Abstract
The chemokine receptor CXCR3 is critical for the function of activated T cells. We studied the molecular mechanisms of CXCR3 signalling. The addition of CXCR3 ligands to normal human T cells expressing CXCR3 led to the tyrosine phosphorylation of multiple proteins. Addition of the same ligands to Jurkat T cells engineered to express CXCR3 induced tyrosine phosphorylation of proteins with molecular weights similar to those in normal cells. Immunoblotting with phosphotyrosine-specific antibodies identified Zeta-associated protein of 70 000 molecular weight (ZAP-70), linker for the activation of T cells (LAT), and phospholipase-C-γ1 (PLCγ1) to be among the proteins that become phosphorylated upon CXCR3 activation. ZAP-70 was phosphorylated on tyrosine 319, LAT on tyrosines 171 and 191, and PLCγ1 on tyrosine 783. The ZAP-70 inhibitor piceatannol reduced CXCR3-mediated tyrosine phosphorylation of ZAP-70, LAT, PLCγ1 and mitogen-activated protein kinase Erk and it reduced CXCL10-mediated chemotaxis of both CXCR3-transfected Jurkat T cells and normal T cells expressing CXCR3. These results are consistent with the involvement of ZAP-70 in CXCR3-mediated protein tyrosine phosphorylation and CXCR3-induced T-cell chemotaxis. Studies with the Lck-deficient Jurkat T-cell line, JCAM1.6, demonstrated that phosphorylation of ZAP-70 after CXCR3 activation is a Lck-dependent process. Finally, stimulating CXCR3-expressing Jurkat T cells and normal T cells expressing CXCR3 through the T-cell receptor attenuated CXCR3-induced tyrosine phosphorylation and CXCR3-mediated T-cell migration, indicating the occurrence of cross-talk between T-cell receptor and CXCR3-signalling pathways. These results shed light on the mechanisms of CXCR3 signalling. Such information could be useful when designing therapeutic strategies to regulate T-cell function.
Keywords: chemokines, chemotaxis, signal transduction, T cells, T-cell receptor
Introduction
Chemokine receptors are transmembrane spanning, G-protein-coupled polypeptides expressed on the surface of various cells.1–6 These receptors bind chemokines, a group of 8000 to 11 000 molecular weight (MW) proteins subdivided into four families (C, CC, CXC and CX3C) according to the position and separation of their first two N-terminal cysteine (C) residues of a four-cysteine motif.1–6 The binding of ligands to chemokine receptors contributes to multiple biological processes, including leucocyte migration, wound healing, angiogenesis, metastasis, inflammation and lymphopoiesis in the bone marrow.7–11 Recently, binding of chemokines to their cognate receptors has been shown to induce intracellular signals including protein phosphorylation, lipid hydrolysis and calcium mobilization.5,11–20
The chemokine receptor CXCR3 is expressed on different cell types, including T cells, B cells, natural killer cells, tubular cells and vascular pericytes.7,8,14,21–24 CXCR3 is scantily expressed on quiescent T cells, but its expression markedly increases in T cells upon activation, indicating that CXCR3 is important for the function of activated T cells.7,8,15,16,22,25–27 Accordingly, CXCR3 and its ligands have been linked to the recruitment of T cells to sites of inflammation.4,7,9
CXCR3 interacts with at least three chemokines: interferon-γ (IFN-γ)-inducible protein (IP-10/CXCL10), monokine induced by IFN-γ (MIG/CXCL9), and IFN-γ-inducible T-cell α-chemoattractant (ITAC/CXCL11).4,6 The level of these chemokines increases markedly during inflammation and tissue injury presumably as a result of the increases in the level of IFN-γ. Binding of these chemokines to CXCR3 has been implicated in regulating T-cell infiltration during inflammation and tissue injury.7,9,28–31
CXCR3 has been of particular interest in organ transplantation. Recent studies indicate that CXCR3 plays an important role in the immune response to allografts.9,10,32 The number of CXCR3-positive T lymphocytes increases markedly in the bronchoalveolar lavage fluid of lung allograft patients experiencing acute rejection.9 Heart allografts transplanted into allogeneic CXCR3 knockout mice, in the absence of immunosuppressive therapy, demonstrate markedly prolonged survival compared to allografts transplanted into wild-type controls.10 Similarly, transplanting hearts from CXCL10–/– donors into wild-type allogeneic strains prolonged allograft survival.10 Accordingly, antibody against CXCR3 prevented graft recruitment of activated T cells, prolonged allograft survival and reversed acute allograft rejection.10
The molecular mechanisms by which CXCR3 regulates T-cell function are not fully understood. Only a few studies have reported on CXCR3-mediated signalling.15–17 CXCR3–ligand interaction induces activation of the p44/p42 mitogen-activated protein kinases (MAPK) and Akt/phosphatidylinositol-3-kinase (PI3K) signalling pathways in T cells.17 Binding of ligands to CXCR3 induces calcium flux in T cells.15 Molecular events linking CXCR3 to MAPK, Akt, PI3K and calcium signalling events are not clear.
Phosphorylation of proteins on tyrosine residues by protein tyrosine kinases is critical for transducing the intracellular signals of various receptors, including chemokine receptors.18,33–35 To explore signalling events linked to CXCR3 activation, we examined if ligands to CXCR3 induced protein tyrosine phosphorylation in normal human T cells. Our results indicate that CXCR3 activation induced rapid tyrosine phosphorylation of several proteins. To dissect CXCR3-mediated protein tyrosine phosphorylation further, we genetically engineered Jurkat T cells to express human CXCR3. CXCR3 activation in these engineered Jurkat T cells induced rapid tyrosine phosphorylation of proteins with molecular weights similar to those in normal cells. We identified three of these proteins to be the signalling molecules Zeta-associated protein of 70 000 MW (ZAP-70), linker for the activation of T cells (LAT) and phospholipase-C-γ1 (PLCγ1).36–39 We also demonstrated that CXCR3–ligand binding induces tyrosine phosphorylation of ZAP-70 on tyrosine residue 319, of LAT on tyrosine residues 171 and 191, and of PLCγ1 on tyrosine 783. Phosphorylation of these tyrosine residues has been shown to be critical for T-cell receptor (TCR) signalling.36,37,40 Inhibition of CXCR3-mediated protein tyrosine phosphorylation and CXCR3-mediated chemotaxis through the use of the ZAP-70 inhibitor piceatannol reflects a critical role for this protein in the function of CXCR3 in T cells.
We also investigated the mechanism of ZAP-70 phosphorylation after CXCR3 activation. Phosphorylation of ZAP-70 at tyrosine residues was not attenuated in JE6.1/CXCR3 cells treated with pertussis toxin. However, in JCAM1.6 cells (Lck-deficient Jurkat T cells) engineered to express CXCR3, CXCR3 activation resulted in an increase of ZAP-70 phosphorylation only when the Src family kinase Lck was present. These results suggest that ZAP-70 phosphorylation at tyrosine residues is a Gαi-independent, Lck-dependent process.
Finally, stimulating Jurkat and normal T cells expressing CXCR3 through the TCR attenuated CXCR3-induced tyrosine phosphorylation as well as CXCR3-mediated T-cell migration, suggesting cross-talk between TCR and CXCR3 signalling pathways. These results shed further light on the molecular mechanism of CXCR3 signalling in T cells.
Materials and methods
Reagents and materials
Culture media and supplements were purchased from Fisher Scientific (Hampton, NH). Purified antibody to phospho-LAT (pY171 and pY191), phospho-ZAP-70 (pY391), phospho-p44/p42 MAPK Erk (pT202/pY204), ZAP-70, phospho-PLCγ1 (pY783), p44/p42 Erk, and PLCγ1 were obtained from Cell Signaling Technology Inc. (Beverly, MA). Antibody to LAT was purchased from Upstate Biotechnology (Lake Placid, NY). The phosphotyrosine antibody was supplied by Transduction Laboratory (San Diego, CA). Monoclonal anti-human CD3 was from Ancell Corporation (Bayport, MN). Phycoerytherin (PE)-conjugated anti-human CD3 antibody, fluorescein isothiocyanate (FITC)-conjugated anti-human TCR-αβ antibody, and cychrome and PE-conjugated anti-human CXCR3 antibody and isotype controls were purchased from BD Biosciences (San Diego, CA). Recombinant human CXCL9 and CXCL10 were obtained from R & D Systems, Inc. (Minneapolis, MN). Phytohaemagglutinin-L (PHA-L) was from Roche Applied Science (Indianapolis, IN) and recombinant human interleukin-2 (IL-2) was from Biosource (Camarillo, CA). Piceatannol, pertussis toxin and recombinant human CXCL11 were from Sigma (St Louis, MO). JCAM1.6 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Lck cDNA in pCMV6-XL4 (TC116770) was supplied by Origene (Rockville, MD). All enzymes for cloning were from NEB (Ipswich, MA) and the 10% Tris–glycine gels were from Invitrogen (Carlsbad, CA). Chemiluminescent substrates were obtained from KPL (Gaithersburg, MD) and Western blot stripping buffer was from Pierce Biotechnology (Rockville, IL). Polyvinylidene difluoride (PVDF) membranes and all other electrophoresis reagents were from Sigma and Fisher Scientific.
Cells and cell culture
Normal human T cells were isolated from the blood of healthy donors as described previously.41 Briefly, peripheral blood was collected in heparin and mononuclear cells were isolated through Ficoll–Hypaque density gradient centrifugation. The lymphocyte-rich layer was removed and cells were washed twice in culture medium (RPMI-1640 with 10% human AB serum, 2 mm glutamine, 50 μg/ml penicillin, 50 μg/ml streptomycin and 0·05 μg/ml amphotericin B), suspended in the same media, and cultured at 37° in 5% CO2. The Jurkat T-cell line JE6.1/CXCR3 (JE6.1 Jurkat T cells stably transfected with the chemokine receptor CXCR3) was cultured at 37° in 5% CO2 in RPMI-1640 with 20% fetal calf serum (FCS), 2 mm glutamine, 50 μg/ml penicillin, 50 μg/ml streptomycin, and 0·05 μg/ml amphotericin B. All JCAM1.6-derived cell lines were cultured according to ATCC guidelines.
Activation of primary T cells for the expression of CXCR3
For biochemical studies, lymphocytes prepared as above were suspended in culture media with PHA-L (500 ng/ml) at 2 × 106 cells/ml for 48 hr at 37°. After incubation, cells were removed from culture and resuspended at the same density in culture media supplemented with recombinant human IL-2 (50 ng/ml). Culture media were changed and IL-2 was re-supplemented every 2 days. T cells in culture were analysed by fluorescence activated cell sorting (FACS) analysis for TCR expression with FITC-conjugated anti-TCR-αβ and for CXCR3 expression with PE-conjugated anti-hCXCR3 antibody. Cells in culture for 10 days were composed of 80–90% T cells and these T cells expressed CXCR3 at high levels. These cells were used to study CXCR3 signalling as described below. For chemotaxis studies, lymphocytes were prepared as above, but were stimulated with a staphylococcal enterotoxin cocktail (SEA, SEB, SEC, SED, and SEE) at 500 pg/ml supplemented with 500 IU/ml IL-2 for 72 hr. Cells were then kept in culture for 19 days before their use in chemotaxis experiments.
