Abstract
CD4+ CD25+ Foxp3+ naturally occurring regulatory T cells (nTreg) are potent inhibitors of almost all immune responses. However, it is unclear how this minor population of cells is capable of exerting its powerful suppressor effects. To determine whether nTreg mediate part of their suppressor function by rendering naive T cells anergic or by converting them to the suppressor phenotype, we cocultured mouse nTreg with naive CD4+ CD25– T cells from T-cell receptor (TCR) transgenic mice on a RAG deficient (RAG–/–) background in the presence of anti-CD3 and interleukin-4 (IL-4) to promote cell viability. Two distinct responder cell populations could be recovered from the cocultures. One population remained undivided in the coculture and was non-responsive to restimulation with anti-CD3 or exogenous IL-2, and could not up-regulate IL-2 mRNA or CD25 expression upon TCR restimulation. Those responder cells that had divided in the coculture were anergic to restimulation with anti-CD3 but responded to restimulation with IL-2. The undivided population was capable of suppressing the response of fresh CD4+ CD25– T cells and CD8+ T cells, while the divided population was only marginally suppressive. Although cell contact between the induced regulatory T cell (iTreg) and the responders was required for suppression to be observed, anti-transforming growth factor-β partially abrogated their suppressive function. The iTreg did not express Foxp3. Therefore nTreg are not only able to suppress immune responses by inhibiting cytokine production by CD4+ CD25– responder cells, but also appear to modulate the responder cells to render them both anergic and suppressive.
Keywords: regulatory T cells, T-cell activation, tolerance, suppression, anergy
Introduction
CD4+ CD25+ Foxp3+ naturally occurring regulatory T cells (nTreg) are crucial for the control of autoimmune disease and the maintenance of immunological homeostasis and self-tolerance.1 Early studies demonstrated that transfer of CD4+ CD25– T cells into nu/nu mouse recipients resulted in the development of organ-specific autoimmune diseases that could be prevented by cotransfer of nTreg.2 The nTreg are potent suppressors of the activation of naive CD4+ CD25– and CD8+ CD25– cells in vitro by inhibiting the induction of interleukin-2 (IL-2) mRNA synthesis in a cytokine-independent, but cell-contact-dependent, manner.3 The biochemical pathways that mediate suppression in vivo or in vitro remain unknown.
It is widely accepted that nTreg must be activated via their T-cell receptor (TCR) to exert their suppressive effects.3 However, this result raises the question of how the very small number of antigen-specific nTreg in the polyclonal populations used to inhibit autoimmune disease can be so efficient in their suppressive effects in vivo. Studies with nTreg from TCR transgenic mice demonstrate that nTreg preferentially accumulate or expand in vivo at sites of immune responses such as inflamed tissues.4 It is possible that the small number of antigen-specific or organ-specific nTreg in a polyclonal population might also be expanded. Activated nTreg might then mediate their effects by acting on antigen-presenting dendritic cells to render them tolerogenic.5,6 Alternatively, it remains possible that nTreg might directly or indirectly (via the dendritic cells) exert potent and permanent effects on effector T cells, rendering them refractory or anergic to stimulation via the TCR. Ermann et al.7 reported several years ago that CD4+ CD25– T cells isolated after a 24-hr coculture with nTreg failed to proliferate or produce IL-2 when restimulated via the TCR although they did respond to exogenous IL-2.
Several studies have demonstrated that coculture of human nTreg with naive CD4+ CD25– T cells converts potential effector T cells into suppressors.8,9 Although the conversion of effectors into suppressors was cytokine-independent, the converted effectors mediated suppression primarily by secreting IL-10 and/or transforming growth factor-β (TGF-β). Different nTreg subpopulations have been proposed to be involved in the induction of effectors that produce distinct profiles of suppressor cytokines.10 The goal of the present study was to develop in vitro a mouse model system to test if coculture of nTreg with effectors rendered them anergic and/or suppressive. One advantage of the mouse model is that the effector cells can be obtained from TCR transgenic mice on a RAG-deficient (RAG–/–) background, which contain no Foxp3+ nTreg,11 ruling out the possibility that the effects of the coculture are secondary to selection of a minor population of CD25– Foxp3+ cells that contaminate the responder T cells. We demonstrate here that coculture of CD4+ CD25– Foxp3– T cells with nTreg, in the presence of IL-4 to promote cell viability, renders them non-responsive to subsequent stimulation via the TCR. The non-responsive state is secondary to their inability to up-regulate both IL-2 and CD25 expression. In addition, the anergic responder T cells can suppress the activation of both fresh naive CD4+ and CD8+ T cells in a cell-contact-dependent manner.
Materials and methods
Mice
Female, 6- to 8-week-old BALB/c mice (H-2Kd) were purchased from the National Cancer Institute (Frederick, MD). B10.A 5CC7 TCR transgenic RAG2–/– mice (H-2Kk)12 and C57BL/6 OT-I mice13 were obtained from Taconic (Germantown, NY) under National Institute of Allergy and Infectious Diseases (NIAID) contract.
