Abstract
T-cell differentiation is driven by a complex network of signals mainly derived from the thymic epithelium. In this study we demonstrate in the human thymus that cortical epithelial cells produce bone morphogenetic protein 2 (BMP2) and BMP4 and that both thymocytes and thymic epithelium express all the molecular machinery required for a response to these proteins. BMP receptors, BMPRIA and BMPRII, are mainly expressed by cortical thymocytes while BMPRIB is expressed in the majority of the human thymocytes. Some thymic epithelial cells from cortical and medullary areas express BMP receptors, being also cell targets for in vivo BMP2/4 signalling. The treatment with BMP4 of chimeric human–mouse fetal thymic organ cultures seeded with CD34+ human thymic progenitors results in reduced cell recovery and inhibition of the differentiation of human thymocytes from CD4− CD8− to CD4+ CD8+ cell stages. These results support a role for BMP2/4 signalling in human T-cell differentiation.
Keywords: bone morphogenetic protein, human thymus, thymic epithelial cells, thymocytes
Introduction
T-cell differentiation involves a highly ordered and strictly regulated maturation programme that requires the specific microenvironment provided by the thymus. The thymus is a lobulated lymphoepithelial organ, every thymic lobule being composed of two distinct zones, the cortex and the medulla. The cortex is separated from the connective capsule by a superficial layer of flattened and tightly joined epithelial cells that form the subcapsule. Each of these thymic compartments provides a different microenvironment appropriate to the specific processes that occur during T-cell development.1–3 In humans, the haematopoietic progenitors that seed the postnatal thymus are CD4– CD8– double-negative (DN) cells that express high levels of the antigen CD34. These cells have the capacity to differentiate into multiple cell lineages including T cells, dendritic cells (DCs), natural killer (NK) cells and monocytes.3,4 Thymic progenitors enter the thymus through the perimedullary cortical venules, and migrate across the cortex before accumulating in the subcapsular zone, undergoing progressive developmental changes during this process.5 T-cell-lineage-committed precursors (CD34+ CD5+ CD1+) give rise to immature single-positive CD34– CD4+ CD8– cells that differentiate into CD4+ CD8+ double-positive (DP) cells, located in the cortex of the thymus. This is a heterogeneous cell population that represents the majority of thymocytes and includes cells that are rearranging their T-cell receptor β (TCR-β) gene as well as others that already express cytoplasmic TCR-β chain.4,6 Upon productive TCR-α gene rearrangement, DP TCR-αβ+ cells die unless they are positively selected after the occurrence of low-affinity recognition of self-peptide–major histocompatibility complex (MHC) complexes expressed by the thymic epithelium. In contrast, those thymocytes showing high affinity for self-peptide–MHC complexes expressed in thymic DCs and B lymphocytes will be deleted in a process known as negative selection.4,7 Positively selected DP cells then differentiate into CD4+ CD8– and CD4– CD8+ single-positive (SP) cells through distinct phenotypic and functionally defined cell stages. SP thymocytes localize in the medulla and eventually emigrate to secondary lymphoid organs.8
The thymus, comparable with every cellular system supporting a differentiation process, requires specific signals that stringently govern phenomena as diverse as the maintenance of the progenitor cell population or the regulation of the number, phenotype and function of the mature cells produced. Considerable progress has been made in understanding the regulation of intrathymic T-cell differentiation and several reports have demonstrated the involvement in this process of the most important families of morphogens, Hedgehog (Hh) proteins, Wnt proteins and, belonging to transforming growth factor-β superfamily, bone morphogenetic protein 2 (BMP2) and BMP4.1,9–13 Morphogens are secreted proteins that form functional gradients from their source. They are signalling molecules that are capable of governing differentiation, expansion and survival of the cells under their influence. Accordingly, they play an essential role both in the embryo, orchestrating morphogenesis, and in the adult, ensuring the regenerative capacity of diverse organs that maintain a stem cell population that continuously differentiates throughout life.14–17
BMP2 and BMP4 belong to the decapentaplegic subgroup of BMP ligands. They signal through a common pathway that requires the hetero-oligomerization of two types of serine/threonine membrane receptor kinases, the type I receptors, BMPRIA or BMPRIB, and the type II receptor, BMPRII. Upon BMP-binding, type II receptors phosphorylate type I receptors, turning on their kinase activity, which initiates the intracellular pathway. The type I receptors activate BMP receptor-regulated SMADs (BR-SMADs: SMAD1, SMAD5 and SMAD8) by phosphorylation. BR-SMADs bind to the common partner SMAD4 (Co-SMAD).18,19 This heteromeric complex translocates into the nucleus where it regulates gene expression using different mechanisms, including direct DNA binding and interaction with transcription factors, or with specific transcriptional co-activators or co-repressors.20,21
BMP signalling is stringently regulated at multiple levels. Different secreted proteins, such as Noggin, Chordin, Chordin-like, Follistatin, FSRP, DAN, Cerberus, Gremlin and Esclerostin, bind selectively to BMPs, impeding their biological activities. A different protein, Twisted gastrulation, promotes or inhibits BMP function depending on the activity of Chordin.22 At the cell surface, the pseudoreceptor BAMBI/Nma, which is structurally similar to type I receptors but lacks the intracellular serine/threonine kinase domain, stably associates with type II receptors, so preventing the formation of active receptor complexes.23 In contrast, BMP co-receptors, DRAGON and RGMa, potentiate BMP signalling.24,25 Inside the cell, the inhibitory SMAD proteins, SMAD6 and SMAD7, prevent the activation of BR-SMADs, or their binding to SMAD4;26 and two factors, Smurf1 and Smurf2, seem to promote the degradation of SMAD proteins and type I receptors.20,21
BMPs were originally described by their capacity to induce bone and cartilage formation.27,28 However, further studies have revealed a much broader range of biological activities of the different components of the BMP family. It is now known that BMPs regulate the proliferation, differentiation, chemotaxis and apoptosis of different cell types17,29–31 and are involved in many developmental processes, including left–right asymmetry,32 patterning of the central nervous system33 and the development of many organs including kidney,34 gut,35 lung,36 skin,37 tooth,31 limb38 and testis.31 The BMP signalling also seems to participate in the proliferation and differentiation of human bone marrow haematopoietic stem cells, in synergy with several cytokines.39,40 In the immune system, we and others have recently described a role for BMP2 and BMP4 in murine T-cell development.41–44 These reports show the intrathymic expression of BMPs, their receptors, molecules involved in their signalling pathway, and regulatory molecules, as well as an inhibitory role of BMPs in murine thymocyte maturation. Recently, the involvement of BMPs in murine thymus organogenesis has also been described.45,46 In the current study we show that BMP2 and BMP4 and all the molecular machinery required for their signalling are expressed in the human thymus and we demonstrate that BMP4 treatment inhibits human T-cell differentiation acting on the maturation of thymic CD34+ cell progenitors.
