Abstract
The current study was aimed at developing a one-way mixed leucocyte culture–enzyme-linked immunospot (MLC-ELISPOT) assay for the study of CD4+ CD25+ regulatory T (Treg) cells and applying this method in the study of antifetal immune reactions during human pregnancy. Twenty-one pregnant women and the corresponding fathers-to-be, and 10 non-pregnant control women and men, participated in the study. CD4+ CD25+ cells were isolated from peripheral blood mononuclear cells (PBMC) by immunomagnetic selection. Maternal/control PBMC were stimulated with paternal or unrelated PBMC in MLC. Secretion of interleukin-4 (IL-4) and interferon-γ (IFN-γ) from responder cells, with or without the presence of autologous Treg cells, was analysed by ELISPOT. PBMC from pregnant women showed increased secretion of IL-4 compared to controls. In pregnant and non-pregnant controls, Treg cells suppressed IFN-γ reactivity against paternal and unrelated alloantigens. Interestingly, Treg cells suppressed IL-4 secretion against paternal but not unrelated alloantigens during pregnancy. We have successfully developed a model for studying Treg cells in antifetal cytokine reactions during pregnancy. Results indicate that Treg cells contribute to strict regulation of both T helper type 1-like and type 2-like antifetal immune reactions. Interestingly, T helper type 2-like cells specific to unrelated alloantigens are able to escape the suppression of Treg cells, which would allow for IL-4, alongside CD4+ CD25+ Treg cells, to control potentially detrimental IFN-γ reactions during pregnancy.
Keywords: human; reproductive immunology; regulatory T cells (Treg); T cells, T helper type 1 and type 2 cells
Introduction
In the mid-1990s, it was discovered that transfer of CD4+ CD25+ cells prevented autoimmune disease and transplant rejection caused by CD25+ cell depletion or neonatal thymectomy in mice.1,2 These CD4+ CD25+ regulatory T (Treg) cells originate in the thymus as natural mediators of self-tolerance3,4 but have also been implicated in alloantigen reactivity,5 allergy6 and infectious diseases.7 In humans, Treg cells are found within the CD4+ CD25bright compartment comprising approximately 1–2% of peripheral CD4+ cells.8,9 Besides expression of the interleukin-2 (IL-2) receptor α-chain CD25, human Treg cells have been shown to constitutively express other markers of activation such as cytotoxic T lymphocyte antigen-4 and glucocorticoid-induced tumour necrosis factor receptor family related gene, none of which are Treg-cell-specific or involved in in vitro suppression.10,11 The forkhead/winged helix transcription factor gene FOXP3 is to date the only specific marker of Treg cells and seems critical for Treg-cell lineage commitment, at least in mice.12–14In vitro, Treg cells are anergic but require IL-2 or IL-15 for maintenance and T-cell receptor engagement for suppression.15,16 Once activated, Treg cells can suppress effector T cells regardless of specificity as well as convey suppressive characteristics to non-regulatory T cells.17–20 Unable to secrete significant levels of cytokines themselves, Treg cells inhibit cytokine secretion and proliferation of CD4+ and CD8+ cells in a cell contact-dependent, cytokine-independent manner.8,10,11,15,17,21–23 However, recent data indicate transforming growth factor-β (TGF-β) to be part of human Treg-cell-suppressive mechanisms.24 Uncertainty regarding cytokine dependence in Treg-cell suppression may, in addition to incongruent results in vitro compared with in vivo, be caused by the simultaneous existence of other CD4+ regulatory subsets, T helper type 3 (Th3) and Treg cell type 1 (Tr1), suppression of which is largely dependent on IL-10 and/or TGF-β16.
More than 50 years ago, Medawar described pregnancy as an immunological paradox because the fetus is normally accepted by the maternal immune system despite expression of paternal alloantigens.25 This intriguing phenomenon is partly explained by a switch in Th-cell cytokine balance, away from the aggressive cell-mediated effects of Th1-like and proinflammatory factors such as interferon-γ (IFN-γ), tumour necrosis factor-α and IL-2.26–28 Indeed, increased secretion of the Th2-like cytokine IL-4, antagonistic to Th1-like immunity,29 and the suppressive cytokine IL-10 have been shown in the blood of pregnant women.30–33 In addition, peripheral leucocytes of pregnant women respond with secretion of IL-4, in addition to IFN-γ, to paternal alloantigens in mixed leucocyte culture (MLC)31. Recently, CD4+ CD25+ Treg cells have been associated with maternal tolerance to the fetus. In humans, successful pregnancy is associated with increased numbers of CD4+ CD25+ Treg cells, at least locally in the decidua,34–37 whereas Treg-cell changes in blood have recently been questioned.38 Mice that are depleted of Treg cells fail to complete full-term pregnancies, whereas normal mice display increased Treg-cell numbers during pregnancy.39–42 In a murine abortion model, Treg cells adoptively transferred from normal pregnant mice prevent fetal demise and induce a battery of pregnancy-favouring factors including IL-10, TGF-β, haemoxygenase-1 and leukaemia inhibitory factor at the fetal–maternal interface.40,43 However, the role for Treg cells in cytokine regulation during human pregnancy remains unclear and is the focus of this work. In addition, it is still unknown if Treg cells differentially affect the immunological response to paternal alloantigens compared with unrelated alloantigens. The current study aimed to develop a Treg-cell MLC enzyme-linked immunospot (ELISPOT) suppression assay for investigation of Treg-cell importance in antifetal cytokine responses during normal second-trimester human pregnancy.
Materials and methods
Subjects
For methodological development, blood was obtained from two healthy non-pregnant women (age 23 and 37 years), employees at the Department of Molecular and Clinical Medicine, Linköping University.
Twenty-one pregnant women with no signs of pregnancy complications (pregnancy week 24–28, age 19–36 years), and the corresponding fathers-to-be (age 18–38 years), visiting the maternity outpatient care unit in Linköping (Kvinnohälsan), were asked to participate in the study. Ten women (age 18–40 years) and men (age 18–55 years), who were all employees/students at the Department of Molecular and Clinical Medicine, Linköping University, or blood donors at Linköping University Hospital, served as control subjects. Informed consent was obtained from all participants. The study was approved by the Local Ethics Committee at Linköping University.