Stable expression of CXCR3 in Jurkat T cells and JCAM1.6 cells
Five million Jurkat JE6.1 cells were resuspended in 0·8 ml growth medium (RPMI-1640 without HEPES) and electroporated at 260 V, 960 μF in a Gene Pulser (Bio-Rad, Hercules, CA) with 20 μg pBJneo/human CXCR3.26,42 After electroporation, cells were placed in growth medium for 48 hr, then changed to growth medium containing 1 mg/ml G418 to select resistant cells. Single cell clones were isolated by dilution cloning into round-bottom, 96-well plates (Costar, Cambridge, MA). Positive clones were identified by staining with anti-CXCR3 antibody [monoclonal antibody (mAb) clone IC6, PE-labelled; BD Biosciences] and analysed by flow cytometry. The CXCR3 clones that were chosen for future use showed a single population of CXCR3-positive cells at moderate cell density. Receptor expression was quantified using QuantiBRITE beads (BD Biosciences). JCAM1.6 cells were engineered to express CXCR3 and Lck as follows. CXCR3 DNA from pBJneo was cloned into the retroviral vector pCMMP-IRES-GFP. Lck cDNA from pCMV6-XL4 was cloned into the retroviral vector pCMMP-IRES-Puro. Retrovirus was produced as described previously.43 JCAM1.6 cells were then infected with pCMMP-CXCR3-IRES-GFP or control retrovirus and sorted based on high GFP expression. The top 20% of the green cells as measured by FACS was collected. These cells were then infected with pCMMP-Lck-IRES-Puro or control retrovirus and cells were selected in 1 μg/ml Puromycin. Lck expression and the expression of CXCR3 were confirmed by Western blotting and FACS, respectively. The three engineered cell lines derived from JCAM1.6 cells were as follows: JCAM1.6 CXCR3–/Lck–, JCAM1.6 CXCR3+/Lck–, and JCAM1.6 CXCR3+/Lck+ cells.
Stimulation of T cells through CXCR3
Ten-day-old normal human T cells were washed three times in 0·001% bovine serum albumin (BSA)/RPMI then suspended at 40 × 106 cells/ml in the same solution; 5 × 106 cells were placed in each reaction tube. JE6.1/CXCR3 cells or engineered JCAM1.6 cell lines were suspended at 80 × 106 cells/ml and 20 × 106 cells were placed in each reaction tube. Normal T cells or engineered Jurkat cells were placed on ice for 5 min, and then an equal volume of 0·001% BSA/RPMI containing CXCL9, CXCL10 or CXCL11 at the concentrations indicated was added to the cell suspensions. Cells were incubated on ice for a further 5 min. After incubation, cells were transferred to a 37° water bath. After stimulation, the cells were lysed using boiling 2×sample buffer (20% sodium dodecyl sulphate, 20% glycerol, 1·0 m Tris–HCl, 0·2 m dithiothreitol, pH 6·8). Cell lysates were used for Western blotting as described below.
For stimulation through the TCR, JE6.1/CXCR3 cells were washed three times in 0·001% BSA/RPMI and suspended at 80 × 106 cells/ml in the same solution; 20 × 106 cells were placed in each reaction tube. Cells were stimulated with anti-CD3 mAb (3 μg/ml) for 5 min in a 37° waterbath. Cells were lysed with boiling 2 × sample buffer. Tyrosine phosphorylated proteins in whole cell lysates were analysed by immunoblotting as described below.
Piceatannol and pertussis toxin studies
JE6.1/CXCR3 cells were suspended in media with 5% FCS at 80 × 106 cells/ml, and 20 × 106 were placed in each reaction tube. Cells were incubated with the indicated concentration of piceatannol for 2 hr at 37° in 5% CO2. For inhibition with pertussis toxin, JE6.1/CXCR3 cells were washed and suspended in 0·01% BSA/RPMI containing the indicated concentrations of pertussis toxin for 4 hr. After incubation, cells were washed twice with 0·001% BSA/RPMI, resuspended in the same solution, and then stimulated with CXCL10 or CXCL11 for the indicated times. After stimulation cells were lysed with 2 × sample buffer. Tyrosine phosphorylated proteins in whole cell lysates were analysed by immunoblotting as described below.
Chemotaxis studies
Treatment of JE6.1/CXCR3 cells and normal T cells with piceatannol or dimethylsulphoxide (DMSO) control before chemotaxis studies was performed as described above. For cross-talk experiments, JE6.1/CXCR3 cells and normal T cells were stimulated with anti-CD3 mAb or mouse immunoglobulin G before conducting chemotaxis studies. Chemotaxis in response to CXCR3 ligands was measured as follows. Chemotaxis plates (5·7-mm diameter, 5-μm pore size, 30-μl well; Neuro Probe Inc., Gaithersburg, MD) were prepared by treating chemotaxis filters with fibronectin (10 μg/ml). The bottom wells of chemotaxis plates were then loaded with assay buffer alone (0·5% BSA/0·5% DMSO/RPMI without phenol red) or with assay buffer containing CXCL10 or CXCL11 in the concentrations indicated. JE6.1/CXCR3 cells were prepared by washing with 0·01% BSA/Hanks' balanced salt solution without phenol red and were then resuspended in the same solution at 1 × 106 cells/ml. Cells were stained with Calcein AM (Molecular Probes Inc., Eugene, OR) for 30 min, washed and resuspended in assay buffer, then loaded on the top of the chemotaxis plate filters at 3 × 105 cells per well. Cells and plates were incubated at 37° for 1 hr. The top of the chemotaxis filter was then washed to remove non-adherent cells and plates were read in a fluorescent plate reader. Chemotactic index was calculated by dividing the fluorescence in wells containing chemoattractant by fluorescence in wells filled with assay buffer alone. Dose–response curves and statistical analyses were performed using prism software (GraphPad Software, Inc.).
TCR/CXCR3 cross-talk studies
JE6.1/CXCR3 cells were first stimulated with anti-CD3 mAb for 5 min as described above. After stimulation, the cells were washed twice in 0·001% BSA/RPMI, suspended in media with 5% FCS, and then incubated for 2 hr at 37° in 5% CO2. Following incubation, cells were again washed twice in 0·001% BSA/RPMI, resuspended in the same solution and then stimulated with CXCL10 or CXCL11. After stimulation, cells were lysed with 2 × sample buffer. Tyrosine phosphorylated proteins in whole cell lysates were analysed by immunoblotting as described below.
Gel electrophoresis, Western transfer and immunoblotting
These techniques were performed as described previously.44 Briefly, proteins in whole cell lysates were subjected to 10% Tris–glycine sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred to PVDF membranes. Free sites on the membranes were blocked by incubating the membranes for 4 hr at room temperature in 5% non-fat dried milk or 4% BSA. After blocking, the membranes were incubated with primary antibody, washed and then incubated with secondary antibody conjugated to horseradish peroxidase. Proteins bands were visualized using a chemiluminescent system. To examine for equal loading, blots were treated with stripping buffer for 45 min at 37° and washed before reblotting.
Flow cytometry/internalization studies
These techniques were performed as described previously.45 CXCR3, CD3 and TCR molecules on primary human T cells and JE6.1/CXCR3 were stained with the antibodies listed above for 30 min at 4°. For CXCR3 down-regulation analysis as described in Fig. 5, JE6.1/CXCR3 cells were first incubated with CXCL10 or CXCL11 (as described above) for the times indicated and subsequently fixed with 1% paraformaldehyde solution on ice for 5 min. Cells were washed with FACS buffer (5% FCS/PBS), resuspended in the same, and stained with FITC–anti-hCD3ε and PE–anti-hCXCR3 antibodies along with appropriate isotype controls. For CXCR3 down-regulation analysis as described in Fig. 9, JE6.1/CXCR3 cells were first stimulated with anti-CD3 mAb before being stained with fluorescent antibody. Anti-CD3-stimulated cells were washed twice in 0·001% BSA/RPMI and then resuspended in media with 5% FCS. Cells were incubated for 2 hr at 37°. Cells were centrifuged and resuspended in FACS buffer (5% FCS/PBS) and evaluated for surface expression of CXCR3 via flow cytometry.
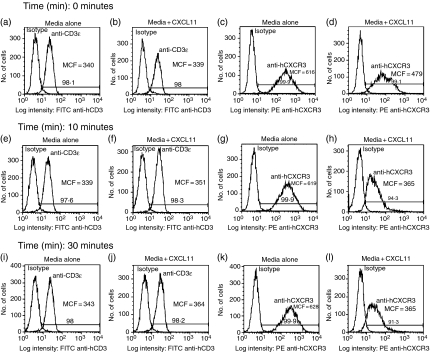
Figure 5.
CXCL11 treatment results in internalization of CXCR3 but not CD3. JE6.1/CXCR3 cells were treated with media alone (0·001% BSA/RPMI) or with media containing CXCL10 or CXCL11. After treatment for the times indicated in a 37° water bath cells were fixed on ice with 1% paraformaldehyde then washed and stained with FITC anti-CD3ε antibody, PE anti-hCXCR3 antibody, and appropriate isotype controls. After staining, CD3 and CXCR3 surface expression were measured by FACS analysis. (a, b, e, f, i and j) represent CD3 expression measured by FACS in untreated (a, e, i) and CXCL11-treated (b, f, j) cells at 0 min (a, b), 10 min (e, f) and 30 min (i. j). Similarly, (c, d, g, h, k and l) represent CXCR3 expression measured by FACS in untreated cells (c, g, k) and CXCL11-treated cells (d, h, l) at 0 min (c, d), 10 min (g, h), and 30 min (k, l). Results presented are representative of two independent experiments performed separately with CXCL10 and CXCL11. CD3 expression as measured by mean channel fluorescence (MCF) was statistically identical for all treatment groups at all time-points as evaluated by both unpaired and paired t-tests. Differences in CXCR3 expression as measured by mean channel fluorescence were statistically non-identical for treated versus untreated cells as evaluated by both unpaired and paired t-tests. These values are listed as follows. CXCL10 (treated versus untreated): 10 min MCF 568·5 ± 0·5 versus 608 ± 7·0, P < 0·05; 30 min MCF 579 ± 13·0 versus 639 ± 17·0, P < 0·05. CXCL11 (treated versus untreated): 0 min, MCF 493·5 ± 14·5 versus 635 ± 19·5, P < 0·05; 10 min, 376 ± 23·06 versus 608 ± 11·0, P = 0·01; 30 min MCF 370 ± 9·5 versus 621·5 ± 6·5, P < 0·005.
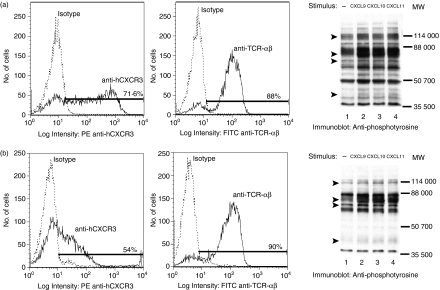
Figure 9.
