Abstract
P-glycoprotein (Pgp) and multidrug resistance protein 1 (MRP1) are members of the ATP-binding cassette (ABC) family of transporter proteins. Both molecules are membrane-associated, energy-dependent efflux pumps with different substrate selectivity and they may play a role in the activation, differentiation and function of haematopoietic cells. Mouse haematopoietic cells are characterized by the expression of the cell surface molecules c-kit and Sca-1. Herein, the presence and activities of Pgp and MRP1 in mouse bone marrow mononuclear cells (BMMC) and their relationship with the proteins c-kit and Sca-1 were evaluated. Pgp and MRP activities were measured based on the extrusion of rhodamine 123 (for Pgp) and Fluo-3 (for MRP). Cell populations were assessed by cytometry using anti-c-kit and anti-Sca1 antibodies. Pgp activity was present in 5% of BMMC while 50% of BMMC cells showed MRP activity. These findings agreed with the proportion of cells expressing the MRP1 surface molecule (51·3 ± 4·17%). About 14% of BMMC were positive for c-kit and/or Sca-1 (9·3% c-kit– Sca-1+, 4·2% c-kit+ Sca-1– and 0·9% c-kit+ Sca-1+). Among these subpopulations only c-kit– Sca-1+ cells presented Pgp activity (21·36%). On the other hand, MRP activity was present in all three subpopulations. Most cells (82·5%) of the c-kit+ Sca-1– subpopulation presented MRP1 activity compared to only 54·1% of c-kit+ Sca-1+ and 38·8% of c-kit– Sca-1+. This study demonstrates the expression and activity of MRP1 in BMMC. While only a small proportion of precursor cells had Pgp activity, MRP1 activity was present among different subpopulations of precursor cells. Further studies are necessary to establish the role of these transporters in haematopoietic cells.
Keywords: c-kit, mouse bone marrow, multidrug resistance protein 1, P-glycoprotein, Sca-1
Introduction
P-glycoprotein (Pgp) and multidrug resistance protein 1 (MRP1) are the two best-characterized ATP-binding cassette (ABC) family transporter proteins involved in resistance to a variety of structurally unrelated anticancer agents. Both Pgp and MRP1 are membrane-associated, energy-dependent efflux pumps that function to reduce the intracellular levels of hydrophobic cytotoxic compounds.1,2
Despite similarities their in drug-resistance profiles, these transporters differ markedly in their substrate selectivity. MRP1 is capable of transporting many lipophilic anions, including conjugates of glutathione, glucuronic acid and sulphate.3 In contrast, P-glycoprotein predominantly transports neutral or mildly cationic molecules. Neither conjugation nor cotransport of drug substrates with physiological molecules is required for the efflux process mediated by Pgp. Both proteins were first described in tumour cells, but they have now been described in many normal tissues such as lung, gut, liver, kidney, brain, testis and placenta (reviewed in ref. 4). These transport proteins play a critical role in the absorption, distribution, metabolism and excretion of drugs and toxins.4 Moreover, these proteins were described in thymocytes,5–7 mature lymphocytes,8,9 dendritic cells,10,11 monocytes12 and macrophages.13
It has been suggested that multidrug transporters may play a role in the cells of the immune system, mediating the efflux of xenobiotics14,15 and regulating cell activation16–18 and migration.10,11
Haematopoietic stem cells (HSC) comprise approximately 0·05% of bone marrow (BM). Mouse HSC express the cell surface molecules c-kit and Sca-1 and lack markers for differentiated peripheral blood cells.19,20 In 1991, Chaudhary and Roninson showed that human CD34+ HSC express Pgp and show activity for this transporter.21 The activity of Pgp is also related to the murine side population (SP), cells that are highly enriched for repopulating activity,22 and over-expression of Pgp in murine BM cells results in the expansion of SP stem cells in vitro and of repopulating cells in vivo.23 Murine SP cells express another ABC transporter known as breast-cancer-resistance protein (BCRP) and this might explain why mice lacking Pgp expression show no difference in their number of progenitor cells.24 The SP cells from these animals also express the mrp1 gene. However, despite all the information on Pgp in BM cells, there is a paucity of knowledge regarding MRP1. The MRP1 has been shown to be present in human BM cells, but there is some controversy about the subpopulation expressing this molecule.9,25
The present work characterizes the expression and activity of MRP1 in murine bone marrow mononuclear cells (BMMC), and relates Pgp and MRP1 activities to the surface molecules c-kit and Sca-1 present in those cells.