Materials and methods
Isolation of human thymocyte subsets
Human thymus samples from patients aged 1 month to 5 years undergoing corrective cardiac surgery were obtained and used according to the guidelines of the Medical Ethics Commission of La Zarzuela and Madrid-MontePríncipe Hospitals (Madrid, Spain). Informed consent was provided according to the Declaration of Helsinki. Thymuses were dissected free of the surrounding connective tissue and then gently disrupted with a Potter homogenizer until completely disaggregated. Thymocyte subpopulations defined by their CD4 CD8 expression were purified from the whole thymic cell suspensions using a CD4 Multisort Kit and CD8-Microbeads (Miltenyi Biotec, Bergisch, Germany). To isolate thymic CD34+ precursors, thymocyte suspensions were enriched in immature thymocytes by using the sheep red blood cell rosetting technique followed by monoclonal antibody (mAb)-coupled magnetic bead treatment (Dynabeads; Dynal, Oslo, Norway) to deplete monocytes and T, B and NK cells as previously described.47 CD34+ cells were then purified by magnetic sorting using a CD34 Progenitor Cell Selection System (Dynal) following the manufacturer's instructions. The purity of the recovered subpopulations was always greater than 98%.
Isolation of human thymic epithelial cells
Thymus fragments were cultured floating on Millipore filters (8-μm pore size) (Millipore Ibérica, Madrid, Spain) in RPMI-1640 (Invitrogen, Grand Island, NY) supplemented with 5% fetal calf serum (Harlan Sera-Laboratory; Leicestershire, UK) and 1·35 mm 2-deoxyguanosine (Sigma España; Madrid, Spain). After 7 days, thymic fragments were trypsinized (0·25% trypsin in 0·02% ethylenediaminetetraacetic acid) (Sigma España) to form a single-cell suspension. Residual thymocytes, DCs and NK, B and myeloid cells were depleted as described above by adding anti-CD34 to the cocktail of purified mAbs.
Cell lines
Human postnatal thymic epithelial cell lines P1.1A3 and P1.4D6 were kindly provided by Dr M. L. Toribio (Centro de Biología Molecular ‘Severo Ochoa’; UAM, Madrid, Spain).48 Cell lines were maintained in RPMI-1640 supplemented with 10% fetal calf serum, 2 mm l-glutamine (Invitrogen), and penicillin/streptomycin (Invitrogen) at 37° in 5% CO2, and were trypsinized before confluence.
Reverse transcription–polymerase chain reaction (RT-PCR) analysis
RNA isolation was performed using a Strataprep Total RNA Miniprep Kit (Stratagene Cloning Systems; La Jolla, CA), including a DNase I digestion step, as recommended by the supplier, to avoid genomic DNA contamination. Total cDNA was synthesized with Superscript II RT polymerase (Invitrogen), according to the instructions of the commercial supplier and then used as target in the PCR amplifications. Amplifications were performed using the following primer sets and annealing conditions: β-actin, forward primer 3′-AGAGATGGCCACGGCTGCTT-5′, reverse primer 5′-ATTTGCGGTGGACGATGGAG-3′ at 61° with a 445-base-pair (bp) product; BMP2 forward primer 3′-TCAGCAGAGCTTCAGGTTTT-5′, reverse primer 5′-TTCCACCCCACGTCACT-3′ at 60° with a 494-bp product; BMP4 forward primer 3′-AAAGGGGCTTCCACCGTAT-5′, reverse primer 5′-CCGCTGTGAGTGCTTAG-3′ at 58° with a 386-bp product; BMPRIA forward primer 3′-CATTCGATGGCTGGTTTTG-5′, reverse primer 5′-TCCATATCGGCCTTTACCAA-3′ at 58° with a 298-bp product; BMPRIB forward primer 3′-CATGCTTTTGCGAAGTGCAG-5′, reverse primer 5′-CAGGCAACCCAGAGTCATCC-3′ at 60° with a 197-bp product; BMPRII forward primer 3′-AAGCGAGGTTGGCACTATCA-5′, reverse primer 5′-TTTGGCACACGCCTATTATGT-3′ at 56° with a 495-bp product; SMAD1 forward primer 3′-GCAAAGTCGGAACTGCAACTA-5′, reverse primer 5′-GAGGTGAACCCATTTGAGTAAGA-3′ at 60° with a 308-bp product; SMAD4 forward primer 3′-CAGCACCACCCGCCTATG-5′, reverse primer 5′-CCAAACATCACCTTCACCTTTACAT-3′ at 60° with a 330-bp product; SMAD5 forward primer 3′-TCCAGCAGTAAAGCGATTGT-5′, reverse primer 5′-TGGAAACGTGGCATTTTG-3′ at 58° with a 487-bp product; SMAD8 forward primer 3′-ACGTCGGGGGAGAGGTGTAT-5′, reverse primer 5′-GGTGACATCCTGGCGATGA-3′ at 60° with a 277-bp product. Primers were designed from sequences available from the GenBank database (accession numbers β-actin, BCO16045; BMP2, NM_001200; BMP4, NM_001202; BMPRIA, NM_004329; BMPRIB, NM001203; BMPRII, NM_033346; SMAD1, NM_005900; SMAD4, NM_005359; SMAD5, NM_005903; SMAD8, NM_005905). All PCR analyses were performed on a Mastercycler gradient machine (Eppendorf; Hamburg, Germany) using AmpliTaqGold DNA polymerase (Applied Biosystems; Foster City, CA) under the following conditions: 3 min at 94°, 40 cycles of 45 seconds at 94°, 45 seconds at each particular annealing temperature and 45 seconds at 72°, followed by 10 min at 72°. PCR products were resolved on a 2% agarose gel and the measured sizes were as expected.