Stimulation through the TCR attenuates CXCL10-induced and CXCL11-induced protein tyrosine phosphorylation. (a) JE6.1/CXCR3 cells were incubated at 37° for 5 min in 0·001% BSA/RPMI alone (lane 1) or with 0·001% BSA/RPMI containing 3 μg/ml anti-CD3 antibody (lane 2). After incubation, the cells were either lysed (a), or were washed and resuspended in 5% FCS/RPMI. The cells were then allowed to rest for 2 hr at 37° in 5% CO2. After resting, the cells were washed and incubated for 5 min on ice in 0·001% BSA/RPMI alone (b–f: lane 1) or in 0·001% BSA/RPMI containing 30 nm CXCL10 (b–d: lanes 2, 3) or 30 nm CXCL11 (e and f: lanes 2, 3). After incubation, the cells were moved to a 37° water bath and incubated for 5 min. After incubation, the cells were lysed and the proteins in the whole cell lysates were separated by SDS–PAGE, transferred to membranes, and immunoblotted with anti-phosphotyrosine mAb (b, e), with antibody specific for phosphotyrosine residue 319 in ZAP-70 (c and f, upper panels), or with the combination of antibodies specific for phosphotyrosine residues 191 and 171 in LAT (d and f, upper panels). After immunoblotting, membranes were stripped and reblotted with antibody against ZAP-70 (c and f, lower panels) or LAT (d and f, lower panels) to test for equal protein loading. Pre-αCD3 indicates cells that were prestimulated with anti-CD3 antibody.
Results
The addition of CXCR3 ligands to normal human T cells and to Jurkat T cells engineered to express CXCR3 induces protein tyrosine phosphorylation
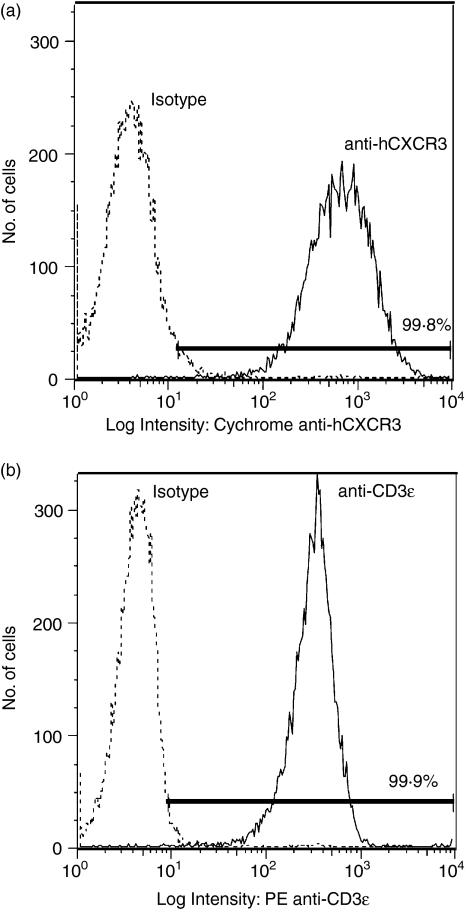
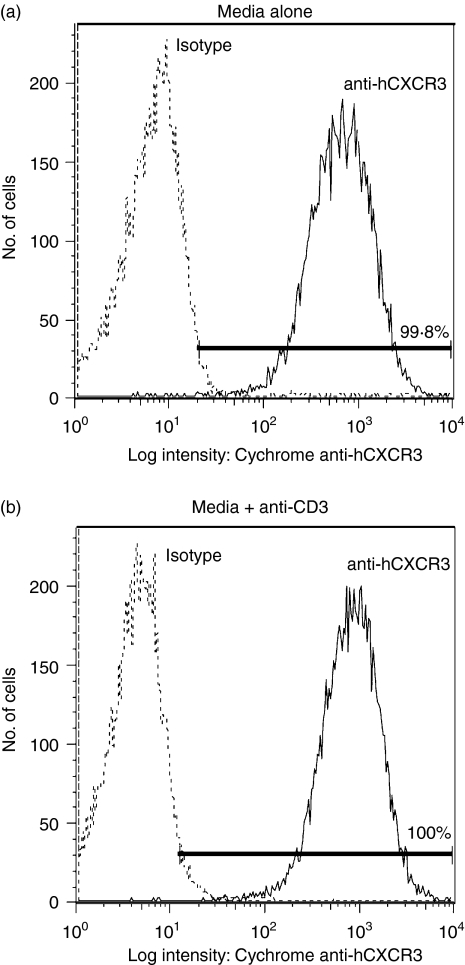
The molecular mechanisms underlying CXCR3 signalling are not clearly understood. Although CXCR3 is scantily expressed on quiescent T cells, it is strongly expressed on activated T cells.7,8,15,16,22,25–27 To induce the expression of CXCR3, T cells isolated from normal human volunteers were stimulated with PHA-L (500 ng/ml) in combination with human IL-2 (50 ng/ml) as described in the Material and methods. This approach reproducibly led to the stable expression of CXCR3 (Fig. 1). At least 90% of the cells expressed TCR (Fig. 1).
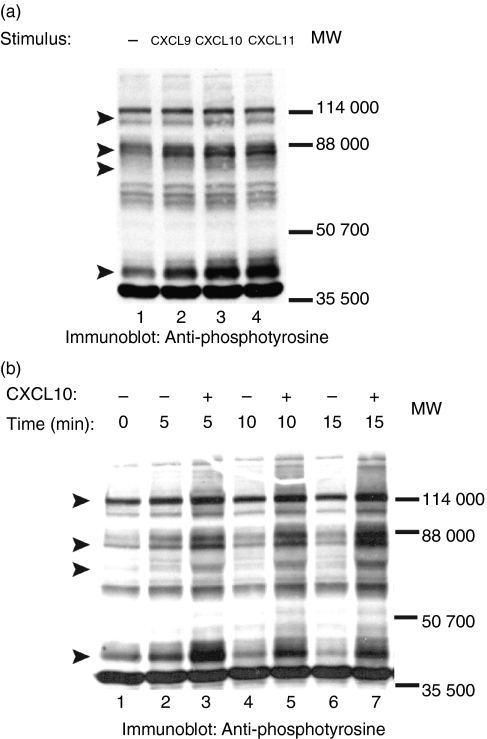
Figure 1.
CXCR3–ligand interaction in normal human T cells induces protein tyrosine phosphorylation. T cells were harvested from the blood of normal volunteers, stimulated with PHA (500 ng/ml) for 48 hr and then supplemented with IL-2 (50 ng/ml). After 10 days in culture, FACS analysis of stimulated cells was performed by labelling cells with PE-conjugated anti-human-CXCR3 and FITC-conjugated anti-TCR-αβ monoclonal antibody along with appropriate isotype controls for 30 min on ice. PI was used to exclude non-living cells from the FACS analysis. Panels represent per cent of cells positive for CXCR3 and TCR-αβ as compared to isotype controls. Purified normal human T cells expressing CXCR3 were incubated on ice for 5 min in 0·001% BSA/RPMI alone (lane 1) or in 0·001% BSA/RPMI containing 100 nm of the CXCR3 ligands CXCL9, CXCL10, or CXCL11 (lanes 2–4). After incubation cells were moved to a 37° water bath for 5 min. Proteins in whole cell lysates were separated by SDS–PAGE, transferred to membranes, and immunoblotted with anti-phosphotyrosine mAb. Arrows indicate proteins showing increased tyrosine phosphorylation after CXCR3–ligand interaction. (a) and (b) represent experiments with T cells harvested from two unique donors.
To study CXCR3-induced phosphorylation of proteins on tyrosines, normal T cells expressing CXCR3 were stimulated with the ligands, CXCL9, CXCL10 or CXCL11. As shown in Fig. 1, incubation of these cells, individually, with each ligand increased the tyrosine phosphorylation of proteins with MW 110 000, 70 000–85 000, 65 000 and 38 000. Although, these results were reproducibly seen in cells from different volunteers, the extent of CXCR3-induced phosphorylation varied (Fig. 1). The reason for the variation in CXCR3-induced phosphorylation of proteins is not clear at this time.
Having demonstrated that CXCR3 activation induces phosphorylation of proteins on tyrosines in normal human T cells, we engineered Jurkat T cells to express CXCR3 (JE6.1/CXCR3 T cells) and used these cells to dissect the mechanism of CXCR3 signalling further. We chose to generate these cells because CXCR3 is sparsely expressed on quiescent T cells, because of the marked variation in the expression of CXCR3 on activated T cells from different individuals, and because of the prolonged culture methods needed to induce the expression of CXCR3 on T cells. As shown in Fig. 2, we successfully expressed CXCR3 on Jurkat T cells (Fig. 2a). These cells also expressed CD3 robustly (Fig. 2b). Cells were routinely subjected to FACS analysis for CD3 and CXCR3 expression; both CD3 and CXCR3 expression remained abundant and stable for at least 6 months (not shown). Transfected Jurkat T-cell clones showing a uniform CXCR3 peak expression at a low cell density were selected to study CXCR3 signalling. To ensure that the experimental results generated were not unique to a single clone or simply the consequence of over-expression of CXCR3, biochemical experiments were carried out with at least two clones, a high-expressing clone (64 000 copies CXCR3 per cell) and a low-expressing clone (32 000 copies CXCR3 per cell) with similar results. Results with these two clones were also confirmed in similar experiments using the entire pool of Jurkat cells successfully transfected with CXCR3, cells which had a broad range of CXCR3 expression.
Figure 2.
JE6.1 Jurkat T cells stably transfected to express CXCR3 have high expression of both CXCR3 and CD3. JE6.1 Jurkat T cells were transfected with plasmid (PBJneo) containing human CXCR3 cDNA. Successfully transfected cells were isolated by selection in media containing 1 mg/ml G-418. After selection, FACS analysis was performed on clones to determine level of surface expression of CXCR3 (a) and CD3 (b).
Also, with regard to any possible differences in chemotaxis between our engineered cells and normal T cells expressing CXCR3, the migratory behaviour of JE6.1/CXCR3 cells and normal T cells expressing CXCR3 was compared before their use in experiments described below. The migratory behaviour of JE6.1/CXCR3 cells and normal T cells expressing CXCR3 was found to be identical, indicating that the chemotaxis of the cells engineered to express CXCR3 reflects that of normal T cells. For example, JE6.1/CXCR3 and normal T cells expressing CXCR3 had identical dose–response curves and EC50 values when exposed to either CXCL9, CXCL10 or CXCL11 in trans-well migration assays (data not shown).
To examine if CXCR3 activation in JE6.1/CXCR3 cells induced phosphorylation of proteins on tyrosines, JE6.1/CXCR3 cells were incubated with CXCL9, CXCL10 and CXCL11. CXCR3–ligand interaction induced tyrosine phosphorylation of multiple proteins in JE6.1/CXCR3 cells compared to untreated controls (Fig. 3a). These results were consistent with those seen in primary T cells. Time–course studies indicated that CXCR3-induced phosphorylation of proteins on tyrosines is rapid; it was apparent within 5 min of CXCR3–ligand interaction (Fig. 3b).
Figure 3.