Antibodies and reagents
Biotin-anti-CD25 (7D4), phycoerythrin (PE)-streptavidin, anti-CD3ε (2C11), PE-anti-H2Kd and anti-CD28 were purchased from BD PharMingen (San Diego, CA). Human recombinant IL-2 was obtained from the Preclinical Repository of the Biological Resources Branch, National Cancer Institute. Anti-TGF-β and IL-4 were purchased from R & D Systems (Minneapolis, MN). Anti-CD8, anti-CD4, anti-PE magnetic microbeads and the CD4+ isolation kit were purchased from Miltenyi Biotec (Auburn, CA). Cells were grown in RPMI-1640 with 10% heat-inactivated fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), 2 mm l-glutamine, 10 mm HEPES, 0·1 mm non-essential amino acids, 1 mm sodium pyruvate and 50 µm 2-mercaptoethanol.
Cell purification
Cells were purified as described previously.3 Briefly, lymph node cells were enriched for T cells on T-cell columns (R & D Systems). The enriched T cells were incubated with biotin-conjugated anti-CD25 and subsequently stained with PE-conjugated streptavidin. The cells were then labelled with anti-PE magnetic microbeads and purified on autoMACs (Miltenyi Biotec). The purity of CD4+ CD25+ regulatory T cells was between 96% and 99%. Naive CD4+ CD25– cells were purified using the CD4+ T-cell isolation kit. T-cell-depleted splenocytes (TdS) were used as antigen-presenting cells (APC) and prepared by depleting CD90+ cells using magnetic antibody cell sorting on the autoMACS.
Coculture of naive CD4+ CD25– T cells and nTreg
Naive CD4+ CD25– T cells obtained from 5CC7 TCR transgenic RAG2–/– mice were prepared on the autoMACS as described above. Cells were then labelled with carboxyfluorescein diacetate succinimidyl ester (CFSE, 5 μm for 5 min). CD4+ CD25– T cells (5 × 104/well) were cultured with CD25+ T cells (5 × 104/well) from BALB/c mice or CD4+ CD25– T cells (5 × 104/well) from BALB/c mice (as control) in the presence of IL-4 (5 ng/ml) in 96-well plates with APC (5 × 104) and 0·5 μg/ml anti-CD3 for 4–5 days. Cells were then harvested and stained with PE-anti-H2Kd, and the CFSEhigh and CFSElow fractions were purified by cell sorting. The induced T cells were > 99·7% H-2Kd negative.
Proliferation assays
Induced cells (5 × 104/well) were cultured in 96-well plates with APC (5 × 104) and 0·5 μg/ml anti-CD3 in the presence or absence of IL-2 (100 U/ml) for 1–3 days. Triplicate wells were pulsed with 1 μCi [3H]thymidine ([3H]TdR) for the last 6 hr of the culture. Freshly isolated CD4+ T cells (2·5 × 104/well) or CD8 T cells (2·5 × 104/well) were CFSE-labelled and cocultured with induced cells (5 × 104/well) in 96-well plates for 66 hr, and CFSE dilution was analysed by flow cytometry.
Quantitative real-time polymerase chain reaction (PCR) for IL-2 mRNA analysis
Total RNA was prepared by the TRIzol method (Invitrogen, Carlsbad, CA) followed by DNase I treatment (Invitrogen). The cDNA was made using Superscript II (Invitrogen) with random primers (Invitrogen) and analysed for IL-2, interferon-γ (IFN-γ) and Foxp3 gene expression by real-time PCR assay using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). An internal control for normalization, 18S ribosomal RNA (rRNA) (Applied Biosystems) was used. Unstimulated CD4+ CD25– cells were given an arbitrary value of 1·0 for the normalization and the remaining samples were plotted relative to that value. All PCRs were performed in triplicate with a TaqMan Universal PCR Master Mix (Applied Biosystems).
Results
NTreg cells anergize naive CD4+ CD25– T cells
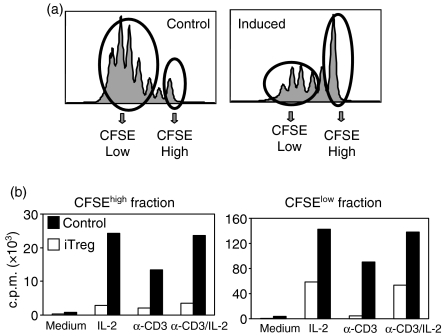
In preliminary experiments, we attempted to determine what effects were induced in CD4+ CD25– Foxp3– T cells following coculture with nTreg. We initially cultured CD4+ CD25– T cells from TCR transgenic mice on a RAG–/– background that lack both conventional CD4+ CD25+ Foxp3+ cells and the newly described CD4+ CD25– Foxp3+ regulatory cells11 with nTreg from conventional mice under conditions in which maximum suppression of proliferation is observed. Unfortunately, very few viable responder T cells were recovered from the cocultures after 4–5 days and the numbers were insufficient for further functional studies. To enhance cell viability in the cocultures, we supplemented them with exogenous IL-4. IL-4 has been previously shown to be a major viability promoting factor for both resting and stimulated T cells.14 We first analysed the proliferation of CFSE-labelled responders in the cocultures in the presence of IL-4 (Fig. 1a). Almost all of the responder population diluted CFSE in the absence of the regulatory cells, although a small peak of undivided cells was always detected. In contrast, when the responders were cultured in the presence of the regulatory cells, a significant percentage of the responders remained in the undivided peak. The number of viable cells recovered from cultures performed in the presence of nTreg was 50% of the number seen in cultures of CD25– cells alone, a result that is consistent with the decrease in proliferation seen in the CFSE studies.