Histology and immunofluorescence
Thymus cryosections (5 μm) were air-dried for 2 hr at room temperature and fixed in acetone for 10 min. Non-specific binding of antibodies and streptavidin was blocked by incubation with diluted donkey serum (Santa Cruz Biotechnology, Santa Cruz, CA) and avidin-biotin (Vector Laboratories, Burlingame, CA). Human thymus sections were incubated with specific goat antibodies against BMP2 (N-14), BMP4 (N-16), BMPRIA (E-16) (Santa Cruz Biotechnology), BMPRII (AF811) (R & D Systems, Minneapolis, MN), and a specific mouse mAb against BMPRIB (88614) (R & D Systems). Then, they were stained with biotin or fluorescein isothiocyanate (FITC)-conjugated Fab′ fragments of donkey anti-goat immunoglobulin G (IgG) or donkey anti-mouse IgG (Jackson Immunoresearch Laboratories, West Grove, PA). The sections incubated with biotin-conjugated antibodies were further stained with Texas Red-conjugated streptavidin (Amersham Biosciences, Munich, Germany). Thymic epithelial cells were detected using specific mouse mAb against human leucocyte antigen (HLA)-DR (G46-6) (BD Biosciences, San Jose, CA), followed by Texas red-conjugated Fab′ fragments of donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories) or using a FITC-conjugated anti-cytokeratin antibody (Sigma España). Thymocytes were stained using a biotin anti-CD5 mAb (CD5-5D7) (Caltag, Burlingame, CA) followed by Texas red–streptavidin. In all the experiments the secondary antibodies employed were solid-phase multiadsorbed and showed minimal cross-reactivity to other species. Slides were mounted in Vectashield (Vector Laboratories) and examined on a Zeiss Axioplan-2 microscope (Zeiss; Oberkochen, Germany) from the Centro de Microscopía y Citometría, Complutense University of Madrid.
Animals
CB-17 scid/scid (severe combined immunodeficiency) mice, originally purchased from Harlan (Harlan Iberica, Barcelona, Spain), were bred in our own pathogen-free breeding facility. To obtain timed pregnancies, female and male mice were mated overnight and the day of the plug was considered day 0. Fetal thymic lobes were dissected from embryos at day 15 of gestation.
Chimeric human–mouse fetal thymic organ cultures (hu-mo FTOC)
Human CD34+ thymic progenitors (1 × 104 to 2 × 104 cells/lobe) were transferred to thymic lobes derived from 15-day-old embryos of SCID mice by the hanging drop method for 48 hr, followed by culture of the recolonized lobes in FTOC as described previously.49 Cultures were supplemented with human recombinant BMP4 at a concentration of 100 ng/ml (R & D Systems) throughout the culture period. Medium was replaced every week.
Flow cytometry
To analyse the differentiation of human cells, mouse thymuses from hu-mo FTOC were dispersed into single-cell suspensions and stained with mAbs specific for human cell surface antigens, CD45 (HI30-phycoerythrin), CD4 (SK3-peridinin chlorophyll protein), CD5 (UCHT2-FITC) and CD8α (SK1-allophycocyanin) (BD Biosciences). Flow cytometric analysis was then performed on electronically gated CD45+ human cells. Cell cycle analysis was performed after surface staining. Cells were fixed overnight using Cellfix (BD Biosciences) and incubated for 30 min in a solution containing 30% ethanol−1% bovine serum albumin and 5 μg/ml Hoechst 3342 (Molecular Probes, Leiden, the Netherlands). Cycling cells were determined, discarding apoptotic cells, as those with a DNA cell content between 2C and 4C. Analyses were conducted in a three-laser BD LSR flow cytometer (BD Biosciences) from the Centro de Microscopía y Citometría UCM, Madrid. For apoptosis assays, cells were stained with Annexin-V-FITC (Boehringer Mannheim, Mannheim, Germany) according to the supplier's instructions. Cells were analysed on a FACScan (Centro de Microscopía y Citometría UCM) and gated according to forward scatter, side scatter, and their ability to exclude propidium iodide. Apoptotic cells were considered to be those that were annexin-V positive and propidium iodide negative.
Results
BMP2 and BMP4 are produced in the human thymus
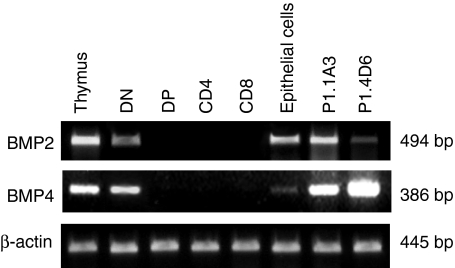
To analyse the expression of BMP2 and BMP4 genes in the human thymus we performed RT-PCR on total RNA samples obtained from thymic tissue fragments. These experiments demonstrated the presence of RNAs encoding for both proteins in the organ (Fig. 1). Further analyses using isolated thymocyte subpopulations defined according to CD4 and CD8 expression showed that BMP2 and BMP4 transcripts were expressed in DN cells, an heterogeneous and minority thymic population that includes CD34+ thymic progenitors. However, we were unable to detect them in DP, CD4+ or CD8+ thymocyte subsets. The presence of specific transcripts for these two proteins was also detected in purified thymic epithelial cells as well as in the human thymic epithelial cell lines P1.1A4 and P1.4D6 (Fig. 1).
Figure 1.
RT-PCR analysis of the expression in the human thymus of BMP2 and BMP4. Primer pairs specific for BMP4 and BMP2 were used to determine their presence in total thymus, DN (CD4– CD8–), DP (CD4+ CD8+), CD4 (CD4+ CD8–) and CD8 (CD4– CD8+) thymocytes, thymic epithelial cells and human thymic epithelial cell lines P1.1A3 and P1.4D6. Band sizes are indicated.
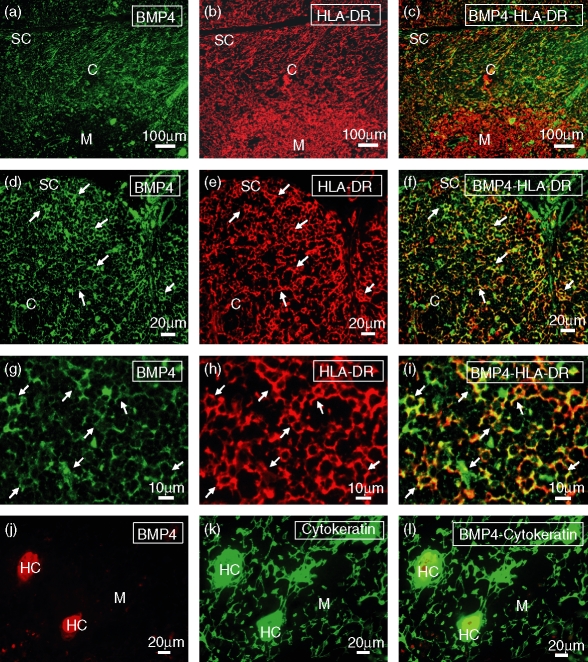
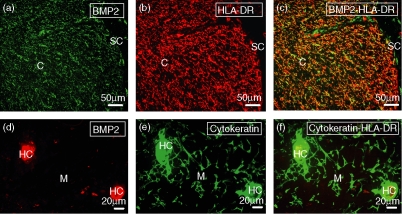
The histological localization of cells producing BMP2 and BMP4 in the human thymus was demonstrated by an immunofluorescence analysis on tissue cryosections. BMP2 and BMP4 showed a very similar expression pattern, being both expressed in the subcapsular area and throughout the thymic cortex as a reticular network corresponding to thymic epithelial cells, as supported by the coexpression of HLA-DR and cytokeratin (Figs 2 and 3). In contrast, in the thymic medulla BMP2/4 expression was mainly restricted to Hassall's corpuscles (Figs 2 and 3).