Separation of peripheral blood mononuclear cells
Approximately 60 ml whole blood was obtained in sodium-heparin vacutainer tubes. Peripheral blood mononuclear cells (PBMC) were separated within 1 hr on Lymphoprep (Nycomed, Norway) according to Boyum44 followed by washing in Hank's balanced salt solution (HBSS; Gibco, BRL, Paisley, UK). PBMC for direct ELISPOT analysis (see below) were resuspended to a density of 106 lymphocytes/ml in T-cell culture medium (TCM) consisting of Iscove's modified Dulbecco's medium (IMDM; Sigma Aldrich, Stockholm, Sweden) supplemented with l-glutamine (292 mg/ml; Sigma), sodium bicarbonate (3024 g/l; Sigma), penicillin (50 IE/ml), streptomycin (50 μg/ml) (PEST from Flow Laboratories, Irvine, UK), 100 × non-essential amino acids (10 ml/l; Invitrogen, Stockholm, Sweden) and 5% heat-inactivated fetal bovine serum (FBS; Sigma). Cells were kept on ice until culturing. For immunomagnetic separation, PBMC were resuspended in HBSS supplemented with 2% FBS (HBSS 2% FBS) to a density of 5 × 106 PBMC/ml.
Immunomagnetic separation of CD4+ CD25+ Treg cells
CD4+ CD25+ cells were separated using the CD4 Positive Isolation kit followed by the CD25-portion (positive selection) of the CD4+ CD25+ Treg-cell kit, both from Dynal Biotech (Oslo, Norway). This allowed retrieval of CD4– and CD4+ CD25– cells to be used as Treg-cell-depleted responder cells. Dynabeads CD4 (mouse monoclonal anti-human CD4 antibody) and Dynabeads CD25 (mouse monoclonal anti-human CD25 antibody) were washed with HBSS 2% FBS before being used according to the manufacturer's instructions.
In a first step, CD4+ cells were separated by positive selection; Dynabeads CD4, corresponding to two beads per PBMC, were added to the PBMC suspension and incubated at 4° for 20 min with gentle tilting and rotation. CD4+ cells were separated using the magnetic particle concentrator MPC-50 (Dynal Biotech) and the CD4+ rosettes were washed four times in HBSS 2% FBS. The CD4– fraction was saved in TCM and kept on ice. CD4+ rosettes were resuspended in 100 μl IMDM 1% FBS per millilitre of the original PBMC suspension. Ten microlitres of Detachabead CD4 (sheep anti-mouse Fab) solution was added per 100 μl rosette-suspension and incubated for 1 hr at room temperature with gentle tilting and rotation. Detached CD4+ cells, from which CD4+ CD25+ cells would be isolated, were magnetically separated from the antibodies and beads using the magnetic device. Beads were washed three times in 500 μl IMDM 1% FBS. Free CD4+ cells were washed once in 10 ml IMDM 1% FBS for 10 min at 400 g. CD4+ cells were resuspended in HBSS 2% FBS to a concentration of 1·5 × 107 cells/ml.
In a second step, CD4+ CD25+ cells were separated from the CD4+ population by positive selection. Dynabeads CD25, corresponding to three beads per CD4+ cell, were added to the CD4+ cell suspension and incubated at 4° for 25 min with gentle tilting and rotation. CD4+ CD25+ cells were separated using the Dynal MPC-50 magnetic device and the CD25+ rosettes were washed three times in HBSS 2% FBS. The CD25– fractions were saved in TCM and kept on ice. CD4+ CD25+ cells were resuspended in 100 μl IMDM 1% FBS per 50 μl CD25 beads originally added. Twenty microlitres of Detachabead CD25 was added per 100 μl of the CD25 rosettes followed by incubation for 1 hr with gentle tilting and rotation. Detached CD4+ CD25+ cells were aspirated from the magnetically separated beads and the beads were washed twice with IMDM 1% FBS in the magnetic device. CD4+ CD25+ cells were washed once in 10 ml IMDM 1% FBS by centrifugation at 400 g for 10 min and resuspended in TCM to a cell density of 1 × 106 cells/ml.
Treg-depleted cells were prepared by pooling the CD4– and CD4+ CD25– fractions and resuspending cells in TCM at 1 × 106 lymphocytes/ml.
Paraformaldehyde treatment of autologous/allogeneic PBMC for MLC
Autologous PBMC (pregnant women/non-pregnant control), allogeneic PBMC (father/male control) and a freeze-thawed alloantigen-pool, consisting of PBMC from 20 healthy donors, were treated with 4% paraformaldehyde (PFA; Sigma) in phosphate-buffered saline (PBS) pH 7·4 (EC Diagnostics AB, Uppsala, Sweden) for 10 min at room temperature. PFA treatment of PBMC has been shown to totally inhibit cytokine secretion, yet rendering cells capable of stimulating responder cells in the MLC.31 Cells were washed twice in PBS by centrifugation at 400 g at 4° for 10 min. Cells were resuspended at 106 PBMC/ml in TCM. The alloantigen-pool was used because, as a result of the many alloantigens present, it increases the probability of reaching an alloresponse. It has been shown that alloresponses theoretically reach a plateau level when the pool consists of 10–15 randomly selected blood donors.31
Flow cytometry
The immunomagnetic selection of Treg cells from control subjects was evaluated on four occasions by flow cytometry. Briefly, PBMC, CD4+ CD25+-depleted PBMC and CD4+ CD25+ cells were incubated with 10 μl mouse isotype controls [immunoglobulin G1 conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or peridinin chlorophyll protein (PerCP)] or mouse anti-human CD25–FITC, CD4–PE and CD3–PerCP (BD Biosciences, Stockholm, Sweden) for 15 min. This was followed by lysing and fixating the cells by incubating samples with 100 μl Optilyse B (Immunotech, Beckman Coulter, Fullerton, CA) for 10 min followed by 1 ml deionized water for 10 min. Cells were then centrifuged at 400 g for 5 min and resuspended in PBS 2% human serum albumin (Pharmacia Biotech, Uppsala, Sweden). Cells were analysed on FACSCalibur using CellQuest Pro version 4·0.2 software (BD Biosciences). Cells were gated for analysis of lymphocytes by side/forward scatter and gating for analysis of T cells was based on CD3 expression. Gates for coexpression of the CD4 and CD25 molecules were set according to isotype controls. The CD25bright gate was adjusted to contain CD4+ cells that expressed CD25 more brightly than CD4– CD25+ cells.