Materials and methods
Reagents
The culture medium RPMI-1640 (Sigma Chemical Co., St Louis, MO) was used for the cell cultures. Fluo-3, obtained from Molecular Probes (Eugene, OR), was dissolved in dimethylsulphoxide (DMSO; Sigma Chemical Co., St Louis, MO) as a 10 mm stock solution and stored at −20°. Rhodamine 123 (Rho 123; Sigma Chemical Co.) was dissolved in saline as a 100 μg/ml stock solution and stored at −20°. The fluorescent probes, Fluo-3 and Rho 123, were diluted in RPMI-1640 medium to a final concentration of 5 μg/ml and 200 ng/ml, respectively, just before use. Indomethacin (INDO; Sigma Chemical Co.) was dissolved as a stock solution in DMSO at a concentration of 30 mm and stored at −20°. Probenecid (PRB; Sigma Chemical Co.) and verapamil (VP; Sigma Chemical Co.) were dissolved in saline as stock solutions of 25 mm and 5 mm, respectively, and stored at −20°. Cyclosporin A (CSA 100 mg/ml, obtained from Novartis, São Paulo, Brazil) was further diluted in medium at a concentration of 1 mm and stored at −20°. MK 571 (Merck, São Paulo, Brazil) was dissolved in distilled water as a 50 mm stock solution and stored at −20°. INDO, PRB, MK 571, VP and CSA were diluted in RPMI-1640 medium just before use. 3-[4,5-dithylthiazol-2- yl]-2,5-diphenyl tetrazolium bromide (MTT), obtained from Sigma, was dissolved in saline at 5 mg/ml, and stored at 4°. Monoclonal antibodies anti-CD117 (tricolour) and anti-ly6 Sca1 (phycoerythrin-conjugated) were obtained from Caltag (Burlingame, CA). Primary rat monoclonal antibody anti-MRP1 was purchased from Alexis Biochemicals (San Diego, CA) and secondary antibody, goat anti-rat immunoglobulin G conjugated with fluorescein isothiocyanate, was obtained from Sigma Chemical Co. Fluorescence activated cell sorter (FACS) lysing solution was obtained from Becton Dickinson (San Jose, CA).
Animals
Inbred male C57BL/6 mice (2–3 months old) were used in all experiments. Animals were housed in a temperature-controlled room and received free access to water and standard rat chow (ad libitium). During all experiments, the animals were treated in accordance with published COBEA regulations for animal laboratory use.
Isolation of BMMC
Mice were anaesthetized with ethyl ether (Reagen, Rio de Janeiro, Brazil) and killed by cervical dislocation. Bone marrow was aspirated from femoral and tibial bones with a syringe containing RPMI-1640 medium. Bone marrow cells were homogenized and centrifuged at 450 g for 10 min and resuspended in 3 ml medium. Afterwards this cell suspension was loaded on a density gradient of Histopaque 1083 (Sigma) and centrifuged at 800 g for 30 min. Cells retained on the Histopaque–medium interface were transferred into a conical tube and washed three times with saline. After that procedure, cells were resuspended in RPMI-1640 with 10% fetal bovine serum (Gibco Life Technologies, Rockville, MD).