Figure 2.
Localization of BMP4-expressing cells in the human thymus. Frozen sections of human thymus were doubled stained with anti-BMP4 (a, d, g, j) and anti-HLA-DR (b, e, h) or anti-cytokeratin (k) antibodies. (a–f) BMP4 expression (green fluorescence; a, d) is mostly restricted to the cortical and subcapsular areas, appearing as a reticular network associated with HLA-DR+ epithelial cells (red fluorescence; b, e). (g–i) BMP4 expression (green fluorescence; g) shows a punctate pattern distributed in the soma and the cellular processes of the cortical HLA-DR+ epithelial cells (red fluorescence; h). (j–l) In the thymic medulla, BMP4 expression (red fluorescence; j) is associated with cytokeratin-positive Hassall's corpuscles (HC; green fluorescence; k). Yellow fluorescence demonstrates the expression of BMP4 in subcapsular and cortical epithelial cells (c, f, i) and medullary Hassall's corpuscles (l). C, cortex; M, medulla; SC, subcapsule.
Figure 3.
Distribution of BMP2-producing cells in the human thymus. Human thymic cryosections were double stained with anti-BMP2 (a, d) and anti-HLA-DR (b) or anti-cytokeratin (e) antibodies. (a–c) BMP2 expression (green fluorescence; a) is found in HLA-DR+ thymic epithelial cells (red fluorescence; b) located in the subcapsule and cortex of the thymus. (d–f) Detail of thymic medulla showing the expression of BMP2 (red fluorescence; d) on cytokeratin-positive epithelial cells from Hassall's corpuscles (HC; green fluorescence; e). Yellow fluorescence demonstrates the expression of BMP2 in subcapsular and cortical epithelial cells (c) and medullary Hassall's corpuscles (f). C, cortex; M, medulla; SC, subcapsule.
Expression of BMP2/4 receptors in the human thymus
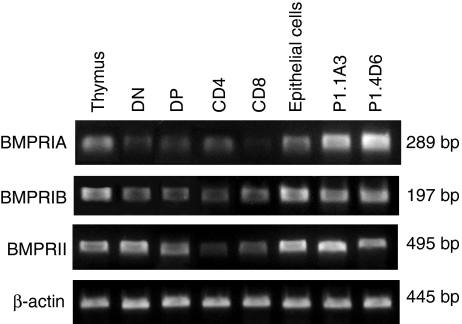
RNAs encoding for BMPRIA, BMPRIB and BMPRII were detected in all thymocyte subpopulations as well as in the thymic epithelium (Fig. 4). P1.1A3 and P1.4D6 thymic epithelial cell lines also expressed the three receptors (Fig. 4).
Figure 4.
RT-PCR analysis of the expression of BMP2/4 receptors in the human thymic cell populations. Expression of BMPRIA, BMPRIB and BMPRII on total thymus, DN (CD4– CD8–), DP (CD4+ CD8+), CD4 (CD4+ CD8–) and CD8 (CD4– CD8+) thymocytes, thymic epithelial cells, and P1.1A3 and P1.4D6 thymic epithelial cell lines. Band sizes are indicated.
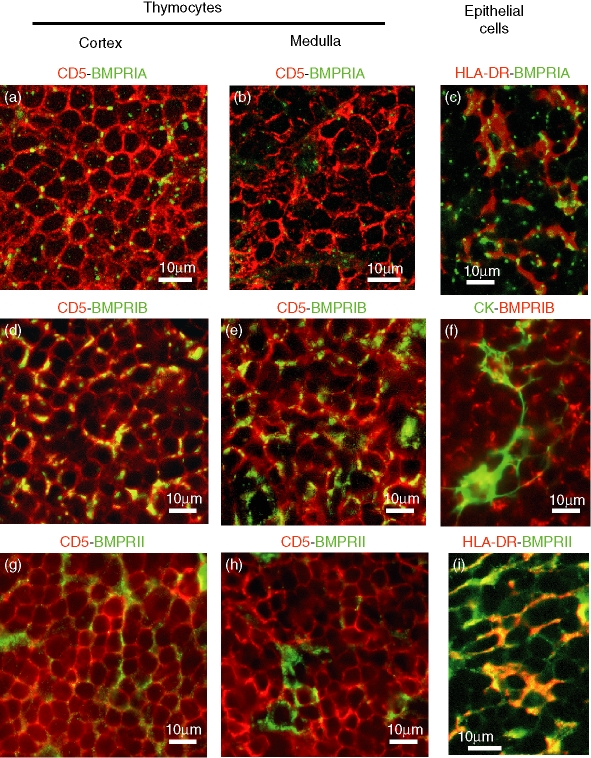
Although these results demonstrated that all the thymic populations analysed expressed BMP2/4 receptors, the immunofluorescent analysis on thymic tissue sections revealed that the cells that expressed these receptors were not homogeneously distributed throughout the thymic parenchyma. We found that BMPRIA-expressing and BMPRII-expressing cells showed a very similar distribution in the organ. In the cortex, BMPRIA-positive and BMPRII-positive cells mostly corresponded to CD5+ thymocytes (Fig. 5a,g). In contrast, the majority of BMPRIA-expressing and BMPRII-expressing cells in the medullary area were CD5– cytokeratin+ epithelial cells (Fig. 5b,h). The expression of BMPRIA in the thymic cells was markedly restricted to punctate, brightly stained areas, whereas BMPRII staining was weaker and confined to larger areas.
Figure 5.