RNA extraction and reverse transcriptase real-time polymerase chain reaction (PCR) for quantification of FOXP3 mRNA expression
Expression of FOXP3 messenger RNA (mRNA) was analysed in PBMC, CD4+ CD25+-depleted PBMC and CD4+ CD25+ cells isolated from two pregnant women. Total RNA was extracted using the RNeasy minikit (Qiagen, Crawley, West Sussex) according to the manufacturer's instructions. RNA was quantified using the ND-1000 NanoDrop spectrophotometer (Nanodrop Technologies, Wilmington, DE) by measuring the absorbance at 260 nm. Approximately 1 μg RNA was converted to complementary DNA (cDNA) using the cDNA high-capacity archive kit (Applied Biosystems, Foster City, CA) with RNase inhibitor (Applied Biosystems) according to the manufacturer's instructions. Reverse transcription was performed on the GeneAmp PCR system 2700 (Applied Biosystems) for 10 min at 25° followed by 2 hr at 37°. For real-time PCR, 1 μl cDNA was mixed with 1 × TaqMan Universal Mastermix (Applied Biosystems) together with primers and probe for FOXP3 (TaqMan Gene Expression assay, Assay ID: Hs00203958_m1) or 18S ribosomal RNA (Assay ID: Hs99999901_s1) (Applied Biosystems). PCR were initiated with denaturation for 2 min at 50° and 10 min at 95° followed by 40 cycles of denaturation for 15 seconds at 95° and annealing/extension for 1 min at 60°. Reactions were performed on the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Expression of the 18S subunit of ribosomal RNA was used for normalization of RNA content in all samples. The absence of genomic DNA amplification was controlled by amplifying one sample of RNA. Data were analysed with the ABI Prism Sequence Detector version 1·7a software (Applied Biosystems). Quantification was determined using the standard curve method. All samples were analysed in duplicate and the variation limit between duplicates was set to < 15%.
ELISPOT assay analysis of cytokine secretion
ELISPOT is a highly sensitive technique for in vitro detection of cell-secreted cytokines at the single-cell level. Nitrocellulose bottomed 96-well MAHAN 4550 microtitre plates (Millipore, Bedford, MA) were incubated overnight at 4° with 100 μl/well of mouse anti-human IFN-γ monoclonal antibody or mouse anti-human IL-4 monoclonal antibody (both from Mabtech, Stockholm, Sweden) diluted in sterile PBS to a final concentration of 15 μg/ml. Coated plates were stored at − 20°. Before use, plates were thawed and emptied by suction using a multiscreen vacuum manifold (Millipore), whereupon they were washed eight times with 100 μl/well PBS. Unspecific binding sites on the nitrocellulose of membrane were blocked by incubation with 100 μl/well TCM for 30 min at 37° 5% CO2.
Cultures were set in ELISPOT plates for direct analysis of cytokine secretion from responder cells in a final volume of 200 μl/well. As negative controls, TCM alone, without cells, was added to some wells. No spots were detected in these wells. As positive controls, responder cells were stimulated with phytohaemagglutinin (Sigma Aldrich, St Louis, MO) to a final concentration of 20 μg/ml, which always generated a strong response with several hundred spots. PFA-treated cells did not respond to phytohaemagglutinin stimulation, confirming their inability to secrete cytokines.
After incubation with cells, plates were emptied and cells were removed by washing four times with PBS, followed by washing twice with PBS Tween (PBS containing 0·05% Tween-20; EC Diagnostics AB). Wells were incubated for 2 hr with 100 μl/well biotinylated mouse anti-human IL-4 or mouse anti-human IFN-γ detection antibodies (both from Mabtech) diluted in PBS-Tween to a final concentration of 1 μg/ml. Following four washes with PBS-Tween, wells were incubated with streptavidin–alkaline phosphatase conjugate (Mabtech) diluted 1/1000 in PBS for 1 hr. Plates were then washed four times with PBS and wells were incubated with alkaline phosphatase substrate 5-bromo-4-chloro-3-indolyl phosphate-nitro blue tetrazolium (BCIP-NBT; Bio-Rad, Solna, Sweden) for approximately 15 min. Plates were washed with excessive amounts of water and allowed to dry upside down overnight. Spots were counted under a dissection microscope.
Methodological development of the MLC-Treg-cell suppression assay
The assay was based on the one-way MLC-ELISPOT method originally described by Ekerfelt et al.31 Initially, the minimum number of allogeneic stimulator cells needed for PBMC stimulation was investigated. PBMC were separated from two healthy blood donors and 105 responder PBMC from both donors were cultured alone or crosswise with PFA-treated autogeneic or allogeneic stimulator PBMC in varying ratios (1 : 1, 1 : 2, 1 : 4, 1 : 8, 1 : 16). Cells were incubated overnight, at 37° in a humidified atmosphere with 5% CO2, in coated ELISPOT wells for direct analysis of IFN-γ secretion from responder cells. All cultures were analysed in triplicates. The ratio 1 : 2 (stimulator : responder) was found to be the lowest number of stimulator cells needed for allogeneic stimulation to exceed the autologous response so this ratio was used in the Treg-cell MLC-ELISPOT assay. The ability of Treg cells to suppress the allogeneic response in MLC was investigated using the same blood donors as in the previous MLC-experiment. IFN-γ secretion was measured from 105 PBMC or 105 Treg-cell-depleted responders cultured alone or with 5 × 104 PFA-treated allogeneic stimulator cells. In addition, 105 allogeneically stimulated Treg-cell-depleted responders (ratio stimulator : responder; 1 : 2) were cultured with Treg cells (ratio responder : Treg cell; 1 : 1 and 2 : 1). An incubation period of approximately 42 hr was found to be optimal. The incubations were performed at 37° in a humidified atmosphere with 5% CO2. All cultures were analysed in triplicate.