CXCR3–ligand interaction induces rapid protein tyrosine phosphorylation in JE6.1/CXCR3 cells. (a) Jurkat T cells stably expressing CXCR3 (JE6.1/CXCR3) were incubated for 5 min on ice in 0·001% BSA/RPMI alone (lane 1) or in 0·001% BSA/RPMI containing 0. 3 nm CXCL9, CXCL10 or CXCL11 (lanes 2–4). After incubation, the cells were moved to a 37° water bath for 5 min. Proteins in whole cell lysates were separated by SDS–PAGE, transferred to membranes, and immunoblotted with anti-phosphotyrosine mAb. (b) Time–course study of CXCR3-induced protein tyrosine phosphorylation. Cells were stimulated for the indicated time with CXCL10 as described in (a) and protein tyrosine phosphorylation was analysed as in (a).
Treatment of JE6.1/CXCR3 cells with CXCR3 ligands induces rapid tyrosine phosphorylation of ZAP-70, LAT and PLCγ1
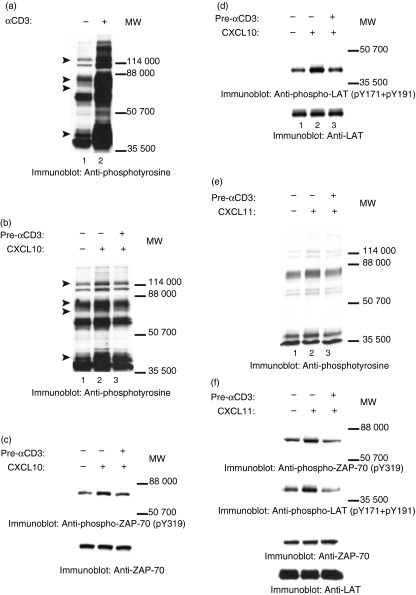
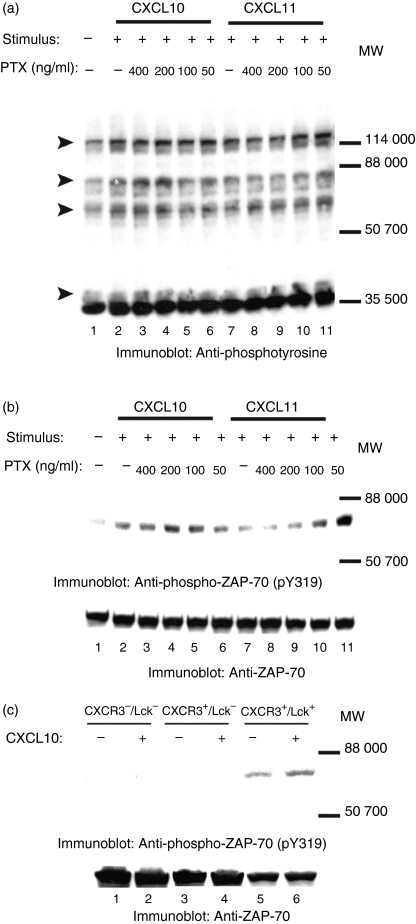
We next identified the proteins that became tyrosine-phosphorylated upon treating JE6·1/CXCR3 cells with the CXCR3 ligands CXCL9, CXCL10, and CXCL11. As shown in Fig. 3(a,b), treating cells with CXCR3 ligands led to the tyrosine phosphorylation of a 70 000 MW protein. To examine if this protein was ZAP-70, a molecule that is critical for T-cell activation and function,36 cell lysates from unstimulated and CXCR3-stimulated cells were immunoblotted with antibody that recognized phosphorylated tyrosine residue 319 (pY319) on ZAP-70. CXCR3 activation led to the tyrosine phosphorylation of Y319 (Fig. 4a, upper panel). Equal protein loading was confirmed by reblotting membranes with anti-ZAP-70 antibody (Fig. 4a, lower panel). CXCR3-induced tyrosine phosphorylation of ZAP-70 was rapid, with an increase in the phosphorylation of ZAP-70 apparent within 5 min of adding ligands to the cells.
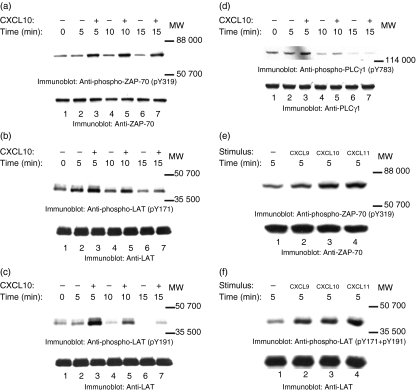
Figure 4.
CXCR3–ligand interaction induces tyrosine phosphorylation of ZAP-70, LAT, and PLCγ1. JE6.1/CXCR3 cells were incubated with 0·001% BSA/RPMI alone (a–d: lanes 1, 2, 4 and 6; e and f: lane 1) or with 0·001% BSA/RPMI containing CXCL9 (e and f: lane 2), CXCL10 (a–d: lanes 3, 5 and 7; e and f: lane 3), or CXCL11 (e and f: lane 4) for 5 min on ice. After incubation, the cells were moved to a 37° water bath for the times indicated. Proteins in whole cell lysates were separated by SDS–PAGE, transferred to membranes, and immunoblotted with antibody specific for phosphotyrosine residue 319 (pY319) in ZAP-70 (a and e, upper panels), with antibody specific for phosphotyrosine residue 171 (pY171) in LAT (b and f, upper panels), with antibody specific for phosphotyrosine residue 191 (pY191) in LAT (c and f, upper panels), or with antibody specific for phosphotyrosine residue 783 (pY783) in PLCγ1 (d, upper panel). After immunoblotting, membranes were stripped and reblotted with antibody against ZAP-70 (a and e, lower panel), LAT (panels b, c and f, lower panel), and PLCγ1 (d, lower panel) to test for equal protein loading.
Another protein of which the tyrosine phosphorylation increased after the addition of ligand had a molecular mass of ∼40 000 (Fig. 3a,b). ZAP-70 has previously been implicated in phosphorylating LAT, a ∼36 000–38 000 protein, in TCR signalling cascades.37 To examine if LAT was involved in the CXCR3 signalling, cell lysates from unstimulated and CXCR3-stimulated cells were immunoblotted with antibodies that recognize phosphorylated tyrosine residues 171 (pY171) or 191 (pY191) in LAT. CXCL10 treatment induced phosphorylation of tyrosine 171 (Fig. 4b, upper panel) and 191 (Fig. 4c, upper panel). Equal protein loading was confirmed by reblotting membranes with anti-LAT antibody (Fig. 4b,c, lower panels). As with CXCR3-induced tyrosine phosphorylation of ZAP-70, CXCR3-induced tyrosine phosphorylation of LAT was rapid; the increase in the phosphorylation of Y171 and Y191 was apparent within 5 min of CXCR3 activation. These results indicate that CXCR3 signalling pathways involve both ZAP-70 and its substrate LAT.
Another potential target for ZAP-70 is PLCγ1.40 For example, optimal TCR-induced tyrosine phosphorylation of PLCγ1 required the phosphorylation and the activation of ZAP-70.38–40 To examine whether PLCγ1 was involved in CXCR3 signalling, cell lysates from unstimulated and from CXCR3-stimulated cells were transferred to membranes and then immunoblotted with antibody that recognizes phosphorylated tyrosine residue 783 in PLCγ1 (pY783). The addition of CXCL10 to the cells induced tyrosine phosphorylation of tyrosine 783 in PLCγ1 (Fig. 4d, upper panel). Equal loading was confirmed by reblotting membranes with anti-PLCγ1 antibody (Fig. 4d, lower panel). As with CXCR3-induced tyrosine phosphorylation of ZAP-70 and LAT, CXCR3-induced tyrosine phosphorylation of PLCγ1 was rapid; the increase in the phosphorylation of Y783 was apparent within 5 min of CXCR3–ligand interaction.
To confirm that our results were related to the activation of CXCR3 in general and were not specific to treatment of cells with CXCL10, we treated cells with CXCL9 and CXCL11 as well. Similar increases in tyrosine phosphorylation of LAT and ZAP-70 were seen whether cells were treated with CXCL9, CXCL10 or CXCL11 (Fig. 4e,f). To ensure that the detected phosphorylation of proteins was the result of ligand interaction with CXCR3 and not the activation of some other surface signalling molecule such as CD3, surface expression of CD3 and CXCR3 was tested by FACS after treatment with CXCR3 ligands. Treatment with CXCR3 ligands reduced CXCR3 surface expression (indicating internalization) by up to 50% in treated cells versus controls but had no effect on CD3 expression levels (Fig. 5a–l). Furthermore, treatment of untransfected Jurkat cells, as well as of cells that did not express CXCR3 but contained the empty transfection vector, with CXCR3 ligands did not result in the phosphorylation of proteins at tyrosine residues either in general or specifically with regards to ZAP-70, LAT and PLCγ1 (data not shown).
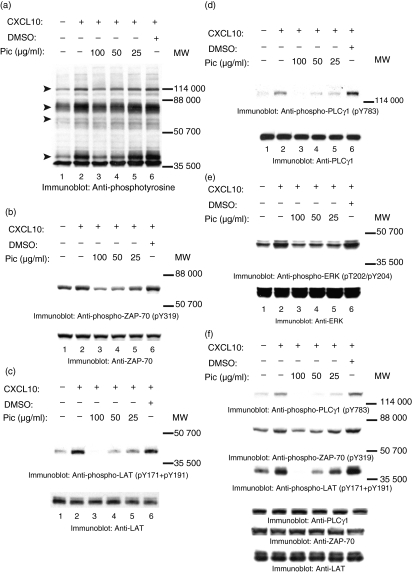
Piceatannol inhibits CXCR-mediated phosphorylation of proteins on tyrosines and CXCR3-mediated T-cell chemotaxis
To examine the role of ZAP-70 in CXCR3 signalling pathways, JE6.1/CXCR3 cells were treated with the indicated concentrations of the Syk/ZAP-70 inhibitor piceatannol.46–49 After incubating with piceatannol, the cells were stimulated with CXCL10 as described above. After incubation, the cells were lysed and cell lysates were transferred to membranes and immunoblotted with anti-phosphotyrosine antibodies. Piceatannol treatment inhibited CXCR3–CXCL10-induced phosphorylation of proteins on tyrosines in a dose-dependent manner (Fig. 6a, lanes 3–5). Piceatannol blocked CXCR3–CXCL10-induced tyrosine phosphorylation of ZAP-70 (Fig. 6b, upper panel; lanes 3–5), LAT (Fig. 6c, upper panel; lanes 3–5), and PLCγ1 (Fig. 6d, upper panel; lanes 3–5) in the JE6.1/CXCR3 cells in a dose-dependent manner. In contrast, treating the cells with the vehicle DMSO (using the volume used to dilute 100 μg piceatannol) had no effect on CXCR3-induced phosphorylation of proteins on tyrosines (Fig. 6a–d, upper panels; lane 6). Equal amounts of ZAP-70, LAT and PLCγ1 were present in each lane (Fig. 6b–d, lower panels). Piceatannol treatment did not affect cell viability; this was indicated by an assay using trypan blue exclusion (not shown). It is noteworthy that although piceatannol has been shown to block the activation of Syk and ZAP-70, Syk is not expressed in JE6.1 cells.50 Previous studies have shown that ligands to CXCR3 increase tyrosine phosphorylation of MAPK Erk.17 Interestingly, piceatannol also blocked CXCR3-induced tyrosine phosphorylation of MAPK Erk (Fig. 6e, upper panel). To confirm that our results were not specific to CXCL10, we treated cells with piceatannol before stimulating them with CXCL11. Treatment of JE6.1/CXCR3 cells with piceatannol inhibited CXCL11-induced protein tyrosine phosphorylation of LAT, ZAP-70 and PLCγ1, to the same extent as seen in experiments with CXCL10 (Fig. 6f, upper panels). These results implicate ZAP-70 as having a role in CXCR3-induced tyrosine phosphorylation of LAT, PLCγ1 and Erk.