Double immunofluorescence analysis of BMP2/4 receptors in human thymocytes and thymic epithelial cells. To analyse the expression of BMP receptors in thymocytes, tissue sections were double-stained with the pan-thymocyte marker CD5 (red fluorescence), and anti-BMPRIA (a, b), anti-BMPRIB (d, e) or anti-BMPRII (g, h) antibodies (all of them in green fluorescence). Yellow fluorescence demonstrates the expression of BMP receptors in thymocytes located in the thymic cortex (a, d, g) or medulla (b, e, h). Thymic sections were also double-stained with anti-HLA-DR (red fluorescence; c, i) or anti-cytokeratin (green fluorescence;f)antibodies, for the identification of thymic epithelial cells, and anti-BMPRIA (green fluorescence; c), anti-BMPRIB (red fluorescence; f) or anti-BMPRII (green fluorescence; i) antibodies. Micrographs show details of the thymic cortical area and the yellow fluorescence demonstrates BMP receptor expression in thymic epithelial cells.
The immunofluorescence analysis also showed that BMPRIB was expressed by most thymocytes, and was located both in the cortex and the medulla (Fig. 5d–f). Although the majority of the BMPRIB-positive cells were CD5+ thymocytes, some cortical and medullary epithelial cells also expressed this receptor. In addition, in all positive cells BMPRIB staining was restricted to localized areas, as punctate patches (Fig. 5d–f).
Expression of the BMP-SMAD transcription factors in the human thymus
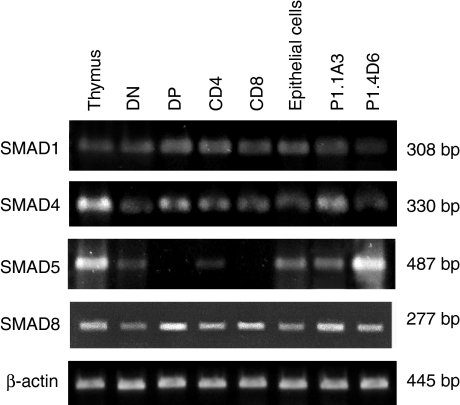
RT-PCR analysis detected the presence of RNAs encoding for SMAD1, SMAD4 and SMAD8 in all thymocyte subpopulations. We also detected SMAD5-encoding RNAs in both DN and CD4+ cell subsets, but not in DP and CD8+ thymocyte subsets. Likewise, the thymic epithelial cell component expressed all the necessary molecular machinery for the BMP2/4 signal transduction, as shown by the RT-PCR analysis performed on isolated thymic epithelial cells and the thymic epithelial cells P1.1A3 and P1.4D6 (Fig. 6).
Figure 6.
RT-PCR analysis of the expression of the specific SMADs that mediate BMP signalling in the human thymus. Expression of BR-SMADs, SMAD1, SMAD5 and SMAD8, and the common-partner SMAD4 on total thymus, DN (CD4– CD8–), DP (CD4+ CD8+), CD4 (CD4+ CD8–) and CD8 (CD4– CD8+) thymocytes, thymic epithelial cells, and P1.1A3 and P1.4D6 thymic epithelial cell lines. Band sizes are indicated.
Effects of BMP4 on human T-cell differentiation
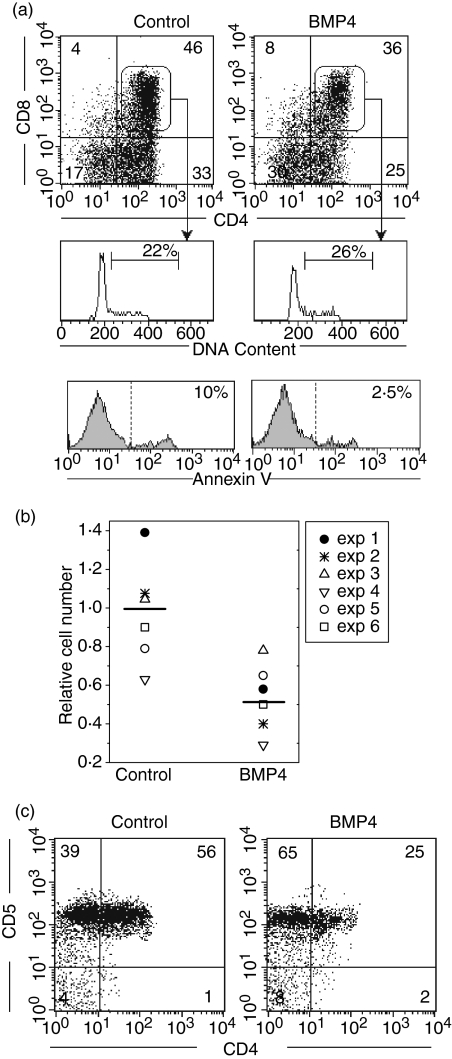
The expression of the components of the BMP2/4 signalling pathway suggested a functional role for these morphogens in human T-cell differentiation. Therefore, we analysed the effects of BMP4 on the differentiation of human CD34+ precursor cells using chimeric hu-mo FTOC. After 12 days of culture in the presence of BMP4 (100 ng/ml), hu-mo FTOCs showed a strong reduction in human cellularity compared to control cultures. Additionally, a multiparametric flow cytometry analysis of the human cells recovered from these cultures showed that BMP4 treatment induced a decrease in the proportions of CD4+ CD8+ and immature CD4+ thymocytes, which correlated with a twofold increase in the proportion of the most immature DN cell subpopulation (Fig. 7a).
Figure 7.
BMP4 inhibits the differentiation of human CD34+ thymic precursors. Chimeric hu-mo FTOC were grown in the absence (Control) or presence of BMP4 (100 ng/ml).(a)Dot plots show CD4 versus CD8 expression on gated human CD45+ cells from 12-day cultures. Histograms represent the percentage of cycling cells (cells in S plus G2 plus M phases of the cell cycle) as well as the proportion of Annexin V-positive cells in gated human CD4+ CD8+ thymocytes obtained in these cultures. Data are representative of three independent experiments where the number of human cells recovered was 81 × 103 and 17 × 103, in control and treated cultures, respectively.(b)The scatter plot shows the human cell recovery from chimeric hu-mo FTOC cultured for 5 days under the same conditions as described above. The number of human cells recovered in each experiment was divided by the control mean number of human cells recovered, calculated from the six data sets obtained in the control cultures, to give the relative cell number of six individual experiments.(c)Dot plots show the expression of CD4 and CD5 on gated human CD45+ cells from hu-mo FTOC cultured for 5 days. Data are representative of three independent experiments.