Figure 1.
CD4+ CD25– T cells cocultured with nTreg become unresponsive to restimulation. nTreg were isolated from BALB/c mice (H-2Kd). Naive CD4+ CD25– T cells were isolated from 5CC7 TCR transgenic RAG–/– mice (H-2Kk), CFSE-labelled, and cultured with IL-4 (5 ng/ml) in the presence (induced) or absence (control) of freshly isolated nTreg at a ratio of 1 : 1 for 4 or 5 days. (a) Proliferation of naive CD4+ CD25– T cells in the cocultures was measured by CFSE dilution. (b) iTreg were separated from the nTreg, sorted into CFSEhigh and CFSElow populations, and restimulated (2·5 × 104/well) with anti-CD3 (0·5 μg/ml) and TdS (5 × 104/well) in the absence or presence of IL-2 (100 U/ml) for 28 hr. Proliferation was measured by [3H]TdR uptake. Results are expressed as the mean of triplicate cultures and are representative of at least three independent experiments.
After removal of the nTreg from the cocultures by cell sorting, we further separated the divided and undivided populations. Both the CFSEhigh and the CFSElow populations from the control cultures responded vigorously to all three stimuli (Fig. 1b). In contrast, the CFSElow cells from the induced cultures responded only to IL-2, but not anti-CD3, while the CFSEhigh cells isolated from the induced cultures were non-responsive to all stimuli. Coculture of naive CD4+ CD25– T cells with nTreg resulted in profound anergy to restimulation via the TCR and differential responses to IL-2 depending on the whether the cells had divided in the initial culture. Although these studies were performed with major histocompatibility complex (MHC) mismatched cell populations, culture of the TCR transgenic CD4+ CD25– T cells with CD4+ CD25– T cells from BALB/c mice had no effect on the response. Thus, it is unlikely that allogeneic effects contribute to the induction of the anergic state. For simplicity we will refer to the T cells recovered from the cocultures as induced T regulatory cells (iTreg, see below).
One trivial explanation for these results is that the iTreg rapidly die in the second cultures. However, when the CFSEhigh fractions of the control cells and the iTreg were kept in IL-2 for at least 2 days, the number of dead cells detected in both groups was similar (21–24% at 24 hr or 28–29% at 48 hr) and the cultured iTreg were still non-responsive to stimulation with IL-2 or anti-CD3 (data not shown).
CFSEhigh iTreg fail to up-regulate both IL-2 mRNA and CD25 on restimulation
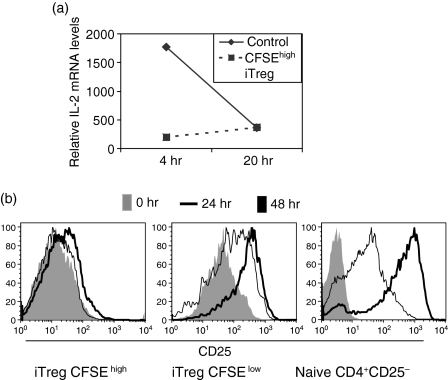
To determine the mechanisms responsible for the non-responsiveness of the CFSEhigh iTreg, we first restimulated them with anti-CD3 and measured their capacity to produce IL-2 mRNA by quantitative real-time PCR (Fig. 2a). While the CFSEhigh cells from the control cultures produced IL-2 mRNA after 4 hr of restimulation, only very low levels of IL-2 mRNA could be detected in cultures of CFSEhigh iTreg after 4 hr or 20 hr of restimulation. As the CFSEhigh iTreg also failed to respond to exogenous IL-2, we analysed their level of CD25 expression immediately after 4 days of coculture or following 24 hr or 48 hr of restimulation with anti-CD3. The CFSEhigh iTreg isolated from the cocultures expressed higher levels of CD25 compared to naive T cells (Fig. 2b, compare left and right panels), but failed to up-regulate expression at any time-point after TCR stimulation. In contrast, CFSElow iTreg expressed higher levels of CD25 following the coculture, which is consistent with their ability to respond to exogenous IL-2 (Fig. 2b, middle panel); they also up-regulated CD25 expression following stimulation with anti-CD3. T cells isolated from the control cultures already expressed high levels of CD25 that did not increase or decreased slightly following restimulation (data not shown).