MRP- and Pgp-related transport activities
MRP- and Pgp-related transport activities were investigated by using the fluorescent probes Fluo-3 and Rho 123 in an efflux assay. BMMC (106 cells/ml) were loaded with 5 μg/ml Fluo-3 or 200 ng/ml Rho 123 in the presence or absence of MRP inhibitors or Pgp inhibitors. PRB (100, 500, 1000 or 2500 μm); INDO (75, 150 or 300 μm) or MK 571 (12·5, 25 or 50 μm) were used to inhibit the MRP activity. CSA (0·1, 0·5 or 1 μm) and VP (5, 10 or 50 μm) were used to inhibit the Pgp activity. Mononuclear cells were incubated in RPMI-1640 with 10% fetal bovine serum for 30 min at 37° in the presence of the fluorescent probes. Following this period, Fluo-3-loaded or Rho 123-loaded cells were washed with cold medium. Cells were re-incubated in medium, with or without MRP or Pgp inhibitors, for a further 90 min at 37°. In some experiments this process was followed by incubating the cells for 30 min at 4° with anti-CD117 (tricolour) and anti-ly6 Sca-1 (phycoerythrin-conjugated) monoclonal antibodies. The cells were then washed with phosphate-buffered saline (PBS), and analysed by flow cytometry using a FACScalibur (Becton Dickinson). One hundred thousand cells were acquired based on forward and side scatter. All flow cytometry analyses were accomplished using winmdi, version 2·8.
MRP1 detection
For the detection of MRP1 at the single-cell level, 106 BMMC were rendered permeable with FACS lysing solution for 10 min at room temperature. Cells were washed with PBS and incubated with anti-MRP, diluted in PBS with 1% BSA (1 : 80) for 30 min at room temperature. This was followed by incubating with the secondary antibody for 20 min at room temperature. Cells were washed with PBS and analysed by flow cytometry. Ten thousand cells were acquired based on forward and side scatter.
Viability assay
The BMMC were seeded in 96-well plates at a concentration of 5 × 105 cells/well. The cells were incubated in the presence or absence of INDO (75, 150 or 300 μm), PRB (0·5, 1·0 or 2·5 mm), MK 571 (12·5, 25 or 50 μm), VP (5, 10 or 50 μm) or CSA (0·1, 0·5 or 1 μm) for 48 hr at 37° in a humidified atmosphere and 5% CO2. Cell viability was assayed by the addition, of 20 μl/well MTT at a concentration of 5 mg/ml. The method is based on the reduction of the tetrazolium salt by mitochondrial dehydrogenases present in viable cells. After incubation for 3 hr at 37°, the plates were centrifuged at 400 g for 5 min, the supernatant was removed and 200 μl DMSO was added to each well to dissolve the formazan crystals. The plates were read on a Thermomax Microplate Reader (Molecular Devices, Menlo Park, CA) at a wavelength of 490 nm. Results represent the mean ± standard deviation (SD) of triplicate determinations.
Statistical analysis
Values are given as mean ± SD. Statistical significance was calculated by one-way analysis of variance (anova) followed by Bonferroni's t-test, and P-values of less than 0·05 were considered significant.
Results
Expression of MRP1 in BMMC
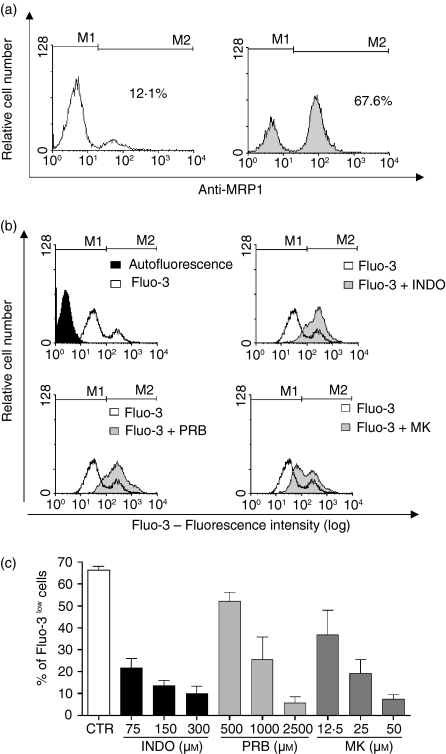
The expression of MRP1 in murine BMMC was analysed. The level of MRP1 expression was determined using anti-MRP1 antibody and measured by flow cytometry (Fig. 1a). It was observed that 51·3 ± 4·2% of the BMMC were positive for MRP1.
Figure 1.
