79:461–70. doi: 10.1099/0022-1317-79-3-461. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe AH, Gaulton GN, McDade KK, Fields BN, Greene MI. Syngeneic monoclonal anti-idiotype can induce cellular immunity to reovirus. J Exp Med. 1984;160:1195–205. doi: 10.1084/jem.160.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaghouani H, Goldstein D, Shah H, Anderson S, Lacroix M, Dionne G, Kennedy R, Bona C. Induction of antibodies to the envelope protein of the human immunodeficiency virus by immunization with monoclonal anti-idiotypes. Proc Natl Acad Sci USA. 1991;88:5645–9. doi: 10.1073/pnas.88.13.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souza EB, Lopes JD, Almeida SR. B and T cell responses elicited by monoclonal anti-idiotypic antibody (Ab2beta) mimicking gp43 from Paracoccidioides brasiliensis. Clin Exp Immunol. 2004;137:123–8. doi: 10.1111/j.1365-2249.2004.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pervin S, Chakraborty M, Bhattacharya-Chatterjee M, Zeytin H, Foon KA, Chatterjee SK. Induction of anti-tumor immunity by an anti-idiotype antibody mimicking carcinoembryonic antigen. Cancer Res. 1997;57:728–34. [PubMed] [Google Scholar]
- 30.Pride MW, Thakur A, Thanavala Y. Mimicry of a determinant of hepatitis B surface antigen by an anti-idiotypic antibody. I. Evaluation in hepatitis B surface antigen responder and nonresponder strains. J Exp Med. 1993;177:127–34. doi: 10.1084/jem.177.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mougneau E, Hugues S, Glaichenhaus N. Antigen presentation by dendritic cells in vivo. J Exp Med. 2002;196:1013–16. doi: 10.1084/jem.20021636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451–4. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 33.Zang YC, Hong J, Rivera VM, Killian J, Zhang JZ. Human anti-idiotypic T cells induced by TCR peptide corresponding to a common CDR3 sequence motif in myelin basic protein-reactive T cells. Int Immunol. 2003;15:1073–80. doi: 10.1093/intimm/dxg105. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Vandevyver C, Stinissen P, Raus J. In vivo clonotypic regulation of human myelin basic protein reactive T cells by T cell vaccination. J Immunol. 1995;155:5868–77. [PubMed] [Google Scholar]
- 35.Mouthan L, Kaveri SV, Spalter SH, Lacroix-Desmazes S, Lefranc C, Desai R, Kazatchkine MD. Mechanisms of action of intravenous immune globulin in immune-mediated diseases. Clin Exp Immunol. 1996;104:3–9. [PubMed] [Google Scholar]
- 36.Shenk RR, Weissberger HZ, Dickler HB. Anti-idiotype stimulation of antigen-specific, antigen-independent antibody responses in vitro. II. Triggering of B lymphocytes by idiotype plus anti-idiotype in the absence of T lymphocytes. J Immunol. 1984;132:2709–14. [PubMed] [Google Scholar]
- 37.Bhattacharya-Chatterjee M, Chatterjee SK, Vasile S, Seon BK, Kohler H. Idiotype vaccines against human T cell leukemia. II. Generation and characterization of a monoclonal idiotype cascade (Ab1, Ab2, and Ab3) J Immunol. 1988;141:1398–403. [PubMed] [Google Scholar]
- 38.Bergenbrant S, Quing YI, Osterborg A, Bjorkholm M, Osby E, Mellstedt H, Lefvert AK, Holm G. Modulation of anti-idiotypic immune response by immunization with the autologous M-component protein in multiple myeloma patients. Br J Haematol. 1996;92:840–6. doi: 10.1046/j.1365-2141.1996.419959.x. [DOI] [PubMed] [Google Scholar]
- 39.Weiss S, Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci USA. 1989;86:282–6. doi: 10.1073/pnas.86.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lens SM, Den Drijver BF, Potgens AJ, Tesselaar K, Van Oers MH, Van Lier RA. Dissection of pathways leading to antigen receptor-induced and Fas/CD95-induced apoptosis in human B cells. J Immunol. 1998;160:6083–92. [PubMed] [Google Scholar]
- 41.Hagiyama H, Adachi T, Yoshida T, Nomura T, Miyasaka N, Honjo T, Tsubata T. Signaling through the antigen receptor of B lymphocytes activates a p53-independent pathway of c-Myc-induced apoptosis. Oncogene. 1999;18:4091–8. doi: 10.1038/sj.onc.1202772. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Wang HG, Srinivasula SM, Alnemri ES, Cooper NR. B cell apoptosis triggered by antigen receptor ligation proceeds via a novel caspase-dependent pathway. J Immunol. 1999;163:2483–91. [PubMed] [Google Scholar]
- 43.Ghosh SK, Chakrabarti D. Immunoregulation by processed immunoglobulin on B-cells. Indian J Biochem Biophys. 1993;30:414–21. [PubMed] [Google Scholar]
- 44.Chakrabarti D, Ghosh SK. Induction of syngeneic cytotoxic T lymphocytes against a B cell tumor. III. MHC class I-restricted CTL recognizes the processed form(s) of idiotype. Cell Immunol. 1992;144:455–64. doi: 10.1016/0008-8749(92)90259-r. [DOI] [PubMed] [Google Scholar]
- 45.Chakrabarti D, Ghosh SK. Induction of syngeneic cytotoxic T lymphocytes against a B cell tumor. II. Characterization of anti-idiotypic CTL lines and clones. Cell Immunol. 1992;144:443–54. doi: 10.1016/0008-8749(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 46.Chakrabarti D, Ghosh SK. Induction of syngeneic cytotoxic T lymphocytes against a B cell tumor. I. Role of idiotypic immunoglobulin. Cell Immunol. 1992;142:54–66. doi: 10.1016/0008-8749(92)90268-t. [DOI] [PubMed] [Google Scholar]
- 47.Christinck ER, Luscher MA, Barber BH, Williams DB. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature. 1991;352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 48.Crotzer VL, Christian RE, Brooks JM, et al. Immunodominance among EBV-derived epitopes restricted by HLA-B27 does not correlate with epitope abundance in EBV-transformed B-lymphoblastoid cell lines. J Immunol. 2000;164:6120–9. doi: 10.4049/jimmunol.164.12.6120. [DOI] [PubMed] [Google Scholar]
- 49.De Haan EC, Moret EE, Wagenaar-Hilbers JP, Liskamp RM, Wauben MH. Possibilities and limitations the rational design of modified peptides for T cell mediated immunotherapy. Mol Immunol. 2005;42:365–73. doi: 10.1016/j.molimm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 50.De Haan EC, Wagenaar-Hilbers JP, Liskamp RM, Moret EE, Wauben MH. Limited plasticity in T cell recognition of modified T cell receptor contact residues in MHC class II bound peptides. Mol Immunol. 2005;42:355–64. doi: 10.1016/j.molimm.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 51.Westall FC. Molecular mimicry or structural mimicry? Mol Immunol. 2006;43:1062–4. doi: 10.1016/j.molimm.2005.06.039. [DOI] [PubMed] [Google Scholar]