Figure 2.
CFSEhigh iTreg express low levels of both IL-2 and IL-2 receptor. Naive CD4+ CD25– T cells (TCR transgenic RAG–/– mice) labelled with CFSE were cultured in presence or absence (control) of freshly isolated CD4+ CD25+ T cells (BALB/c) at a ratio of 1 : 1 in the presence of IL-4 (5 ng/ml) for 4 days. The TCR transgenic iTreg were then separated from the CD4+ CD25+ T cells and were sorted into CFSEhigh and CFSElow populations. (a) CFSEhigh iTreg were restimulated for 4 hr and 20 hr. Quantitative PCR for IL-2 mRNA was performed on CFSEhigh iTregs. Samples were normalized to 18S rRNA and a relative value of 1·0 was given to unstimulated CD4+ CD25– cells. (b) CFSEhigh and CFSElow iTreg were restimulated with anti-CD3 (0·5 μg/ml) and irradiated TdS for 0, 24 and 48 hr. CD25 expression was measured by flow cytometry. The results are representative of at least three independent experiments.
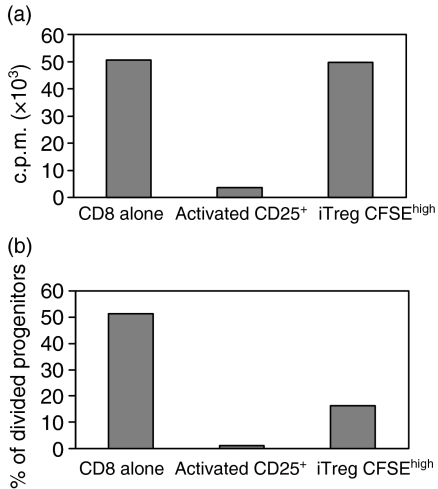
iTreg suppress the activation of both CD4+ CD25– and CD8+ responders
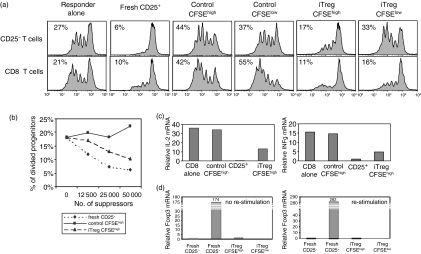
To evaluate whether iTreg would also exhibit suppressor activity, we separated them from the CD4+ CD25+ T cells after 4 days of coculture and evaluated their ability to suppress the response of freshly isolated CD4+ CD25– or CD8+ T cells. Significant suppression of the proliferative responses of both CD4+ and CD8+ T cells was seen in cultures containing CFSEhigh iTreg. CFSElow iTreg exhibited marginal suppressive effects on the response of CD4+ cells, but still suppressed CD8+ cells (Fig. 3a). In multiple experiments of this type, CFSEhigh iTreg also suppressed the responses of CD8+ T cells to a greater extent than CD4+ T cells. Neither CFSEhigh nor CFSElow cells from the control cultures exhibited suppressive activity and they frequently augmented proliferative responses because they produced IL-2 upon restimulation. Although CFSEhigh iTreg reproducibly suppressed T-cell activation, they were never as potent on a per cell basis as freshly isolated CD4+ CD25+ nTreg (Fig. 3b, CD8+ T cells as responders). The iTreg could be maintained in IL-2 for 3 days and remained anergic and suppressive (data not shown).
Figure 3.
CFSEhigh iTreg inhibit the responses of CD4 and CD8 T cells. (a) CFSEhigh iTreg, CFSElow iTreg or freshly isolated CD4+ CD25+ T cells (5 × 104/well) were cocultured with CFSE-labeled CD4+ CD25– or CD8+ T cells (2·5 × 104/well) and stimulated with anti-CD3 (0·5 μg/ml) and TdS. Proliferation of responder cells was measured by CFSE dilution at 66 hr. (b) CFSE dilution is represented as percentage of divided progenitors when the indicated numbers of Tregs are added to the coculture. The percentage of divided responder cells among the initial progenitors was calculated using flowjo software. (c) CD8+ T cells were cocultured with CFSEhigh iTreg or control cells for 24 hr. CD8+ T responder cells were separated from the coculture and quantitative PCR for IL-2 mRNA and IFN-γ mRNA was performed (d). CFSEhigh and CFSElow iTreg were left unstimulated or were restimulated with anti-CD3 and TdS. Quantitative PCR for Foxp3 was performed. Samples were normalized to 18S rRNA and a relative value of 1·0 was given to unstimulated CD8+ or CD4+ CD25– T cells. The results are representative of at least three independent experiments.