Figure 6.
Piceatannol (Pic) inhibits CXCR3-induced protein tyrosine phosphorylation. JE6.1/CXCR3 cells were incubated for 2 hr at 37° with 5% CO2 in 5% FCS/RPMI in the absence (a–f: lanes 1 and 2) or presence of the inducted concentrations of piceatannol (Pic; a–f: lanes 3–5). Control cells were incubated in 5% FCS/RPMI containing DMSO (a–f: lane 6). After incubation, the cells were washed and incubated in 0·001% BSA/RPMI alone (a–f: lane 1) or in 0·001% BSA/RPMI containing 30 nm CXCL10 or 30 nm CXCL11 (f: lanes 2–6) for 5 min on ice. After incubation, cells were moved to a 37° water bath for 5 min. Proteins in whole cell lysates were separated by SDS–PAGE, transferred to membranes, and immunoblotted with anti-phosphotyrosine mAb (a), anti-pY319 ZAP-70 antibody (b and f, upper panels), the combination of anti-pY171 LAT antibody and anti-pY191 LAT antibody (c and f, upper panels), with anti-p783 PLCγ1 antibody (d and f, upper panels), or with anti-phospho-Erk (pT202/pY204) antibody (e, upper panel). After immunoblotting, membranes were stripped and reblotted with anti-ZAP-70 antibody (b and f, lower panels), anti-LAT antibody (c and f, lower panels), with anti-PLCγ1 (d and f, lower panels), or with anti-Erk (e, lower panel).
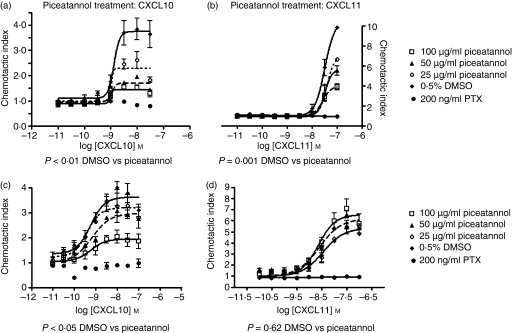
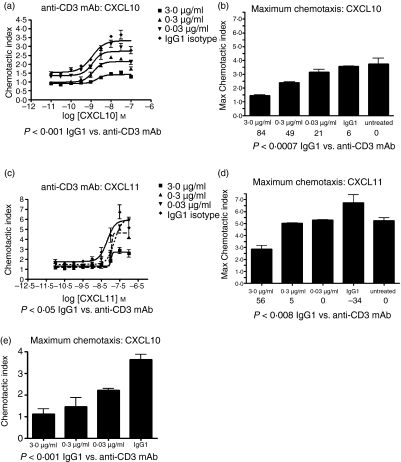
Published data also indicate that ZAP-70 activation is critical for chemokine-induced migration in T cells.19,20 We examined the role of ZAP-70 in CXCR3-mediated T-cell chemotaxis by pretreating cells with piceatannol before their incubation with the CXCR3 ligands CXCL10 and CXCL11 in a transwell chemotaxis assay. JE6.1/CXCR3 cells treated with piceatannol demonstrated a reduced chemotactic index in response to CXCL10 or CXCL11 as compared to cells treated with DMSO alone (Fig. 7a,b). The reduction of chemotaxis was dose-dependent. Furthermore, this reduction in chemotaxis was statistically significant when dose–response curves were compared using both anova and t-tests for non-paired data: P < 0·01 DMSO treatment versus piceatannol for CXCL10 chemotaxis assay, P < 0·001 DMSO treatment versus piceatannol for CXCL11 chemotaxis assay. Treatment of cells with pertussis toxin completely blocked JE6.1/CXCR3 chemotaxis, suggesting that the cell chemotaxis seen in this assay was mediated by CXCR3 activation.
Figure 7.
Piceatannol differentially inhibits CXCR3-mediated T-cell chemotaxis. JE6.1/CXCR3 cells (a, b) and normal T cells expressing CXCR3 (c, d) were treated with piceatannol as described above before being subjected to a trans-well chemotaxis assay. Chemotaxis filters were treated with 10 μg/ml fibronectin. Lower wells were filled with assay buffer alone or assay buffer containing CXCL10 (a, c) or CXCL11 (b, d) at the concentrations indicated. Cells were stained with Calcein AM and then loaded at 3 × 105 cells per well. Plates were incubated at 37° for 1 hr, after which, filter inserts were washed to remove non-adherent cells. Fluorescence was measured in a plate reader (485 nm excitation, 530 nm emission) and the chemotactic index was calculated by comparing the fluorescence of wells with chemoattractant to those with assay buffer alone. Dose–response curves based on chemotactic index were calculated for DMSO and piceatannol-treated cells and plotted: (a, b) represent data obtained with JE6.1/CXCR3 cells and (c, d) represent data obtained from normal T cells. Dose–response curves were compared using one-way anova and non-paired t-tests. Results of statistical analyses are indicated below each panel.
However, when these same studies were performed in normal T cells expressing CXCR3, treatment with piceatannol dose-dependently reduced chemotaxis in response to CXCL10 (Fig. 7c) but not CXCL11 (Fig. 7d). This finding suggests that although CXCL10 or CXCL11 treatment of T cells can result in tyrosine phosphorylation of ZAP-70, the role of ZAP-70 differs in the CXCL10 pathway compared to the CXCL11 pathway. Together, these results indicate that ZAP-70 plays a role in CXCR3-mediated T-cell chemotaxis but that this role depends on which ligand binds to CXCR3.
ZAP-70 phosphorylation at tyrosine residues following CXCR3 activation is a Gαi-independent, Lck-dependent process
Having found that ZAP-70 was involved in CXCR3-mediated signalling and in CXCL10-mediated T-cell chemotaxis, we investigated what mechanisms might be responsible for ZAP-70 phosphorylation at tyrosine 319 following CXCR3 activation. One possibility is that CXCR3-mediated ZAP-70 phosphorylation is a Gαi-dependent process. Chemokine receptors signal through Gαi-coupled G proteins and Gαi proteins can activate Src family kinases directly.1–4,13,51 Activation of the Src family kinase Lck expressed in T cells is necessary for phosphorylation of ZAP-70 at tyrosine residues after stimulation of the TCR.33–36 Thus, ligand binding to CXCR3 could result in Gαi-dependent activation of Lck, and activated Lck could in turn phosphorylate ZAP-70 at tyrosines. To investigate the role of Gαi in CXCR3-mediated ZAP-70 phosphorylation, we treated JE6.1/CXCR3 cells with the Gαi-inhibitor pertussis toxin before stimulating them with CXCL10 or CXCL11. We found that pertussis toxin had no effect on CXCL10-mediated or CXCL11-mediated phosphorylation of proteins at tyrosines in general (Fig. 8a, lanes 2–11) or on CXCL10-mediated or CXCL11-mediated phosphorylation of ZAP-70 at tyrosine 319 (Fig. 8b, lanes 2–11, upper panel). These results suggest that phosphorylation of ZAP-70 at tyrosine 319 after CXCR3 activation is a Gαi-independent process.
Figure 8.
CXCR3-mediated phosphorylation of ZAP-70 at tyrosine 319 is a Gαi-independent, Lck-dependent process. JE6.1/CXCR3 cells were treated with pertussis toxin (PTX) with the concentrations indicated (a and b: lanes 3–6, 8–11) or left untreated (a and b: lanes 2 and 7) before stimulation with CXCL10 (a and b: lanes 2–6) or CXCL11 (a and b: lanes 7–11). Lysates in (a) and (b), lane 1, are from untreated, unstimulated JE6.1/CXCR3 cells. In (c), the Lck-deficient Jurkat T cell line JCAM1.6 was enginereed to express CXCR3. A subset of these cells was then reconstituted with Lck. Three cell lines were generated: CXCR3– Lck– cells (contain vector control for both constructs; c: lanes 1 and 2), CXCR3+ Lck– cells (express CXCR3 and have vector control for Lck construct; c: lanes 3 and 4), and CXCR3+ Lck+ cells (express CXCR3 and Lck; c: lanes 5 and 6). These cells were stimulated with CXCL10 as described earlier. After stimulation with either CXCL10 or CXCL11, JE6.1/CXCR3 cells or JCAM1.6-derived cell lines were lysed. Whole cell lysates were separated by SDS–PAGE, transferred to membranes, and immunoblotted with anti-phosphotyrosine mAb (a) or with antibody specific for phosphotyrosine residue 319 in ZAP-70 (b and c, upper panels). After immunoblotting, membranes were stripped and reblotted with antibody against ZAP-70 (b and c, lower panels) to test for equal protein loading.
However, Lck could still be involved in CXCR3-mediated ZAP-70 phosphorylation at tyrosine 319. Beta arrestins can be recruited to ligand-bound G-protein-coupled receptors in a G-protein-independent manner and can serve as signalling scaffolds upon which Src family kinases can bind and become activated.52–54 So Lck could be activated and phosphorylate ZAP-70 at tyrosine 319 in a Gαi-independent fashion. To investigate this potential role of Lck in CXCR3-mediated ZAP-70 phosphorylation at tyrosine 319 we engineered the Lck-deficient Jurkat T-cell line JCAM1.6 to express CXCR3. A subset of these cells was then reconstituted with Lck. These engineered cell lines JCAM1.6 CXCR3–/Lck–, JCAM1.6 CXCR3+/Lck–, and JCAM1.6 CXCR3+/Lck+ were treated with CXCL10. An increase in ZAP-70 phosphorylation at tyrosine 319 after CXCL10 treatment was only apparent in JCAM1.6 cells expressing both CXCR3 and Lck (CXCR3+/Lck+, Fig. 8c, compare lanes 5 and 6, upper panel). The presence of CXCR3 alone or of vector controls was not sufficient to induce CXCL10-mediated ZAP-70 tyrosine phosphorylation (Fig. 8c, lanes 1–4, upper panel). In total, these results suggest that Lck is involved in CXCR3-mediated ZAP-70 tyrosine phosphorylation and that Lck activation and ZAP-70 phosphorylation following CXCR3 activation are Gαi-independent events.