MRP1 expression and activity in murine bone marrow mononuclear cells. (a) Cells were permeabilized, and labelled with rat anti-MRP1. Cells were then washed and stained with goat anti-rat IgG FITC. Cells were washed and acquired by flow cytometry. Left panel: control, cells were labelled only with the secondary antibody. Right panel: gray histogram represents cells labelled with both antibodies. M1 indicates the autofluorescence region and M2 indicates the labelled region. The value in the figure indicates the percentual of bone marrow mononuclear cells expressing MRP1. This figure is representative of three different experiments. (b) Histograms showing the accumulation of Fluo-3 by the cells, in the presence or absence of MRP inhibitors: INDO – indomethacin (75 μm), PRB – probenecid (1000 μm) and MK – MK571 (25 μm). (c) Dose-response curve of the effect of inhibitors: INDO, PRB or MK in MRP1 activity. Results are expressed as mean ± SD of at least 3 independent experiments.
MRP-related transport activity
To confirm that approximately 50% of the BMMC present an active protein, the extrusion capacity of the pump was evaluated. It has been reported that the fluorescent anion Fluo-3 can be used to monitor MRP1 activity.17 In the present experiments, nearly half of the BMMC population was able to transport Fluo-3 (Fig. 1b,c). To evaluate whether this effect was related to MRP activity, cells were incubated with Fluo-3 in the presence of MRP inhibitors: INDO (75–300 μm), PRB (500–2500 μm) and MK 571 (12·5–50 μm) (Fig. 1c). A concentration-dependent increase in intracellular fluorescence was observed in the presence of inhibitors with a consequent decrease in the numbers of Fluo-3low cells. These results provide evidence that BMMC expressed an active MRP1 protein.
MRP activity and cell viability
To evaluate the influence of inhibitors of MRP1 activity on cellular viability, BMMC were incubated with the MRP inhibitors used in this study: INDO (75, 150 or 300 μm), PRB (500, 1000 or 2500 μm), and MK 571 (12·5, 25 or 50 μm) for 48 hr. After this incubation period, the cells remained alive in the presence of low concentrations of MRP inhibitors. However, with high concentrations of the inhibitors there was a loss of cellular viability, INDO (150 and 300 μm) viability decreased to 42% and 22%, respectively, PRB (2500 μm) fell to 23% and MK 571 (50 μm) decreased to 55%. These results suggest that this protein is not essential for BMMC survival because other concentrations of inhibitors, producing inhibition of MRP1 activity in at least 50% of the Fluo-3low cells, did not alter the cell viability. Furthermore, it is possible in these cells to use concentrations of 75 μm INDO, 1000 μm PRB and 25 μm MK 571 without observing a general toxicity.
Pgp-related transport activity
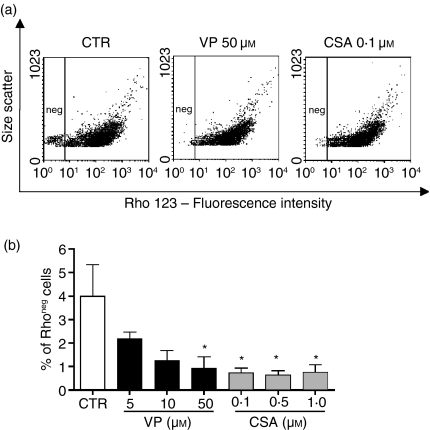
The presence of Pgp activity has been described in BMMC, and it has been suggested that this population corresponds to HSC. The function of Pgp was investigated using the efflux of the fluorescent substrate Rho 123. We observed that around 4% of BMMC were able to transport Rho 123 (Fig. 2), and this population was considered Rho negative. To confirm that this activity was related to Pgp, cells were incubated with Rho 123 in the presence of Pgp inhibitors, VP (5, 10 or 50 μm) and CSA (0·1, 0·5 or 1 μm), and it was observed that in the presence of Pgp inhibitors this population accumulated the dye (Fig. 2).
Figure 2.
Pgp-related transport activity in murine bone marrow mononuclear cells. (a) Representative dotplots showing the amount of Rho 123 negative cells (left hand side region). (b) Dose-response curve of Pgp inhibitors: VP (verapamil) and CSA (cyclosporin A). Results are expressed as mean ± SD of 3 independent experiments. *Significantly different from control P < 0·05.