The hallmarks of the suppressive effects of CD4+ CD25+ nTreg in vitro are suppression of IL-2 mRNA induction in CD4+ CD25– and CD8+ responders as well as the suppression of the production of effector cytokines such as IFN-γ. CFSEhigh iTreg, but not control CFSEhigh cells, suppressed the ability of CD8+ responders to produce both IL-2 and IFN-γ mRNA in cocultures as measured by real-time PCR (Fig. 3c). Taken together these studies suggest that CFSEhigh iTreg resemble nTreg in many of their properties. However, we could not detect significant expression of Foxp3 as measured by real-time PCR immediately after separation or after restimulation with anti-CD3 (Fig. 3d), while nTreg as expected expressed high levels of Foxp3 mRNA. Foxp3 could also not be detected by intracellular staining in CFSEhigh iTreg (data not shown).
The generation of iTreg can be mediated by preactivated nTreg in the absence of TCR restimulation
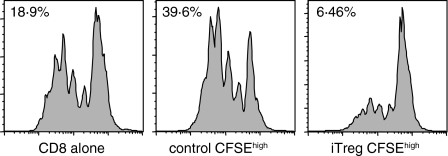
Previous studies15,16 have demonstrated that CD4+ CD25+ nTreg can be expanded in short-term cultures by stimulation with anti-CD3 and IL-2. Such preactivated nTreg are capable of mediating potent suppressor activity in an antigen-non-specific manner without a requirement for restimulation via their TCR. It was therefore of interest to determine if preactivated nTreg were also capable of inducing iTreg in an antigen-non-specific manner in the absence of TCR stimulation. nTreg from normal BALB/c mice were preactivated with anti-CD3 and IL-2 for 4–5 days, washed, and then cocultured with naive CD4+ CD25– T cells from mice expressing a transgenic TCR specific for a cytochrome C peptide on a RAG–/– background in the presence of APC and their cognate peptide. Following 4 days of coculture the responder cells were isolated and tested for their ability to suppress the proliferative response of CD8+ T cells to anti-CD3 (Fig. 4). CFSEhigh iTreg, but not control CFSEhigh cells, markedly suppressed the response of CD8+ T cells. This result suggests that once nTreg are activated via their TCR they are capable of both directly suppressing the responses of any type of responder T cells and of inducing the responders to develop into iTreg in the absence of a requirement for restimulation of the preactivated nTreg via their TCR.
Figure 4.
Induction of iTreg by preactivated nTreg is not antigen-specific. CD4+ CD25+ T cells were isolated from BALB/c mice and were activated by plate-bound anti-CD3 (0·5 μg/ml) and IL-2 (100 U/ml) for 4 or 5 days. CFSE-labelled CD4+ CD25– T cells (5 × 104/well) from 5CC7 TCR transgenic RAG–/– mice were stimulated with peptide (0·05 μm) and TdS (BALB/c, 5 × 104/well) and IL-4 (5 ng/ml) in the presence (iTreg) or absence (control) of preactivated CD4+ CD25+ T cells (5 × 104/well). After 4 or 5 days coculture, iTreg were separated from the CD4+ CD25+ T cells and were sorted into CFSEhigh and CFSElow populations. CFSEhigh iTreg and control cells (5 × 104/well) were then cocultured with CFSE-labelled CD8+ responder cells (2·5 × 104/well) stimulated with anti-CD3 (0·5 μg/ml) and TdS for 66 hr. The results are representative of at least three independent experiments.
iTreg require TCR stimulation to mediate suppression
In the studies above, all the experiments involved stimulation of the induction of the iTreg via the TCR and TCR restimulation in the assays of their suppressive function. To determine whether they resembled preactivated nTreg, which do not require TCR restimulation to mediate suppression, we generated iTreg from 5CC7 TCR transgenic RAG–/– mice as described above by anti-CD3 stimulation of cocultures, and then tested their suppressive function in cultures of CD8+ OT-I cells stimulated with SIINFEKL-pulsed T-depleted spleen cells. We failed to observe suppression when the iTreg were cocultured with OT-I T cells stimulated by antigen (SIINFEKL) (Fig. 5a), but suppression was manifest when the same cultures were stimulated with anti-CD3 (Fig. 5b). In Fig. 5(a), it was difficult to reliably observe suppression in CFSE dilution assays under the given conditions because some of the OT-I cells divided very rapidly before suppression was manifest. Subsequent cell divisions are more easily inhibited and detected in the [3H]TdR incorporation assay. Thus, in contrast to preactivated nTreg, iTreg must be restimulated via their TCR to mediate their suppressive effects.
Figure 5.
iTreg require TCR restimulation to mediate their suppressive function in vitro. CFSE-labelled CD8+ T cells were isolated from OT-I mice and stimulated with peptide pulsed TdS (a) or soluble anti-CD3 (0·5 μg/ml) and TdS (b) in the absence or presence of CFSEhigh iTreg. Proliferation was measured by [3H]TdR incorporation (a), or by the percentage of divided responder cells among the progenitors calculated by CFSE dilution peaks (b). The results are representative of at least three independent experiments.