Stimulation through TCR attenuates CXCR3-induced protein tyrosine phosphorylation and CXCR3-mediated T-cell migration
Our results showed that CXCR3–ligand interaction induces the tyrosine phosphorylation of ZAP-70, LAT and PLCγ1. These molecules also become phosphorylated on tyrosines upon TCR ligation.36–39,55 Thus, both TCR and CXCR3 signalling appear to involve, at least in part, the activation and/or tyrosine phosphorylation of the same molecules. To test the possibility that these receptors affect each others' signalling, we examined whether the simultaneous activation of these receptors induced synergistic phosphorylation of ZAP-70, LAT or PLCγ1. However, we did not observe a significant increase in the phosphorylation of these proteins upon coactivating CXCR3 and TCR (not shown). We also examined whether stimulating either of these receptors would modulate subsequent stimulation through the other receptor as follows: JE6.1/CXCR3 cells were left untreated or were stimulated through the TCR with anti-CD3 mAb. After treatment, the cells were either lysed (Fig. 9a), or were washed and then rested for 2 hr at 37° to allow for TCR-induced protein tyrosine phosphorylation to subside to baseline levels. After resting, the cells were incubated in media alone or in media containing CXCL10. After incubation, cell lysates were transferred to membranes and immunoblotted with anti-phosphotyrosine antibody. Stimulation through the TCR reduced CXCR3-induced protein tyrosine phosphorylation (Fig. 9b, compare lanes 2 and 3). Similarly, stimulation through the TCR reduced CXCR3-induced protein tyrosine phosphorylation of ZAP-70 (Fig. 9c, upper panel, compare lanes 2 and 3) and LAT (Fig. 9d, upper panel, compare lanes 2 and 3) as compared to untreated cells. Equal protein loading was confirmed by reblotting membranes with anti-ZAP-70 and anti-LAT antibodies. To confirm that this result was not specific to CXCL10 alone, JE6.1/CXCR3 cells were incubated with CXCL11 after stimulation of the TCR with anti-CD3 mAb. Treatment of cells with anti-CD3 mAb attenuated CXCL11-induced protein tyrosine phosphorylation to the same extent as seen in experiments conducted with CXCL10 (Fig. 9e,f). Previous stimulation through CXCR3 with either CXCL9, CXCL10 or CXCL11, however, had no effect on TCR-induced protein tyrosine phosphorylation (not shown). These results demonstrate that TCR stimulation can attenuate CXCR3 signalling, indicating that TCR and CXCR3 signalling pathways are probably interdependent.
Internalization of chemokine receptors on T cells can occur after stimulation through the TCR.56 To test the possibility that TCR ligation is attenuating CXCR3 signalling by inducing the internalization of CXCR3, JE6.1/CXCR3 cells were left untreated (Fig. 10a) or were stimulated with anti-CD3 mAb (Fig. 10b). After resting, cells were stained with PE-conjugated anti-CXCR3 antibody. The surface expression of CXCR3 was measured by FACS analysis. Stimulation with anti-CD3 mAb did not affect CXCR3 surface expression as compared to untreated cells (compare Fig. 10b with Fig. 10a): mean ± SE channel fluorescence was 577 ± 80·2 for media alone and 588 ± 75·8 for media + anti-CD3 antibody (n = 3; P = 0·44 as indicated by anova and Fisher's exact test). This indicates that TCR-induced attenuation of CXCL10-mediated or CXCL11-mediated protein tyrosine phosphorylation in JE6.1/CXCR3 cells is not a result of down-regulation of CXCR3 surface expression.
Figure 10.
Stimulation through the TCR does not affect CXCR3 expression in JE6.1/CXCR3 cells. JE6.1/CXCR3 cells were incubated with 0·001% BSA/RPMI alone (a) or with 0·001% BSA/RPMI containing 3 µg/ml anti-CD3 mAb (b). After treatment, the cells were moved to a 37° water bath for 5 min. Cells were removed from the water bath, washed, resuspended in 5% FCS/RPMI, and incubated for 2 h at 37°, 5% CO2. After incubation, FACS analysis was performed to determine the level of surface CXCR3 surface expression on unstimulated cells (a) or on cells stimulated with anti-CD3 mAb (b).
Having found that TCR stimulation could attenuate CXCR3-mediated signalling, we investigated the effects of TCR activation on CXCR3-mediated T-cell migration. JE6.1/CXCR3 cells and normal T cells expressing CXCR3 were treated with anti-CD3 mAb (0·03, 0·3 or 3 μg/ml) or mouse immunoglobulin G1 (3 μg/ml) before incubation with CXCL10 or CXCL11 in a trans-well chemotaxis assay. Initial experiments conducted with JE6.1/CXCR3 cells demonstrated that stimulation of the TCR with anti-CD3 mAb attenuated maximal T-cell chemotaxis in response to CXCL10 (Fig. 11e). These results were reconfirmed in a single experiment with CXCL11 (data not shown). However, given the differences in chemotaxis after piceatannol treament in normal T cells expressing CXCR3 compared with our engineered cell lines we decided to proceed with more extensive studies in normal T cells. Treatment of normal T cells expressing CXCR3 with anti-CD3 mAb attenuated T-cell chemotaxis in response to CXCL10 (Fig. 11a,b) or CXCL11 (Fig. 11c,d). This effect was dose-dependent (Fig. 11b,d). The effect was statistically significant whether comparing dose–response curves or maximal chemotactic index (Fig. 11). In total, our results suggest that TCR activation and CXCR3-mediated T-cell chemotaxis are closely linked, that activation of the TCR or CXCR3 induces similar signalling events, and that regulation of these signalling events involves common regulatory mechansims.
Figure 11.
Stimulation of the TCR attenuates CXCR3-mediated T-cell chemotaxis. Normal T cells expressing CXCR3 (a–d) or JE6.1/CXCR3 cells (e) were stimulated with varying concentrations of anti-CD3 mAb or with immunoglobulin G1 before being subjected to a trans-well chemotaxis assay as described above. Dose–response curves to CXCL10 (a) or CXCL11 (c) were calculated and statistically compared. In addition, per cent inhibition of maximal chemotaxis was also compared between treatment groups for both CXCL10 (b and e) and CXCL11 (d). Per cent inhibition of maximal chemotaxis for each treatment is indicated below each bar graph. Results of statistical analyses using one-way anova and non-paired t-tests are reported below each panel.
Discussion
The chemokine receptor CXCR3 plays an important role in regulating the immune response by controlling T-cell migration and function; yet, the molecular mechanisms of CXCR3 signalling in the T cell are not clear. Studying CXCR3 signalling has been hampered, at least in part, by the limited expression of CXCR3 on quiescent T cells, by the variation in the expression of CXCR3 on activated T cells from different individuals, and by the prolonged culture methods needed to induce the expression of CXCR3 on T cells. We overcame these problems by stably transfecting CXCR3 in Jurkat T cells, which are widely used as a model for T-cell activation. We showed that the interaction between CXCR3 and its ligands induces the tyrosine phosphorylation of several proteins, including the T-cell signalling molecules ZAP-70, LAT and PLCγ1.
To maximize signalling through chemokine receptors, previous studies used activation protocols in which cells were serum-starved for extended periods before engaging the chemokine receptor with its ligand.17 We found that incubating normal human T cells expressing CXCR3 on ice for 5 min, adding the ligands, and then incubating the cells for an additional 5 min on ice before transferring the cells to 37° led to a stronger CXCR3-induced protein tyrosine phosphorylation compared to CXCR3-induced protein tyrosine phosphorylation in cells that were not preincubated on ice (not shown). The reason for this difference may be that CXCR3 was fully occupied by ligand while cells were on ice, but that signalling was inhibited. Transfer of cells from ice to 37° may have allowed for synchronized signalling through CXCR3, resulting in a stronger signal. The addition of CXCR3 ligands to JE6.1/CXCR3 T cells yielded similar results regardless of whether the cells were preincubated on ice or not (not shown). This was true in all biochemical studies conducted with JE6.1/CXCR3 cells (stimulation, piceatannol and cross-talk studies). To be consistent, we chose to stimulate JE6.1/CXCR3 T cells under the same conditions that were used to stimulate normal T cells.
ZAP-70 is a protein-tyrosine kinase (PTK) expressed primarily in T cells, and is critical for T-cell development and function.36 Previous studies have also implicated ZAP-70 in playing a role in chemokine receptor signalling.18–20 For example, activation of CXCR4, a chemokine receptor constitutively expressed on T cells, with its ligand SDF-1α induced tyrosine phosphorylation of ZAP-70 in T cells.18,20 Furthermore, the CXCR4-induced tyrosine phosphorylation of ZAP-70 was critical for CXCR4-mediated T-cell migration.20 Our studies showed that CXCR3 activation induced a rapid increase in ZAP-70 tyrosine phosphorylation and that inhibition of ZAP-70 with piceatannol reduced both CXCR3-mediated protein tyrosine phosphorylation and CXCR3-mediated T-cell chemotaxis. These results indicate a role for ZAP-70 in CXCR3-signalling.
The activation of ZAP-70 is known to require phosphorylation by a PTK.57,58 CXCR3 has no intrinsic PTK activity. Thus, the phosphorylation of ZAP-70 upon CXCR3 activation must be brought about by a PTK that becomes activated following ligand binding. The binding of certain chemokines to their receptors induces activation of Src PTK in T cells.59,60 For example, the binding of SDF-1α to CXCR4 induces the phosphorylation and activation of Lck in T cells;59 Lck is a PTK that phosphorylates ZAP-70.61,62 We showed that the addition of CXCR3 ligands induces the phosphorylation of ZAP-70 on tyrosine 319. Phosphorylated tyrosine 319 on ZAP-70 is critical for ZAP-70–Lck protein–protein interactions; phosphorylated tyrosine 319 acts as a docking site for the SH2 domain of Lck.61 Our studies with JCAM1.6 (Lck-deficient) Jurkat T cells engineered to express CXCR3 suggest that Lck is involved in CXCR3-mediated phosphorylation of ZAP-70 at tyrosine residues and that the activation of Lck and phosphorylation of ZAP-70 after CXCR3 stimulation are Gαi-independent processes. At first glance, these results appear inconsistent with our functional studies in which both the Gαi-inhibitor pertussis toxin and ZAP-70-inhibitor piceatannol attenuated migration of JE6.1/CXCR3 cells in response to CXCL10. However, it seems likely that ZAP-70 and Gαi contribute differently to CXCR3-mediated T-cell chemotaxis. ZAP-70 is involved in lymphocyte function-associated antigen 1 (LFA-1)-dependent migration of T-cell hybridomas and ZAP-70 phosphorylation is enhanced upon LFA-1 ligation in these cells.48,63 CCR5 activation results in the phosphorylation of ZAP-70 at tyrosines and leads to its coprecipitation with focal adhesion kinase in Jurkat T cells.19 These reports indicate that ZAP-70 has a role in integrin- and chemokine-induced T-cell adhesion. ZAP-70 could similarly regulate CXCL10-mediated T-cell adhesion and so inhibition of ZAP-70 by piceatannol would lead to decreased CXCL10-induced T-cell chemotaxis. This process is likely independent of Gαi-initiated signalling events that also contribute to T-cell chemotaxis in response to CXCL10. Thus, signalling through both ZAP-70 and Gαi would be necessary but individually insufficient to support successful T-cell chemotaxis after the activation of CXCR3 by CXCL10; however, inhibiting the signalling activity of either molecule would result in a decreased chemotactic response to CXCL10, as we saw in our studies.