Pgp activity and cell viability
Another important aspect of Pgp activity is the importance of this function to cell survival because some of the substrates transported by this pump could regulate the viability of adjoining cells. Thus, we have investigated whether Pgp activity was vital for BMMC survival. Cells were incubated with Pgp inhibitors: VP (5–50 μm) and CSA (0·1–1 μm) for 48 hr, and, after this period, the cells were still alive. Only in the presence of high concentrations of CSA (1 μm) was a loss of viability observed, and even then 64% of the cells were still alive. These results suggested that Pgp activity was not essential for cell survival of total BMMC and that the inhibitors could be used without an undesirable toxicity.
MRP-related and Pgp-related transport activity and progenitor cells
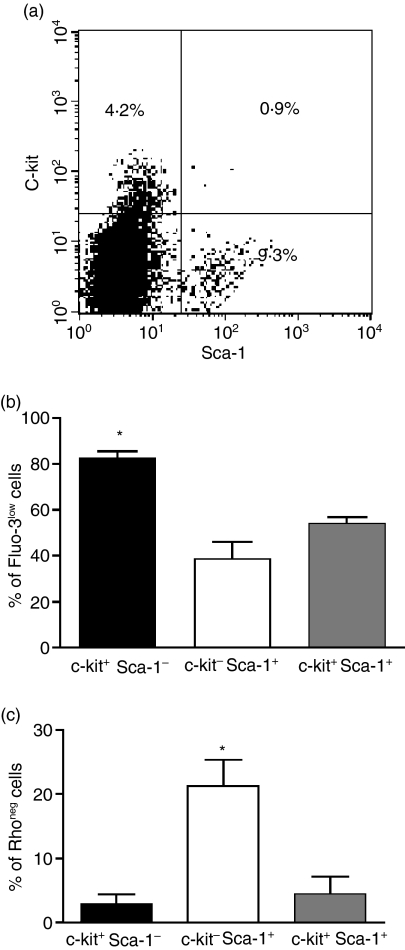
Our results demonstrated that BMMC express MRP1 and present MRP and Pgp activity. The relationship of MRP and Pgp transport activity and progenitor cells was therefore studied. BMMC were labelled with antibodies that recognize the membrane proteins present in more undifferentiated cells, such as c-kit and Sca-1. It was found that among BMMC approximately 14% express these proteins, being 9·30 ± 2·0% c-kit– Sca-1+, 4·19 ± 0·7% c-kit+ Sca-1– and 0·89 ± 0·4 c-kit+ Sca-1+(Fig. 3a).
Figure 3.
(a) Distribution of progenitor cells subpopulations among bone marrow mononuclear cells. Cells were incubated with anti-c-kit TC and anti-Sca-1 PE antibodies. Cells were then washed and acquired by flow cytometry. Representative dotplot. (b) MRP1 transport activity. Bars represent the percentage of cells extruding Fluo-3 (MRP activity) among c-kit+Sca-1− (black bar), c-kit−Sca-1+ (white bar) and c-kit+Sca-1+ (gray bar). (c) Pgp transport activity. Bars represent the percentage of cells extruding Rho 123 (Pgp activity) among c-kit+Sca-1− (black bar), c-kit−Sca-1+ (white bar) and c-kit+Sca-1+ (gray bar). Results are expressed as mean ± SD of 3 independent experiments. *Significantly different from the other two groups P < 0·05.
Among the murine BMMC that presented MRP activity 14% expressed c-kit and/or Sca-1, while among the population that presented Pgp activity approximately 35% were precursors.
Moreover, MRP was present in 82·52 ± 5·3% of the c-kit+ Sca-1– subpopulation, while only 38·81 ± 12·5% of the c-kit– Sca-1+ cells showed MRP activity. Among the c-kit+ Sca-1+ cells, 54·14 ± 4·5% showed an active protein (Fig. 3b). On the other hand, when Pgp activity was analysed, it was observed that among the c-kit+ Sca-1– and c-kit+ Sca-1+ subpopulations only a negligible activity for this protein was present compared with 21·36 ±6·83% of the c-kit– Sca-1+ subpopulation exhibit Pgp activity (Fig. 3c).
