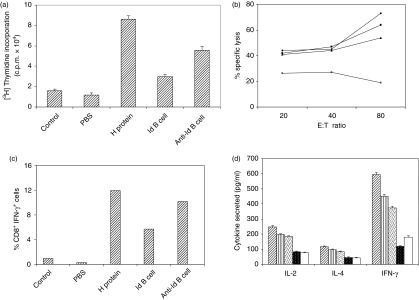
Figure 6.
Generation of memory T-cell response by anti-idiotypic B cells. Mice were immunized with H protein (50 μg/mouse). After 72 days, a booster dose was given with H protein, irradiated H-specific idiotypic B cells (Id; 2 × 107), anti-idiotypic B cells (anti-Id; 2 × 107), normal B cells (2 × 107) or only PBS in Freund's incomplete adjuvant. After 1 week (a) in vitro proliferation of splenocytes was performed in the presence of H protein (50 μg/ml). Data shown are the mean counts/min of triplicate wells. The result shown is representative of three independent experiments (b) Draining lymph nodes were collected from the above mentioned immune mice 1 week after booster. Lymph node cells (4 × 106 cells/well) (boosted with antigen, ♦; anti-Id, ▪; Id, ▴; normal cells, ○) were restimulated in vitro with irradiated P815-H cells and IL-2 (100 units/ml) as mentioned in the Materials and methods. A CTL assay was performed using these as effector cells and P815-H cells as targets. (c) Lymph node cells (4 × 106 cells/well) were restimulated in vitro with P815 cells expressing H protein or P815 cells (5 × 106 cells/well) transfected with vector alone for 8 hr in complete medium. Brefeldin-A was added in the last 6 hr. Surface staining for CD8 and intracellular cytokine staining for IFN-γ were performed and analysed by fluorescence-activated cell sorting. The results shown are the percentages of CD8 and IFN-γ double-positive cells. (d) From the in vitro proliferated splenocyte cultures (crossed bar, H; striped bar, anti-Id antibody; dotted bar, Id antibody; closed dotted bar, normal cells; open bar, PBS-boosted) cell-free supernatants were collected and were assayed for IL-2, IL-4 and IFN-γ. Data shown are means of triplicate wells and the amount of cytokine (pg/ml) was calculated using cytokine standards.