Importance of Treg cells in pregnancy: application of the Treg-cell MLC-ELISPOT suppression assay
PBMC and Treg-cell-depleted cells from 21 pregnant women and 10 non-pregnant women were stimulated with PFA-treated PBMC from the corresponding fathers-to-be or control males. In addition, responder PBMC and Treg-cell-depleted cells were stimulated with autoantigens or pooled alloantigens. The stimulator : responder cell ratio was set to 1 : 2 (25 000 : 50 000 cells) based on the initial experiments. Stimulated PBMC and Treg-cell-depleted responder cells were then cultured with Treg cells in responder : Treg cell ratios of 1 : 1 or 2 : 1, depending on cell yield. All cultures were analysed in triplicate and occasionally in duplicate.
Treg-cell MLC-cultures were analysed for responder cell secretion of IFN-γ and IL-4 by ELISPOT. Ten pregnancy samples were analysed for secretion of IL-4 and 11 for secretion of IFN-γ. For the non-pregnant controls, IFN-γ secretion was investigated in 10 samples because the normal allogeneic response is predominantly a Th1 situation with no, or very low, IL-4 secretion.45,46 To confirm this, IL-4 was analysed in six of the 10 control samples. However, Treg cells were not reconstituted to these cultures. Autologously stimulated PBMC were used as a methodological control for the addition of PFA-treated cells to the wells. Cytokine secretion from these cells did not significantly differ from the unstimulated secretion so unstimulated secretion was chosen as a measure of the spontaneous secretion.
Statistical analysis
The statistical guidance resource at Linköping University was consulted for the statistical analyses. Differences within the pregnancy/non-pregnant control groups were analysed by Friedman's test followed by Bonferroni-corrected Wilcoxon-signed rank test as a post hoc test to adjust for the number of pairwise comparisons within each group. Differences between pregnant and non-pregnant controls were analysed by Mann–Whitney U-test corrected for the number of pairwise comparisons between groups by Bonferroni correction. Significance level was set to 5%, i.e. P < 0·05 was considered significant. All statistical analyses were performed using GraphPad Prism version 4 software (GraphPad Software Inc., San Diego, CA).
Results
Immunomagnetic separation of Treg cells from PBMC
The yield of cells from the immunomagnetic method was determined by direct microscopic cell counting following each selection step. CD4+ separation resulted in median yields of 38% and 37% CD4+ cells of lymphocytes in pregnant subjects (n = 21) and non-pregnant controls (n = 10), respectively. CD25+ separation resulted in median yields of 0·9% and 1·0% CD4+ CD25+ of yielded CD4+ cells from pregnant subjects and non-pregnant controls, respectively.
Flow cytometry
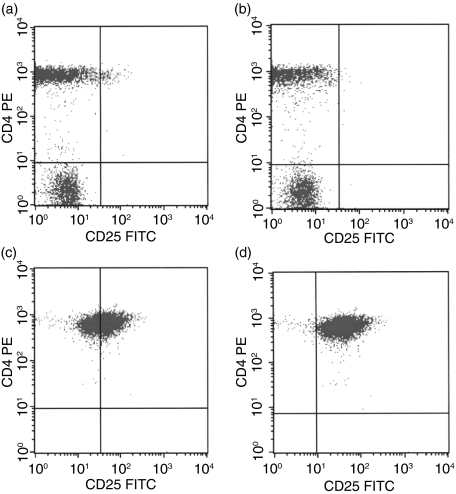
Figure 1 shows typical flow cytometric scatterplots of the immunomagnetically selected populations (Fig. 1). The median proportion of CD4+ CD25bright cells in PBMC was 2% of CD4+ cells (range 1·3–2·7%; Fig. 1a). When the PBMC population was depleted of CD4+ CD25+ cells, the median proportion of CD4+ CD25bright of CD4+ fell to 0·6% (range 0·4–0·8%; Fig. 1b). The selected Treg-cell population showed a median of 53% CD4+ CD25bright of CD4+ (range 27–68%; Fig. 1c) and 91% CD4+ CD25+ of CD4+ (range 67–99%; Fig. 1d).
Figure 1.
Flow cytometric analysis of (a) total PBMC, unselected (CD25bright gate), (b) CD4+ CD25+ depleted cells (CD25bright gate), (c) immunomagnetically selected CD4+ CD25+ cells (CD25bright gate), and (d) immunomagnetically selected CD4+ CD25+ cells (CD25+ gate). The CD25+ gate was set according to isotype controls and the CD25bright gate was adjusted to contain CD4+ cells that expressed CD25 more brightly than CD4– CD25+ cells. The x-axis shows expression of CD25 and the y-axis represents expression of CD4. (c, d) show that the immunomagnetically selected CD4+ CD25+ cells contained both CD25bright and CD25dim cells.
FOXP3 expression in immunomagnetically separated cell populations
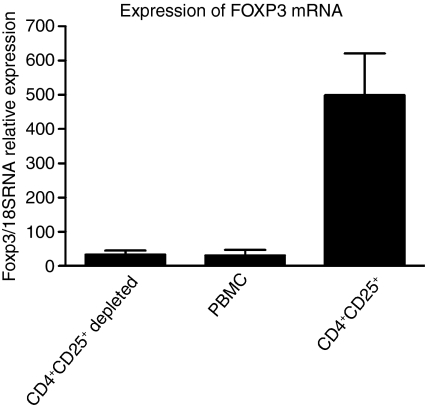
CD4+ CD25+ cells expressed approximately 15 times more FOXP3 mRNA than PBMC and CD4+ CD25+-depleted PBMC. No difference in FOXP3 expression was detected between PBMC and CD4+ CD25+-depleted PBMC (Fig. 2).
Figure 2.