Discussion
In the present study the expression and activity of the ABC transporters Pgp and MRP1 were investigated in normal murine BM cells. We found that 5% of murine BMMC were capable of Rho 123 efflux, related to Pgp activity, and our results match those from studies that reported Pgp activity in 4–7% of these cells.26 Furthermore, we demonstrated for the first time in murine BMMC that approximately 50% of the cells presented an active MRP1 protein, measured by their Fluo-3 efflux capacity. When ABC transport activity was analysed in relation to phenotypic markers present in immature haematopoietic cells it was found that 35% of cells with Pgp activity were precursors, and approximately 14% of MRP1 cells expressed the c-kit and/or Sca-1 phenotypes.
Pgp activity has been described in stem cells21 and, in some instances, this activity has been ascribed to those cells with enriched repopulation capacity.22,23 In the present work, Pgp activity in immature cells was restricted to the c-kit– Sca-1+ subpopulation. A lineage negative population with a similar phenotype, c-kit– Sca-1+ incorporating low levels of the dye Rho 123, was found not to have the capacity to reconstitute and to be present in the normal murine BM.27 Because these cells incorporated low levels of Rho 123 and were small, it has been suggested that they might be a population of resting stem cells. In our work, the low incorporation of Rho 123 could be reversed by VP, indicating that the dye was being effluxed from the cells via Pgp and not that the low uptake represented decreased mitochondrial activity.
Nevertheless, it was possible that these c-kit– Sca-1+ cells represented a subpopulation of stem cells that was incapable of producing or secreting growth factors and that depended on a different subpopulation of cells to provide them with the necessary stimulus.27 In this context, it is interesting that leukotrienes potentiate colony stimulating factor (CSF)-induced colony formation from freshly isolated normal bone marrow cells28,29 and immature myeloid cells present the necessary enzymes to metabolise leukotrienes.30 Furthermore, leukotriene inhibitors affect cellular proliferation.31 Leukotriene C4 is secreted via MRP132 and the regulation of this transport activity could therefore affect normal myelopoiesis. Our results indicated that in murine BM nearly half of BM cells exhibited MRP1 activity, but inhibition of MRP1 activity for 48 hr using INDO, PRB or MK 571 did not result in decreased cellular viability, which is in agreement with the observation that mice lacking mrp1 are viable and there is no apparent change in their haematopoiesis.33 The expression of MRP1 molecules has already been described in human normal and malignant haematopoietic cells.9,25,34 When the immature subpopulations of murine BMMC were studied, MRP activity was present mainly in c-kit+ cells (82% of the c-kit+ Sca1– and 54% of the c-kit+Sca-1+cells), compared to 38·8% of the c-kit– Sca1+. It was not possible with the technique used in this study to determine if the same cell could present both Pgp and MRP1 activities simultaneously.
Studying human peripheral blood, Laupeze et al.25 described how peripheral CD34+ haematopoietic cells had higher MRP-related efflux compared to mature leucocytes. It has been suggested that, among the murine BM population, c-kit+ cells may be more immature than c-kit– Sca-1+ cells because they are capable of originating c-kit– cells in reconstitution experiments.27 Therefore, in an analogous manner to the results obtained when human haematopoietic cells were used, our experiments have shown that c-kit+ subpopulation has a higher proportion of cells with MRP1 transport activity.
The function of Pgp and MRP1 transporters has been, for many years, associated with a protective role. However, physiologically, they may exhibit many other functions, including secretion of mediators and hormones.35 It is possible that among the immature population in the BM, cells with different efflux capacities are capable of regulating their growth and the growth of other subpopulations based on the accumulation and/or transport of substances essential for their activation and differentiation.
In summary, this study indicates that Pgp activity is present mainly in one subpopulation of immature cells, whereas MRP1 activity varies among the different subpopulations, further studies will be necessary to establish the role of these transporters in the differentiation of these cells.
Acknowledgments
We would like to thank Dr Ottilia Affonso Mitidieri for critically reading the manuscript. This work was supported by Brazilian National Research Consul (CNPq), and PRONEX. F.K.C receives a grant from CNPq and J.E.-L. is supported by a fellowship from CNPq.