This effect could be the result of inhibition of the differentiation from DN to DP thymocytes. However, another possible explanation for the reduction of the DP subpopulation in the BMP4-treated hu-mo FTOC could be that this treatment affects the expansion or survival of DP thymocytes. To test this possibility, we analysed the proliferative and apoptotic rates in human DP cells from chimeric FTOC combining CD45, CD4 and CD8 expression with the analysis of the DNA cell content. These experiments showed that there was a decrease of apoptosis in DP thymocytes of BMP4-treated cultures, relative to the control cultures. About 10% of DP thymocytes were apoptotic in control cultures whereas only 2–3% of them were apoptotic in the treated cultures (Fig. 7a). Moreover, in control cultures the proliferation rate of DP thymocytes was around 20% compared with 25% in the cultures grown in the presence of BMP4 (Fig. 7a). Therefore, the reduction in the proportion of DP thymocytes could not be explained by a decrease or an increase in their proliferative and apoptotic rates, respectively, which suggests that BMP4 may be inhibiting the maturation of thymic precursor cells, as supported by the accumulation of DN cells seen after BMP4 addition (Fig. 7a).
To confirm the capacity of BMP4 for blocking thymocyte maturation, we analysed hu-mo FTOC after 5 days of culture, when no thymocyte has reached the DP cell differentiation stage and immature CD4+ thymocytes are starting to appear. In these cultures, BMP4 treatment also induced a reduction of human cellularity (Fig. 7b) and a marked inhibition of thymocyte maturation. As shown in Fig. 7(c), about 60% of human thymocytes had reached the immature CD4+ cell differentiation stage in control cultures. In contrast, only 20–25% of thymocytes expressed CD4 in the presence of BMP4, which correlated to an accumulation of CD4– CD5+ cells (Fig. 7c), most of which expressed CD34 antigen (data not shown).
Discussion
In this study we demonstrate that in the human thymus both BMP4 and BMP2 are mainly produced by the epithelial cell network distributed throughout the cortical and subcapsular areas, a similar expression pattern to that described for BMP4 in the murine thymus.42 Immature CD4+ cells and mainly DP thymocytes, the majority T-cell developmental stage in the thymus, which are undergoing their TCR rearrangements and intrathymic selection processes,3,4,6,50 are located in those thymic regions. In addition, as previously reported in mice,5 one would be likely to find in those areas the scarce CD34+ thymic precursors which transmigrate across the cortex, from the blood vessels located at the perimedullary cortex to the subcapsular zone, while undergoing progressive developmental changes. Therefore, the wide distribution of BMP2- and BMP4-expressing cells mainly in the outer thymic regions suggests that intrathymic precursor cells and immature thymocytes could be under the permanent influence of a high BMP2/4 pathway activation. However, the complex molecular machinery that regulates the BMP pathway both intra- and extracellularly20,22,51 could involve the existence of spatially discrete thymic microenvironments, where a range of BMP2/4 signalling doses could differentially modulate the diverse processes that are accomplished by the thymocytes located in the cortex and subcapsule. A similar situation could occur in the medulla where SP thymocytes could be exposed to different levels of BMP2/4 derived from Hassall's corpuscles. In addition, it is not unlikely that mature thymocytes could also be influenced by BMP2/4 emanating from cortical epithelial cells.
Since BMP2 and BMP4 share a common signalling pathway, the coincidence in the pattern of distribution of these secreted proteins could suggest a redundant function for these BMPs in the human thymus. However, these proteins are encoded by two genes with two independent systems of regulation52,53 and, moreover, in other developmental systems BMP4 and BMP2 function together but induce distinct responses.54,55 Therefore, in the human thymus, the same doses of BMP2 and BMP4 could result in quantitatively and/or qualitatively different responses of thymocytes, as previously described in human bone marrow for CD34+ haematopoietic progenitors.39
In most developmental processes, BMPs do not function alone but frequently interact with other morphogen families, mainly Hedgehog and Wnt proteins, regulating one another's expression and function during the development of many tissues.56–60 Recently, we have described in the human thymus the expression and function of Sonic hedgehog, the most pleiotropic member of the Hh family of morphogens.11,47 Remarkably, Sonic hedgehog is produced by thymic subcapsular and medullary epithelial cells, indicating that the subcapsule and medulla are thymic regions where BMPs and Hh proteins could be interacting to regulate some steps of thymocyte maturation. A similar regulatory network could be functioning in the cortical region, where some thymic epithelial cells produce the other two Hh proteins, Indian and Desert Hh.11 Furthermore, we cannot discard the possibility that Sonic hedgehog may diffuse from the subcapsule or medulla to the cortex, joining the cortical regulatory signalling network.
BMP signalling requires the oligomerization of two homodimers formed by type I and type II receptor chains. In the case of BMP2 and BMP4, there are two possible type I receptors, BMPRIA and BMPRIB, that can oligomerize with the type II receptor, BMPRII.18,19 Our RT-PCR analyses demonstrate transcripts encoding for the three receptors in all the populations of human thymocytes defined by the expression of CD4 and CD8 markers. Immunofluorescent staining revealed that BMPRIA and BMPRII are largely expressed in thymocytes located in the cortical zone, most of them DP cells, while only a small proportion of SP cells, located in the medulla, express these receptors. In contrast, BMPRIB is homogeneous and widely distributed in all the thymic compartments. These results indicate that there would be SP thymocytes expressing BMPRI but not BMPRII. Therefore, these cells would not be able to initiate BMP2/4 signalling but, with the contribution of other type II receptors, could signal for other members of the transforming growth factor-β family.61,62
The immunofluorescence analysis also showed that the expression of BMP receptors, and mainly BMPRIA, was restricted to bright, punctate areas in both thymocytes and epithelial cells. These data suggest that BMP receptors exhibit a specific localization in cell membrane subdomains, probably associated to lipid rafts, as has been reported in other cell lineages.63,64 Recently, two glycosyl-phosphatidylinositol-anchored proteins, Dragon and RGMa, have been described as BMP co-receptors in mammals. They promote BMP4 and BMP2 signalling in a ligand-dependent manner, probably acting as stabilizers of the receptor complex, in a mechanism that would depend on their localization on lipid rafts.24,25 Therefore, BMP receptors could be considered as new members of the group of signalling receptors that are spatially segregated and organized in lipid rafts in T cells and that are involved in many aspects of T-cell biology, such as activation, adhesion, cytoskeleton reorganization, cell polarity maintenance, or interaction with infectious organisms.65,66
We also demonstrate the existence of a complete BMP/SMAD signalling pathway in some thymocyte subpopulations. Thus, whereas DN and CD4+ SP thymocytes express all the BR-SMAD, DP and CD8+ SP cells are devoid of SMAD5 transcripts, indicating distinct BMP signalling requirements during T-cell maturation.