FOXP3 mRNA expression in CD4+ CD25+ depleted PBMC, PBMC and CD4+ CD25+ cells determined by real-time RT-PCR (n = 2). Results are presented as median FOXP3/18S rRNA relative expression and error bars represent the highest value.
The alloantigen response in nonpregnant controls
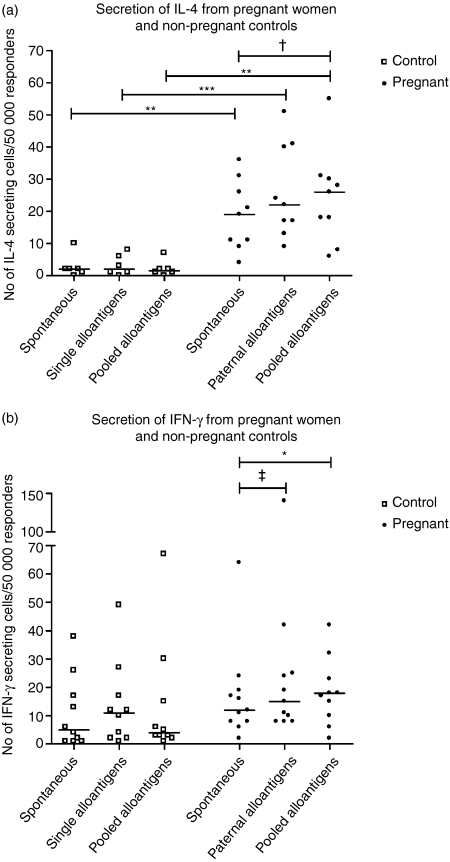
IL-4 secretion from control PBMC was not significantly affected compared with the spontaneous secretion, by stimulation with the single alloantigens or the alloantigen pool (Fig. 3a). Notably, the IL-4 response ranged from zero to a maximum of 12 spots/50 000 responder cells regardless of stimulation. Stimulation with single or pooled alloantigens did not significantly increase the secretion of IFN-γ from control PBMC compared to the spontaneous secretion (Fig. 3b).
Figure 3.
Number of IL-4-secreting and IFN-γ-secreting cells in PBMC from pregnant women and non-pregnant controls. Results are displayed as plots with medians marked with a line. (a) Secretion of IL-4 from pregnant women (n = 10) and non-pregnant control women (n = 6) determined by ELISPOT. Differences between controls and pregnant women were always statistically significant (Bonferroni-corrected Mann–Whitney U-test; **P < 0·01, ***P < 0·001). In pregnant women, the difference between spontaneous and pooled alloantigen stimulated secretion was almost statistically significant (Friedman's test (P < 0·05) followed by Bonferroni-corrected Wilcoxon test; †P = 0·06). (b) Secretion of IFN-γ from pregnant women (n = 11) and non-pregnant control women (n = 10) determined by ELISPOT. In pregnant women, the difference between spontaneous and pooled alloantigen-stimulated secretion reached statistical significance while the difference between spontaneous and paternal alloantigen-stimulated secretion was almost statistically significant (Friedman's test; P < 0·05, followed by Bonferroni-corrected Wilcoxon test; *P < 0·05, ‡P = 0·06).
The alloantigen response in pregnant women
In pregnant women, secretion of IL-4 from PBMC was not significantly stimulated by paternal alloantigens compared to spontaneous secretion (Fig. 3a). The increase in IL-4 secretion against the alloantigen pool was nearly significant (P = 0·06). Secretion of IFN-γ from PBMC was significantly increased upon stimulation with the alloantigen pool compared to the spontaneous secretion (Fig. 3b; P < 0·05). The IFN-γ secretion against paternal alloantigens was nearly significantly increased compared to the spontaneous secretion (P = 0·06).
Unstimulated and alloantigen reponses in pregnant women compared with non-pregnant controls
When comparing the IFN-γ response of pregnant women with non-pregnant controls, no statistical differences could be detected regardless of stimulus (Fig. 3b). In contrast, both the spontaneous and stimulated secretion of IL-4 was always significantly higher in pregnant woman than in non-pregnant controls (Fig. 3a). In addition, the IL-4 : IFN-γ ratio was significantly higher in PBMC from pregnant women compared with that from non-pregnant women, both when stimulated with the paternal/single alloantigens and the alloantigen pool (Bonferroni-corrected Mann–Whitney U-test; P < 0·05; data not shown). The spontaneous IL-4 : IFN-γ ratio was almost significantly higher in PBMC from pregnant women compared with non-pregnant women (Bonferroni-corrected Mann–Whitney U-test; p = 0·05; data not shown).
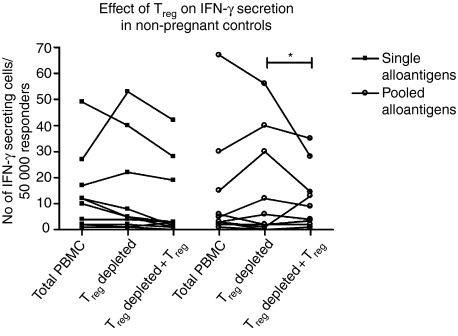
Treg-cell suppression of alloantigen reactivity in non-pregnant controls
Figure 4 shows the effects of Treg cells in the IFN-γ response to alloantigens in 10 non-pregnant control women. Treg-cell depletion did not significantly increase the secretion of IFN-γ from single and pooled alloantigen-stimulated cells compared to the IFN-γ secretion in total PBMC. However, when Treg cells were re-added to Treg-cell-depleted cultures, Treg-cell suppression of pooled alloantigen response reached statistical significance (*P < 0·05).
Figure 4.
Effect of Treg cells on the number of IFN-γ-secreting cells from non-pregnant controls (n = 10). Secretion was significantly inhibited by re-addition of Treg cells in the presence of pooled alloantigens (Friedman's test; P < 0·05, followed by Bonferroni-corrected Wilcoxon test; *P < 0·05).