Abbreviations
- ABC
ATP-binding cassette family
- BM
bone marrow
- BCRP
breast-cancer-resistance protein
- BMMC
bone marrow mononuclear cells
- CSA
cyclosporin A
- DMSO
dimethyl sulphoxide
- FACS
fluorescence-activated cell sorter
- HSC
haematopoietic stem cells
- INDO
indomethacin
- MRP1
multidrug resistance protein 1
- MTT
(3-[4,5-dithylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide)
- PBS
phosphate-buffered saline
- Pgp
P-glycoprotein
- PRB
probenecid
- Rho 123
rhodamine 123
- SP
side population
- VP
verapamil
References
- 1.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer. The early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 2.Cole SPC, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 3.Deeley RG, Cole SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1) FEBS Lett. 2006;580:1103–11. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins. Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–37. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald HPR, Bommhardt U, Cerottini JC. Developmentally regulated expression of P-glycoprotein (multidrug resistance) activity in mouse thymocytes. Eur J Immunol. 1995;25:1457–60. doi: 10.1002/eji.1830250549. [DOI] [PubMed] [Google Scholar]
- 6.Pilarski LM, Paine D, Mcelhaaney JE, Cass CE, Belvh AR. Multidrug transporter P-glycoprotein 170 as a differentiation antigen on normal human lymphocytes and thymocytes: modulation with differentiation stage and during aging. Am J Hematol. 1995;49:323–35. doi: 10.1002/ajh.2830490411. [DOI] [PubMed] [Google Scholar]
- 7.Leite DFP, Echevarria-Lima J, Salgado LT, Capella MAM, Calixto JB, Rumjanek VM. In vivo and in vitro modulation of MDR molecules in murine thymocytes. Int Immunopharmacol. 2006;6:204–15. doi: 10.1016/j.intimp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Drach D, Zhao S, Drach J, Mahadevia R, Gattringer C, Huber H, Andreeff M. Subpopulations of normal peripheral blood and bone marrow cells express a functional multidrug resistant phenotype. Blood. 1992;80:2729–34. [PubMed] [Google Scholar]
- 9.Legrand O, Perrot JY, Tang R, Simonin G, Gurbuxani S, Zittoun R, Marie JP. Expression of the multidrug resistance-associated protein (MRP) mRNA and protein in normal peripheral blood and bone marrow haemopoietic cells. Br J Haematol. 1996;94:23–33. doi: 10.1046/j.1365-2141.1996.d01-1776.x. [DOI] [PubMed] [Google Scholar]
- 10.Randolph GJ, Beaulieu S, Pope M, Sugawara I, Hoffman L, Steinman RM, Muller WA. A physiologic function for P-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci USA. 1998;95:1624–9. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–68. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 12.Vellenga E, Tuyt L, Wierenga BJ, Muller M, Dokter W. Interleukin-6 production by activated human monocytic cells is enhanced by MK-571, a specific inhibitor of the multi-drug resistance protein-1. Br J Pharmacol. 1999;127:441–8. doi: 10.1038/sj.bjp.0702577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puddu P, Fais S, Luciani F, et al. Interferon-gamma up-regulates expression and activity of P-glycoprotein in human peripheral blood monocyte-derived macrophages. Laboratory Invest. 1999;79:1299–309. [PubMed] [Google Scholar]
- 14.Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20:11–20. doi: 10.1002/stem.200011. [DOI] [PubMed] [Google Scholar]
- 15.Elliott JI, Raguz S, Higgins C. Multidrug transporter activity in lymphocytes. Br J Pharmacol. 2004;143:899–907. doi: 10.1038/sj.bjp.0705940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohoff M, Prechtl S, Sommer F, et al. A multidrug-resistance protein (MRP) -like transmembrane pump is highly expressed by resting murine T helper (Th) 2, but not Th1 cells, and is induced to equal expression levels in Th1 and Th2 cells after antigenic stimulation in vivo. J Clin Invest. 1998;101:703–10. doi: 10.1172/JCI824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prechtl S, Roellinghoff M, Scheper R, Cole SP, Deeley RG, Lohoff M. The multidrug resistance protein 1: a functionally important activation marker for murine Th1cells. J Immunol. 2000;164:754–61. doi: 10.4049/jimmunol.164.2.754. [DOI] [PubMed] [Google Scholar]