ZAP-70 tyrosine phosphorylates various signalling molecules in T cells, including LAT and PLCγ1.37,40,64 LAT is a linker protein linking ZAP-70 with downstream signalling events in T cells.37,55,64 We showed that CXCR3 activation induces rapid phosphorylation of tyrosine residues 171 and 191 in LAT. Interestingly, these tyrosine residues become phosphorylated upon TCR ligation, and are critical for TCR function.37,55,64 LAT has previously been shown to be a substrate for ZAP-70 because LAT became markedly phosphorylated on tyrosine residues when coexpressed with ZAP-70 in 293T cells.37 Thus, it is possible that ZAP-70 is the PTK, or at least one of the PTKs, that phosphorylates LAT after CXCR3 activation. The phosphorylation of tyrosine residues 171 and 191 in LAT has been shown to be important for linking ZAP-70 with downstream signalling molecules, including PLCγ1, in the TCR signalling.37,55,64 Our results showing that CXCR3–CXCL10 interaction induces the phosphorylation of tyrosine residue 783 in PLCγ1 suggest that a similar chain of signalling events might also be involved in transducing CXCR3 signalling. The phosphorylation of tyrosine residue 783 in PLCγ1 has been shown to be critical for the activation of PLCγ1.65 Because activated PLCγ1 has been implicated in calcium mobilization in T cells, it is tempting to speculate that PLCγ1 is involved, at least in part, in CXCR3-induced calcium flux in T cells.15
The studies with piceatannol, a ZAP-70-inhibitor, further implicate ZAP-70 in the tyrosine phosphorylation of LAT and PLCγ1. Interestingly, piceatannol also blocked CXCR3-induced MAPK Erk tyrosine phosphorylation; so ZAP-70 appears to function upstream of Erk in the CXCR3 signalling cascade.
The role played by ZAP-70 in CXCR3-mediated chemotaxis appears to be dependent on which ligands bind to CXCR3. The inhibition of CXCL10-induced, but not CXCL11-induced, normal T-cell chemotaxis after treatment of these cells with piceatannol suggests that these two ligands can produce CXCR3-mediated T-cell chemotaxis through different signalling mechanisms. Since both CXCL10 and CXCL11 treatment of JE6.1/CXCR3 cells resulted in tyrosine phosphorylation of ZAP-70, ZAP-70 probably has a role in CXCL11-induced signalling that remains undefined.
Recent studies have reported cross-talk between chemokine receptors and other surface molecules.56,66,67 For example, TCR ligation strongly inhibited CXCR4-mediated T-cell chemotaxis, a process that appears dependent on molecules that are important for initiating TCR signalling such as Lck.56,59,66 Interestingly, CXCR4–ligand interaction attenuated TCR-induced phosphorylation of ZAP-70 and LAT, suggesting that CXCR4 regulates the threshold for TCR-mediated T-cell activation.56 However, Nanki et al. reported that CXCR4 acted as a costimulator for TCR-mediated proliferation and cytokine production.67 The reason for the discrepancy between the studies of Nanki67 and of Peacock56 is not clear at this time. Nevertheless, these studies suggest an interaction between TCR and chemokine receptors. Our data suggest that CXCR3 and TCR may cross-talk.
Both TCR and CXCR3 induced tyrosine phosphorylation of ZAP-70, LAT, and PLCγ1.
Both receptors induced tyrosine phosphorylation of the same tyrosine residues in ZAP-70, LAT, and PLCγ1.
Previous TCR ligation strongly attenuated CXCR3-induced protein tyrosine phosphorylation and CXCR3-mediated T-cell migration.
However, we did not detect any additive or synergistic increase in protein tyrosine phosphorylation upon costimulating CXCR3 and TCR (not shown). Thus, the mechanisms by which TCR ligation attenuates CXCR3 signalling and CXCR3-mediated T-cell migration are not clear; but they could be attributed to TCR-activated tyrosine phosphatases because TCR ligation has been shown to activate several tyrosine phosphatases.68–70 This issue awaits further investigation. In contrast, we did not detect any attenuation in TCR signalling when the cells were prestimulated through CXCR3. This finding might be attributed to the fact that CXCR3-induced signalling events are weaker than that induced by TCR ligation; so the regulatory mechanisms elicited by CXCR3–ligand interaction might not be strong enough to down-regulate TCR-induced signals in this experimental system.
Our data suggest that a regulatory relationship exists between T-cell activation and CXCR3-mediated effects on T-cell function. Other in vitro and in vivo evidence, though, suggests a costimulatory role for CXCR3; it has been well documented that T-cell activation by major histocompatibility complex–antigen occurs while in close juxtaposition to antigen-presenting cells, leading to the formation of specialized contact zones, a reorganization of signalling components and an assembly of supramolecular activation clusters.71 These complexes, termed immunological synapses, function in part to control the strength and duration of costimulatory signals.72,73 Recent data suggest that chemokine receptors are recruited to the immunological synapse, where secretion of their cognate ligands by antigen-presenting cells, not only provide costimulatory signals to T cells, but also helps to sustain T-cell–antigen-presenting cell interactions.74 Data in support of a costimulatory role for CXCR3 in this regard come from the study of CXCR3 knockout mice, which have decreased mixed lymphocyte reaction responses, but normal T-cell proliferative responses to mitogen.10 An additional level of complexity exists in that the contribution of CXCR3-mediated signals to T-cell activation may be T-cell subset specific. For example, CXCR3 signalling has been shown to be more important for efficient activation of CD8+ than CD4+ T cells, especially those of the CD44low naive phenotype.27
Antithetically, some chemokines have been proposed to suppress T-cell activation by overriding major histocompatibility complex–antigen-mediated ‘stop signals’, allowing T cells to migrate away before the formation of an immunological synapse.75 Interestingly, in one in vitro system, CXCL10 has been shown to have this effect.76 Thus, while the data presented here define, at the molecular and in vitro functional levels, the potential for cross-talk between TCR and CXCR3 on both recombinant and normal T cells, future studies will focus on the signalling interplay between these receptors on specific T-cell subsets, in a setting of immune responses initiated by antigen-presenting cells.
In summary, we have shown for the first time that CXCR3 activation induces protein tyrosine phosphorylation in normal human T cells and in Jurkat T cells stably expressing CXCR3. We also demonstrate that ZAP-70, LAT and PLCγ1 are involved in CXCR3 signalling and that ZAP-70 plays a role in CXCR3-mediated chemotaxis. Our data suggest that more than one signalling pathway may be involved in CXCR3-mediated T-cell chemotaxis. Finally, we present data on the attenuation of CXCR3 signalling and CXCR3-mediated T-cell chemotaxis upon previous TCR ligation, indicating a close and complex relationship between T-cell activation and CXCR3 activation. These results shed new light on the molecular mechanisms of CXCR3 signalling in T cells.
Acknowledgments
We wish to thank Dr. Bill Sugden of the McArdle Laboratory for Cancer Research at the University of Wisconsin-Madison and Dr. Kathleen Sullivan, Dr. Julie A. DeMartino, and Sandra L. Gould of Merck Research Labs for their invaluable discussions and support. This research was conducted with support from the National Institutes of Health grant numbers AI-051–724 awarded to S. Knechtle and CA-22443 awarded to B. Sugden.
Glossary
Abbreviations:
- BSA
bovine serum albumin
- DMSO
dimethyl sulphoxide
- FACS
fluorescence activated cell sorting
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- IFN-γ
interferon-γ
- IL
interleukin
- LAT
linker for the activation of T cells
- LFA-1
lymphocyte function-associated antigen-1
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MCF
mean channel fluorescence
- MW
molecular weight
- PE
phycoerythrin
- PHA-L
phytohaemagglutinin-L
- PI3K
phosphatidylinositol-3-kinase
- PLCγ1
phospholipase-C-γ1
- PTX
pertussis toxin
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- SEA–SEE
staphylococcal enterotoxins A to E
- TCR
T-cell receptor
- ZAP-70
Zeta-associated protein of 70 000 MW
References
- 1.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 3.Yoshie O, Imai T, Nomiyama H. Novel lymphocyte-specific CC chemokines and their receptors. J Leukoc Biol. 1997;62:634–44. doi: 10.1002/jlb.62.5.634. [DOI] [PubMed] [Google Scholar]
- 4.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 5.Wong MM, Fish EN. Chemokines: attractive mediators of the immune response. Semin Immunol. 2003;15:5–14. doi: 10.1016/s1044-5323(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 6.Colvin BL, Thomson AW. Chemokines, their receptors, and transplant outcome. Transplantation. 2002;74:149–55. doi: 10.1097/00007890-200207270-00001. [DOI] [PubMed] [Google Scholar]
- 7.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–9. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostini C, Calabrese F, Rea F, et al. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–11. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–20. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandis AZ, Cherla RP, Ganju RK. Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45. J Biol Chem. 2003;278:9536–43. doi: 10.1074/jbc.M211803200. [DOI] [PubMed] [Google Scholar]
- 12.Curnock AP, Logan MK, Ward SG. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105:125–36. doi: 10.1046/j.1365-2567.2002.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–34. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 14.Bonacchi A, Romagnani P, Romanelli RG, et al. Signal transduction by the chemokine receptor CXCR3. Activation of Ras/ERK Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem. 2001;276:9945–54. doi: 10.1074/jbc.M010303200. [DOI] [PubMed] [Google Scholar]
- 15.Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162:3840–50. [PubMed] [Google Scholar]
- 16.Jinquan T, Anting L, Jacobi HH, et al. CXCR3 expression on CD34(+) hemopoietic progenitors induced by granulocyte-macrophage colony-stimulating factor. II. Signaling pathways involved. J Immunol. 2001;167:4405–13. doi: 10.4049/jimmunol.167.8.4405. [DOI] [PubMed] [Google Scholar]
- 17.Smit MJ, Verdijk P, van der Raaij-Helmer EM, Navis M, Hensbergen PJ, Leurs R, Tensen CP. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood. 2003;102:1959–65. doi: 10.1182/blood-2002-12-3945. [DOI] [PubMed] [Google Scholar]
- 18.Kremer KN, Humphreys TD, Kumar A, Qian NX, Hedin KE. Distinct role of ZAP-70 and Src homology 2 domain-containing leukocyte protein of 76 kDa in the prolonged activation of extracellular signal-regulated protein kinase by the stromal cell-derived factor-1 alpha/CXCL12 chemokine. J Immunol. 2003;171:360–7. doi: 10.4049/jimmunol.171.1.360. [DOI] [PubMed] [Google Scholar]