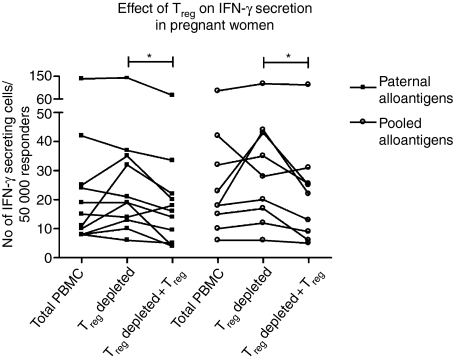
Treg-cell suppression of paternal alloantigen reactivity in pregnant women
Figure 5 shows the effects of Treg cells in the maternal IFN-γ response towards paternal and unrelated, pooled alloantigens. Depletion of Treg cells did not significantly increase the secretion of IFN-γ compared to total PBMC, whereas reconstitution of this population significantly suppressed the IFN-γ secretion in both paternal and unrelated alloantigen-stimulated cultures (*P < 0·05).
Figure 5.
Effect of Treg cells on the number of IFN-γ-secreting cells from pregnant women (n = 11 for all groups except pooled alloantigen stimulated Treg-cell-depleted + Treg cells n = 9). Secretion was significantly inhibited by the re-addition of Treg cells both in the presence of paternal and pooled alloantigens (Friedman's test; P < 0·05, followed by Bonferroni-corrected Wilcoxon test; *P < 0·05).
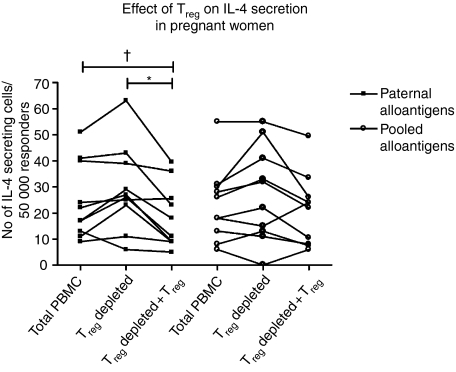
Treg-cell-depletion of paternal and unrelated alloantigen-stimulated PBMC did not significantly increase the secretion of IL-4 (Fig. 6). However, when Treg cells were re-added to the cultures, secretion of IL-4 against paternal alloantigens was significantly inhibited compared to Treg-cell-depleted cultures (P < 0·05) and was almost significantly lowered in comparison to total PBMC (P = 0·06). In contrast, the IL-4 response in the presence of pooled alloantigens was not significantly suppressed by re-adding Treg cells to the cultures.
Figure 6.
Effect of Treg cells on the number of IL-4-secreting cells from pregnant women (n = 10). Secretion was significantly, or almost significantly, inhibited by the re-addition of Treg cells in the presence of paternal alloantigens in comparison to Treg-cell-depleted cultures and total PBMC, respectively (Friedman's test; P < 0·01, followed by Bonferroni-corrected Wilcoxon test; *P < 0·05, †P = 0·059).
Discussion
Maintenance of normal pregnancy is based on the ability to tolerate fetal alloantigens. The pregnant state resembles that of transplantation and involves adaptation of the maternal immune system. The importance of regulatory CD4+ CD25+ T cells in peripheral tolerance against both foreign and self antigens sheds light on the possibility of Treg cells playing a role in fetal tolerance. In the present study, we have developed an in vitro method for studying the role of Treg cells in maternal tolerance towards fetal and unrelated alloantigens.
This study is the first to apply the sensitive ELISPOT technique for investigation of Treg-cell suppressivity. In addition to the high sensitivity of the ELISPOT method, analysing cytokine secretion rather than proliferation seems to be a more sensitive approach for analysis of Treg-cell suppression.47 Furthermore, looking at different immune response outcomes, i.e. Th1-like and Th2-like reactions, adds additional information about Treg-cell function. In this study, single and pooled alloantigens did not always significantly increase the IL-4 and IFN-γ responses. This suboptimal stimulation, with a responder : stimulator ratio of 2 : 1, was deliberately chosen for this assay. It has convincingly been shown that in situations of high antigen-specific, as well as unspecific, stimulation T cells become less sensitive to the regulatory effects of Treg cells.48,49
Although the alloantigenic stimulation was suboptimal in this study, we confirmed previous reports by our group and others showing that during pregnancy, alloreactivity is biased towards a Th2-like response with higher secretion of IL-4 compared to that in non-pregnant controls.26,31–33 In contrast to previous data,31 pregnant women showed significantly higher Th2-like responses against both paternal and unrelated pooled alloantigens compared to non-pregnant controls. In addition, pregnant women showed both spontaneous and stimulated increased IL-4/IFN-γ ratios compared to non-pregnant women. This suggests that the Th2-like deviation seen during pregnancy is not specific to paternal/fetal antigens but is more general and is driven by pregnancy itself. Interestingly, pregnant women did not alter their Th1-like activity compared to non-pregnant women. Nevertheless, pregnant women, but not non-pregnant controls, displayed significantly, or almost significantly, increased IFN-γ secretion against unrelated and paternal alloantigens compared to the spontaneous secretion. This possibly reflects an overall increased priming sensitivity of Th1-like immunity during pregnancy compared to the non-pregnant state. Since the effects of IL-4 have been shown to govern those of IFN-γ 50 this would allow for a Th1-like response to occur when necessary under the control of the meliorated Th2-like activity.
To investigate the purity of the immunomagnetically selected CD4+ CD25+ regulatory T cells, surface expression of CD25 and mRNA expression of FOXP3 were analysed by flow cytometry and real-time PCR, respectively. High expression of CD25 (CD25bright) was used to define Treg cells and this gate was adjusted to contain CD4+ cells that expressed CD25 more brightly than the CD4– CD25+ cells. The median number of peripheral CD4+ CD25bright in PBMC from the non-pregnant control individuals was 2% of CD4+, which is in agreement with previous reports.9 Since CD25 is a marker of activation, it is highly possible that an unknown amount of CD4+ CD25+, potentially activated, T cells contaminated the immunomagnetically selected Treg-cell population. Despite recent reservations, the transcription factor FOXP3 is still the best marker of CD4+ CD25+ Treg cells and the expression of this factor was investigated in the different populations. Real-time PCR revealed that the selected CD4+ CD25+ cells expressed considerably more FOXP3 mRNA than CD4+ CD25+-depleted PBMC or total PBMC, indicating an enrichment of Treg cells in this fraction. This was further supported by the flow cytometric analyses showing a median of 53% CD25bright cells of the selected CD4+ CD25+ cells. Conversely, CD4+ CD25+-depleted PBMC and total PBMC showed 0·6% and 2% CD4+ CD25bright of CD4+, respectively. Furthermore, no difference in FOXP3 expression was seen between these two latter populations. Taken together, the selected Treg-cell population seemed to be relatively pure whereas the CD4+ CD25+-depleted population still contained a considerable proportion of potentially regulatory FOXP3 mRNA-expressing and CD25bright-expressing cells. This should be taken into consideration when interpreting the results of this study. To further improve the purity of the subpopulations, flow-cytometry-based sorting strategies may be preferred.