BMP4 treatment of chimeric hu-mo FTOCs leads to a decrease in the human cell yield and an inhibition of human T-cell differentiation affecting the maturation of CD34+ CD5+ thymocyte precursors. Similar effects on murine T-cell differentiation have been demonstrated,41,42,44 where BMP2/4 signalling arrests thymocyte differentiation at the CD44+ CD25– DN stage, before T-cell lineage commitment, and at the transition from DN to DP. This inhibitory effect observed in mice is mediated both directly by BMP signalling on CD44+ CD25– DN thymic progenitors and indirectly by acting on epithelial cell function.42,44 Our current results cannot discriminate between the direct effects on DN thymocytes and those that result from a modification of epithelial physiology because human thymic epithelial cells also express the BMP receptors, as well as the BMP/SMAD signalling pathway, which suggests an autocrine role of BMP proteins on thymic epithelium. Interestingly, our data also suggest that when thymocytes reach the DP cell stage the BMP2/4 signalling pathway can positively regulate their proliferation and survival, which would correlate to the fact that BMP2/4 proteins are broadly expressed by the cortical epithelial cell network where DP cells are localized. The mechanisms through which BMP signalling can differentially regulate human T-cell differentiation therefore need further investigation.
Acknowledgments
We thank Dr F. Villagrá and the Paediatric Cardiosurgery Units from La Zarzuela and Madrid-MontePríncipe Hospitals for the thymus samples, and Dr M.L. Toribio for the generous gift of thymic epithelial cell lines. This work was supported by grants BMC2003-01901, BFU2004-03132 and BFU2006-00651/BMC from the Ministerio de Educación y Ciencia, and CAM-910552 from the Comunidad Autónoma de Madrid.
Abbreviations
- BMP
bone morphogenetic protein
- bp
base pair
- DC
dendritic cell
- DN
double-negative CD4– CD8–
- DP
double-positive CD4+ CD8+
- FITC
fluorescein isothiocyanate
- Hh
Hedgehog
- hu-mo FTOC
chimeric human–mouse fetal thymic organ cultures
- HLA
human leucocyte antigen
- IgG
immunoglobulin G
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- NK
natural killer
- RT-PCR
reverse transcription–polymerase chain reaction
- SP
single-positive CD4+ CD8–/CD4– CD8+ TCR-β, T-cell receptor β
References
- 1.Anderson G, Jenkinson WE, Jones T, et al. Establishment and functioning of intrathymic microenvironments. Immunol Rev. 2006;209:10–27. doi: 10.1111/j.0105-2896.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Crivellato E, Vacca A, Ribatti D. Setting the stage: an anatomist's view of the immune system. Trends Immunol. 2004;25:210–17. doi: 10.1016/j.it.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Blom B, Spits H. Development of human lymphoid cells. Annu Rev Immunol. 2006;24:287–320. doi: 10.1146/annurev.immunol.24.021605.090612. [DOI] [PubMed] [Google Scholar]
- 4.Spits H. Development of alphabeta T cells in the human thymus. Nat Rev Immunol. 2002;2:760–72. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 5.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–34. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco YR, Navarro MN, de Yebenes VG, Ramiro AR, Toribio ML. Regulation of surface expression of the human pre-T cell receptor complex. Semin Immunol. 2002;14:325–34. doi: 10.1016/s1044-5323(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Sprent J. Proving negative selection in the thymus. J Immunol. 2005;174:3841–2. doi: 10.4049/jimmunol.174.7.3841. [DOI] [PubMed] [Google Scholar]
- 8.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–72. [PubMed] [Google Scholar]
- 9.Varas A, Hager-Theodorides AL, Sacedon R, Vicente A, Zapata AG, Crompton T. The role of morphogens in T-cell development. Trends Immunol. 2003;24:197–206. doi: 10.1016/s1471-4906(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt TM, Zuniga-Pflucker JC. Thymus-derived signals regulate early T-cell development. Crit Rev Immunol. 2005;25:141–59. doi: 10.1615/critrevimmunol.v25.i2.40. [DOI] [PubMed] [Google Scholar]
- 11.Sacedon R, Varas A, Hernandez-Lopez C, Gutierrez-d, eFrias C, Crompton T, Zapata AG, Vicente A. Expression of hedgehog proteins in the human thymus. J Histochem Cytochem. 2003;51:1557–66. doi: 10.1177/002215540305101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staal FJ, Meeldijk J, Moerer P, Jay P, van de Weerdt BC, Vainio S, Nolan GP, Clevers H. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001;31:285–93. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Staal FJ, Clevers HC. WNT signalling and haematopoiesis: a WNT–WNT situation. Nat Rev Immunol. 2005;5:21–30. doi: 10.1038/nri1529. [DOI] [PubMed] [Google Scholar]
- 14.Adachi-Yamada T, O'Connor MB. Mechanisms for removal of developmentally abnormal cells: cell competition and morphogenetic apoptosis. J Biochem (Tokyo) 2004;136:13–17. doi: 10.1093/jb/mvh099. [DOI] [PubMed] [Google Scholar]
- 15.Eldar A, Shilo BZ, Barkai N. Elucidating mechanisms underlying robustness of morphogen gradients. Curr Opin Genet Dev. 2004;14:435–9. doi: 10.1016/j.gde.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–12. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Wall NA, Hogan BL. TGF-beta related genes in development. Curr Opin Genet Dev. 1994;4:517–22. doi: 10.1016/0959-437x(94)90066-c. [DOI] [PubMed] [Google Scholar]
- 19.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–41. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 20.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–63. doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–9. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–50. [PubMed] [Google Scholar]
- 23.Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–5. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- 24.Babitt JL, Zhang Y, Samad TA, et al. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:29820–7. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 25.Samad TA, Rebbapragada A, Bell E, et al. DRAGON, a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:14122–9. doi: 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- 26.Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol. 2005;38:9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- 27.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 28.Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci USA. 1988;85:9484–8. doi: 10.1073/pnas.85.24.9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305–12. doi: 10.1002/jcb.10309. [DOI] [PubMed] [Google Scholar]