- 19.Bacon KB, Szabo MC, Yssel H, Bolen JB, Schall TJ. RANTES induces tyrosine kinase activity of stably complexed p125FAK and ZAP-70 in human T cells. J Exp Med. 1996;184:873–82. doi: 10.1084/jem.184.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ticchioni M, Charvet C, Noraz N, Lamy L, Steinberg M, Bernard A, Deckert M. Signaling through ZAP-70 is required for CXCL12-mediated T-cell transendothelial migration. Blood. 2002;99:3111–18. doi: 10.1182/blood.v99.9.3111. [DOI] [PubMed] [Google Scholar]
- 21.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 22.Romagnani P, Beltrame C, Annunziato F, et al. Role for interactions between IP-10/Mig and CXCR3 in proliferative glomerulonephritis. J Am Soc Nephrol. 1999;10:2518–26. doi: 10.1681/ASN.V10122518. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Yue TL, Ohlstein EH, Sung CP, Feuerstein GZ. Interferon-inducible protein-10 involves vascular smooth muscle cell migration, proliferation, and inflammatory response. J Biol Chem. 1996;271:24286–93. doi: 10.1074/jbc.271.39.24286. [DOI] [PubMed] [Google Scholar]
- 24.Kanmaz T, Feng P, Torrealba J, et al. Surveillance of acute rejection in baboon renal transplantation by elevation of interferon-gamma inducible protein-10 and monokine induced by interferon-gamma in urine. Transplantation. 2004;78:1002–7. doi: 10.1097/01.tp.0000134397.55564.71. [DOI] [PubMed] [Google Scholar]
- 25.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–43. [PubMed] [Google Scholar]
- 26.Weng Y, Siciliano SJ, Waldburger KE, et al. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem. 1998;273:18288–91. doi: 10.1074/jbc.273.29.18288. [DOI] [PubMed] [Google Scholar]
- 27.Ogasawara K, Hida S, Weng Y, et al. Requirement of the IFN-alpha/beta-induced CXCR3 chemokine signalling for CD8+ T cell activation. Genes Cells. 2002;7:309–20. doi: 10.1046/j.1365-2443.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- 28.Saetta M, Mariani M, Panina-Bordignon P, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–9. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 29.Xie JH, Nomura N, Lu M, et al. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003;73:771–80. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita T, Akamatsu H, Tomitaka A, Ogawa Y, Sugawara N, Matsunaga K. IP-10 in atopic dermatitis. Allergy. 2003;58:261. doi: 10.1034/j.1398-9995.2003.00062_2.x. [DOI] [PubMed] [Google Scholar]
- 31.Giustizieri ML, Mascia F, Frezzolini A, De Pita O, Chinni LM, Giannetti A, Girolomoni G, Pastore S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J Allergy Clin Immunol. 2001;107:871–7. doi: 10.1067/mai.2001.114707. [DOI] [PubMed] [Google Scholar]
- 32.Hancock WW, Wang LYeQ, Han R, Lee I. Chemokines and their receptors as markers of allograft rejection and targets for immunosuppression. Curr Opin Immunol. 2003;15:479–86. doi: 10.1016/s0952-7915(03)00103-1. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Weiss A. T cell receptor signalling. J Cell Sci. 2001;114:243. doi: 10.1242/jcs.114.2.243. [DOI] [PubMed] [Google Scholar]
- 34.Kurosaki T. Functional dissection of BCR signaling pathways. Curr Opin Immunol. 2000;12:276–81. doi: 10.1016/s0952-7915(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 35.Hamawy MM, Mergenhagen SE, Siraganian RP. Protein tyrosine phosphorylation as a mechanism of signalling in mast cells and basophils. Cell Signal. 1995;7:535–44. doi: 10.1016/0898-6568(95)00024-j. [DOI] [PubMed] [Google Scholar]
- 36.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 38.Weiss A, Koretzky G, Schatzman RC, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci USA. 1991;88:5484–8. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Secrist JP, Karnitz L, Abraham RT. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J Biol Chem. 1991;266:12135–9. [PubMed] [Google Scholar]
- 40.Williams BL, Irvin BJ, Sutor SL, et al. Phosphorylation of Tyr319 in ZAP-70 is required for T-cell antigen receptor-dependent phospholipase C-gamma1 and Ras activation. EMBO J. 1999;18:1832–44. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamawy MM, Tsuchida M, Manthei ER, Dong Y, Fechner JE, Knechtle JS. Activation of T lymphocytes for adhesion and cytokine expression by toxin-conjugated anti-CD3 monoclonal antibodies. Transplantation. 1999;68:693–8. doi: 10.1097/00007890-199909150-00016. [DOI] [PubMed] [Google Scholar]
- 42.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–54. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy G, Komano J, Sugden B. Epstein–Barr virus provides a survival factor to Burkitt's lymphomas. Proc Natl Acad Sci USA. 2003;100:14269–74. doi: 10.1073/pnas.2336099100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho CS, Elkahwaji J, Chang Z, Scheunemann TL, Manthei ER, Hamawy MM. Modulation of the electrophoretic mobility of the linker for activation of T cells (LAT) by the calcineurin inhibitors CsA and FK506: LAT is a potential substrate for PKC and calcineurin signaling pathways. Cell Signal. 2003;15:85–93. doi: 10.1016/s0898-6568(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 45.Hamawy MM, Tsuchida M, Cho CS, Manthei ER, Fechner JH, Knechtle SJ. Immunotoxin FN18-CRM9 induces stronger T cell signaling than unconjugated monoclonal antibody FN18. Transplantation. 2001;72:496–503. doi: 10.1097/00007890-200108150-00023. [DOI] [PubMed] [Google Scholar]
- 46.Ferrigni NR, McLaughlin JL, Powell RG, Smith CR., Jr Use of potato disc and brine shrimp bioassays to detect activity and isolate piceatannol as the antileukemic principle from the seeds of Euphorbia lagascae. J Nat Prod. 1984;47:347–52. doi: 10.1021/np50032a019. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchida M, Knechtle SJ, Hamawy MM. CD28 ligation induces tyrosine phosphorylation of Pyk2 but not Fak in Jurkat T cells. J Biol Chem. 1999;274:6735–40. doi: 10.1074/jbc.274.10.6735. [DOI] [PubMed] [Google Scholar]
- 48.Soede RD, Wijnands YM, Van Kouteren-Cobzaru I, Roos E. ZAP-70 tyrosine kinase is required for LFA-1-dependent T cell migration. J Cell Biol. 1998;142:1371–9. doi: 10.1083/jcb.142.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell Fc epsilon R1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. 1994;269:29697–703. [PubMed] [Google Scholar]
- 50.Fargnoli J, Burkhardt AL, Laverty M, Kut SA, van Oers NS, Weiss A, Bolen JB. Syk mutation in Jurkat E6-derived clones results in lack of p72syk expression. J Biol Chem. 1995;270:26533–7. doi: 10.1074/jbc.270.44.26533. [DOI] [PubMed] [Google Scholar]
- 51.Ma YC, Huang J, Ali S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:635–46. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 52.Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–33. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 53.Luttrell LM, Ferguson SS, Daaka Y, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–61. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 54.Miller WE, Maudsley S, Ahn S, Khan KD, Luttrell LM, Lefkowitz RJ. β-arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC. Role of β-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J Biol Chem. 2000;275:11312–19. doi: 10.1074/jbc.275.15.11312. [DOI] [PubMed] [Google Scholar]
- 55.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–26. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 56.Peacock JW, Jirik FR. TCR activation inhibits chemotaxis toward stromal cell-derived factor-1. Evidence for reciprocal regulation between CXCR4 and the TCR. J Immunol. 1999;162:215–23. [PubMed] [Google Scholar]
- 57.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wange RL, Guitian R, Isakov N, Watts JD, Aebersold R, Samelson LE. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–3. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 59.Inngjerdingen M, Torgersen KM, Maghazachi AA. Lck is required for stromal cell-derived factor 1 alpha (CXCL12)-induced lymphoid cell chemotaxis. Blood. 2002;99:4318–25. doi: 10.1182/blood.v99.12.4318. [DOI] [PubMed] [Google Scholar]
- 60.Chernock RD, Cherla RP, Ganju RK. SHP2 and cbl participate in alpha-chemokine receptor CXCR4-mediated signaling pathways. Blood. 2001;97:608–15. doi: 10.1182/blood.v97.3.608. [DOI] [PubMed] [Google Scholar]
- 61.Pelosi M, Di Bartolo V, Mounier V, Mege D, Pascussi JM, Dufour E, Blondel A, Acuto O. Tyrosine 319 in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J Biol Chem. 1999;274:14229–37. doi: 10.1074/jbc.274.20.14229. [DOI] [PubMed] [Google Scholar]
- 62.Denny MF, Patai B, Straus DB. Differential T-cell antigen receptor signaling mediated by the Src family kinases Lck and Fyn. Mol Cell Biol. 2000;20:1426–35. doi: 10.1128/mcb.20.4.1426-1435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soede RD, Driessens MH, Ruuls-Van Stalle L, Van Hulten PE, Brink A, Roos E. LFA-1 to LFA-1 signals involve zeta-associated protein-70 (ZAP-70) tyrosine kinase: relevance for invasion and migration of a T cell hybridoma. J Immunol. 1999;163:4253–61. [PubMed] [Google Scholar]
- 64.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–50. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 65.Sekiya F, Poulin B, Kim YJ, Rhee SG. Mechanism of tyrosine phosphorylation and activation of phospholipase C-gamma 1. Tyrosine 783 phosphorylation is not sufficient for lipase activation. J Biol Chem. 2004;279:32181–90. doi: 10.1074/jbc.M405116200. [DOI] [PubMed] [Google Scholar]
- 66.Ptasznik A, Urbanowska E, Chinta S, et al. Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells. J Exp Med. 2002;196:667–8. doi: 10.1084/jem.20020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nanki T, Lipsky PE. Cutting edge: stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J Immunol. 2000;164:5010–14. doi: 10.4049/jimmunol.164.10.5010. [DOI] [PubMed] [Google Scholar]
- 68.Frearson JA, Alexander DR. The phosphotyrosine phosphatase SHP-2 participates in a multimeric signaling complex and regulates T cell receptor (TCR) coupling to the Ras/mitogen-activated protein kinase (MAPK) pathway in Jurkat T cells. J Exp Med. 1998;187:1417–26. doi: 10.1084/jem.187.9.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furukawa T, Itoh M, Krueger NX, Streuli M, Saito H. Specific interaction of the CD45 protein-tyrosine phosphatase with tyrosine-phosphorylated CD3 zeta chain. Proc Natl Acad Sci USA. 1994;91:10928–32. doi: 10.1073/pnas.91.23.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gervais FG, Veillette A. The unique amino-terminal domain of p56lck regulates interactions with tyrosine protein phosphatases in T lymphocytes. Mol Cell Biol. 1995;15:2393–401. doi: 10.1128/mcb.15.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–83. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 72.Dustin ML, Shaw AS. Costimulation: building an immunological synapse. Science. 1999;283:649–50. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- 73.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 74.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, Martinez AC, Manes S, Viola A. T cell costimulation by chemokine receptors. Nat Immunol. 2005;6:465–71. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 75.Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21:305–14. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 76.Bromley SK, Peterson DA, Gunn MD, Dustin ML. Cutting edge. hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J Immunol. 2000;165:15–19. doi: 10.4049/jimmunol.165.1.15. [DOI] [PubMed] [Google Scholar]