Keeping in mind the incomplete depletion of Treg cells, this was most probably the reason why depletion of CD4+ CD25+ cells did not cause a significant elevation of the cytokine response in the Treg-MLC-ELISPOT assay regardless of stimuli, in either samples from pregnant women or non-pregnant controls compared to total PBMC. In addition, many more Treg cells were re-added than removed, which would also explain why differences were seen between Treg-cell-depleted and reconstituted cultures but not total PBMC and Treg-cell-depleted cultures. In non-pregnant controls, Treg cells suppressed the IFN-γ response when re-added to Treg-cell-depleted cultures in the presence of pooled alloantigens but not single alloantigens. This shows that pooled alloantigens seem to be a better model stimulus than single alloantigens when studying suppression of alloantigenic Th1-like reactions in the Treg-MLC-ELISPOT assay during normal conditions. Since non-pregnant controls displayed very low secretion of IL-4, Treg-cell suppression of Th2-like responses could not be compared between pregnant women and non-pregnant controls in this study. Treg cells from both pregnant and non-pregnant women suppressed IFN-γ secretion against pooled alloantigens when re-added to Treg-cell-depleted cultures. Hence, during pregnancy, Treg-cell suppression of Th1-like alloantigen reactions does not seem to be dramatically altered compared to non-pregnant controls. Several studies have suggested an increase in the peripheral CD4+ CD25+ Treg-cell pool during pregnancy.34–36 However, a very recent study does not support these results.38 In our study, we did not find any evidence for altered Treg numbers or function during pregnancy because no considerable change in suppression of Th1-like immunity was seen when compared to non-pregnant individuals. However, further investigations, i.e. involving titration of the number of Treg cells in the MLC-ELISPOT assay, have to be performed to draw such conclusions.
In pregnant women, Treg cells were able to significantly suppress both Th1-like and Th2-like reactions against paternal alloantigens when re-added to Treg-depleted cultures. Interestingly, in the presence of unrelated alloantigens, Treg cells from pregnant women significantly suppressed the Th1-like, but not the Th2-like, reaction. In line with our results, Cosmi and colleagues reported that human Th1 and Th2 clones have different susceptibilities to the suppressive effects of thymic Treg cells.51 Th2 clones were less sensitive to Treg-cell suppression than Th1 clones, especially in the presence of IL-4 or IL-9. Wing et al. showed that in birch-allergic individuals, Treg cells become less suppressive of Th2-like, but not Th1-like, antigen-specific reactions, during the birch pollen season.52 We speculate that in pregnant women, Treg cells specific for paternal alloantigens are activated and/or expanded in vivo. When reactivated in vitro by paternal alloantigens, Treg cells limit both Th1-like and Th2-like reactions against paternal alloantigens. However, in the presence of unrelated alloantigens, fewer Treg cells are activated. We show that during pregnancy Th2-like activity is increased, as illustrated by increased IL-4 secretion and increased IL-4/IFN-γ ratio compared to non-pregnant women. This possibly lowers the overall Treg suppression of Th2-like responses. In addition, because no immunological memory exists against the unrelated alloantigens, fewer Treg cells are activated in vitro by these alloantigens. Supposing that Th2 cells are in fact less susceptible to Treg-cell suppression, the Th1 reaction against unrelated alloantigens would be preferentially suppressed. In contrast, in pregnant women, the paternal specific Treg cells may be proportionally more abundant and are hence able to suppress both Th1 and Th2 reactions. These results were somewhat surprising because it could be expected that Treg cells would support Th2 responses against paternal alloantigens during pregnancy. Importantly, our results suggest that natural CD4+ CD25+ Treg cells are not responsible for this differential regulation but that they are still important in controlling immune responses during pregnancy. However, our observations do not exclude the possibility of other regulatory T-cell subsets playing a role in the Th2 deviation seen during pregnancy.
In conclusion, we have successfully developed a model for studying Treg cells in antifetal (antipaternal) cytokine reactions during pregnancy. We show that Th2-like activity is increased during pregnancy and that Treg cells are able to suppress both Th1-like and Th2-like immunity against paternal/fetal alloantigens. These antigens are obviously constantly present during pregnancy and immune responses against them need to be strictly regulated. We hypothesize that when encountering unrelated alloantigens, Th2-like cells seem able to escape the suppression of Treg cells, which would allow for IL-4, in conjunction with CD4+ CD25+ Treg cells, to control potentially detrimental IFN-γ reactions during pregnancy.
Acknowledgments
We express our most sincere gratitude to the personnel, especially Lotta Lind Åstrand and Pia Cederholm, at the Women's Hospital and Kvinnohälsan, respectively, at Linköping University Hospital, for excellent sampling of patients. We wish to thank the personnel, especially Karin Backteman, Jeannette Svartz and colleagues at the Department of Clinical Immunology and Transfusion Medicine, Linköping University Hospital for enthusiastically performing the flow cytometric analyses. We also thank Olle Eriksson at Linköping University for statistical advice. This study was supported by grants from the Swedish Foundation for Strategic Research, the Swedish Research Council (project 73X-15335–01A) and the Health Research Council in the South-east of Sweden (FORSS).