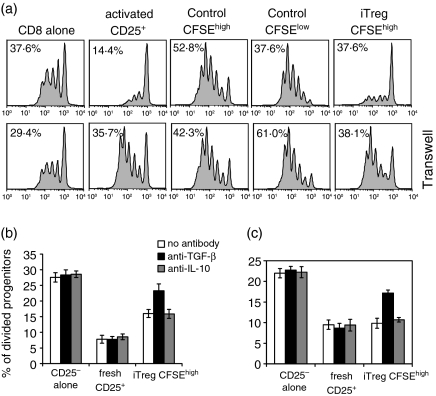
The generation of iTreg is independent of IL-10 and TGF-β
As a number of studies17,18 have demonstrated that regulatory T cells can be generated from naive CD4+ CD25– precursors in the presence of TGF-β and/or IL-10, we determined whether these cytokines play a role in the induction of iTreg in our model. We added anti-TGF-β and anti-IL-10 to the cocultures, isolated the iTreg after 4 days and tested their ability to suppress the proliferative responses of CD4+ CD25– or CD8+ responders. Neither anti-TGF-β nor anti-IL-10, separately or together (data not shown), had any effect on the generation of iTreg (Fig. 6).
Figure 6.
Anti-TGF-β and anti-IL-10 do not inhibit the generation of iTreg. Naive CD4+ CD25– T cells (5 × 104/well) purified from TCR transgenic RAG–/– mice were cocultured with CD4+ CD25+ T cells isolated from BALB/c mice (5 × 104/well) and IL-4 (5 ng/ml) in the presence or absence of anti-TGF-β (a) or anti-IL-10 (b), and anti-CD3 (0·5 μg/ml) and TdS (BALB/c (5 × 104/well). After 4 or 5 days, the coculture was sorted for CFSEhigh and CFSElow iTreg. Freshly isolated CFSE-labelled CD4+ CD25– T cells or CD8+ T cells were stimulated with soluble anti-CD3 (0·5 μg/ml) and TdS in the presence or absence of CFSEhigh iTreg for 66 hr. Proliferation was measured as the percentage of divided responder cells. The experiment was repeated at least three times, a representative result was chosen.
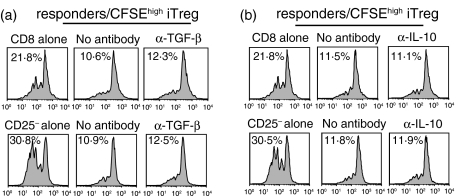
The suppressive effects of iTreg are cell-contact-dependent and partially TGF-β-dependent
Previous studies7–9 have demonstrated that iTreg generated from cocultures of human nTreg and CD4+ CD25– T cells exert their suppressive effects, in part, by secreting TGF-β and/or IL-10. To determine if soluble factors played a role in the suppressor effector function of iTreg in our model, we first determined if suppression could be observed when the iTreg were separated from the responder population across a semipermeable membrane in a trans-well. Both cell populations were stimulated in the presence of APC and soluble anti-CD3. Suppression was only observed when both populations were cocultured in the bottom well (Fig. 7a). This result strongly suggested that suppression by iTreg was cell-contact-dependent, which we have previously shown for the nTreg.3 However, when anti-TGF-β was added to cocultures of the iTreg with either CD4+ CD25– (Fig. 7b) or CD8+ (Fig. 7c), the responders partial reversal of the suppressive effects of the iTreg was observed, suggesting that TGF-β plays some role in this model.
Figure 7.
The suppressive function of CFSEhigh iTreg is cell contact-dependent, but anti-TGF-β partially abrogates suppression. (a) CD8+ T cells were stimulated with anti-CD3 (0·5 μg/ml) and TdS. CFSEhigh iTreg were added directly to the culture (upper panels) or the transwell (lower panels) in the presence of TdS at a ratio of 1 : 2 responder cells to iTreg. CFSEhigh iTreg were cultured with CFSE-labelled CD4+ CD25– T cells (b) or CD8+ T cells (c) and stimulated with soluble anti-CD3 (0·5 μg/ml) and TdS in the presence or absence of anti-TGF-β or anti-IL-10 antibodies. Proliferation was measured by CFSE dilution of responder cells. Two independent experiments were performed and the data are expressed as an average of the two experiments.
Discussion
We initially attempted to duplicate the protocol used for the induction of regulatory T cells by human nTreg8,9 by simply coculturing nTreg with naive responder T cells. We believed it was important to achieve maximum suppression of responder T-cell activation by nTreg to observe the induction of anergy or suppressive activity in the responders and therefore set up the cocultures as a ratio of nTreg : responder of 1 : 1. Using these conditions, recovery of viable responder cells was negligible after 72–96 hr of coculture. We have previously shown that nTreg induce a G1-S arrest in the cell cycle in responder cells and such an arrest is most often followed by cell death.19 As an alternative approach, we added IL-4 to the cocultures to maintain cell viability. Although IL-4 partially masks the suppression of cell proliferation induced by the nTreg,20 the induction of IL-2 mRNA in the responder cells was still completely inhibited.21 Thus, suppression is maintained in the presence of proliferation of both the responders and suppressors. After 96 hr of culture, viability of the recovered responders was similar to that seen in cultures of responders alone. It is also possible that IL-4 played some role in the induction of the iTreg. However, we could not recover cells in the absence of IL-4 and cell recovery was also low when IL-7 was used in place of IL-4.