- 30.Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–26. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- 31.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 32.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–78. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Hall AK, Miller RH. Emerging roles for bone morphogenetic proteins in central nervous system glial biology. J Neurosci Res. 2004;76:1–8. doi: 10.1002/jnr.20019. [DOI] [PubMed] [Google Scholar]
- 34.Martinez G, Bertram JF. Organisation of bone morphogenetic proteins in renal development. Nephron Exp Nephrol. 2003;93:e18–22. doi: 10.1159/000066649. [DOI] [PubMed] [Google Scholar]
- 35.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 36.Warburton D, Bellusci S. The molecular genetics of lung morphogenesis and injury repair. Paediatr Respir Rev. 2004;5(Suppl. A):S283–7. doi: 10.1016/s1526-0542(04)90052-8. [DOI] [PubMed] [Google Scholar]
- 37.Botchkarev VA. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J Invest Dermatol. 2003;120:36–47. doi: 10.1046/j.1523-1747.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- 38.Capdevila J, Izpisua Belmonte JC. Patterning mechanisms controlling vertebrate limb development. Annu Rev Cell Dev Biol. 2001;17:87–132. doi: 10.1146/annurev.cellbio.17.1.87. [DOI] [PubMed] [Google Scholar]
- 39.Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189:1139–48. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Detmer K, Walker AN. Bone morphogenetic proteins act synergistically with haematopoietic cytokines in the differentiation of haematopoietic progenitors. Cytokine. 2002;17:36–42. doi: 10.1006/cyto.2001.0984. [DOI] [PubMed] [Google Scholar]
- 41.Graf D, Nethisinghe S, Palmer DB, Fisher AG, Merkenschlager M. The developmentally regulated expression of Twisted gastrulation reveals a role for bone morphogenetic proteins in the control of T cell development. J Exp Med. 2002;196:163–71. doi: 10.1084/jem.20020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hager-Theodorides AL, Outram SV, Shah DK, Sacedon R, Shrimpton RE, Vicente A, Varas A, Crompton T. Bone morphogenetic protein 2/4 signaling regulates early thymocyte differentiation. J Immunol. 2002;169:5496–504. doi: 10.4049/jimmunol.169.10.5496. [DOI] [PubMed] [Google Scholar]
- 43.Nosaka T, Morita S, Kitamura H, et al. Mammalian twisted gastrulation is essential for skeleto-lymphogenesis. Mol Cell Biol. 2003;23:2969–80. doi: 10.1128/MCB.23.8.2969-2980.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai PT, Lee RA, Wu H. BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood. 2003;102:3947–53. doi: 10.1182/blood-2003-05-1657. [DOI] [PubMed] [Google Scholar]
- 45.Bleul CC, Boehm T. BMP signaling is required for normal thymus development. J Immunol. 2005;175:5213–21. doi: 10.4049/jimmunol.175.8.5213. [DOI] [PubMed] [Google Scholar]
- 46.Patel SR, Gordon J, Mahbub F, Blackburn CC, Manley NR. Bmp4 and Noggin expression during early thymus and parathyroid organogenesis. Gene Expr Patterns. 2006;6:794–9. doi: 10.1016/j.modgep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Gutierrez-Frias C, Sacedon R, Hernandez-Lopez C, Cejalvo T, Crompton T, Zapata AG, Varas A, Vicente A. Sonic hedgehog regulates early human thymocyte differentiation by counteracting the IL-7-induced development of CD34+ precursor cells. J Immunol. 2004;173:5046–53. doi: 10.4049/jimmunol.173.8.5046. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez E, Vicente A, Zapata A, Brera B, Lozano JJ, Martinez C, Toribio ML. Establishment and characterization of cloned human thymic epithelial cell lines. Analysis of adhesion molecule expression and cytokine production. Blood. 1994;83:3245–54. [PubMed] [Google Scholar]
- 49.Hernandez-Lopez C, Varas A, Sacedon R, Jimenez E, Munoz JJ, Zapata AG, Vicente A. Stromal cell-derived factor 1/CXCR4 signaling is critical for early human T-cell development. Blood. 2002;99:546–54. doi: 10.1182/blood.v99.2.546. [DOI] [PubMed] [Google Scholar]
- 50.Prockop S, Petrie HT. Cell migration and the anatomic control of thymocyte precursor differentiation. Semin Immunol. 2000;12:435–44. doi: 10.1006/smim.2000.0267. [DOI] [PubMed] [Google Scholar]
- 51.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–35. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 52.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 54.Maguer-Satta V, Bartholin L, Jeanpierre S, Ffrench M, Martel S, Magaud JP, Rimokh R. Regulation of human erythropoiesis by activin A, BMP2, and BMP4, members of the TGFbeta family. Exp Cell Res. 2003;282:110–20. doi: 10.1016/s0014-4827(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 55.Martinez G, Mishina Y, Bertram JF. BMPs and BMP receptors in mouse metanephric development: in vivo and in vitro studies. Int J Dev Biol. 2002;46:525–33. [PubMed] [Google Scholar]
- 56.Liem KF, Jr, Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127:4855–66. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- 57.Patten I, Placzek M. Opponent activities of Shh and BMP signaling during floor plate induction in vivo. Curr Biol. 2002;12:47–52. doi: 10.1016/s0960-9822(01)00631-5. [DOI] [PubMed] [Google Scholar]
- 58.Callahan CA, Oro AE. Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic hedgehog signaling. Curr Opin Genet Dev. 2001;11:541–6. doi: 10.1016/s0959-437x(00)00230-6. [DOI] [PubMed] [Google Scholar]
- 59.Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, Vortkamp A. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development. 2001;128:4523–34. doi: 10.1242/dev.128.22.4523. [DOI] [PubMed] [Google Scholar]
- 60.Marcelle C, Stark MR, Bronner-Fraser M. Coordinate actions of BMPs, Wnts, Shh and noggin mediate patterning of the dorsal somite. Development. 1997;124:3955–63. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- 61.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 62.Brederlau A, Faigle R, Elmi M, Zarebski A, Sjoberg S, Fujii M, Miyazono K, Funa K. The bone morphogenetic protein type Ib receptor is a major mediator of glial differentiation and cell survival in adult hippocampal progenitor cell culture. Mol Biol Cell. 2004;15:3863–75. doi: 10.1091/mbc.E03-08-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J Cell Sci. 2005;118:643–50. doi: 10.1242/jcs.01402. [DOI] [PubMed] [Google Scholar]
- 64.Ramos M, Lame MW, Segall HJ, Wilson DW. The BMP type II receptor is located in lipid rafts, including caveolae, of pulmonary endothelium in vivo and in vitro. Vascul Pharmacol. 2006;44:50–9. doi: 10.1016/j.vph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 65.Pizzo P, Viola A. Lipid rafts in lymphocyte activation. Microbes Infect. 2004;6:686–92. doi: 10.1016/j.micinf.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Horejsi V. Lipid rafts and their roles in T-cell activation. Microbes Infect. 2005;7:310–16. doi: 10.1016/j.micinf.2004.12.004. [DOI] [PubMed] [Google Scholar]