Glossary
Abbreviations:
- BCIP-NBT
5-bromo-4-chloro-3-indolyl phosphate-nitro blue tetrazolium
- cDNA
complementary DNA
- ELISPOT
enzyme-linked immunospot assay
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HBSS
Hank's balanced salt solution
- IFN-γ
interferon-γ
- IL-2
interleukin-2
- IMDM
Iscove's modified Dulbecco's medium
- MLC
mixed leucocyte culture
- mRNA
messenger RNA
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PE
phycoerythrin
- PerCP
peridinin chlorophyll protein
- PEST
penicillin/streptomycin
- PFA
paraformaldehyde
- rRNA
ribosomal RNA
- TCM
T-cell culture medium
- TGF-β
transforming growth factor-β
- Th cell
T helper cell
- Treg
regulatory T cell
References
- 1.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 2.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity. production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 4.Coutinho A, Caramalho I, Seixas E, Demengeot J. Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection. Curr Top Microbiol Immunol. 2005;293:43–71. doi: 10.1007/3-540-27702-1_3. [DOI] [PubMed] [Google Scholar]
- 5.Hauben E, Bacchetta R, Roncarolo MG. Utilizing regulatory T cells to control alloreactivity. Cytotherapy. 2005;7:158–65. doi: 10.1080/14653240510018154. [DOI] [PubMed] [Google Scholar]
- 6.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116:961–8. doi: 10.1016/j.jaci.2005.09.004. quiz 9. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 8.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 9.Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–9. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levings MK, Sangregorio R, Roncarolo MG. Human CD25(+) CD4(+) T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–46. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 14.Allan SE, Passerini L, Bacchetta R, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–84. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+) CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levings MK, Roncarolo MG. Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr Top Microbiol Immunol. 2005;293:303–26. doi: 10.1007/3-540-27702-1_14. [DOI] [PubMed] [Google Scholar]
- 17.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+) CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25– cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–21. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 19.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+) CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196:247–53. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4(+) CD25(+) cells. a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 22.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+) CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4(+) CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–31. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 25.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–38. [Google Scholar]
- 26.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, Clerici M. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 28.Wilczynski JR. Th1/Th2 cytokines balance – yin and yang of reproductive immunology. Eur J Obstet Gynecol Reprod Biol. 2005;122:136–43. doi: 10.1016/j.ejogrb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Paludan SR. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand J Immunol. 1998;48:459–68. doi: 10.1046/j.1365-3083.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 30.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164:5721–8. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- 31.Ekerfelt C, Matthiesen L, Berg G, Ernerudh J. Paternal leukocytes selectively increase secretion of IL-4 in peripheral blood during normal pregnancies: demonstrated by a novel one-way MLC measuring cytokine secretion. Am J Reprod Immunol. 1997;38:320–6. doi: 10.1111/j.1600-0897.1997.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 32.Matthiesen L, Ekerfelt C, Berg G, Ernerudh J. Increased numbers of circulating interferon-gamma- and interleukin-4-secreting cells during normal pregnancy. Am J Reprod Immunol. 1998;39:362–7. doi: 10.1111/j.1600-0897.1998.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 33.Matthiesen L, Khademi M, Ekerfelt C, Berg G, Sharma S, Olsson T, Ernerudh J. In-situ detection of both inflammatory and anti-inflammatory cytokines in resting peripheral blood mononuclear cells during pregnancy. J Reprod Immunol. 2003;58:49–59. doi: 10.1016/s0165-0378(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–53. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 35.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136:373–8. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Ramon S, Navarro AJ, Aristimuno C, Rodriguez-Mahou M, Bellon JM, Fernandez-Cruz E, de Andres C. Pregnancy-induced expansion of regulatory T-lymphocytes may mediate protection to multiple sclerosis activity. Immunol Lett. 2005;96:195–201. doi: 10.1016/j.imlet.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Tilburgs T, Roelen DL, van der Mast BJ, et al. Distribution of CD4(+) CD25(bright) and CD8(+) CD28(–) T-cells in decidua and maternal blood during human pregnancy. Placenta. 2006;27(suppl. A):S47–53. doi: 10.1016/j.placenta.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Zenclussen AC, Gerlof K, Zenclussen ML, et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–22. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 42.Darasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4(+) CD25(+) regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–9. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Zenclussen AC, Gerlof K, Zenclussen ML, et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal–maternal interface. Eur J Immunol. 2005;36:82–94. doi: 10.1002/eji.200535428. [DOI] [PubMed] [Google Scholar]
- 44.Boyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7. [PubMed] [Google Scholar]
- 45.Svenvik M, Ekerfelt C. Increased IFN-gamma response to transplantation antigens measured by cytokine MLC. indications for a bi-phasic response pattern. Transpl Immunol. 2003;11:101–5. doi: 10.1016/S0966-3274(02)00146-6. [DOI] [PubMed] [Google Scholar]
- 46.Danzer SG, Kirchner H, Rink L. Cytokine interactions in human mixed lymphocyte culture. Transplantation. 1994;57:1638–42. [PubMed] [Google Scholar]
- 47.Wing K, Larsson P, Sandstrom K, Lundin SB, Suri-Payer E, Rudin A. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005;115:516–25. doi: 10.1111/j.1365-2567.2005.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baecher-Allan C, Viglietta V, Hafler DA. Inhibition of human CD4(+) CD25(+high) regulatory T cell function. J Immunol. 2002;169:6210–17. doi: 10.4049/jimmunol.169.11.6210. [DOI] [PubMed] [Google Scholar]
- 49.George TC, Bilsborough J, Viney JL, Norment AM. High antigen dose and activated dendritic cells enable Th cells to escape regulatory T cell-mediated suppression in vitro. Eur J Immunol. 2003;33:502–11. doi: 10.1002/immu.200310026. [DOI] [PubMed] [Google Scholar]
- 50.Rocken M, Racke M, Shevach EM. IL-4-induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:225–31. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 51.Cosmi L, Liotta F, Angeli R, et al. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood. 2004;103:3117–21. doi: 10.1182/blood-2003-09-3302. [DOI] [PubMed] [Google Scholar]
- 52.Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin Exp Allergy. 2004;34:1364–72. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]