In contrast to control cultures, where 80–90% of the responders proliferated, two distinct populations of cells were isolated from the cocultures performed in the presence of IL-4. About one-third of the responders had diluted CFSE, while two-thirds remained in a resting state. Most importantly, both cell populations were completely unresponsive to restimulation via the TCR in the presence of APC. The population of CFSEhigh cells also failed to respond to exogenous IL-2, while the responder cells that had divided in the cocultures responded normally. The proportion of CFSEhigh and CFSElow cells did not change when the cocultures were performed for longer time periods. One important question that must be addressed is whether the anergic state of the undivided cells was actually the result of their interaction with nTreg or simply due to anergy induced by TCR occupancy in the absence of proliferation as described several years ago.22 The vast majority of responders in both the control and cocultures expressed CD69 consistent with their receipt of a TCR signal. We therefore isolated the minor population of undivided cells from the control cultures. This cell population responded normally to stimulation via their TCR, indicating that the anergic state of the responders recovered from the cocultures was secondary to their exposure to nTreg. The anergic state of the undivided cells from the cocultures was characterized by their failure to synthesize IL-2 mRNA upon restimulation. Although the CFSEhigh subset expressed low levels of CD25 on their cell surface following the coculture, they failed to up-regulate CD25 upon restimulation via the TCR. This failure to up-regulate CD25 expression closely resembles the defect induced in CD8+ T cells by nTreg that also fail to up-regulate CD25 expression when cocultured with nTreg.23 Similar results were obtained by Duthoit et al.24 who found that CD4+ T cells isolated from cocultures with preactivated nTreg uniformly expressed high levels of CD25, but failed to respond to exogenous IL-2. Although cells isolated from our cocultures performed in the presence of exogenous IL-4 after 4–5 days were anergic when restimulated via the TCR in the presence of APC, Duthoit et al.24 found that responder cells isolated after only 24 hr of coculture (in the absence of added cytokines) proliferated normally when restimulated via the TCR; their ability to both make and respond to IL-2 was restored. There are numerous differences in the protocols used by Duthoit et al. and by us that could account for these differences.
Our ability to readily induce T-cell anergy by coculture of nTreg with naive responder cells in the presence of IL-4 differs markedly from recent studies21 that claimed that IL-4 actually prevented the induction of anergy when nTreg were cocultured with CD4+ CD25– T cells. In these studies, only responder cells from IL-4Rα–/– mice that could not respond to IL-4 failed to respond to restimulation with IL-2 or anti-CD3, while responder cells from wild-type mice responded normally. There are several differences between our studies and those of Pace et al.21 First, we separated the responder T cells into those that underwent cell division (CFSElow) in the first culture and those that did not divide (CFSEhigh). Both populations were anergic to stimulation via the TCR, while only the latter were anergic to restimulation with IL-2. Second, the magnitude of the response to anti-CD3 stimulation in the Pace et al. study was so low, even in the control cultures, that it was very difficult to conclude that anergy had actually been induced. As the restimulation assays in these studies were performed after 3 days of restimulation, it is likely that the peak response that occurs after 24 hr of culture was missed (unpublished observations).