- 18.Frank MH, Denton MD, Alexander SI, Khoury SJ, Sayegh MH, Briscoe DM. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J Immunol. 2001;166:2451–9. doi: 10.4049/jimmunol.166.4.2451. [DOI] [PubMed] [Google Scholar]
- 19.Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Nishikawa S, Miura Y, Suda T. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood. 1991;78:1706–12. [PubMed] [Google Scholar]
- 20.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 22.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunting KD, Zhou S, Lu T, Sorrentino BP. Enforced P-glycoprotein pump function in murine bone marrow cells results in expansion of side population stem cells in vitro and repopulating cells in vivo. Blood. 2000;96:902–9. [PubMed] [Google Scholar]
- 24.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–34. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 25.Laupeze B, Amiot L, Payen L, Drenou B, Grosset JM, Lehne G, Fauchet R, Fardel O. Multidrug resistance protein (MRP) activity in normal mature leukocytes and CD34-positive hematopoietic cells from peripheral blood. Life Sci. 2001;68:1323–31. doi: 10.1016/s0024-3205(00)01026-2. [DOI] [PubMed] [Google Scholar]
- 26.Li CL, Johnson GR. Murine hematopoietic stem and progenitor cells. I. Enrichment and biologic characterization. Blood. 1995;85:1472–9. [PubMed] [Google Scholar]
- 27.Randall TD, Weissman IL. Characterization of a population of cells in the bone marrow that phenotypically mimics hematopoietic stem cells: resting stem cells or mystery population? Stem Cells. 1998;16:38–48. doi: 10.1002/stem.160038. [DOI] [PubMed] [Google Scholar]
- 28.Miller AM, Weiner RS, Ziboh VA. Evidence for the role of leukotrienes C4 and D4 as essential intermediates in CSF-stimulated human myeloid colony formation. Exp Hematol. 1986;14:760–5. [PubMed] [Google Scholar]
- 29.Stenke L, Mansour M, Reizenstein P, Lindgren JA. Stimulation of human myelopoiesis by leukotrienes B4 and C4: interactions with granulocyte–macrophage colony-stimulating factor. Blood. 1993;81:352–6. [PubMed] [Google Scholar]
- 30.Tornhamre S, Stenke L, Granzelius A, Sjolinder M, Nasman-Glaser B, Roos C, Widell S, Lindgren JA. Inverse relationship between myeloid maturation and leukotriene C4 synthase expression in normal and leukemic myelopoiesis-consistent overexpression of the enzyme in myeloid cells from patients with chronic myeloid leukemia. Exp Hematol. 2003;31:122–30. doi: 10.1016/s0301-472x(02)01026-3. [DOI] [PubMed] [Google Scholar]
- 31.Snyder DS, Castro R, Desforges JF. Antiproliferative effects of lipoxygenase inhibitors on malignant human hematopoietic cell lines. Exp Hematol. 1989;17:6–9. [PubMed] [Google Scholar]
- 32.Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994;269:27807–10. [PubMed] [Google Scholar]
- 33.Lorico A, Rappa G, Finch RA, Yang D, Flavell RA, Sartorelli AC. Disruption of the murine MRP (multidrug resistance protein) gene leads to increased sensitivity to etoposide (VP-16) and increased levels of glutathione. Cancer Res. 1997;57:5238–42. [PubMed] [Google Scholar]
- 34.Abbaszadegan MR, Futscher BW, Klimecki WT, List A, Dalton WS. Analysis of multidrug resistance-associated protein (MRP) messenger RNA in normal and malignant hematopoietic cells. Cancer Res. 1994;54:4676–9. [PubMed] [Google Scholar]
- 35.Loe DW, Almquist KC, Cole SP, Deeley RG. ATP-dependent 17 beta-estradiol 17-(beta-d-glucuronide) transport by multidrug resistance protein (MRP). Inhibition by cholestatic steroids. J Biol Chem. 1996;271:9683–9. doi: 10.1074/jbc.271.16.9683. [DOI] [PubMed] [Google Scholar]