In addition to the induction of anergy in the CD4+ CD25– responders, coculture with nTreg also induced suppressor function in the responders. There are a number of similarities and differences between the suppressive function of nTreg and iTreg. First, the iTreg fail to express any detectable Foxp3 mRNA or intracellular Foxp3 protein (data not shown), while Foxp3 is readily detected at high levels in nTreg. The suppressive activity of the iTreg is never as potent as that observed with an equivalent number of nTreg. The nTreg are, in general, more effective suppressors of CD8+ responders, and this is also true of the iTreg. Activated nTreg will exhibit potent non-specific suppressor effector function in the absence of restimulation via their TCR. In contrast, iTreg, although activated, must be restimulated via the TCR to mediate suppression. There are several possibilities for the modest level of suppression seen with the iTreg. Although the anergic state of these cells appears to be profound, it is likely that we have only converted a subpopulation of these cells to manifest suppressor function. The iTreg that had divided in the induction culture were much less suppressive than the undivided iTreg. It is also possible that modifying the induction culture (ratio of nTreg : responders) may result in more potent suppressor activity. The suppressive activity of both nTreg and iTreg appeared to require cell contact because suppression was not observed when the suppressors and responders were separated in a transwell culture. In contrast to our failure to neutralize suppression in the cocultures of nTreg and responders with anti-IL-10 or anti-TGF-β, we did observe moderate neutralization of the suppressive activity of iTreg with anti-TGF-β, but not anti-IL-10. It is possible that cell surface TGF-β25 may be mediating some of the suppressive effects of the iTreg, but we failed to detect TGF-β on iTreg by fluorescence-activated cell sorting (data not shown). As the magnitude of the suppressive effects of the iTreg is not high, it remains possible that neutralization of TGF-β produced by the responder cells in the cocultures simply raises their threshold for suppression by the iTreg. Some of the activity of human iTreg appears to be mediated by TGF-β but this appears to be secreted by the iTreg.10 Although the iTreg failed to express Foxp3, they may mediate suppression via the same, as yet uncharacterized, mechanism used by nTreg. Cell contact-dependent suppression has also been observed with Foxp3-like, Tr1-like cells induced with IL-10, even though the cells are capable of producing IL-10.26
Our results are compatible with the view that some of the potent effects of nTreg in the suppression of immune responses in vivo may be secondary to conversion of potentially autoreactive effector cells to anergic non-pathogenic cells or to induction of cells with the capacity to actively suppress. We intentionally used naive responder T cells from TCR transgenic RAG–/– mice as responders in all studies to definitively rule out the possibility that the cells isolated from our cocultures were derived from Foxp3+ CD25– T cells, which comprise about 10% of the Foxp3+ population in the mouse. Some of the iTreg in earlier studies8–10 may have been derived from this Foxp3+ CD25– subset. We have not yet tested whether iTreg can be generated from memory cells or from T helper type 1 and 2 effector populations. Neither IL-10 nor TGF-β appeared to be required for the induction of iTreg, although Foxp3+ regulatory T cells have been generated in a TGF-β-dependent fashion in vivo from completely Foxp3– precursors27 and IL-10 has a well-established role in the generation of Foxp3– Tr-1 cells.27,29 The critical question that remains to be addressed is whether iTreg are also induced in vivo in the presence of nTreg. Although an infectious mechanism of action of nTreg is attractive in explaining how a small number of cells can exert such potent effects, it is still unclear whether the immune suppression induced by nTreg in vivo is permanent or requires the continuous presence of the nTreg. Only one study has specifically addressed this question and it appeared that nTreg-mediated protection from autoimmune oophoritis was completely abolished when the nTreg were depleted from a protected host.30 At least in this model, it did not appear that the induction of iTreg played a role in preventing the activation of autoimmune effectors. Studies in different models of nTreg function including tumour immunology and infectious disease are needed to resolve whether they mediate infectious tolerance.
Glossary
Abbreviations:
- APC
antigen-presenting cells
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- iTreg
induced T regulatory cell
- nTreg
naturally occurring regulatory T cells
- TdS
T-depleted spleen cells
References
- 1.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 3.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–91. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge. Human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 7.Ermann J, Szanya V, Ford GS, Paragas V, Fathman CG, Lejon K. CD4(+) CD25(+) T cells facilitate the induction of T cell anergy. J Immunol. 2001;167:4271–5. doi: 10.4049/jimmunol.167.8.4271. [DOI] [PubMed] [Google Scholar]
- 8.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+) CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J Exp Med. 2002;196:247–53. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stassen M, Fondel S, Bopp T, et al. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303–11. doi: 10.1002/eji.200324656. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Seder RA, Paul WE, Davis MM, de St Groth BF. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–8. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogquist KA, Jameson SC, Heath WR, Howare JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 14.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 15.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 16.Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol. 2005;175:3053–9. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- 17.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashwell JD, Cunningham RE, Noguchi PD, Hernandez D. Cell growth cycle block of T cell hybridomas upon activation with antigen. J Exp Med. 1987;165:173–94. doi: 10.1084/jem.165.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge. IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–23. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 21.Pace L, Rizzo S, Palombi C, Brombacher F, Doria G. Cutting edge. IL-4-induced protection of CD4+CD25– Th cells from CD4+CD25+ regulatory T cell-mediated suppression. J Immunol. 2006;176:3900–4. doi: 10.4049/jimmunol.176.7.3900. [DOI] [PubMed] [Google Scholar]
- 22.DeSilva DR, Urdahl KB, Jenkins MK. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J Immunol. 1991;147:3261–7. [PubMed] [Google Scholar]
- 23.Piccirillo CA, Shevach EM. Cutting edge. control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 24.Duthoit CT, Mekala DJ, Alli RS, Geiger TL. Uncoupling of IL-2 signaling from cell cycle progression in naive CD4+ T cells by regulatory CD4+CD25+ T lymphocytes. J Immunol. 2005;174:155–63. doi: 10.4049/jimmunol.174.1.155. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+) CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira PL, Christensen JR, Minaee S, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–93. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 27.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–10. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 28.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 29.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–8. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+ CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–81. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]